Abstract
Neuropsychiatric disorders share susceptibility genes, suggesting a common origin. One such gene is CNTNAP2 encoding contactin-associated protein 2 (CASPR2), which harbours mutations associated to autism, schizophrenia, and intellectual disability. Antibodies targeting CASPR2 have also been recently described in patients with several neurological disorders, such as neuromyotonia, Morvan’s syndrome, and limbic encephalitis. Despite the clear implication of CNTNAP2 and CASPR2 in neuropsychiatric disorders, the pathogenic mechanisms associated with alterations in CASPR2 function are unknown. Here, we show that Caspr2 is expressed in excitatory synapses in the cortex, and that silencing its expression in vitro or in vivo decreases the synaptic expression of α‐amino‐3‐hydroxy‐5‐methylisoxazole‐4‐propionic acid (AMPA) receptors and the amplitude of AMPA receptor-mediated currents. Furthermore, Caspr2 loss of function blocks synaptic scaling in vitro and experience-dependent homoeostatic synaptic plasticity in the visual cortex. Patient CASPR2 antibodies decrease the dendritic levels of Caspr2 and synaptic AMPA receptor trafficking, and perturb excitatory transmission in the visual cortex. These results suggest that mutations in CNTNAP2 may contribute to alterations in AMPA receptor function and homoeostatic plasticity, and indicate that antibodies from anti-CASPR2 encephalitis patients affect cortical excitatory transmission.
Keywords: AMPA receptors, Autoantibodies, CNTNAP2/CASPR2, experience-dependent plasticity, homoeostatic synaptic scaling, neuropsychiatric disorders
Introduction
Psychiatric disorders are often associated with manifestations of cognitive dysfunction, abnormal thoughts, and behavioural deficits. Despite a complex aetiology, these disorders present common defective features in brain structure and wiring and share susceptibility risk genes, suggesting a similar neurodevelopmental origin. One such gene is CNTNAP2, in which several disease-associated mutations have been identified (Verkerk et al. 2003; Strauss et al. 2006; Belloso et al. 2007; Alarcon et al. 2008; Arking et al. 2008; Bakkaloglu et al. 2008; Friedman et al. 2008; Vernes et al. 2008; Zweier et al. 2009; O’Dushlaine et al. 2011; Smogavec et al. 2016). Patients carrying CNTNAP2 mutations display seizures, language impairments, intellectual disability, and varying autistic-core behaviours (Penagarikano and Geschwind 2012; Rodenas-Cuadrado et al. 2014; Poot 2017). Cntnap2 knockout (KO) mice develop epileptic and autism-related behavioural phenotypes that parallel patients’ symptoms (Penagarikano et al. 2011), thus emphasising the relevance of the CNTNAP2 gene in brain function.
The CNTNAP2-encoded protein contactin-associated protein 2 (CASPR2) has been recently identified as an antigen in autoimmune synaptic encephalitis (Irani et al. 2010; Lancaster et al. 2011). Serum and cerebrospinal fluid autoantibodies targeting CASPR2 (CASPR2-Abs) have been associated with diverse neurological syndromes including neuromyotonia, Morvan’s syndrome, and limbic encephalitis (Bastiaansen et al. 2017; van Sonderen et al. 2017). These syndromes frequently overlap, presenting with peripheral disturbances but also central nervous system dysfunctions such as sleep alterations, seizures, memory impairment, cognitive deficits, and psychosis (Irani et al. 2010; Lancaster et al. 2011; Somers et al. 2011; Klein et al. 2013; Sunwoo et al. 2015; Joubert et al. 2016; van Sonderen et al. 2016; Bien et al. 2017). Circulating CASPR2-Abs were also detected during pregnancy in mothers of children with autism spectrum disorder (Brimberg et al. 2016) or intellectual disability (Coutinho, Jacobson et al. 2017), hinting that gestational transfer of maternal CASPR2-Abs to the foetus contributes to the development of neuropsychiatric disorders in the progeny, and recent studies reveal that offspring mice exposed in utero to CASPR2-Abs develop neuropsychiatric phenotypes (Brimberg et al. 2016; Coutinho, Menassa et al. 2017).
CASPR2 is a neurexin-related cell-adhesion molecule expressed in axons, where it acts as a membrane scaffold for the clustering of Shaker-like K+ channels (VGKC) at the juxtaparanodal region of myelinated axons (Poliak et al. 1999; Scott et al. 2017). However, roles for CASPR2 in regulating the excitation/inhibition balance and neuronal migration have been suggested (Penagarikano et al. 2011). More recently, a synaptic function for Caspr2 has been proposed, since abnormal spine development and a reduced number of excitatory and inhibitory synapses (Anderson et al. 2012; Gdalyahu et al. 2015), as well as accumulation of cytoplasmic aggregates of α‐amino‐3‐hydroxy‐5‐methylisoxazole‐4‐propionic acid (AMPA)-type glutamate receptors (AMPARs) (Varea et al. 2015) were observed upon loss of Caspr2.
Despite the relevant roles of the CNTNAP2 gene and its encoded protein CASPR2 for human brain function and their implication in disease mechanisms, the cellular and molecular mechanisms mediated by CASPR2 remain unclear. In the present study, we address the hypothesis that Caspr2 regulates glutamatergic function by testing whether Caspr2 loss of function affects activity-dependent changes in the trafficking of AMPARs, crucial for the expression of synaptic plasticity mechanisms, and by evaluating a potential pathogenic effect of patients CASPR2-Abs in glutamatergic transmission.
Materials and Methods
Animals
For electrophysiological studies, C57BL/6J mice (Jackson Laboratories) were raised under a normal 12-h light/dark cycle until the beginning of experiments. For primary cultures of cortical neurons, pregnant Wistar rats were used. For primary cultures of hippocampal neurons, either Wistar pups at postnatal Day 0 (P0) or pregnant Cntnap2 KO mice [B6.129(Cg)Cntnap2tm1Pele/J; Jackson Laboratory] were used. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee at Johns Hopkins University, Baltimore, USA, or by the Portuguese National Authority for Animal Health (DGAV).
Human Samples and IgG Purification
Plasma exchange samples of a 72-year-old male patient with anti-CASPR2 encephalitis were used in this study. The patient presented with Morvan’s syndrome secondary to high levels of CASPR2 autoantibodies, with progressive neuropathic pain, lack of perception with goosebumps, and memory complaints, with no indication of paraneoplastic causes. Serum from a healthy age- and sex-matched subject was kindly donated. Written informed consent was obtained from both patient and healthy control. Plasma antibody titre was determined through a live cell-based assay as previously reported (Coutinho, Menassa et al. 2017). End-point titration at which a positive signal was still observed for patient antibodies was 1:6400. Human immunoglobulins (IgGs) were purified from healthy or patient plasma samples as previously described (Coutinho, Menassa et al. 2017), and their concentration determined: healthy pIgG—21.2 mg/mL; patient pIgG—15.1 mg/mL. Details can be found in Supplementary Material.
Primary Neuronal Cultures, Neuronal Transfection and In vitro Paradigms
Primary cultures of cortical and hippocampal neurons were prepared as previously described in Santos et al. (2012); Coutinho, Menassa et al. (2017), and kept in culture until DIV 13–18. DNA plasmids (Anderson et al. 2012) for neuronal transfection to deplete Caspr2 expression were a kind gift from Dr Thomas Südhof (Stanford University, USA), and were recombinantly expressed in DIV 7 cortical cultures using a previously described protocol (Santos et al. 2012). To induce synaptic scaling, neurons were treated with 1 μM Tetrodotoxin (TTX) or TTX and 100 μM d-5-aminophosphonovalerate (APV) for 48 h at DIV 11. To study CASPR2-Abs, hippocampal or cortical neurons were incubated either with diluted patient serum at 1:100 or with serum-purified human IgGs for 1–7 h. All biochemical and immunocytochemical assays were performed as previously described in Santos et al. (2012); Mele et al. (2014); Coutinho, Menassa et al. (2017). Details can be found in Supplementary Material.
Stereotactic Surgeries
Stereotactic surgeries were performed as previously described (Petrus et al. 2015). Briefly, C57BL/6J mice were injected onto the primary visual cortex (V1) with either recombinant lentivirus or 200 ng of purified human IgGs in a stereotactic apparatus. To specifically target V1-layer 2/3, calculated stereotactic coordinates were: bregma −3.6 mm, lateral 2.5 mm, depth 0.36 mm. Mice injected with human IgGs were recovered for 7 h before sacrifice. Lentiviral-infected mice were returned to the animal colony for 4–5 weeks post-infection before experimental paradigms were initiated. Details can be found in Supplementary Material.
Visual Deprivation Paradigms
Visual deprivation was induced in the form of dark exposure (DE), as described previously (Goel and Lee 2007). Briefly, mice were kept in a dark room for 2 days, and cared for using infra-red vision goggles under dim infra-red light. Age-matched control (normally reared [NR]) animals were continuously raised in normal light conditions for the same duration. Details can be found in Supplementary Material.
Electrophysiology
Acute primary visual cortical slices and electrophysiological methods have been previously described (Goel et al. 2006). AMPA receptor-mediated miniature excitatory postsynaptic currents (mEPSCs) were pharmacologically isolated with 1 μM TTX, 20 μM bicuculline and 100 μM APV and measured in whole-cell patch-clamp configuration. Cells were held at −80 mV, digitised at 10 kHz with a data acquisition board and acquired through custom software. At least, 200 consecutive mEPSCs for each cell were analysed with a detection threshold set at 3 times the root mean square noise level. Details can be found in Supplementary Material.
Data Analysis and Statistics
All results are presented as normalised mean ± SEM. Biochemical and immunocytochemistry data are presented from at least 3 different experiments, performed in independent preparations. Mann–Whitney test was used for 2-group comparisons. Comparisons across multiple groups were performed with the Kruskal–Wallis analysis of variance followed by Dunn’s Multiple Comparison test, or with a 2-way ANOVA analysis followed by Tukey’s multiple comparison test. Significance of cumulative distributions was calculated with the Kolmogorov–Smirnov test. For all tests, P < 0.05 was considered statistically significant.
Results
Caspr2 Regulates the Trafficking and Synaptic Content of AMPARs
We immunolabelled cultured rat cortical neurons for Caspr2, and found the Caspr2 signal to be significantly distributed along both axons and dendrites (Fig. 1A). Given the high expression of Caspr2 in axons, to evaluate the dendritic localisation of Caspr2 without interference from axonal staining, we sparsely transfected cortical neurons with a construct encoding HA-tagged human CASPR2 and observed expression of HA-CASPR2 in axons and MAP2-labelled dendrites (Supplementary Fig. S1A). We then co-stained cultured neurons for Caspr2, for the postsynaptic scaffolding protein PSD95 and for the presynaptic vesicular glutamate transporter vGluT1 (Fig. 1A); the signal overlap of Caspr2 with synaptic clusters positive for both PSD95 and vGluT1 was determined (Fig. 1A, B). We found that 45.5 ± 3.6% of all excitatory synapse clusters contained Caspr2 (Fig. 1B). Furthermore, Caspr2 is expressed in PSD95-enriched postsynaptic densities (PSD) isolated from the total mouse brain (Fig. 1C), or from mouse cortex (Fig. 1D). In agreement with previous studies showing altered inhibitory function in the cortex of Cntnap2−/− mice (Bridi et al. 2017), we found that Caspr2 is also expressed in 61.2 ± 3.2% of inhibitory synapses, detected by the overlap of clusters positive for both gephyrin and vGAT (Supplementary Fig. S1B, C).
Figure 1.
Caspr2 is required to regulate synaptic clusters of surface AMPARs. (A) Immunolabelling of Caspr2, PSD95, and vGlut1 in cortical neurons. Scale bars = 5 μm. (B) Number of glutamatergic synapses (PSD95+/vGlut1+) per 10 μm dendritic length that contain Caspr2 (PSD95+/vGlut1+/Caspr2+). [Independent experiments (N) ≥ 3, number of cells per condition (n) ≥ 30 cells]. (C, D) Representative blots of subcellular fractionations of mouse whole brain (C) and cortical (D) lysates (Bl), plasma membrane (Pm) fractions, and purified PSD95-enriched postsynaptic densities (PSD). (E) Immunolabelling of surface GluA1 and PSD95 in mCherry-expressing cortical neurons transfected with a control empty-vector or the Caspr2-shRNA, or co-transfected with the Caspr2-shRNA and a shRNA-resistant Caspr2 mutant. Scale bars = 5 μm. (F) Fluorescence intensity of total and PSD95-co-localised synaptic clusters of surface GluA1 was analysed. (N ≥ 3, n ≥ 30 cells); in (F) Kruskal–Wallis test, Dunn’s post hoc test, *P < 0.05, **P < 0.01 compared with control, and #P < 0.05, ##P < 0.01 relative to Caspr2-shRNA. In (B) and (F), results are presented as mean ± SEM.
Based on the localisation of Caspr2 at excitatory synapses, and on the role of Caspr1, a homologous family member of Caspr2, in regulating AMPAR traffic in hippocampal neurons (Santos et al. 2012), we hypothesised that Caspr2 may regulate excitatory synaptic function. To investigate if Caspr2 affects AMPAR trafficking, we transfected cortical neurons with a specific shRNA sequence previously validated to knockdown (KD) Caspr2 expression (Anderson et al. 2012), and evaluated the cell surface and synaptic distribution of GluA1-containing AMPARs (Fig. 1E). Quantitative immunofluorescence analysis of mCherry-expressing transfected neurons revealed that shRNA-mediated loss of Caspr2 resulted in decreased intensity of total and synaptic cell surface GluA1 clusters (Fig. 1F). To exclude the contribution of off-target effects of the Caspr2-shRNA, neurons were co-transfected with the Caspr2-shRNA and a shRNA-resistant mutant construct of Caspr2 (Anderson et al. 2012). Expression of this mutant rescued the KD-mediated decrease in total and synaptic GluA1 cluster intensity, indicating that the observed defects were specifically caused by the loss of Caspr2 (Fig. 1E, F). Caspr2 depletion did not affect PSD95 dendritic clustering (Supplementary Fig. S2A, C) nor the intensity of GluA2 clusters (Supplementary Fig. S2A, B). These observations indicate that Caspr2 regulates the trafficking of GluA1-AMPARs to the cell surface and into synapses.
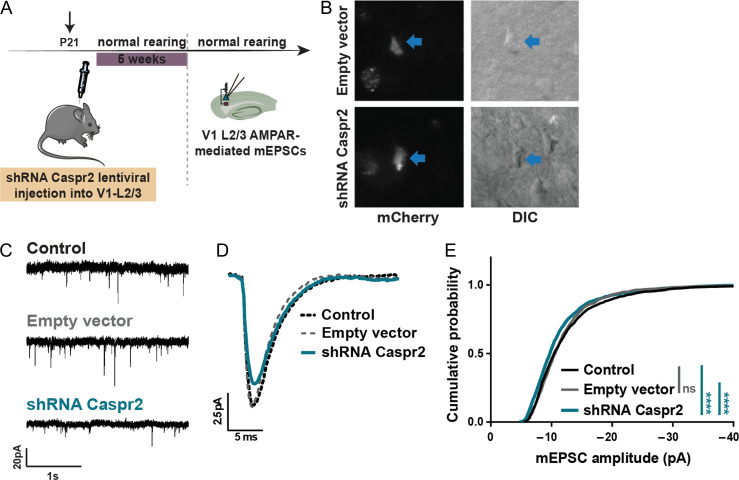
Caspr2 is Required for In vivo Regulation of AMPAR Function
To understand if Caspr2 is relevant for AMPAR function in vivo, we injected lentivirus encoding the Caspr2-shRNA and co-expressing mCherry into layer 2/3 of the mouse primary visual cortex (V1) (Fig. 2A). Infected pyramidal neurons in V1-layer 2/3 of injected animals at P60 were identified by the pyramid-shaped soma with the apical dendrite pointing to the pia, and expression of mCherry fluorescence (Fig. 2B). We measured AMPAR-mediated mEPSCs (Fig. 2C). There was a marked decrease in the amplitude of average trace of mEPSCs recorded from Caspr2 shRNA-infected animals (Fig. 2D—blue trace), and the cumulative distribution of Caspr2 shRNA-mEPSC amplitudes was significantly shifted towards smaller values (Fig. 2E—blue trace), with no changes in the current frequency (Supplementary Table S1). These observations indicate that in vivo loss of Caspr2 impairs basal AMPAR-mediated synaptic transmission in the mouse visual cortex.
Figure 2.
Caspr2 regulates AMPAR function in vivo in the mouse primary visual cortex. (A) Caspr2-shRNA or empty vector-encoding lentivirus were injected into the mouse V1-L2/3, and AMPAR-mediated mEPSCs were recorded from V1-L2/3 pyramidal neurons. (B) Representative image of mCherry-expressing V1-L2/3 pyramidal neurons from lentiviral-infected mice. (C, D) Comparison of representative mEPSC traces (C) and average mEPSC traces (D) recorded from either control mice (black, n = 13 cells), empty vector-infected (grey, n = 9 cells) or shRNA Caspr2-infected mice (blue, n = 12 cells). (E) Cumulative distribution of mEPSC amplitudes of empty vector-(grey) and shRNA Caspr2-infected mice (blue) plotted against control littermates (Kolmogorov–Smirnov test, ****P < 0.0001; ns—non-significant). No changes in the mEPSC frequency or kinetics were observed. See also Supplementary Table S1.
CASPR2 Autoantibodies Impair AMPAR Function
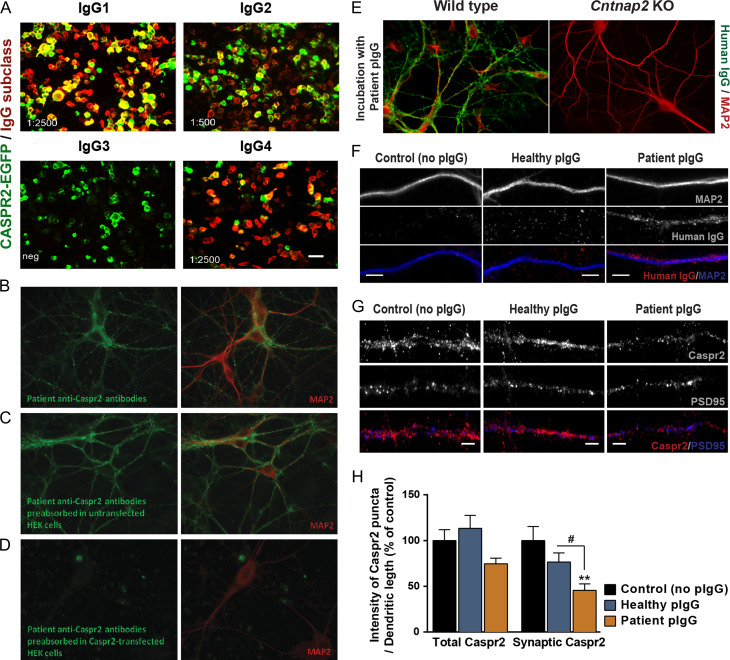
Autoantibodies targeting CASPR2 have been recently described in encephalitis patients with severe cognitive defects and psychiatric symptoms, but their pathogenesis is not fully characterised. To explore the mechanisms underlying the pathogenesis of CASPR2 autoantibodies, we used human IgGs preparations from a patient with CASPR2-Ab autoimmune encephalitis, and tested their specificity of binding to CASPR2 (Coutinho, Menassa et al. 2017). Human IgGs in the plasma of the patient have high titre of both CASPR2-IgG1 and -IgG4 subtypes (Fig. 3A), and bind strongly to hippocampal neurons, which did not occur when plasma was previously pre-absorbed in human CASPR2-transfected HEK293 cells (Fig. 3B–D). Moreover, purified IgGs (pIgGs) from the patient plasma failed to bind to hippocampal neurons isolated from Cntnap2 KO mice (Fig. 3E), indicating specific detection of Caspr2. We incubated cortical neurons for 7 h with pIgGs, which specifically bind to dendritic compartments (Fig. 3F), and evaluated their effect on the dendritic and synaptic distribution of Caspr2 (Fig. 3G). Quantitative analysis revealed that incubation with IgGs purified from the patient plasma (patient pIgG; 200 ng/mL) decreased the intensity of total and synaptic dendritic clusters of Caspr2, as determined using an antibody against the intracellular C-terminus of the protein, when compared with control cells or neurons incubated with IgGs purified from a healthy control (healthy pIgGs; Fig. 3H). We further tested whether CASPR2-Abs can be internalised over time. Cultured cortical neurons were incubated during 1 or 7 h with either healthy or patient pIgGs, fixed and then immunolabelled with an excess concentration of an anti-human fluorophore-tagged secondary antibody to stain human IgGs bound to the cell surface. After permeabilization, cells were immunolabelled with a second anti-human secondary antibody, tagged with a different fluorophore, to stain potentially internalised human IgGs, and further stained against MAP2 to visualize dendritic processes. This analysis showed significant intracellular presence of anti-human antibodies in neurons incubated with the patient CASPR2-Abs, supporting the possibility that CASPR2-Abs and associated Caspr2 are internalised (Supplementary Fig. S3).
Figure 3.
CASPR2 autoantibodies, predominantly of the IgG1 and IgG4 subclasses, fail to bind to Cntnap2 KO hippocampal neurons and significantly alter the expression of Caspr2 in cortical dendrites. (A) Immunolabeling of human CASPR2-EGFP-transfected HEK293 cells (green) incubated for 1 h with the plasma of a patient with anti-CASPR2 encephalitis. Human immunoglobulins (IgGs; red) are immunolabelled with anti-human IgG antibodies specific for each IgG subclass. End-point titration for each subclass is given in the lower left corner of each panel. (B) Immunolabelling of human IgGs (green) and the neuronal marker MAP2 (red) in hippocampal neurons incubated for 1 h with the patient plasma. (C, D) To confirm the specificity of patient IgGs for CASPR2, and test for the presence of autoantibodies against other neuronal protein targets, patient plasma was pre-absorbed in (C) untransfected HEK293 cells, or (D) HEK293 cells expressing human CASPR2, and incubated with hippocampal neurons. Plasma pre-absorbed in CASPR2-expressing cells fails to stain hippocampal neurons. (E) Immunolabelling of human IgGs (green) and the neuronal marker MAP2 (red) in either wild-type or Cntnap2 KO hippocampal neurons incubated for 1 h with IgGs purified from the patient plasma (patient pIgGs). Patient pIgGs bind strongly to the surface of WT, but not of Cntnap2 KO hippocampal neurons. (F) Immunolabelling of human IgGs (red) and the dendritic neuronal marker MAP2 (blue) in cortical neurons incubated for 7 h with IgGs purified from either the patient plasma (patient pIgGs) or from healthy control (healthy pIgGs). Scale bars = 5 μm. (G) Immunolabelling of Caspr2 clusters in cortical neurons incubated for 7 h with healthy or patient pIgGs. Scale bars = 5 μm. (H) Fluorescence intensity of total and PSD95-co-localised synaptic clusters of Caspr2 was quantified. N = 3, n ≥ 30 cells; Kruskal–Wallis test, Dunn’s post hoc test, **P < 0.01 compared with control, #P < 0.05 relative to healthy pIgG. Results are presented as mean ± SEM.
We then evaluated whether CASPR2-Abs might impact the distribution of cell surface GluA1-AMPARs (Fig. 4A). In fact, incubation of cortical neurons with patient pIgGs significantly decreased the intensity of surface GluA1 total and synaptic clusters (Fig. 4B), without affecting the intensity of PSD95 dendritic clusters (Fig. 4C). This result suggests that CASPR2 autoantibodies may perturb the role of Caspr2 in the regulation of AMPAR synaptic traffic, possibly through changes in the molecular mechanisms underlying receptor endocytosis and/or recycling. To further explore this, we started by evaluating the effect of CASPR2-Abs on AMPAR internalisation. Since internalisation is expected to be fast following antibody exposure, we first tested whether 1 h incubation with patient CASPR2-Abs is enough to lead to decreased GluA1 surface levels. We found that in fact after 1 h incubation with patient pIgGs surface GluA1 levels are decreased (Supplementary Fig. S4A, B) to a similar extent as with the 7 h incubation time-point (Fig. 4A, B). We also tested total GluA1 levels, and found them to be unchanged in neurons incubated for 1 h with patient pIgGs when compared with both control or healthy pIgG-incubated cells (Supplementary Fig. S4C, D), further arguing in favour of increased AMPAR endocytic rates sustaining the decrease in surface AMPAR levels upon CASPR2-Ab incubation. To test this, we performed antibody-feeding experiments, in which neurons were first incubated for 10 min at RT with an antibody against an extracellular epitope in AMPAR subunits, followed by 1 h incubation with either healthy or patient pIgGs at 37 °C to induce internalisation of antibody-bound AMPARs. We analysed the surface and internalised signal of AMPAR clusters (Fig. 4D), and calculated the receptor internalisation ratio (internalised/total GluA, Fig. 4E). We observed that AMPARs are significantly internalised following 1 h incubation at 37 °C, and found that, in neurons incubated for 1 h with patient pIgGs, the internalisation ratio of AMPARs is significantly higher than that of either control (no pIgG) or healthy pIgG-incubated neurons (Fig. 4E). These findings indicate that CASPR2-Abs decrease the synaptic content of surface AMPAR clusters by promoting AMPAR internalisation from the cell surface.
Figure 4.
CASPR2 autoantibodies decrease the synaptic expression of surface AMPAR clusters by increasing their internalisation from the cell surface. (A) Immunolabelling of surface GluA1, Caspr2, and PSD95 clusters in cortical neurons incubated with healthy or patient pIgGs. Scale bars = 5 μm. (Caspr2 and PSD95 representative image panels are identical to Fig. 3G). (B, C) Fluorescence intensity of (B) total and PSD95-co-localised synaptic clusters of surface GluA1, and (C) PSD95 clusters was quantified. (N = 3, n ≥ 30 cells; in (B), Kruskal–Wallis test, Dunn’s post hoc test, *P < 0.05, **P < 0.01 compared with control, #P < 0.05, ##P < 0.01 relative to healthy pIgGs). (D) Immunolabelling of surface and internalised AMPAR clusters following a 10-min incubation of live neurons with an antibody against an extracellular epitope of GluA subunits (“feeding”). Neurons were then incubated with 200 ng/mL of either healthy or patient pIgGs for up to 1 h at 37 °C to promote internalisation of antibody-bound AMPARs. Scale bars = 5 μm. (E) Fluorescence intensity of surface and internalised clusters of AMPARs was analysed and the receptor internalisation ratio (internalised/total GluA) was calculated. N = 2, n ≥ 40 cells; Kruskal–Wallis test, Dunn’s post hoc test: ***P < 0.001, ****P < 0.0001 when comparing incubated cells after 1 h of internalisation to the control (0 min) condition; #P < 0.05, ##P < 0.01 when multiple comparisons were done between conditions after 1 h of internalisation. In (B), (C), and (E), results are presented as mean ± SEM.
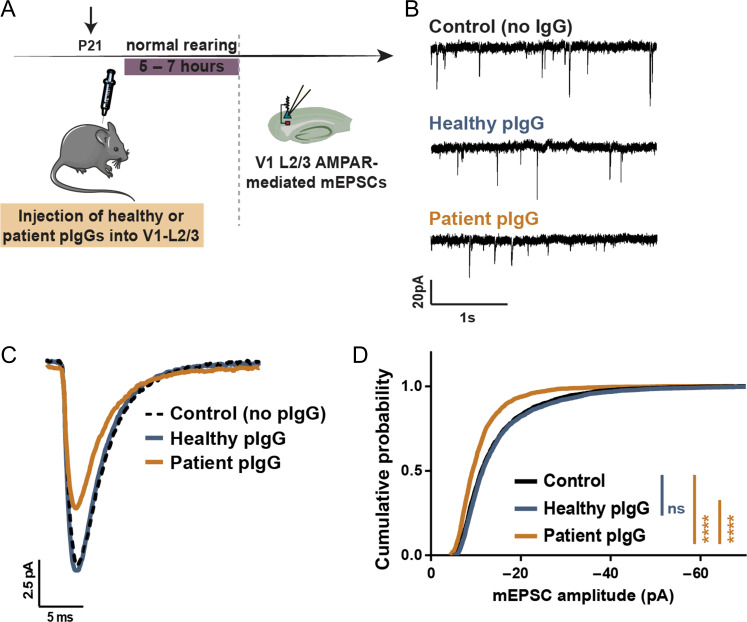
To investigate in vivo if the disruptive effect of CASPR2-Abs in the regulation of AMPAR trafficking is relevant for AMPAR function, we injected patient or healthy IgGs (200 ng) into mouse V1-layer 2/3 (Fig. 5A) and measured AMPAR-mediated mEPSCs in V1-layer 2/3 pyramidal neurons (Fig. 5B). The average mEPSC trace of mice injected with patient pIgGs was strikingly smaller than control or healthy pIgG traces (Fig. 5C—yellow trace), and the cumulative distribution of patient pIgG-mEPSC amplitudes was significantly shifted towards smaller values (Fig. 5D; patient pIgG: yellow). No changes were detected in mEPSC frequency or current kinetics (Supplementary Table S2). These observations reveal that CASPR2-Abs impair the localisation of Caspr2 to excitatory synapses and perturb AMPAR-mediated synaptic transmission in vivo.
Figure 5.
CASPR2 autoantibodies perturb AMPAR function in vivo in the mouse primary visual cortex. (A) Healthy or patient pIgGs were injected into L2/3 of the mouse V1, and AMPAR-mediated mEPSCs recorded from V1-L2/3 pyramidal neurons. (B, C) Representative mEPSC traces (B), and average mEPSC traces (C) recorded from control animals (n = 7 cells—black), or from mice injected with either healthy (n = 10 cells—blue), or patient pIgGs (n = 9 cells—yellow). (D) Cumulative distribution of mEPSC amplitudes of mice injected with healthy (blue) or patient pIgGs (yellow) plotted against control mice (black). (Kolmogorov–Smirnov test, ****P < 0.0001, ns—non-significant). No changes in mEPSC frequency or kinetics were observed. See also Supplementary Table S2.
Caspr2 is Essential for Homoeostatic Synaptic Scaling
Activity-dependent regulation of AMPAR traffic is fundamental for several modalities of synaptic plasticity (Shepherd and Huganir 2007). Synaptic scaling is a form of homoeostatic plasticity that adjusts the overall synaptic strength of a neuron through compensatory changes in the synaptic accumulation of AMPARs, to maintain neuronal networks in-balance when perturbations in activity occur (Turrigiano 2008; Fernandes and Carvalho 2016). Failure in neuronal synaptic homoeostasis is considered an underlying pathology in several cognitive disorders (Ramocki and Zoghbi 2008; Wondolowski and Dickman 2013; Mullins et al. 2016). We asked whether the role of Caspr2 in the regulation of AMPARs impinges on mechanisms of neuronal homoeostasis. To evaluate if Caspr2 expression is itself regulated by neuronal activity, we treated cortical neurons for 48 h with either the voltage-gated Na+-channel blocker TTX (1 μM) to inhibit action potential firing, or TTX together with APV (100 μM) for additional blockade of NMDAR function. As previously described (Ju et al. 2004; Wierenga et al. 2005), we observed that TTX or TTX + APV treatment resulted in a homoeostatic upregulation of the GluA1 subunit of AMPARs, which was paralleled by an increase in total Caspr2 (Fig. 6A, B). A significant increase in the intensity of total and synaptic GluA1 clusters following blockade of neuronal activity with TTX + APV was detected (Fig. 6C–E). Importantly, when transformed by a scaling factor, the scaled TTX + APV distribution completely superimposed with the control cumulative distribution, indicating a multiplicative effect (a defining characteristic of synaptic scaling). Furthermore, we observed that TTX + APV treatment significantly increased the fluorescence intensity of Caspr2 clusters (Fig. 6F, G), and the intensity and number of cell surface GluA1 clusters co-localised with Caspr2 (Fig. 6H).
Figure 6.
Caspr2 is regulated by neuronal activity, and its loss prevents synaptic upscaling of GluA1-containing AMPARs. (A) Representative blot of GluA1 and Caspr2 levels from cortical neurons treated for 48 h with TTX or TTX and APV. (B) Total protein levels of GluA1 and Caspr2 were determined. (N = 4; Kruskal–Wallis test, Dunn’s post hoc test, *P < 0.05 compared with control). (C) Immunolabelling of surface GluA1, PSD95, and vGlut1 clusters in cortical neurons treated for 48 h with TTX + APV. Scale bars = 5 μm. (D) Fluorescence intensity of total and synaptic (co-localised with PSD95 and vGlut1) clusters of surface GluA1 was quantified. (N ≥ 3, n ≥ 30 cells; Mann–Whitney test, *P < 0.05, ****P < 0.0001 compared with control). (E) Cumulative distribution of GluA1 cluster intensities of TTX + APV-treated neurons (blue) plotted against control values (black). The original TTX + APV distribution was transformed by the best-fit equation and plotted (blue dashed line). (Kolmogorov–Smirnov test, ****P < 0.0001; ns—non-significant). (F) Immunolabelling of Caspr2, surface GluA1, and PSD95 clusters in cortical neurons treated for 48 h with TTX + APV. Scale bars = 5 μm. (G, H) Fluorescence intensity of total and synaptic clusters of Caspr2 (G), and the intensity and number of surface GluA1 clusters that co-localise with Caspr2 (H) were quantified. (N ≥ 3, n ≥ 30 cells; Mann–Whitney test, ***P < 0.001, ****P < 0.0001 compared with control). (I) Immunolabelling of surface GluA1 and PSD95 in cortical neurons transfected with a control empty vector or the Caspr2-shRNA, or co-transfected with the Caspr2-shRNA and a shRNA-resistant Caspr2 mutant, and treated with TTX + APV for 48 h. Scale bars = 5 μm. (Representative image panels of transfection genotypes in basal conditions of activity are identical to Fig. 1E). (J, K) Fluorescence intensity of total (J) and synaptic (K) clusters of surface GluA1 was analysed. (N ≥ 3, n ≥ 30 cells; 2-way ANOVA test, Tukey’s post hoc test, ****P < 0.0001 compared with control, ns relative to control Caspr2-shRNA, ####P < 0.0001 compared with TTX + APV Caspr2-shRNA, $P < 0.05, $$P < 0.01 relative to control Caspr2-shRNA+rescue). In (B), (D), (G), (H), (J), and (K), results are presented as mean ± SEM.
We then investigated if Caspr2 is required for the expression of synaptic scaling. In fact, neurons expressing the Caspr2-shRNA failed to trigger the homoeostatic scaling of GluA1 (Fig. 6I–K), which was fully restored by the expression of the shRNA-resistant form of Caspr2 (Fig. 6I–K). Overall, these findings indicate that Caspr2 is regulated by neuronal activity and necessary for the synaptic scaling of GluA1-AMPARs.
Caspr2 is Required for Experience-Dependent Homoeostatic Plasticity
We asked whether Caspr2 function in vivo impinges on mechanisms of experience-dependent homoeostatic plasticity that are fundamental for sensory processing (Whitt et al. 2014). Such mechanisms have been characterised in the mouse visual cortex through manipulations of visual experience: 2 days of visual deprivation (in the form of dark exposure – DE, Fig. 7A) causes a homoeostatic increase of AMPAR-mediated mEPSCs in V1-layer 2/3 pyramidal neurons (Goel et al. 2011) that is accompanied by a specific upregulation in protein levels of the GluA1 subunit of AMPARs (Goel et al. 2011) (Fig. 7B, C), suggesting that a prolonged absence of visually-driven activity scales up excitatory synapses in V1-layer 2/3. We evaluated if Caspr2 expression can be regulated by visual experience in vivo, and observed that dark exposing (DE) P60 mice for 2 days significantly increased total protein levels of Caspr2 in the V1 when compared with normal reared (NR) mice (Fig. 7B, D), whereas it did not change Caspr2 expression in P21-30 mice (Supplementary Fig. S5), indicating that this effect is developmentally regulated.
Figure 7.
Paradigms of visual deprivation significantly increase Caspr2 levels in the mouse V1, and loss of Caspr2 in V1 prevents visual experience-dependent homoeostatic upscaling of AMPAR-mediated mEPSCs. (A) Normal reared P60 mice were subjected to 2 days of visual deprivation. Whole V1 lysates were prepared for WB analysis. (B) Representative blots of GluA1 and Caspr2 levels in V1 lysates of NR or DE mice. (C, D) Total protein levels of GluA1 (C) and Caspr2 (D) were determined. (GluA1: N ≥ 5; Caspr2: N ≥ 7; Mann–Whitney test, **P < 0.01 relative to NR). (E) shRNA Caspr2 or empty vector-encoding lentivirus were injected into V1-L2/3 of normal reared mice. Mice were then dark exposed for 2 days, and AMPAR-mediated mEPSCs from V1-L2/3 pyramidal neurons recorded. (F) Representative mEPSC traces of non-infected controls, or empty vector- or shRNA Caspr2-infected mice that were either NR (left) or subjected to visual deprivation (DE—right). (Representative mEPSC traces of the different genotypes in normal reared conditions are identical to Fig. 2C). (G, H) Average mEPSC traces (G), and cumulative distribution of mEPSC amplitudes (H) recorded from NR (black) or DE (green) control mice (NR, n = 13 cells; DE, n = 8 cells). (I, J) Average mEPSC traces (I), and cumulative distribution of mEPSC amplitudes (J) recorded from NR (black) or DE (pink) empty vector-infected mice (NR, n = 9 cells; DE, n = 12 cells). (K, L) Average mEPSC traces (K), and cumulative distribution of mEPSC amplitudes (L) recorded from NR (black) or DE (blue) shRNA Caspr2-infected mice (NR, n = 12 cells; DE, n = 9 cells). In (H), (J), and (L), Kolmogorov–Smirnov test, ****P < 0.0001.
To investigate whether Caspr2 is required for visually-driven experience-dependent homoeostatic plasticity in P60 animals, mice infected for expression of the Caspr2-shRNA were dark exposed for 2 days (Fig. 7E) and AMPAR-mediated mEPSCs of V1-layer 2/3 pyramidal neurons were recorded (Fig. 7F). Both in non-infected control or empty vector-infected mice, DE increased the amplitude of average mEPSC traces and significantly shifted the cumulative distribution of mEPSC amplitudes to larger values when compared with NR (Fig. 7G, H—control; Fig. 7I, J—empty vector). Average frequency and kinetics of mEPSCs remained unaltered following DE of both control and empty vector-infected mice (Supplementary Table S1). Importantly, dark exposure of Caspr2 shRNA-mice failed to upscale the average mEPSC trace (Fig. 7K), and the cumulative distribution of DE amplitudes instead showed a small but statistically significant shift towards smaller values than that of NR Caspr2-shRNA (Fig. 7L), suggesting a downscaling effect. Overall, these observations indicate that levels of Caspr2 in the V1 are regulated by visual experience, and that loss of Caspr2 hinders the scaling up of AMPAR function induced by visual deprivation, suggesting that Caspr2 is essential for visually-driven experience-dependent homoeostatic plasticity.
Discussion
We report that Caspr2 is present in approximately half of all excitatory synapses in rat cortical neurons, and enriched in PSD fractions from the mouse brain. Silencing of Caspr2 expression decreases the cell surface expression of GluA1-AMPARs in vitro at cortical synapses, and the amplitude of AMPAR-mediated mEPSCs in vivo in the mouse visual cortex. These findings are in agreement with reports indicating that Caspr2 is present in dendritic spines (Varea et al. 2015) and enriched in the synaptic plasma membrane (Bakkaloglu et al. 2008), and suggest an impairment in AMPAR trafficking and AMPAR-mediated synaptic transmission upon Caspr2 loss of function, accordant with the cytoplasmic AMPAR aggregates detected in Cntnap2 KO cortical neurons (Varea et al. 2015).
AMPAR trafficking and localisation to synapses is dynamically modulated by several AMPAR-interacting intracellular (e.g., SAP97 and other MAGUKs, PICK1, GRIP1, protein 4.1 N) and transmembrane (e.g., stargazin and other TARPs, cornichons, SynDIG1) proteins, which regulate receptor membrane insertion, membrane lateral diffusion and anchoring to the PSD (Shepherd and Huganir 2007). We have previously identified Caspr1, the homologous family member of Caspr2, as an interactor of GluA1-AMPARs that regulates their trafficking and synaptic content (Santos et al. 2012). Although the mechanisms whereby Caspr2 regulates AMPAR trafficking are still unclear, one possibility is that Caspr2 might also be included in the AMPAR macrocomplex. Importantly, the effect of Caspr2 short-term depletion on AMPAR synaptic content that we report is independent of the role of Caspr2 on regulating synapse density/maturation (Anderson et al. 2012; Gdalyahu et al. 2015; Varea et al. 2015), since it does not affect synapse number as determined by the density of PSD95 clusters in cortical neurons (Supplementary Fig. S2A, C), or the frequency of mEPSCs in layer 2/3 V1 neurons (Supplementary Table S1). We detected a decrease in the surface and synaptic content of GluA1 (Fig. 1E,F), but not GluA2 (Supplementary Fig. S2A, B), upon Caspr2 silencing in cultured cortical neurons. Given that we found no changes in mEPSC current kinetics in layer 2/3 V1 neurons depleted of Caspr2 (Supplementary Table S1), it is possible that GluA1/2-AMPAR synaptic expression is impacted by Caspr2, whereas the GluA2/3-AMPAR population is spared. The decrease in GluA1/2-AMPA receptors at the surface and synapses is probably not sufficient to detect a decrease in the global population of cell surface GluA2-containing AMPA receptors.
Rapid changes in AMPAR trafficking and function at the synapse occur in response to different patterns of neuronal activity, which account for the expression of various forms of synaptic plasticity. Interestingly, the molecular players underlying such trafficking mechanisms are often themselves activity-regulated (Shepherd and Huganir 2007; Fernandes and Carvalho 2016). Herein, we demonstrate that the expression of Caspr2 is regulated by neuronal activity in vitro and by visual experience in the mouse visual cortex. Furthermore, our findings reveal that loss of Caspr2 prevents homoeostatic synaptic scaling of AMPARs and hinders experience-dependent plasticity in vivo following prolonged visual deprivation. To our knowledge, we establish for the first time a relevant role for Caspr2 in the modulation of synaptic plasticity phenomena, in particular of neuronal synaptic homoeostasis. Homoeostatic plasticity mechanisms are thought to be of particular importance during periods of heightened plasticity, such as during development, sensory processing or adaptation to sensory environment changes, when massive remodelling of synaptic contacts and neuronal circuits occurs (Turrigiano 2008; Fernandes and Carvalho 2016). A relevant role for homoeostatic plasticity was just recently established in the regulation of sleep/wake physiology (Hengen et al. 2016; de Vivo et al. 2017; Diering et al. 2017), considered to be fundamental for memory consolidation and learning (Cirelli 2017). Most important, it has been proposed that a failure in neuronal synaptic homoeostasis may result in symptoms of several psychiatric disorders (Ramocki and Zoghbi 2008; Mullins et al. 2016). Moreover, some of the strongest susceptibility genes implicated in such disorders are important molecular players involved in the regulation of homoeostatic plasticity mechanisms (e.g., stargazin, MeCP2, FMRP) (Fernandes and Carvalho 2016). Indeed, emerging evidence from disease animal models shows alterations in homoeostatic signalling (Wondolowski and Dickman 2013), which is in accord to the findings uncovered in the present study. Herein, we propose that defects in homoeostatic plasticity are an underlying pathology in CNTNAP2/CASPR2-related neuropsychiatric disorders.
One phenotype reported in Cntnap2 KO animal models is the loss of GABAergic interneurons and impaired GABAergic inhibition (Penagarikano et al. 2011; Anderson et al. 2012; Jurgensen and Castillo 2015; Hoffman et al. 2016; Bridi et al. 2017; Vogt et al. 2018). In particular, in layer 2/3 pyramidal neurons of the primary visual cortex, where we have found effects of Caspr2 depletion on excitatory currents (Figs 2, 5), Cntnap2 KO mice showed reduced phasic inhibition and decreased GABAA-receptor-mediated tonic inhibition (Bridi et al. 2017). In combination with the present study, and other reports on altered excitatory function upon Caspr2 manipulation (Anderson et al. 2012; Gdalyahu et al. 2015; Varea et al. 2015), these studies suggest that Caspr2 regulates the excitatory/inhibitory balance in different brain regions.
CASPR2 is a synaptic target of autoantibody-binding in autoimmune encephalitis, but the mechanisms underlying patients’ symptoms remain elusive. A recent study found that autoantibodies against CASPR2 cause pain-related hypersensitivity in the periphery, and enhanced DRG cell excitability through regulation of Kv1 channel expression (Dawes et al. 2018). In vitro, it was found that IgG4-enriched CASPR2 antibodies inhibit the interaction of Caspr2 with contactin-2, but do not affect surface expression of Caspr2 in cultured hippocampal neurons (Patterson et al. 2018). We now find that a mixture of CASPR2-Abs of the IgG1 and IgG4 subtypes affects the neuronal distribution of Caspr2 in dendrites, significantly decreasing its synaptic expression (Fig. 3G, H). Additionally, we observed significant intracellular clusters of anti-human antibodies in neurons incubated with the patient CASPR2-Abs (Supplementary Fig. S3), supporting the possibility that CASPR2-Abs and associated Caspr2 are internalised. To our knowledge, this is the first report of a direct effect of CASPR2-Abs on endogenous Caspr2 levels, and, together with previous evidence in the literature, it is suggestive of different pathogenic mechanisms being triggered by CASPR2-Abs of different subclasses. Strikingly, we found a significant loss of surface AMPARs at the synapse in cortical neurons incubated with CASPR2-Abs (Fig. 4A, B), but no effects on synapse number (Fig. 4C), and in vivo injection of CASPR2-Abs in the mouse visual cortex significantly decreases AMPAR-mediated currents in layer 2/3 pyramidal neurons (Fig. 5A). These unsuspected data suggest that CASPR2-Abs may exert their pathogenicity by impeding the function of Caspr2 in regulating AMPAR synaptic traffic. Indeed, incubation with CASPR2-Abs increased the ratio of AMPA receptor internalisation in cultured cortical neurons (Fig. 4D, E). It is likely that CASPR2-Abs, by destabilising the synaptic localisation of Caspr2, affect AMPAR synaptic trafficking by increasing the rates of AMPAR endocytosis. One further possibility is that the interaction between Caspr2 and contactin-2 found to be perturbed by CASPR2-Abs (Patterson et al. 2018) is required for the role of Caspr2 in regulating AMPAR traffic. Importantly, our findings implicate dysfunction of glutamatergic transmission in the pathogenesis of anti-CASPR2 encephalitis, which may explain the severity of cognitive and psychiatric symptoms presented by patients, and may enable the development of more specific and efficient therapies for this type of encephalitis.
Altogether, the present study provides compelling evidence for a fundamental role of Caspr2 in the regulation of AMPAR synaptic traffic and function, as well as in the modulation of neuronal synaptic homoeostasis. Importantly, these mechanisms are disrupted in consequence of both CNTNAP2 sequence variations and CASPR2 autoantibodies and most likely contribute to the pathogenesis of CASPR2-related neuropsychiatric disorders.
Supplementary Material
Notes
Conflict of Interest: The authors declare no conflict of interest.
Authors’ Contributions
D.F., S.D.S., J.L.W., E.C, N.B., and T.R. performed experiments. E.C., M.I.L., and C.B. provided new reagents. D.F., S.D.S., J.L.W., and E.C. analysed data. D.F., H.-K.L., and A.L.C. designed experiments. D.F. and A.L.C. wrote the paper.
Funding
This work was financed by the European Regional Development Fund (ERDF), through the Centro 2020 Regional Operational Programme under project CENTRO-01-0145-FEDER-000008:BrainHealth 2020, and through the COMPETE 2020—Operational Programme for Competitiveness and Internationalisation and Portuguese national funds via FCT--Fundação para a Ciência e a Tecnologia, I.P. and FCT Portugal are the same funding agency, under projects POCI-01-0145-FEDER-007440 and POCI-01-0145-FEDER-PTDC/SAU-NMC/4888/2014 and POCI-01-0145-FEDER-29452. DF was funded by FCT Portugal: SFRH/BD/51682/2011.
References
- Alarcon M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Sebat J, Wigler M, Martin CL, Ledbetter DH, et al. 2008. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 82(1):150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GR, Galfin T, Xu W, Aoto J, Malenka RC, Sudhof TC. 2012. Candidate autism gene screen identifies critical role for cell-adhesion molecule CASPR2 in dendritic arborization and spine development. Proc Natl Acad Sci USA. 109(44):18120–18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, Rea A, Guy M, Lin S, Cook EH, et al. 2008. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 82(1):160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkaloglu B, O’Roak BJ, Louvi A, Gupta AR, Abelson JF, Morgan TM, Chawarska K, Klin A, Ercan-Sencicek AG, Stillman AA, et al. 2008. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am J Hum Genet. 82(1):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen AEM, van Sonderen A, Titulaer MJ. 2017. Autoimmune encephalitis with anti-leucine-rich glioma-inactivated 1 or anti-contactin-associated protein-like 2 antibodies (formerly called voltage-gated potassium channel-complex antibodies). Curr Opin Neurol. 30(3):302–309. [DOI] [PubMed] [Google Scholar]
- Belloso JM, Bache I, Guitart M, Caballin MR, Halgren C, Kirchhoff M, Ropers HH, Tommerup N, Tumer Z. 2007. Disruption of the CNTNAP2 gene in a t(7;15) translocation family without symptoms of Gilles de la Tourette syndrome. Eur J Hum Genet. 15(6):711–713. [DOI] [PubMed] [Google Scholar]
- Bien CG, Mirzadjanova Z, Baumgartner C, Onugoren MD, Grunwald T, Holtkamp M, Isenmann S, Kermer P, Melzer N, Naumann M, et al. 2017. Anti-contactin-associated protein-2 encephalitis: relevance of antibody presentation and outcome. Eur J Neurol. 24(1):175–186. [DOI] [PubMed] [Google Scholar]
- Bridi MS, Park SM, Huang S. 2017. Developmental disruption of GABAAR-meditated inhibition in Cntnap2 KO mice. eNeuro. 4(5):ENEURO.0162-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimberg L, Mader S, Jeganathan V, Berlin R, Coleman TR, Gregersen PK, Huerta PT, Volpe BT, Diamond B. 2016. Caspr2-reactive antibody cloned from a mother of an ASD child mediates an ASD-like phenotype in mice. Mol Psychiatry. 21(12):1663–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C. 2017. Sleep, synaptic homeostasis and neuronal firing rates. Curr Opin Neurobiol. 44:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho E, Jacobson L, Pedersen MG, Benros ME, Norgaard-Pedersen B, Mortensen PB, Harrison PJ, Vincent A. 2017. CASPR2 autoantibodies are raised during pregnancy in mothers of children with mental retardation and disorders of psychological development but not autism. J Neurol Neurosurg Psychiatry. 88(9):718–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho E, Menassa DA, Jacobson L, West SJ, Domingos J, Moloney TC, Lang B, Harrison PJ, Bennett DLH, Bannerman D, et al. 2017. Persistent microglial activation and synaptic loss with behavioral abnormalities in mouse offspring exposed to CASPR2-antibodies in utero. Acta Neuropathol. 134(4):567–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes JM, Weir GA, Middleton SJ, Patel R, Chisholm KI, Pettingill P, Peck LJ, Sheridan J, Shakir A, Jacobson L, et al. 2018. Immune or genetic-mediated disruption of CASPR2 causes pain hypersensitivity due to enhanced primary afferent excitability. Neuron. 97(4):806–822 e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vivo L, Bellesi M, Marshall W, Bushong EA, Ellisman MH, Tononi G, Cirelli C. 2017. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science. 355(6324):507–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diering GH, Nirujogi RS, Roth RH, Worley PF, Pandey A, Huganir RL. 2017. Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science. 355(6324):511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes D, Carvalho AL. 2016. Mechanisms of homeostatic plasticity in the excitatory synapse. J Neurochem. 139(6):973–996. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Vrijenhoek T, Markx S, Janssen IM, van der Vliet WA, Faas BH, Knoers NV, Cahn W, Kahn RS, Edelmann L, et al. 2008. CNTNAP2 gene dosage variation is associated with schizophrenia and epilepsy. Mol Psychiatry. 13(3):261–266. [DOI] [PubMed] [Google Scholar]
- Gdalyahu A, Lazaro M, Penagarikano O, Golshani P, Trachtenberg JT, Geschwind DH. 2015. The autism related protein contactin-associated protein-like 2 (CNTNAP2) stabilizes new spines: an in vivo mouse study. PLoS One. 10(5):e0125633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Jiang B, Xu LW, Song L, Kirkwood A, Lee HK. 2006. Cross-modal regulation of synaptic AMPA receptors in primary sensory cortices by visual experience. Nat Neurosci. 9(8):1001–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Lee HK. 2007. Persistence of experience-induced homeostatic synaptic plasticity through adulthood in superficial layers of mouse visual cortex. J Neurosci. 27(25):6692–6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Xu LW, Snyder KP, Song L, Goenaga-Vazquez Y, Megill A, Takamiya K, Huganir RL, Lee HK. 2011. Phosphorylation of AMPA receptors is required for sensory deprivation-induced homeostatic synaptic plasticity. PLoS One. 6(3):e18264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengen KB, Torrado Pacheco A, McGregor JN, Van Hooser SD, Turrigiano GG. 2016. Neuronal firing rate homeostasis is inhibited by sleep and promoted by wake. Cell. 165(1):180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EJ, Turner KJ, Fernandez JM, Cifuentes D, Ghosh M, Ijaz S, Jain RA, Kubo F, Bill BR, Baier H, et al. 2016. Estrogens suppress a behavioral phenotype in zebrafish mutants of the autism risk gene, CNTNAP2. Neuron. 89(4):725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani SR, Alexander S, Waters P, Kleopa KA, Pettingill P, Zuliani L, Peles E, Buckley C, Lang B, Vincent A. 2010. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain. 133(9):2734–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert B, Saint-Martin M, Noraz N, Picard G, Rogemond V, Ducray F, Desestret V, Psimaras D, Delattre JY, Antoine JC, et al. 2016. Characterization of a subtype of autoimmune encephalitis with anti-contactin-associated protein-like 2 antibodies in the cerebrospinal fluid, prominent limbic symptoms, and seizures. JAMA Neurol. 73(9):1115–1124. [DOI] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. 2004. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 7(3):244–253. [DOI] [PubMed] [Google Scholar]
- Jurgensen S, Castillo PE. 2015. Selective dysregulation of hippocampal inhibition in the mouse lacking autism candidate gene CNTNAP2. J Neurosci. 35(43):14681–14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein CJ, Lennon VA, Aston PA, McKeon A, O’Toole O, Quek A, Pittock SJ. 2013. Insights from LGI1 and CASPR2 potassium channel complex autoantibody subtyping. JAMA Neurol. 70(2):229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster E, Huijbers MG, Bar V, Boronat A, Wong A, Martinez-Hernandez E, Wilson C, Jacobs D, Lai M, Walker RW, et al. 2011. Investigations of caspr2, an autoantigen of encephalitis and neuromyotonia. Ann Neurol. 69(2):303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mele M, Ribeiro L, Inacio AR, Wieloch T, Duarte CB. 2014. GABA(A) receptor dephosphorylation followed by internalization is coupled to neuronal death in in vitro ischemia. Neurobiol Dis. 65:220–232. [DOI] [PubMed] [Google Scholar]
- Mullins C, Fishell G, Tsien RW. 2016. Unifying views of autism spectrum disorders: a consideration of autoregulatory feedback loops. Neuron. 89(6):1131–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dushlaine C, Kenny E, Heron E, Donohoe G, Gill M, Morris D, International Schizophrenia C, Corvin A. 2011. Molecular pathways involved in neuronal cell adhesion and membrane scaffolding contribute to schizophrenia and bipolar disorder susceptibility. Mol Psychiatry. 16(3):286–292. [DOI] [PubMed] [Google Scholar]
- Patterson KR, Dalmau J, Lancaster E. 2018. Mechanisms of Caspr2 antibodies in autoimmune encephalitis and neuromyotonia. Ann Neurol. 83(1):40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, et al. 2011. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 147(1):235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagarikano O, Geschwind DH. 2012. What does CNTNAP2 reveal about autism spectrum disorder? Trends Mol Med. 18(3):156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrus E, Rodriguez G, Patterson R, Connor B, Kanold PO, Lee HK. 2015. Vision loss shifts the balance of feedforward and intracortical circuits in opposite directions in mouse primary auditory and visual cortices. J Neurosci. 35(23):8790–8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, Trimmer JS, Shrager P, Peles E. 1999. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron. 24(4):1037–1047. [DOI] [PubMed] [Google Scholar]
- Poot M. 2017. Intragenic CNTNAP2 deletions: a bridge too far? Mol Syndromol. 8(3):118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramocki MB, Zoghbi HY. 2008. Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature. 455(7215):912–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenas-Cuadrado P, Ho J, Vernes SC. 2014. Shining a light on CNTNAP2: complex functions to complex disorders. Eur J Hum Genet. 22(2):171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SD, Iuliano O, Ribeiro L, Veran J, Ferreira JS, Rio P, Mulle C, Duarte CB, Carvalho AL. 2012. Contactin-associated protein 1 (Caspr1) regulates the traffic and synaptic content of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors. J Biol Chem. 287(9):6868–6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R, Sanchez-Aguilera A, van Elst K, Lim L, Dehorter N, Bae SE, Bartolini G, Peles E, Kas MJH, Bruining H, et al. 2017. Loss of Cntnap2 causes axonal excitability deficits, developmental delay in cortical myelination, and abnormal stereotyped motor behavior. Cereb Cortex. 29(2):586–597. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. 2007. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 23:613–643. [DOI] [PubMed] [Google Scholar]
- Smogavec M, Cleall A, Hoyer J, Lederer D, Nassogne MC, Palmer EE, Deprez M, Benoit V, Maystadt I, Noakes C, et al. 2016. Eight further individuals with intellectual disability and epilepsy carrying bi-allelic CNTNAP2 aberrations allow delineation of the mutational and phenotypic spectrum. J Med Genet. 53(12):820–827. [DOI] [PubMed] [Google Scholar]
- Somers KJ, Lennon VA, Rundell JR, Pittock SJ, Drubach DA, Trenerry MR, Lachance DH, Klein CJ, Aston PA, McKeon A. 2011. Psychiatric manifestations of voltage-gated potassium-channel complex autoimmunity. J Neuropsychiatry Clin Neurosci. 23(4):425–433. [DOI] [PubMed] [Google Scholar]
- Strauss KA, Puffenberger EG, Huentelman MJ, Gottlieb S, Dobrin SE, Parod JM, Stephan DA, Morton DH. 2006. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N Engl J Med. 354(13):1370–1377. [DOI] [PubMed] [Google Scholar]
- Sunwoo JS, Lee ST, Byun JI, Moon J, Shin JW, Jeong DE, Lee GH, Jeong SH, Shin YW, Jung KH, et al. 2015. Clinical manifestations of patients with CASPR2 antibodies. J Neuroimmunol. 281:17–22. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. 2008. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 135(3):422–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Sonderen A, Arino H, Petit-Pedrol M, Leypoldt F, Kortvelyessy P, Wandinger KP, Lancaster E, Wirtz PW, Schreurs MW, Sillevis Smitt PA, et al. 2016. The clinical spectrum of Caspr2 antibody-associated disease. Neurology. 87(5):521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Sonderen A, Petit-Pedrol M, Dalmau J, Titulaer MJ. 2017. The value of LGI1, Caspr2 and voltage-gated potassium channel antibodies in encephalitis. Nat Rev Neurol. 13(5):290–301. [DOI] [PubMed] [Google Scholar]
- Varea O, Martin-de-Saavedra MD, Kopeikina KJ, Schurmann B, Fleming HJ, Fawcett-Patel JM, Bach A, Jang S, Peles E, Kim E, et al. 2015. Synaptic abnormalities and cytoplasmic glutamate receptor aggregates in contactin associated protein-like 2/Caspr2 knockout neurons. Proc Natl Acad Sci USA. 112(19):6176–6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerk AJ, Mathews CA, Joosse M, Eussen BH, Heutink P, Oostra BA, Tourette Syndrome Association International Consortium for G . 2003. CNTNAP2 is disrupted in a family with Gilles de la Tourette syndrome and obsessive compulsive disorder. Genomics. 82(1):1–9. [DOI] [PubMed] [Google Scholar]
- Vernes SC, Newbury DF, Abrahams BS, Winchester L, Nicod J, Groszer M, Alarcon M, Oliver PL, Davies KE, Geschwind DH, et al. 2008. A functional genetic link between distinct developmental language disorders. N Engl J Med. 359(22):2337–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt D, Cho KKA, Shelton SM, Paul A, Huang ZJ, Sohal VS, Rubenstein JLR. 2018. Mouse Cntnap2 and human CNTNAP2 ASD alleles cell autonomously regulate PV+ cortical interneurons. Cereb Cortex. 28(11):3868–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt JL, Petrus E, Lee HK. 2014. Experience-dependent homeostatic synaptic plasticity in neocortex. Neuropharmacology. 78:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CJ, Ibata K, Turrigiano GG. 2005. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci. 25(11):2895–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wondolowski J, Dickman D. 2013. Emerging links between homeostatic synaptic plasticity and neurological disease. Front Cell Neurosci. 7:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier C, de Jong EK, Zweier M, Orrico A, Ousager LB, Collins AL, Bijlsma EK, Oortveld MA, Ekici AB, Reis A, et al. 2009. CNTNAP2 and NRXN1 are mutated in autosomal-recessive Pitt-Hopkins-like mental retardation and determine the level of a common synaptic protein in Drosophila. Am J Hum Genet. 85(5):655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.