Abstract
Subplate (SP) neurons exhibit spontaneous plateau depolarizations mediated by connexin hemichannels. Postnatal (P1–P6) mice show identical voltage pattern and drug-sensitivity as observed in slices from human fetal cortex; indicating that the mouse is a useful model for studying the cellular physiology of the developing neocortex. In mouse SP neurons, spontaneous plateau depolarizations were insensitive to blockers of: synaptic transmission (glutamatergic, GABAergic, or glycinergic), pannexins (probenecid), or calcium channels (mibefradil, verapamil, diltiazem); while highly sensitive to blockers of gap junctions (octanol), hemichannels (La3+, lindane, Gd3+), or glial metabolism (DLFC). Application of La3+ (100 μM) does not exert its effect on electrical activity by blocking calcium channels. Intracellular application of Gd3+ determined that Gd3+-sensitive pores (putative connexin hemichannels) reside on the membrane of SP neurons. Immunostaining of cortical sections (P1–P6) detected connexins 26, and 45 in neurons, but not connexins 32 and 36. Vimentin-positive glial cells were detected in the SP zone suggesting a potential physiological interaction between SP neurons and radial glia. SP spontaneous activity was reduced by blocking glial metabolism with DFLC or by blocking purinergic receptors by PPADS. Connexin hemichannels and ATP release from vimentin-positive glial cells may underlie spontaneous plateau depolarizations in the developing mammalian cortex.
Keywords: development, gadolinium, lanthanum, lindane, probenecid
Introduction
During the critical period of development, sensory experiences generate electrical activity which is essential for the tuning of neural circuits in the cerebral cortex (Erzurumlu and Gaspar 2012; Espinosa and Stryker 2012). Prior to this critical period and before sensory impulses can reach cortical layers, endogenous spontaneous electrical activity (precritical activity) drives several fundamental processes of corticogenesis such as neuronal migration, neurotransmitter specification, insertion of ion channels, axon myelination, synaptogenesis, and formation of functional neuronal circuits (Rakic and Komuro 1995; Spitzer et al. 2004; Moody and Bosma 2005; Luhmann et al. 2016). Major neuropsychiatric diseases (autism and schizophrenia) are, in principle, genetic diseases. Affected individuals acquire strong predispositions for autism and schizophrenia through neurodevelopmental abnormalities occurring in the mother’s womb (Lewis and Levitt 2002). Neural genes are strongly regulated by electrical activity (Spitzer et al. 2000; West et al. 2002), therefore, environmental factors affecting early electrical activity have a potential to trigger or facilitate genetic abnormalities (Shorter and Miller 2015). A better understanding of the cellular mechanisms underlying endogenous electrical activity, and the identification of environmental agents (e.g. drugs) that modulate such activity, may benefit preventive strategies for neurodevelopmental brain disorders.
In the developing human cortex, while newborn pyramidal neurons are still migrating from the subventricular zones into the cortical plate (CP) (Clowry et al. 2010), endogenous spontaneous electrical activity (Moore et al. 2011) occurs in a specialized zone of primordial cortex (subplate [SP] zone) inhabited by the ontogenetically oldest cortical neurons called SP neurons (Kostovic and Rakic 1980; Kanold and Luhmann 2010; Moore et al. 2011; Duque et al. 2016). SP electrical activity has an essential role in governing the path-finding of corticopetal and corticofugal axonal projections, and the establishment of thalamocortical connections and cortical columns (Ghosh et al. 1990; Wess et al. 2017). Disruption in SP neurons specifically, has been implicated in several neurodevelopmental disorders such as autism, schizophrenia, and cerebral palsy (Volpe 1996).
Whole-cell recordings performed on the human pioneer SP neurons in brain slices, revealed unprovoked plateau depolarizations (Moore et al. 2011), which are very sensitive to the blocker of connexin-based hemichannels (Moore et al. 2014). Ethical and technical constraints prevent further experimentation on human fetal material (Moore et al. 2012). Here, experiments in neonatal mice were designed to answer questions (Q1–Q7) which had not been answered in experiments on human material (Moore et al. 2014). (Q1) Is the strong effect of lanthanum (La3+) on spontaneous depolarization in SP neurons due to a blockage of voltage-gated calcium channels (VGCCs)? Does La3+ block VGCCs in SP neurons? (Q2) What is the contribution of VGCCs to endogenous spontaneous plateau depolarizations in SP neurons? (Q3) Was the La3+-induced loss of spontaneous plateau depolarizations in human SP neurons due to blocking channels located on neurons or on glia? (Q4) Do pannexin hemichannels contribute to spontaneous electrical activity in SP neurons? (Q5) Are SP neurons hosting purinergic receptors capable of depolarizing the somatic membrane by ~20 mV above resting? (Q6) Does purinergic receptor antagonist affect spontaneous (unprovoked) depolarizations in SP neurons? (Q7) Is the pharmacological profile (e.g. sodium channels, glutamate, glycinergic, and GABAergic receptors) of endogenous spontaneous depolarizations in the human SP different from that in the mouse? In the current study, dose–response curve for La3+ and introduction of drugs which block VGCCs ruled out the possibility that La3+ blocks spontaneous activity in SP neurons via its action on VGCCs. Intracellular (IC) injection of gadolinium (Gd3+), a more specific antagonist of hemichannels, indicated that connexin hemichannels are located on the membrane of SP neurons. We identified thick bundles of vimentin-positive glial fibers inside the SP zone and showed that radial glial cells play a role in spontaneous electrical activity by supplying SP neurons with ATP. Ionotropic purinergic receptors (P2X) produce inward membrane currents, which prolong and sustain electrical activity in neonatal mouse SP neurons. Finally, we performed the entire battery of pharmacological experiments to show the exact level of physiological overlap between human and mouse SP. Our present study reveals that cellular processes underlying inherent spontaneous electrical activity in mouse and human SP are nearly identical.
Materials and Methods
Brain Slice and Electrophysiology
Swiss Webster mice of both sexes were anesthetized with isoflurane inhalation, decapitated and brains were extracted with the head immersed in ice-cold, artificial cerebrospinal fluid (ACSF), according to an animal protocol approved by the Center for Laboratory Animal Care, University of Connecticut. In all experiments, neonatal mice (P1–P6) were used, unless specified differently. ACSF contained (in mM) 125 NaCl, 26 NaHCO3, 10 glucose, 2.3 KCl, 1.26 KH2PO4, 1.2 CaCl2, and 1 MgSO4. Coronal slices (300 μm) were cut from the somatosensory cortex of postnatal pups, incubated at 37 °C for 30 min and then at room temperature prior to experimental recordings. All experimental measurements were performed at 32–34 °C. Acute brain slices were transferred to an Olympus BX51WI upright microscope and perfused with aerated (5% CO2/95% O2) ACSF at 32 °C. Individual cells in the SP were selected by using infrared differential interference contrast video microscopy. Patch pipettes (~7 MΩ) were filled with an IC solution containing (in mM) 135 K gluconate, 10 HEPES, 2 MgCl2, 3 ATP-Na2, 0.3 GTP-Na2, 0.5 EGTA, and 10 phosphocreatine Na2 (pH 7.3 adjusted with KOH, osmolality = 300 mOsm/kg). Whole-cell patch-clamp recordings and voltage and current clamp configurations were used to determine the basic electrophysiological properties of each individual cell. For voltage clamp recordings, SP neurons were held at −70 mV. The voltages were then stepped from −110 to +20 mV (step 10 mV), for a duration of 50 ms, to determine the presence of voltage-gated transmembrane currents. Cells were then switched to current clamp configuration to determine the resting membrane potential and action potential (AP) firing. In case the cell was depolarized, the membrane potential was maintained at −60 mV by injecting DC negative bias current (range from −1 to −20 pA). A series of current steps (−50 to +70 pA in 10 pA steps) were then applied to the cell for a duration of 900 ms. Electrical signals were amplified with Multiclamp 700B and digitized with 2 input boards: 1) Digidata Series 1400 A (Molecular Devices, Union City, CA, USA) and 2) Neuroplex (RedShirtImaging, Decatur, GA, USA). Only cells with a membrane potential more negative than −50 mV (not corrected for junction potential) were included in this study.
Recordings of Spontaneous Activity
Spontaneous activity was continuously monitored for 5 min in regular (1.2 mM Ca2+) ACSF (Control). A cell was identified as being spontaneously active, based on periods of depolarization (amplitude > 2.5 mV, duration > 25 ms) either with or without AP firing. Following control measurements (5 min), drug was introduced via perfusion at a rate of 2–3 mL/min. Recordings of the same duration (5 min) were performed 3–10 min following introduction of the drug into the recording chamber. All drugs were purchased from Sigma unless stated otherwise. Stock solutions of tetrodotoxin (TTX), tetraethylammonium chloride (TEA), 4-aminopyridine (4-AP), mibefradil, diltiazem, lanthanum(III) chloride, gadolinium(III) chloride, and pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) tetrasodium salt hydrate (PPADS) were dissolved in H2O; (+)-bicuculline in ethanol and H2O; DL-2-amino-5-phosphonopentanoic acid (APV) in H2O and NaOH; strychnine, 6,7-Dinitroquinoxaline-2,3(1 H,4 H)-dione (DNQX), and lindane in dimethyl sulfoxide (DMSO), and probenecid in DMSO or in NaOH. Stock solutions were stored at 4°C and diluted in ACSF on the day of the experiment. Because La3+ and Gd3+ precipitate in ACSF, we used a modified Krebs–Ringers solution for both control recordings and in the presence of La3+ and Gd3+. The Krebs–Ringers solution contained (in mM) 140 NaCl, 5.4 KCl, 1 MgCl2, 1.2 CaCl2, 10 HEPES, 20 glucose, pH 7.4 adjusted with NaOH. To prepare 0.1 mM of DL-fluorocitrate (DLFC; method adopted from Voloboueva et al. (2007)), 50 mg DLFC barium salt was dissolved in 625 μL of 1 mM HCl by vortexing. To this, 3.75 mL of 0.1 mM Na2SO4 was added and mixed by vortexing. The following suspension was centrifuged at 4000 rpm for 7 min. Pellet was discarded and the supernatant was added to 250 mL of ACSF. Working solutions of DLFC were stored at 4°C, and used within the next 2–3 days.
Synaptic Stimulation
Stimulation electrodes were pulled from borosilicate glass with filament (1.5 mm outer diameter; 0.8 inner diameter). Synaptic stimulation pipettes (7 MΩ) were filled with ACSF. A stimulus isolation unit, IsoFlex (A.M.P.I., Jerusalem, Israel), was used to generate current pulses for synaptic stimulation. The stimulation pipettes were positioned 80–100 μm away from the cell body. Navigation of the glass pipettes through the slice tissue was achieved with the aid of a “fourth axis” (concomitant engagement of both X- and Z-axes), available on Sutter Instruments MP-200 motorized micromanipulator. Three shocks (0.2 ms pulse duration at 20 Hz frequency) were delivered to the slice with current pulse intensities ranging from 40 to 150 pA.
Calcium Imaging
For single-cell calcium imaging, neurons were injected (via patch pipette) with an IC solution containing: calcium-sensitive fluorescent indicator Oregon green 488 Bapta-1 (OGB1, 150 μM) and fluorescent dye Alexa Fluor 594 (AF-594, 40 μM). For multisite calcium imaging, the neurons were loaded extracellularly by pressure ejecting a cell permeant OGB1-AM (150 μM) from a glass micropipette above the surface of the brain slice. All 3 fluorescent dyes were purchased from Invitrogen, Carlsbad, CA, USA. An epi-illumination system based on metal halide lamp and shutter was used to excite the dyes. Optical filters were purchased from Chroma Technology (Rockingham, VT, USA) and Omega Optical (Brattleboro, VT, USA). Filters for AF-594 were a Chroma exciter HQ580/20× (570–590 nm bandpass), dichroic Q595LP, and emitter HQ630/60 m (600–660 nm bandpass). The filter cube for OGB1 contained an Omega exciter 500AF25, dichroic 525DRLP, and emitter 530ALP. Calcium-dye signals were sampled at 40 Hz full frame rate with NeuroCCD camera (80 × 80 pixel configuration, RedShirtImaging, Decatur, GA). Analysis of optical data, including spatial averaging, high-pass and low-pass filtering, was conducted with Neuroplex v. 8.0.0 (RedShirtImaging). Optical signal amplitudes are expressed as ΔF/F, where, F represents the resting fluorescence intensity at the beginning of the optical trace (baseline), and ΔF represents intensity change from the baseline fluorescence during the biological signal.
Microiontophoresis
A sharp glass pipette (40 ± 10 MΩ) filled with 200 mM glutamate or 100 mM ATP, attached to the motorized micromanipulator was positioned in the SP zone using infrared video microscopy. An IsoFlex (A.M.P.I., Israel) stimulus isolation unit, triggered by data acquisition software Clampex 10.2 (Molecular Devices, CA) was used to deliver a single negative current pulse (5 ms duration, and 10–35 nA amplitude) to drive glutamate or ATP out.
Quantification of Spontaneous Activity and Data Analysis
Spontaneous activity was quantified in Clampfit (Version 10.2; Molecular Devices, CA) by analyzing 4 parameters: number of events, duration, surface area, and peak amplitude of the events. For each neuron, spontaneous activity was recorded and APs were filtered out by low-pass filtering before quantification. Data analysis was performed offline in Clampfit, Excel (Microsoft), and SigmaStat (Version 3.2). Quantified data are expressed as mean ± standard error of the mean (SEM). For determining statistical significance, we used Student’s t-test (if data sets passed normality test). If the normality test failed, the statistical significance was determined with the nonparametric Mann–Whitney U test. Results were considered statistically significant if P ≤ 0.05.
Immunohistochemistry
Brains from postnatal pups (P2) were fixed overnight in 4% (wt/vol) paraformaldehyde (PFA) in phosphate buffer saline (PBS) followed by overnight treatment in 30% sucrose at 4 °C. Each brain was embedded in Tissue-Tec OCT mounting medium in dry ice, and sectioned on a cryostat to 30 μm sections. Sections were mounted onto glass slides and treated for antigen retrieval in citrate buffer (pH 6) at 80 °C for 20 min. Sections were then blocked and permeabilized with 10% normal goat serum (NGS) and 0.05% Triton X-100 dissolved in PBS. Antibodies for Cx32 and Cx43 were purchased from Invitrogen. Rabbit polyclonal antibodies for Cx26, Cx36, and Cx45 were purchased from Alomone Labs (Jerusalem, Israel). Antibody for GFAP was purchased from DAKO (Carpinteria, CA), anti-NeuN from EMD Millipore (Darmstadt, Germany), while the antibody for vimentin was kindly donated by Dr Zecevic (UConn Health, CT). Primary antibodies were applied overnight (4 °C) at the following concentrations in PBS and 5% NGS: Cx32 rabbit polyclonal (1:1000), Cx26 rabbit polyclonal (1:1000), Cx43 rabbit polyclonal (1:1000), Cx36 rabbit polyclonal (1:1000), Cx45 rabbit polyclonal (1:1000), GFAP rabbit polyclonal (1:1000), vimentin rabbit polyclonal (1:200), and NeuN mouse monoconal (1:500). Sections were rinsed 3 × 10 min in PBS and secondary antibodies (Alexa Fluor 594 goat antirabbit and Alexa Flour 488 goat antimouse), both from Invitrogen (Carlsbad, CA) were applied at concentration of 1:1000, at room temperature for 1 h. Sections were rinsed 3 × 10 min with PBS, treated with Hoechst stain (1:2000) for 10 min and mounted with 70% glycerol. Stained sections were visualized by using Axiovert 200 M microscope with an XYZ motorized stage and Apotome for optical sectioning (Zeiss, Jena, Germany).
Results
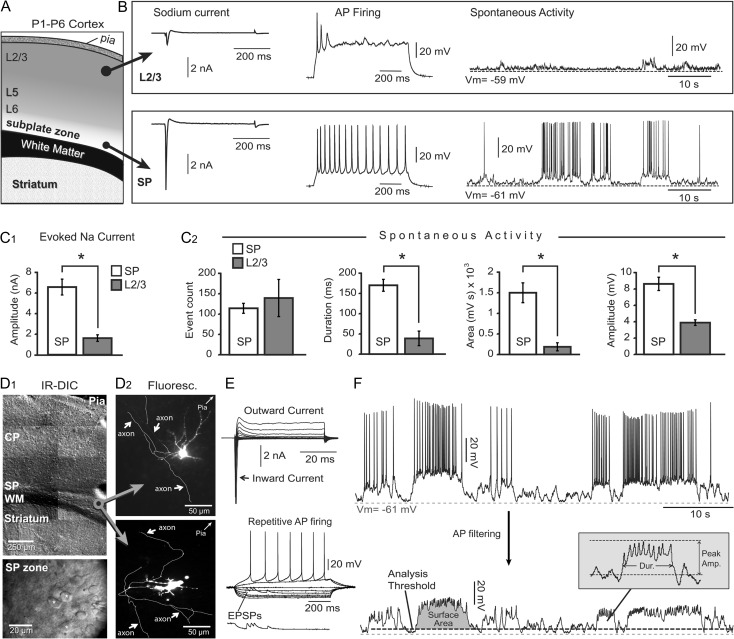
To establish the criteria for identifying SP neurons in acute brain sections of P2 mice, we performed whole-cell patch clamp recordings from SP neurons (near the white matter, WM) and neurons near the pia—putative layer 2/3 pyramidal neurons (Fig. 1A). The command voltage was stepped from −70 to +20 mV (in 10 mV increments) to trigger fast inward Na+ current (Fig. 1B, Sodium current). The peak amplitude of the evoked Na+ current in SP neurons (6.58 ± 0.77 nA, mean ± SEM; n = 6) was significantly larger (P < 0.0001) than the Na+ current in L2/3 cells (1.61 ± 0.32 nA; n = 6, Fig. 1C1). While all SP neurons (6 out of 6) exhibited repetitive AP firing, none of the L2/3 cells (0 out of 6) were able to sustain repetitive firing (Fig. 1B, AP firing). In each L2/3 and SP cell, we continuously monitored spontaneous electrical activity for 5 min (Fig. 1B, spontaneous activity). Layer 2/3 cells spent significantly less time in a depolarized state (duration), experienced a significantly smaller membrane charge transfer (surface area), and their voltage transients were of a lower amplitude than those measured in SP neurons (Fig. 1C2, spontaneous activity). These data are consistent with the previously published notions that SP neurons are the oldest (pioneer) cells of the cerebral cortex, first to appear and first to achieve electrical maturity (Kanold and Luhmann 2010). Newborn L2/3 pyramidal neurons, on the other hand, are born much later than SP neurons. They arrive in superficial layers in a relatively immature state characterized by weak sodium current and broad APs (Moody and Bosma 2005). Identical distributions of “strong” electrical properties in the SP zone and “weak” electrical features of cells in the CP zone near the pia (putative layer 2/3) were previously found in brain slices from human fetuses during midgestation (Moore et al. 2009).
Figure 1.
Subplate neurons versus L2/3 neurons. (A) Schematic drawing of coronal section through neonatal mouse cortex. (B) Whole-cell measurements in L2/3 cells (top row) and SP cells (bottom row). (C1) Comparison of the peak sodium current in 2 layers. In this and all following figures asterisk indicates P < 0.05. (C2) Comparisons of the spontaneous electrical activity parameters in 2 layers. (D1) DIC images of P2 mouse cerebral cortex at 2 magnifications. CP = cortical plate; SP = subplate; WM = white matter. (D2) Alexa Fluor-filled SP neuron with axonal arborization (white arrows). (E) Upper: membrane currents in response to voltage commands from −70 to +20 mV. Lower: repetitive action potential firing upon direct current injection. (F) Upper: recording of spontaneous electrical activity in a mouse subplate neuron. Lower: same trace after low-pass filtering of action potentials. Surface area, duration and peak amplitude are marked on traces. Dashed gray line—resting potential. Dashed black line—event threshold.
Selection of SP Neurons for Inclusion in Experiments
We used infrared video microscopy to identify the mouse SP zone positioned between the CP and the WM (Fig. 1D1, lower magnification). In the SP zone, we patched neurons with large ovoid cell bodies (Fig. 1D1, higher magnification) and injected fluorescent dyes intracellularly to reveal dendritic arborizations (Fig. 1D2). Mouse SP neurons possess well developed axons with many axon collaterals (Fig. 1D2, axon) that project laterally into the same lamina (SP zone), into the WM, or into the CP. In all experiments of the current study, upon establishing whole-cell configuration, we determined 2 electrophysiological parameters at the beginning of the recording session. Each neuron was first tested for Na+ current and then for AP firing pattern. Only neurons with peak Na+ current greater than 2 nA and capable of triggering repetitive full-size APs were identified as mature SP neurons and used in the present study (Fig. 1E). Spontaneous (unprovoked) electrical activity of identified SP neurons exhibited depolarizing plateau potentials with plateau amplitude of ~20 mV, lasting several hundred milliseconds to several seconds (Fig. 1F, upper). In order to focus on the slow plateau depolarizations, prior to each numerical analysis we filtered out APs using a digital low-pass filter (Fig. 1F, lower).
APs and Glutamate
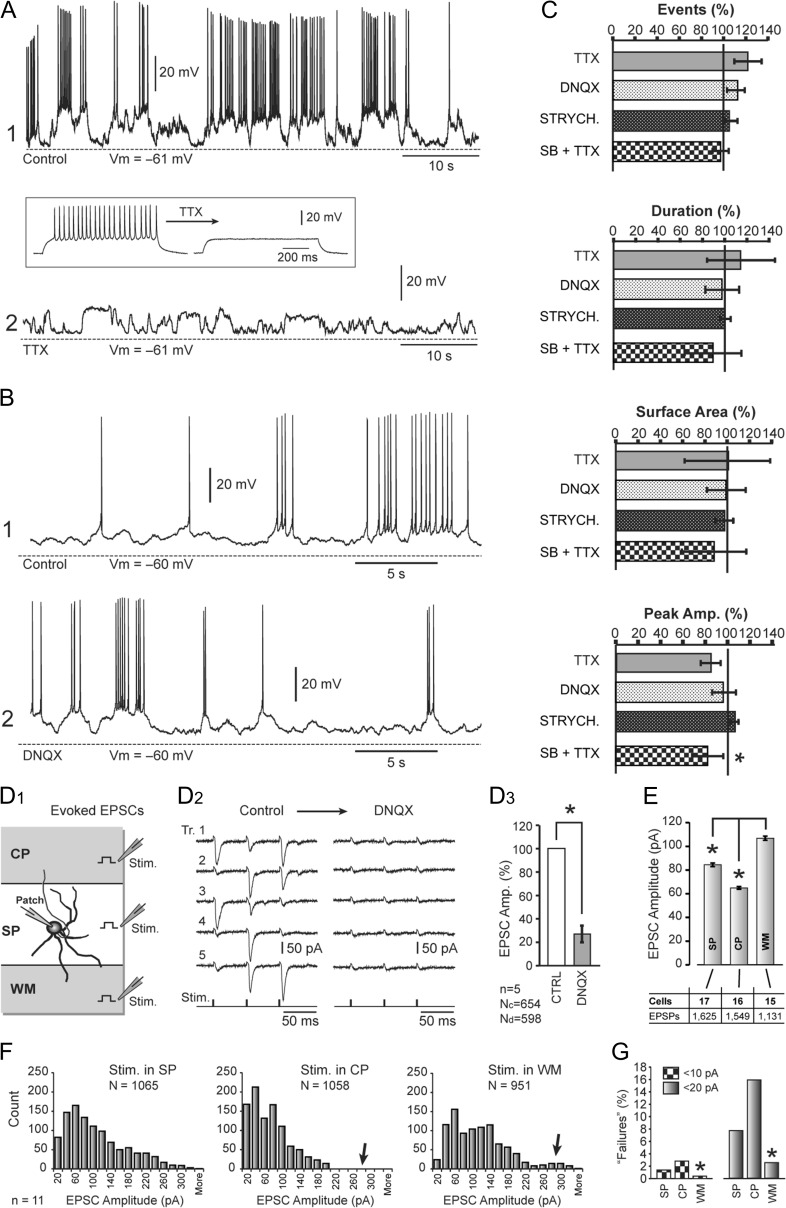
We hypothesized that spontaneous activity in P1–P6 mouse SP neurons was driven by AP firing in afferent neurons—network activity. To test this, spontaneous activity was recorded in the same neuron before (Fig. 2A, control) and after application of sodium channel antagonist TTX (1 μM) (Fig. 2A, TTX). Before recording spontaneous activity, the drug effect was confirmed by absence of APs following current injections (Fig. 2A, inset). Four parameters used for the characterization of spontaneous plateau depolarizations, event count, duration, area, and amplitude, were modestly affected by TTX (Fig. 2C, TTX, n = 5), suggesting that plateau depolarizations in SP neurons are not governed by AP-mediated release of neurotransmitters.
Figure 2.
TTX and synaptic blockers. (A) Spontaneous activity before and after bath application of TTX (1 μM). Inset: The efficacy of TTX is tested in the same neuron by direct current injection. (B) Spontaneous activity before and after application of DNQX (20 μM). (C) Quantification of the TTX, DNQX, and strychnine data revealed no significant changes in the 4 parameters: event count, duration, surface area and peak amplitude (n = 5 for each drug). SB + TTX is a cocktail comprising 1 μM TTX, 20 μM DNQX, 20 μM APV, 20 μM bicuculline, and 20 μM strychnine. (D1) Schematic drawing depicts 3 positions of the synaptic stimulation pipette. (D2) Evoked EPSCs recorded in SP neuron before (left) and after (right) application of 20 μM DNQX. (D3) EPSC amplitude upon treatment with 20 μM DNQX compared with control. n = 5 cells, “Nc” and “Nd” indicate the total number of EPSCs acquired in control and drug condition, respectively. (E) The mean values of EPSC amplitudes recorded in SP neurons when synaptic stimulation electrodes were positioned in 3 locations shown in D1. Asterisk indicates ANOVA followed by Student’s t-test at P < 0.01. (F) Frequency histograms of EPSC amplitudes with stimulation in the SP, CP, and WM. (G) In 11 SP neurons, all 3 stimulations sites were successfully tested in the same neuron and these data presented in the amplitude frequency diagrams. The meaning of the labels: stimulation in SP, CP, and WM, respectively.
Since neurons and glia are capable of releasing glutamate by means other than AP-mediated vesicular release, we tested the contribution of glutamatergic currents to spontaneous electrical activity. In this experimental series, unprovoked neuronal activity was recorded before and after bath application of the AMPA receptor antagonist DNQX (20 μM) (Fig. 2B). Spontaneous plateau depolarizations were not affected by DNQX (Fig. 2C, DNQX, n = 5), suggesting that plateau depolarizations in SP neurons are not mediated by glutamate release. This result was somewhat unexpected, so we tested the efficacy of our batch of DNQX on evoked synaptic currents (Fig. 2D1). The same drug from the same vial effectively blocked synaptically evoked currents in SP neurons (Fig. 2D2). The average amplitude of excitatory postsynaptic currents (EPSCs) before the application of DNQX (38.9 ± 3.9 pA, n = 654 EPSCs in 5 neurons) was significantly reduced in the presence of the AMPA receptor antagonist DNQX (20 μM) to 11.0 ± 3.5 pA (n = 598 EPSCs in 5 neurons, unpaired t test, P < 0.001, Fig. 2D3). These data indicated that our batch of DNQX was effective, therefore, slow plateau depolarizations (Fig. 2B) are not mediated via activation of AMPA receptors.
SP neurons are thought to receive axonal fibers from 3 sources, the thalamus, the SP zone and the CP zone. Axon fibers entering the SP zone via the WM are known to originate in the thalamus (thalamocortical projections). Axon fibers from neighboring SP neurons (intralaminar projections) enter the SP zone horizontally, and axon fibers from young pyramidal neurons populating the developing CP descend radially from the CP into the SP zone. We sought to determine if afferent axons which project onto SP neurons from these 3 general directions (SP, CP, and WM) exhibit similar functional properties. Synaptic shocks were delivered in 3 locations: SP, CP, and WM zone, as indicated in the cartoon (Fig. 2D1, stim), while recording evoked synaptic currents via patch pipette on a SP neuron (Fig. 2D1, patch). The average amplitude of EPSCs at the SP, CP, and WM stimulation sites was 84.5 ± 1.5 pA (n = 1625 stimulations in 17 neurons from 7 animals), 64.9 ± 1.1 pA (n = 1549 stimulations in 16 neurons from 7 animals) and 106.8 ± 1.8 pA (n = 1131 stimulations in 15 neurons from 5 animals), respectively (Fig. 2E). The ANOVA showed that variations between groups were statistically significant (P < 0.00001), with the greatest EPSC amplitudes detected when the stimulation electrodes were positioned in the WM (Fig. 2E).
In eleven SP neurons (from 5 animals), we were able to evoke multiple EPSCs while delivering synaptic shocks of the same intensity and duration in all 3 locations: SP, CP, and WM (Fig. 2D1). In this series of experiments, the average amplitude of EPSCs evoked at the SP, CP, and WM stimulation sites were 97.7 ± 2.1 pA (n = 1065 stimulations in 11 neurons), 70.57 ± 1.6 pA (n = 1058 stimulations in 11 neurons), and 105.2 ± 2.1 pA (n = 951 stimulations in 11 neurons), respectively. A frequency histogram of EPSC amplitudes (Fig. 2F) showed some variations between the 3 stimulus locations. One notable difference was the distribution of small amplitude compound EPSCs (≤20 pA, Fig. 2G), here dubbed “synaptic failures,” because the amplitude of the compound (multiunit) EPSCs decreases with an increase in the number of synaptic failures in individual contributing units. The percentage of failures (EPSCs ≤20 pA) evoked in the SP, CP, and WM stimulation sites were 7.7 % (82 out of 1065), 15.8% (168 out of 1058), and 2.5% (24 out of 951 synaptic stimulations), respectively. Chi-square analysis showed that the difference in the fraction of failures between the groups was statistically significant (P < 0.00001). In order to determine if there was anything exclusive about the 20 pA threshold, we performed an identical data analysis setting the synaptic failure thresholds to 10 pA (Fig. 2G, ≤10 pA), 30 pA (data not shown) or 40 pA (data not shown). At each threshold (10, 20, 30, or 40 pA), the fraction of failures was the lowest in the WM group and the difference between the groups was statistically significant (chi-square, P < 0.00001). These data indicate that afferent axons entering the SP zone from the WM (putative thalamocortical projections) have the most reliable synapses, with the fewest failures, consistent with the notion that the major role of SP neurons is the maintenance of thalamocortical projections while cortical pyramidal cells are still migrating (Kanold et al. 2003).
Chemical Synapses
SP neurons in rodents receive spontaneous synaptic inputs during postnatal days P0-P3 mediated by AMPA, NMDA, glycine, and GABAA receptors (Hanganu et al. 2001, 2002). In the present study, we tested the contributions of AMPA, NMDA, GABA-A, and glycinergic receptor currents towards unprovoked endogenous electrical activity of SP neurons in acute brain slices. Bath application of the glycine receptor blocker, strychnine (20 μM) produced small changes in the number of depolarizing events to 103.9 ± 0.1%, duration to 100.1 ± 0.1%, surface area to 97.9 ± 0.1%, and average peak amplitude to 106.3 ± 0.1%, compared with the controls obtained in the same neuron. These changes were not statistically significant (P > 0.05, n = 7 cells, Fig. 2C, STRYCH). In the next series of experiments, we blocked voltage-gated sodium channels, AMPA, NMDA, GABA-A, and glycinergic receptors by bath application of a drug cocktail (synaptic blockers, SB) containing: 1 μM TTX, 20 μM DNQX, 20 μM APV, 20 μM bicuculline, and 20 μM strychnine. The combined blockade of channels and receptors significantly reduced the peak amplitude of the spontaneous depolarizations to 79.0 ± 4.8% (P = 0.012, n = 5 cells, Fig. 2C, SB + TTX). Although their peak amplitude was reduced by ~20%, the depolarizing transients were still present in the recordings at similar numbers and durations as before drug application. That is, the cocktail of synaptic blocker drugs did not significantly change the number of spontaneous depolarizing events, their durations or surface area (P > 0.05, n = 5 cells, Fig. 2C, SB + TTX). These data indicate that 3 major subtypes of amino acid synapses, glutamatergic, GABAergic, and glycinergic, or any other type of AP-mediated neurotransmitter release, are not responsible for slow plateau-like depolarizations occurring spontaneously in SP neurons of the developing mouse cortex.
Gap Junctions and Hemichannels
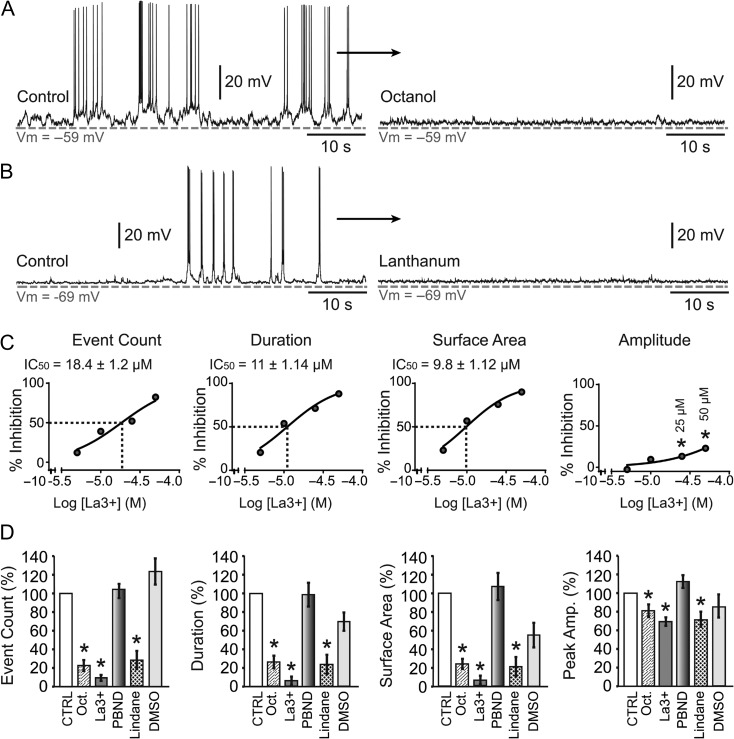
Connexin proteins have a potential to influence the neuronal electrical activity (Deans et al. 2001). Opening of the connexin-based hemichannels may depolarize neurons directly, while opening of the gap junctions bring in depolarizations from the neighboring cells. To test these possibilities in the mouse SP zone, we challenged the spontaneous electrical activity of SP neurons with bath application of the gap junction blocker, octanol (1 mM), which resulted in a radical decrease of spontaneous electrical activity (Fig. 3A, octanol); more radical than any change observed with synaptic blocker drugs (Fig. 2). Octanol significantly reduced the number of depolarizing events, duration, surface area and peak amplitude to 25.7 ± 2.1%, 20.6 ± 4.1%, 17.5 ± 4.2%, and 82.1 ± 5.7%, respectively (P < 0.05; n = 9 cells), compared with control recordings obtained in the same neuron (Fig. 3D, compare Oct. vs. Control). Octanol is a nonselective antagonist of pannexin and connexin pores including gap junctions and hemichannels (Kawamura et al. 2010). During embryonic and postnatal cortical development in rodents, pannexins (PANX1 and 2), and connexins (Cx26, Cx36, Cx37, Cx43, and Cx45) are present in most neurons and exist in hemichannel formation (Nadarajah et al. 1997; Vogt et al. 2005; Cina et al. 2007) suggesting that hemichannels may play a role in facilitating synapse-independent electrical activity in SP neurons. We challenged the spontaneous electrical activity of mouse SP neurons with bath application of a pannexin hemichannels blocker, probenecid (2.5 mM) dissolved in NaOH (0.1 mM). Probenecid-NaOH failed to induce any changes in the 4 activity parameters (P > 0.05, n = 4 cells, data not shown). We next used probenecid dissolved in DMSO. Probenecid-DMSO (2.5 mM) again failed to induce any changes in the spontaneous electrical activity parameters used in the quantitative analysis (P > 0.05, n = 6 cells, Fig. 3D, PBND).
Figure 3.
Gap junctions and hemichannel antagonists. (A) Spontaneous electrical activity in SP neuron before and after application of gap junction blocker octanol (1 mM). (B) Connexin hemichannel blocker lanthanum (50 μM) inhibits the spontaneous activity in SP neuron. (C) Dose response curve for lanthanum (0, 5, 10, 25, and 50 μM) obtained for each of the 4 parameters used in the analysis of spontaneous depolarizations. (D) 2.5 mM probenecid (PBND) and vehicle DMSO have no effect on the 4 parameters. Octanol (1 mM), lanthanum (50 μM), and lindane (50 μM), each significantly reduced event counts, duration, surface area, and peak amplitude of spontaneous plateau depolarizations.
To determine if hemichannels play any role in spontaneous depolarization we used a rare-earth element lanthanum (La3+), which blocks connexin hemichannels but not gap junctions (John et al. 1999; Kondo et al. 2000). Bath application of La3+ (50 μM) produced a notable decrease of spontaneous activity in every neuron tested (Fig. 3B). La3+ significantly reduced the number of depolarizing events, duration, surface area, and peak amplitude of the plateaus to 9.1 ± 2.9%, 5.7 ± 4.4%, 5.7 ± 4.5%, and 69.6 ± 4.6%, respectively (P < 0.05, n = 8), compared with the control recordings obtained in the same neuron (Fig. 3D, La3+). Dose–response curve revealed the effective concentration at which La3+ was able to reduce the number of depolarizing events down to 50% (IC50 = 18.4 ± 1.2 μM, n = 8). The La3+ IC50 values for duration and the surface area were 11 ± 1.14 μM and 9.8 ± 1.12 μM, respectively (Fig. 3C). La+3-induced amplitude reduction at 50 and 25 μM was statistically significant (P < 0.05, n = 8, Fig. 3C, amplitude). In addition to La3+, we also tested lindane (γ-hexachlorocyclohexane), a component of some commercially available lotions and shampoos. Lindane has been previously shown to block connexin hemichannels (Chi et al. 2014). Perfusion of mouse brain slices with lindane (50 μM) produced a statistically significant reduction in the number of events, duration, surface area and peak amplitude down to 27.7 ± 9.76%, 23.1 ± 10.2%, 20.8 ± 10.3%, and 70.1 ± 8.1%, respectively (P < 0.05, n = 5) (Fig. 3D, lindane). Control experiments with vehicle DMSO (0.125%) changed the number of events, duration, surface area and peak amplitude to 123 ± 13.9%, 68.45 ± 9.67%, 54.35 ± 13.1%, and 83.6 ± 12.2%, respectively. The DMSO-induced changes were not statistically significant (P > 0.05, n = 3, Fig. 3D, DMSO).
Lanthanum’s Mode of Action
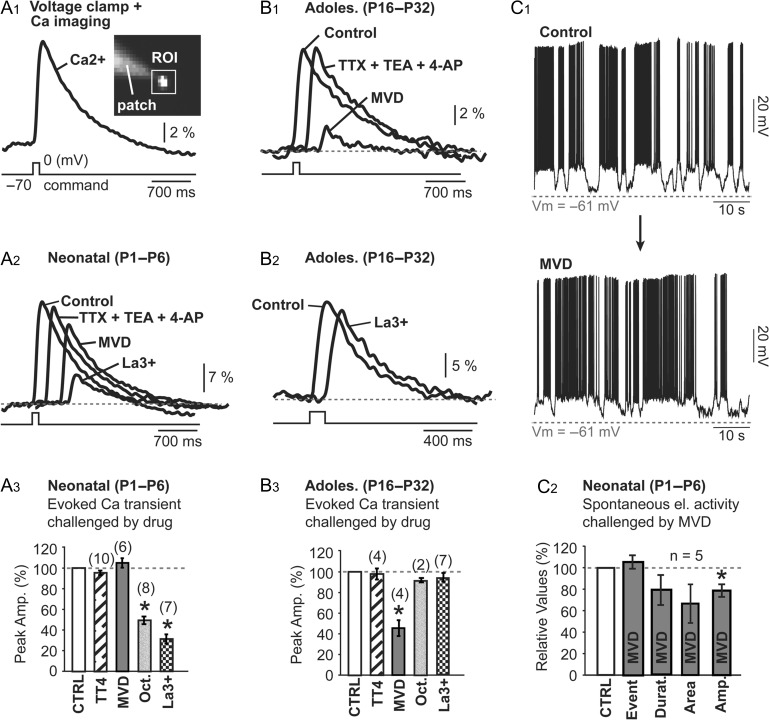
Lanthanum is not an ideal selective antagonist of connexin hemichannels, as it also inhibits VGCCs at concentration of 30 μM (Reichling and MacDermott 1991; Mlinar and Enyeart 1993; Block et al. 1998). In order to determine the La3+ mode of action for inhibiting spontaneous activity in the neonatal mouse (Fig. 3B), we performed experiments in 2 age groups, neonatal mice (P1–P6) and adolescent mice (P16–P32). In neonatal mice (P1–P6), SP neurons injected with calcium-sensitive dye OGB1 were voltage clamped from −70 to 0 mV (duration = 100 ms) and calcium influx was detected in the cell body by calcium imaging (Fig. 4A1). To block voltage-gated Na+ and K+ channels, we used a cocktail of drugs including TTX (1 μM), TEA (10 mM); and 4-AP (5 mM), here denoted “TT4.” Upon TT4 application, calcium signal decreased to 95.4 ± 2.2%, however, this change was not statistically significant (P < 0.05, n = 10, Fig. 4A3). Next we used a cocktail of VGCC blockers (mibefradil, verapamil, and diltiazem, each at 20 μM), here denoted “MVD,” to block several subtypes of Ca2+ channels (Bezprozvanny and Tsien 1995). The mean signal amplitude in MVD was 105.3 ± 4.5% of the control value obtained in the same cell and these changes were not statistically significant (P > 0.05, n = 6 cells, Fig. 4A3, MVD). Bath applications of connexin gap junction antagonist (octanol, 1 mM) or connexin hemichannel antagonists (La3+, 100 μM) in neonatal mice reduced voltage-evoked calcium signal down to 49.2 ± 3.7%, n = 8 and 31.2 ± 4.5%, n = 7, respectively, and both changes were statistically significant P < 0.05 (Fig. 4A3).
Figure 4.
Lanthanum mode of action. (A1) Patch pipette filled with calcium-sensitive dye OGB1 was used to fill a SP neuron. Voltage clamp step (shown below Ca2+) from −70 to 0 mv (duration 100 ms) was used to trigger calcium influx measured by calcium imaging inside the region of interest (ROI) covering the cell body. (A2, A3) In P1–P6 mice, several drug combinations have small effect on the voltage-evoked calcium transient: TTX (1 μM) + TEA (10 mM) + 4AP (5 mM); Mibefradil (20 μM) + Verapamil (20 μM) + Diltiazem (20 μM). However, 100 μM La3+ inhibits calcium flux in neonatal mice. In adolescent mice (P16–P32), on the other hand, MVD inhibits calcium current (B1) while La3+ has no effect (B2, B3). Voltage clamp step was synchronized with the first Ca2+ trace (Control). All subsequent traces were shifted to the right for clarity. (C1) Spontaneus activity before and after MVD (Mibefradil [90 μM] + Verapamil [20 μM] + Diltiazem [20 μM]). (C2) MVD has no effect on 3 parameters: event count, duration, and area.
The same experiments described for neonatal P1–P6 mice (Fig. 4A3) were also performed in layer 6 pyramidal cells of adolescent mice (P16–32), but with a very different outcome. In the adolescent mice, application of MVD greatly reduced the somatic Ca2+ signal (Fig. 4B1, MVD), while application of La3+ (100 μM) had no effect (Fig. 4B2, La3+). More specifically, in layer 6 pyramidal neurons of adolescent mice, changes of the somatic Ca2+ transient induced by TTX + TEA + 4AP (TT4, n = 4), or gap junction blocker (1 mM octanol; n = 2 cells), or hemichannels blocker (100 μM La3+; n = 7 cells), were small and not statistically significant (P > 0.05) (Fig. 4B3). However, the application of VGCC blockers (MVD) significantly reduced peak amplitude of the somatic Ca2+ transients down to 45.6 ± 7.7% of the control value obtained in the same cell (P < 0.05; n = 4 cells, Fig. 4B3). These data indicate that voltage-evoked influx of Ca2+ is mediated by connexin hemichannels in neonatal mice, while in adolescent mice the voltage-evoked influx of Ca2+ is mediated by VGCCs. For that reason, the neonatal calcium flux is very sensitive to hemichannel antagonists, La3+, but insensitive to VGCC antagonists. This finding is completely opposite from the results obtained in adolescent mice. In adolescent mice, voltage-induced calcium flux is not sensitive to hemichannel antagonists (La3+), but very sensitive to VGCC antagonists, MVD (Fig. 4A3,B3).
In the next set of experiments, we directly assessed the contribution of VGCCs in spontaneous activity of SP neurons in neonatal (P1–P6) mice using the same mixture of VGCC blockers (MVD) as in Figure 4A,B, except mibefradil was increased to 90 μM; a concentration at which mibefradil blocks T-type, L-Type, N-Type, Q-Type, and R-Type calcium channels (Viana et al. 1997). Bath application of MVD did not eliminate spontaneous activity in neonatal SP (Fig. 4C1). Changes in the number of depolarizing plateau events, duration, and surface area, before and after the MVD treatment were not statistically significant (P > 0.05; n = 5 cells, Fig. 4C2). However, the peak amplitude of the depolarizations was significantly decreased to 77.6 ± 4.6% compared with the control value obtained in the same neuron (P < 005; n = 5 cells, Fig. 4C2). The simplest explanation of these data is that sparsely expressed VGCCs in SP neurons and VGCCs in afferent axon terminals (which mediate the release of neurotransmitters) contribute ~20% of the total inward current to SP neurons during spontaneous in vitro activity. VGCCs improve the amplitude but they do not affect the total number of depolarizing events or their duration.
Connexin Hemichannels in the Neuronal Plasmalemma
Connexins have been shown to be expressed by both glial and neuronal cell types (Thompson and Macvicar 2008). To determine if connexin hemichannels are present in the membranes of SP neurons, we employed an IC application of hemichannel blocker Gd3+ via patch electrode. To prevent leakage of Gd3+ into extracellular space surrounding the SP neuron, the tip of the patch pipette was filled with Gd3+-free IC solution. A drug-free solution in the tip of the pipette prevented the leakage of Gd3+ into the extracellular environment during formation of the gigaohm seal. This strategy was previously adopted for perforated patch (Linley 2013) or IC injection of lipophilic dyes (Antic 2003).
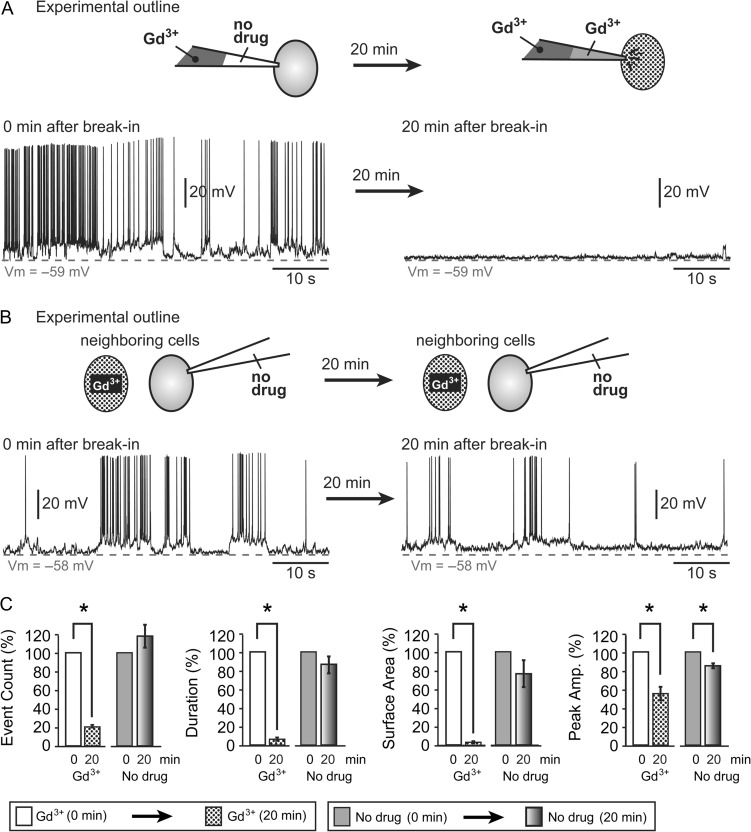
Spontaneous electrical activity was recorded immediately after the whole-cell break-in (Fig. 5A, 0 min), before Gd3+ had a chance to diffuse into the pipette tip (Fig. 5A, cartoon), and again 20 min later, when Gd3+ had diffused into the cell (Fig. 5A, 20 min). IC application of 25 μM Gd3+ thus produced a statistically significant reduction in the number of events, duration, surface area, and peak amplitude down to 20.5 ± 1.4%, 6.3 ± 1.8%, 3.5 ± 1.2%, and 54.8 ± 7.2%, respectively, compared with control recordings performed in the same cell before Gd3+ entered the cell (P < 0.05; n = 3 cells) (Fig. 5C, Gd3+). Next, a neighboring untreated SP neuron was patched with a drug-free IC solution (Fig. 5B, no drug). This was done for 2 reasons. First, to test if during the previous experiment Gd3+ leaked into the extracellular space and blocked neuronal membrane hemichannels from the outside. Second, to test if 20 min recordings caused a rundown of spontaneous activity by IC dialysis (Fig. 5B, cartoon). We did not find any significant changes in the number of events, duration or surface area in the “No drug” group (P > 0.05, n = 3 cells, Fig. 5C, no drug). The peak amplitude of depolarizing events decreased to 84.5 ± 2.6% and the difference was statistically significant (P = 0.027; n = 3 cells). Our data indicate that Gd3+-sensitive pores exist in the neuronal plasma membrane. A Gd3+-induced block of membrane channels causes a severe reduction in the number, duration, and amplitude of spontaneously occurring plateau depolarizations in mouse SP neurons.
Figure 5.
Intracellular application of Gd3+. (A) The cartoon depicts SP neuron in a whole-cell configuration. Patch pipette contains 25 μM Gd3+. The tip of the pipette contains drug-free solution. And 20 min later, Gd3+ diffuses into the cell body. Two traces were recorded from the same SP neuron, immediately after the membrane break-in and 20 min later. (B) The cartoon depicts 2 SP neurons, ~20 μm apart. SP neuron which did not receive Gd3+ injection was patched with a drug-free pipette. Two traces were recorded from the same untreated SP neuron, immediately after the membrane break-in and 20 min later. (C) Intracellular gadolinium significantly reduced the number of events, duration, surface area and peak amplitude when compared with control recordings obtained in the same neuron before Gd3+ diffused into the cell body. Neurons patched with IC solution only (no drug) show a small decrease in plateau depolarization amplitude after 20 min of patching.
Connexin Expression in Developing Mouse Cortex
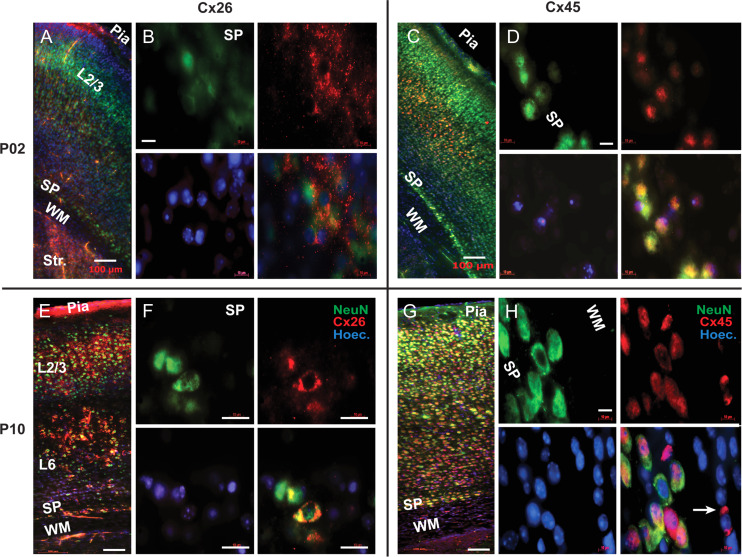
In mouse brain sections, we evaluated the presence of connexin isoforms Cx26, Cx32, Cx36, Cx43, and Cx45 via fluorescence immunolabeling. At age P2, we did not find any expression of Cx32, Cx36, and Cx43 in the mouse cerebral cortex. At age P2, labeling for Cx26 was observed in the striatum (Str), SP zone, and CP (Fig. 6A). Within the SP zone, individual cell bodies and the neuropil surrounding them were labeled in a punctate manner (Fig. 6B). A number of nuclei stained by Hoechst in the SP zone were negative for both NeuN (neuronal marker) and Cx26, indicating a presence of a non-neuronal lineage which does not host Cx26. At age P2, sparse labeling of Cx45 was observed in layer 2/3 and layer 6, while stronger labeling was observed in layer 5, and in the SP zone (Fig. 6C). In the SP zone in particular, Cx45-positive cells were colabeled by NeuN (Fig. 6D). These double-labeled neurons were identified as “SP neurons” based on the location inside the SP zone, large cell diameter and strong expression of NeuN marker, indicative of a mature neuron.
Figure 6.
Connexin 26 and 45 immunolabeling. (A) P2 mouse. Composite image (Zeiss Mosaic) comprised of multiple tiles under 20× objective. Double immunolabeling of Cx26 (red) and NeuN (green) in P2 animals, showing weak expression of Cx26 in SP neurons at this age. (B) 100× objective. Colocalization of NeuN (green) and Cx26 (red) in the SP neurons at P2 age. (C) P2 mouse. Composite image comprised of multiple tiles under 20X objective. Double immunolabeling with Cx45 (red) and NeuN (green) showing expression of Cx45 in all cortical layers and the SP zone at P2 age. (D) 100× objective. Colocalization of NeuN (green) and Cx45 (red) in the SP neurons at P2 age. (E) P10 mouse. Composite image comprised of multiple tiles under a 20× objective. At P10 age, Cx26 (red) expression increases in layers 2, 3, 5 and in SP zone, compared with age P2. (F) Images obtained with 100× lens show colocalization of NeuN (green) and Cx26 (red) in the SP neurons at P10. (G) P10 mouse. Composite image comprised of multiple tiles under 20× objective. At P10, Cx45 (red) expression increases throughout the cortex and SP zone, compared with P2 mice. (H) 100× objective. Colocalization of NeuN (green) and Cx45 (red) in the SP neurons, at P10. Cx45 also expresses in non-neuronal cells of the white matter (arrow). Scale bars in A, C, E, and G = 100 μm; in B, D, F, and H = 20 μm. SP = subplate; WM = white matter. Str. = Striatum; Hoechst nuclei stain (blue).
Brain sections of age P10 mice were treated in the same way as P2 mice, using the same batch of antibodies. At age P10, the Cx26 expression was considerably stronger than in the P2 mice. Cx26 expression was observed in striatum, cortical layers 2/3, 5 and in the SP zone (Fig. 6E). Within the SP zone, individual cell bodies of Cx26-positive cells were co-labeled with NeuN (Fig. 6F). At age P10, the expression of Cx45 was found in the striatum, SP zone and CP (Fig. 6G). In the mouse SP zone the expression of Cx45 was stronger at P10 compared with P2 mice. Within the SP zone of P10 mice, large neurons, putative SP neurons, were co-expressing both neuronal marker NeuN and Cx45 (Fig. 6H). In non-neuronal cells, NeuN-negative cells located inside the WM zone, putative oligodendrocytes were labeled by Cx45 at age P10, but not at age P2 (Fig. 6H, arrow).
Glial Cells
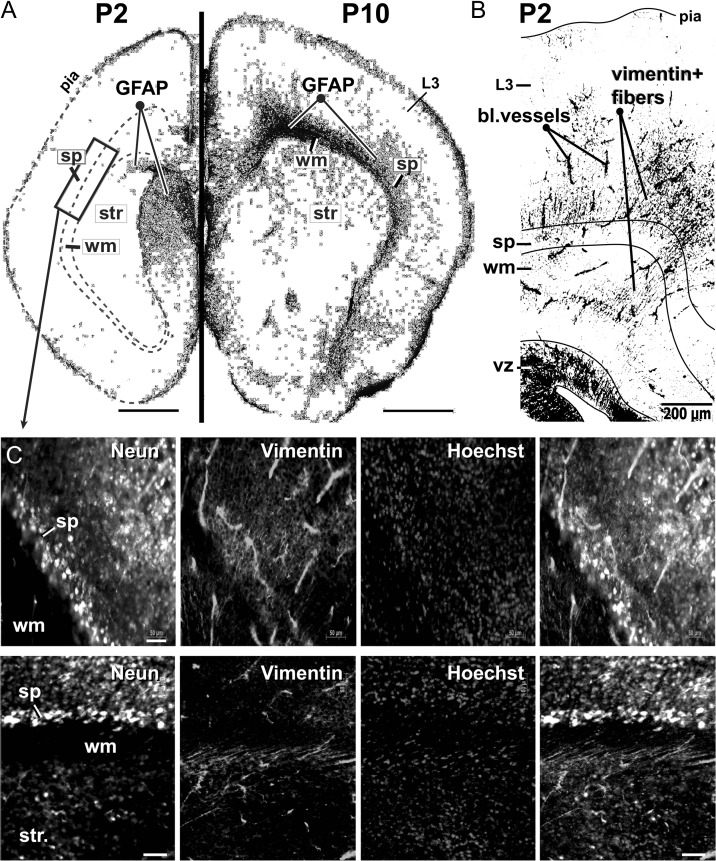
One of the mechanisms by which glial cells release ATP in the extracellular environment is via the opening of connexin hemichannels (Stout et al. 2002; Orellana et al. 2011). We used dual immunolabeling for NeuN (neuronal marker) and GFAP (glial fibrillary acidic protein) to test for the presence of glial cells in our experimental preparation. At age P2, the GFAP signal was very weak in the striatum, SP zone and CP (Fig. 7A, P2). The only notable collection of GFAP-positive cells was found surrounding the ventricle (Fig. 7A, P2, GFAP). At age P10, the expression of GFAP in the cerebral cortex increased markedly (Fig. 7A, P10). GFAP-positive cells were found in the WM, Str, SP, and some scattered GFAP-positive cells were located in cortical layer 3 (Fig. 7A, P10, L3). The highest concentration of GFAP-positive cells was found in the WM. There was a striking difference between P10 WM and P2 WM in terms of GFAP labeling (Fig. 7A, compare P2 dashed line and P10 wm). These data indicate that very few astrocytes are present in the SP zone 2 days after birth (P2). Around age P10, clusters of young astrocytes radiate to the cerebral cortex by means of the white matter (Fig. 7A, P10, wm).
Figure 7.
GFAP and vimentin in the SP zone. (A) Coronal sections of P2 (left) and P10 mouse brain (right), immunolabeled with GFAP (astrocyte marker—black). Each section is a composite of multiple tiles under a 10× objective lens. Images are inverted and equalized. Scale bar = 1 mm. Note that, GFAP is largely absent in P2 cerebral cortex. Rectangle indicates the cortical area used for the acquisition of images at higher magnifications, such as those shown in C. (B) Coronal section of a P2 mouse brain, immunolabeled with Vimentin (black). Scale bar = 200 μm. (C) Subplate zone immunolabeled with NeuN, Vimentin and Hoechst stain. The fourth image is a merge. A high density of vimentin-positive fibers resides inside the SP zone. Scale bar = 50 μm. SP = subplate; WM = white matter; Str = striatum.
Next, we performed double immunolabeling against a glial marker vimentin and neuronal marker NeuN. In contrast to the results obtained with GFAP labeling; the vimentin signal was strong in the cerebral cortex of P2 mice (Fig. 7B). Vimentin-positive fibers projected through the white matter of P2 mice and invaded both the SP and CP (Fig. 7B,C). The vimentin-positive fibers did not acquire any NeuN labeling, suggesting their non-neuronal, glial origin. The antigenic properties (vimentin-positive and NeuN-negative), together with location, cellular morphology and orientation of the fibers, suggested that these are radial glia cells (Clowry et al. 2010). Taken together the immunolabeling data indicate that glial population in the SP zone of P2 mice is dominated by radial glial cells with occasional GFAP-positive astrocytes. The presence of glial cells in the SP zone opens a question of whether glial cells and pioneer SP neurons engage in physiological interactions related to spontaneous electrical activity.
Purinergic Receptors
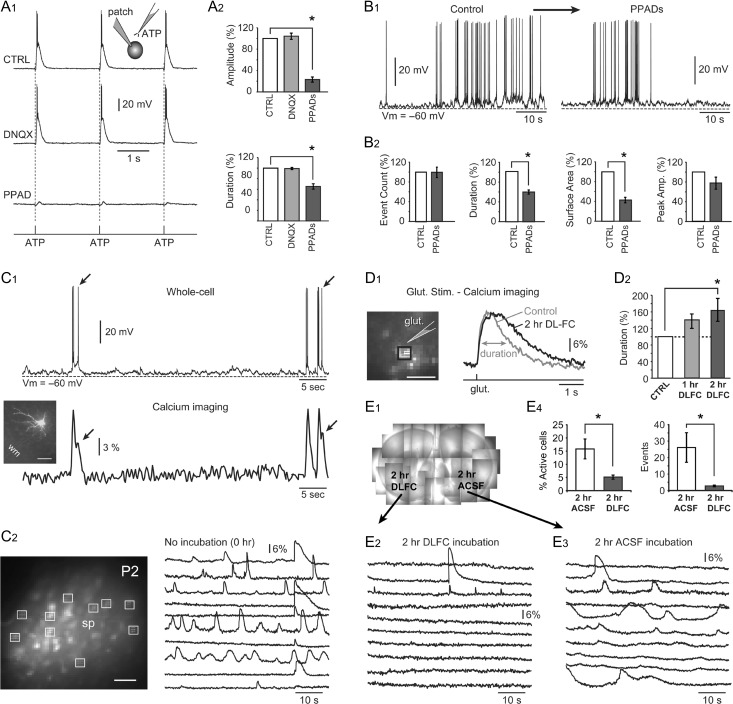
Recent studies have shown connexin-mediated ATP release during early mouse brain development (Goodenough and Paul 2003; Weissman et al. 2004). We attempted to detect the presence of functional purinergic receptors on the mouse SP neurons using electrophysiological measurements. Positively identified SP neurons were patched and glass micropipettes filled with 100 mM ATP were placed ~20 μm away from the cell body (Fig. 8A, inset). Three iontophoretic pulses of ATP (pulse duration = 5 ms, pulse frequency = 0.5 Hz) were delivered near the cell while recording membrane potential. In response to each ATP application, SP neurons generated plateau depolarizations with amplitudes greater than 20 mV and durations greater than 200 ms (Fig. 8A1, CTRL). Since mouse SP neurons receive glutamatergic synapses (Fig. 2) (Hanganu et al. 2002), it is possible that ATP iontophoresis pulses were stimulating glutamatergic release near the target SP cell and the observed plateau depolarizations were mediated by glutamatergic receptor currents. To test for this possibility, we repeated ATP iontophoresis in the presence of the AMPA receptor blocker DNQX (5 μM). DNQX did not produce significant changes in the amplitude or duration (half-width) of the evoked plateau depolarizations (P > 0.05; n = 5 cells, Fig. 8A2). Antagonist of purinergic P2X receptors, PPADS (10 μM), on the other hand, significantly reduced the amplitudes and durations of the evoked plateau depolarizations down to 23.1 ± 4.8% and 65.3 ± 5.0% (P < 0.05; n = 5 cells, Fig. 8A2). The ability of the SP neurons to give plateau depolarizations upon ATP stimulation and the sensitivity of these plateau potentials to the purinergic receptor blocker, PPADS, indicated the presence of functional purinergic P2X receptors on the membrane of SP neurons.
Figure 8.
Purinoreceptors and glial production of ATP. (A1) Inset: Experimental outline comprising whole-cell recording and ATP microiontophoresis in control and drug conditions. In control condition (no drug) and in the presence of glutamate receptor antagonist (DNQX), an SP neuron experiences 3 plateau depolarizations in response to 3 pulses of ATP (pulse duration = 5 ms). (A2) The amplitudes and durations of the ATP-induced plateau depolarizations are insensitive to DNQX but highly sensitive to the P2X receptor antagonist, PPADS. (B1) Spontaneous electrical activity before (control) and after application of PPADS. (B2) PPADS significantly decreased the duration and the surface area of spontaneous plateau depolarizations. (C1) At the border between the SP zone and WM, an SP neuron is loaded with a calcium-sensitive dye (OGB1) via patch pipette (inset, scale bar = 50 μm). Calcium transients accompany spontaneous action potential firing. Individual spikes are reflected in the calcium signal (arrows). (C2) Fluorescent spots mark cells in the SP zone, which took up the calcium-sensitive dye, OGB-1AM. Scale bar = 50 μm. Spontaneous calcium signals from the selected ROIs. (D1) Fluorescent spots mark cells in the SP zone, which took up the calcium-sensitive dye, OGB-1AM. Scale bar = 50 μm. Calcium signals were evoked by glutamate microiontophoresis (5 ms duration) before and after application of DLFC for 2 h. (D2) Half-width (duration) of glutamate-evoked calcium transients increased significantly 2 h following DLFC introduction. (E1) Composite photograph of 2 brain slices, anchored by nylon strings, inside the recording chamber. One slice was treated by DLFC for 2 h (left), while the other slice spent 2 h in drug-free ACSF. Spontaneous calcium transients in treated (E2) and untreated (E3) slice. (E4) Percentage of spontaneously active cells (% active cells) and the total number of calcium transients per visual field (events) are significantly reduced by the drug (DLFC).
We next investigated if purinergic receptors contribute to spontaneous plateau depolarizations in neonatal P2 mice (Fig. 8B1). Application of PPADS (10 μM) did not significantly change the number of depolarizing events or their amplitudes (P > 0.05; n = 5 cells). However, it significantly decreased the duration and surface area of the depolarizing events down to 59.1 ± 4.1% and 43.0 ± 5.4%, respectively (P < 0.05; n = 5 cells, Fig. 8B2). These data indicate that during in vitro spontaneous electrical activity, ATP is released into extracellular space surrounding SP neurons. ATP binds to purinergic receptors present on the SP neurons and causes ionic currents that prolong plateau depolarizations.
Calcium Transients and the Role of Glia
We performed simultaneous whole cell and optical recordings from SP neurons using intracellularly applied calcium-sensitive dye OGB1. Spontaneous electrical events consisting of plateau depolarizations topped with APs produced robust calcium transients. In some cases, single APs were detected in optical traces (Fig. 8C1, arrows). Next, a membrane permeable calcium indicator dye, OGB1-AM, was pressure ejected into the brain slice at the SP zone. The image of fluorescently labeled cells was projected onto the CCD camera (Fig. 8C2, SP) and fluorescence intensity recorded for 120 s. In these experiments (n = 10), we were able to monitor spontaneous calcium signal from 30 to 50 cells in the SP zone simultaneously for the duration of 120 s.
To determine if glial cells of the SP zone supply ATP for the purinergic signaling which mediates spontaneous activity in pioneer SP neurons, we blocked ATP production in glial cells using DL-Fluorocitrate (DLFC). At low concentration, DLFC inhibits Krebs’ cycle in glial cells but not in neurons (Hassel et al. 1992). Since DLFC is readily taken up by glial cells, it is used for selectively inhibiting glial metabolism (Swanson and Graham 1994; Wenker et al. 2010). Because a prolonged blockade of glial metabolism could potentially cause neuronal death, we first determined the time required by DLFC to exhibit its pharmacological effect. Glial glutamate transporters remove extracellular glutamate from the cortical neuropil, consuming ATP in the process. Thus, the efficacy of the glial glutamate uptake reflects the status of the glial ATP production. In this experimental series, brief iontophoretic pulses (duration = 5 ms) of glutamate were used to evoke calcium plateaus, which were detected by fast calcium imaging (Fig. 8D1). Glutamate-evoked calcium transients were measured before DLFC application (Control), at 1 h and at 2 h from the start of DLFC (100 μM) perfusion. Upon 1 hr of drug application, the average half-width of glutamate-evoked calcium signals increased to 140 ± 17.3%, however, this change was not statistically significant (P > 0.05, n = 3, Fig. 8D2, 1 h). Upon 2 h of drug perfusion, the average duration of glutamate-evoked calcium signals increased to 162.7 ± 28% and this change was statistically significant compared with the control measurements obtained in the same region of interest (P < 0.05, n = 3, Fig. 8D2, 2 h). This set of experiments established that bath application of DLFC required 2 h to significantly diminish glutamate uptake (glial ATP production) in acute brain slices.
In order to determine the effect of the glial ATP production (glial metabolism) on spontaneous calcium activity in the SP zone of neonatal mice, we designed the following experiments. Brain slices were harvested from the same animal, separated in 2 groups and kept for 2 h in bubbling ACSF. One group of slices was kept in a drug free ACSF for 2 hr (“2 h ACSF”). The other group received the glial metabolism inhibitor DLFC (100 μM) dissolved in ACSF (“2 h DLFC”). At the end of the 2 h incubation period, slices were washed and transferred to the recording chamber. To assure identical recording conditions (postmortem period, perfusion, temperature, etc.), slices from both groups were placed together, side by side, into the same recording chamber (Fig. 8E1). Spontaneous calcium transients were recorded from the SP zone of both brain slices in an alternating fashion using 2 min long recording sweeps. We found that spontaneous activity of brain slices incubated in DLFC for 2 h (Fig. 8E2) was notably weaker than the activity recorded in slices incubated in drug-free ACSF for 2 hr (Fig. 8E3). The percentage of spontaneously active cells in the “2 h DLFC” group (5.1 ± 0.7%, n = 6) was significantly smaller than in the “2 h ACSF” group (15.8 ± 3.78 %, P < 0.05, n = 6, Fig. 8E4). The number of calcium events (calcium transients) per visual field was also significantly smaller in “2 h DLFC” group (2.78 ± 0.5) compared with the “2 hr ACSF” group (26.1 ± 8.9, Fig. 8E4). These experimental data indicate that intact glial metabolism (ATP production in glial cells) stimulates spontaneous physiological activity in the SP zone of neonatal mice.
Discussion
Limitations of the Current Study
By filtering out APs and fast synaptic potentials, the data analysis used in the current study is biased toward large (> 2.5 mV) and prolonged (25–20 000 ms) depolarizations. This procedure underestimates the synaptic content and synaptic activity of SP (Kostovic and Rakic 1990) and enhances the statistical impact of plateau depolarizations. One additional major concern is a potentially weak translational value of the current study. It may be that an in vitro study on tiny mouse SP (thickness < 0.2 mm, the current study) has very limited value for functional organization of a thick (> 1.5 mm) long-lasting SP in humans (from 8 weeks of gestation to the end of the first year of life) (Duque et al. 2016). Although this is a valid concern, we would like to point out several findings of the present study suggesting that mouse SP is a reasonable preparation for modeling cellular physiological processes occurring in the human SP zone. In P2 mice, we found that layer 2/3 neurons have significantly smaller sodium currents, weaker APs and less spontaneous electrical activity than the large SP neurons, consistent with the whole-cell recordings performed in the SVZ, IZ, SP, and CP of human fetus (Moore et al. 2009), and consistent with the notion that SP neurons are the pioneer cortical neurons, first to be born and first to mature in the mammalian cerebral cortex (Kanold and Luhmann 2010). A significantly greater amount of spontaneous electrical activity in SP neurons, compared with CP cells, is due to better developed membrane (greater density of functional voltage-gated and ligand-gated channels), rich dendritic and axonal trees, capable of receiving excitatory synaptic inputs (Moody and Bosma 2005). It was previously established that rodent SP neurons receive spontaneous synaptic inputs mediated by glutamate, GABA and glycine (Hanganu et al. 2001, 2009; Corlew et al. 2004; Wess et al. 2017). In the present study, we stimulated axons arriving into the SP zone from white matter (ascending), from the CP (descending), as well as axons projecting laterally through the SP zone, while measuring evoked synaptic currents in SP neurons (Fig. 2E). Amplitudes of excitatory currents were very similar among these 3 categories of afferent fibers (Fig. 2F,G). The only difference was in the number of failures (Fig. 2G), suggesting that thalamic projections are more reliable than the projections from SP and CP networks. Previous recordings of evoked synaptic potentials in human SP neurons have determined that only 35% of human SP neurons responded to synaptic stimulation (Moore et al. 2011). In the present study, every mouse SP neuron tested (100%) produced synaptic currents, robustly and repetitively (Fig. 2E). The most likely reason for this difference is a substantially longer postmortem period in the human study (~3 h) causing deterioration of axons and synapses.
Comparisons With Other Forms of Precritical Electrical Activity
The critical period in brain development is the one period of life in which sensory inputs strongly guide the normal development of the brain. Interestingly, the neural connections and precise topography of the receptive fields exist before the critical period, and are most likely based on the precritical forms of spontaneous (sensory input-independent) electrical activity (Tritsch et al. 2007). Precritical spontaneous electrical activity in SP neurons (Fig. 1F) has a very similar voltage waveform (plateau depolarization crowned by APs) to the cortical early network oscillations (cENOs) and giant depolarizing plateau (GDPs) found in rodent cortex and hippocampus during the precritical period of brain development (Ben-Ari et al. 1989; Garaschuk et al. 1998; Crepel et al. 2007; Allene et al. 2008). The cENOs are mediated by NMDA receptors, while the GDPs are mediated by the neurotransmitter GABA, which is excitatory during development (Allene et al. 2008). In the current study, blocking network interactions mediated by AP-mediated release of neurotransmitters (by TTX) did not eliminate spontaneous depolarizing events (Fig. 2A). In addition, despite functional synaptic inputs on SP neurons (Fig. 2D), the spontaneous plateau depolarizations were only modestly affected by blockers of glutamatergic, GABAergic and glycinergic transmission, indicating that: 1) network activity is not the sole driver of SP depolarizations; and 2) SP activity is different from cENOs or GDPs. However, there are interesting physiological similarities between developing retina and SP before the onset of sensory perception. Both entities experience spontaneous bursts of APs riding on a large depolarizing wave, initiated apparently at random in different regions, and occurring at long time intervals lasting tens of seconds (Moody and Bosma 2005). Both retina and SP maintain their spontaneous activity in the presence of AMPA, NMDA, glycine, and GABA-A receptor antagonists (Blankenship and Feller 2010). Both are strongly reduced by pharmacological blockade of connexin-based membrane pores and adenosine receptors (Figs 3 and 8) (Stellwagen et al. 1999; Beamer et al. 2017).
Gap Junctions and Hemichannels
One SP neuron makes on average 9 gap junction couplings with neighboring SP neurons and neurons in the CP (Dupont et al. 2006). Spontaneous network activity patterns in developing cochlea, retina, and neocortex can be suppressed by pharmacological blockade of gap junctions (Peinado et al. 1993; Syed et al. 2004; Tritsch et al. 2007). The sensitivity of spontaneous electrical activity to octanol may initially suggest the involvement of gap junction (Fig. 3A), but octanol is known for not differentiating between gap junctions and hemichannels. Probenecid, a very selective antagonist of pannexin hemichannels did not change spontaneous electrical activity in SP neurons (Fig. 3D, PBND). Extracellular lanthanum at low doses (<20 μM) and IC gadolinium (25 μM), both used as antagonists of connexin hemichannels with no effect on connexin gap junction communication (John et al. 1999; Kondo et al. 2000; Contreras et al. 2002), produced the most profound decline of spontaneous depolarizations (Fig. 3B). We showed that La3+ did not affect VGCCs in adolescent (P16–P32) layer 6 pyramidal neurons (Fig. 4B2), and blocking of VGCCs by a mixture of mibefradil, verapamil and diltiazem (MVD) did not affect spontaneous depolarizations in young (P1–P6) mice (Fig. 4C). The same combination of drugs (MVD) effectively blocked voltage-evoked calcium flux in L6 pyramidal neurons of P16–P32 mice (Fig. 4B1) confirming the potency of our batch of drugs. These data (Table 1) converge on a conclusion that connexin (but not pannexin) hemichannels play a major role in the generation of endogenous spontaneous electrical activity in SP neurons, as previously determined in human SP (Moore et al. 2014). Connexin hemichannels can open either spontaneously or due to changes in pH, electrolytes (ions), O2, and CO2 levels (Ripps et al. 2002). Spontaneous flickering of connexin hemichannels also occurs in developing mouse cochlea (Tritsch et al. 2007; Johnson et al. 2017), developing retina (Pearson et al. 2005) and rodent ventricular zone (Weissman et al. 2004).
Table 1.
Overview of different pharmaco-substances used, cellular-transmission targets and their major effect on spontaneous activity
| Drug | Concentration | Function | Effect on SP spontaneous activity | |
|---|---|---|---|---|
| 01 | TTX | 1 μM | Blocks voltage-gated sodium channels | Complete block of action potentials. No effect on plateau depolarizations |
| 02 | DNQX | 20 μM | Blocks glutamatergic AMPA receptors | No significant effect on plateau depolarizations |
| 03 | Strychnine | 20 μM | Blocks glycinergic receptors | No significant effect on plateau depolarizations |
| 04 | Cocktail of 5 drugs dubbed “Syn. Block” (DNQX, APV, bicuculline, strychnine, and TTX) | DNQX = 20 μM; APV = 20 μM; Bicucull. = 20 μM; strychnine = 20 μM; TTX = 1 μM | Blocks glutamatergic (AMPA and NMDA), GABAergic (GABA-A), and glycinergic receptors, as well as the voltage-gated sodium channels |
|
| 05 | Octanol | 1 mM | Blocks gap junctions and hemichannels | Potently blocks plateau depolarizations |
| 06 | Lanthanum | 50 μM | Blocks connexin hemichannels | Potently blocks plateau depolarizations |
| 07 | Probenecid | 2.5 mM | Blocks pannexin hemichannels | No effect on plateau depolarizations |
| 08 | Lindane | 50 μM | Blocks connexin hemichannels | Potently blocks the number and duration of plateau depolarizations. Small but significant decrease of the plateau amplitude |
| 09 | Cocktail of 3 drugs dubbed “MVD” (Mibefradil, Verapamil and Diltiazem) | Mibefradil = 90 μM; Verapamil = 20 μM; Diltiazem = 20 μM | Blocks voltage-gated calcium channels | No effect on the number of depolarizing events, or their duration. A small but statistically significant decrease in plateau amplitude |
| 10 | Gadolinium | 25 μM | Blocks connexin hemichannels | Potently blocks plateau depolarizations |
| 11 | PPADS | 10 μM | Blocks purinergic receptors | Decreases the duration of the plateau depolarizations, causing a significant change in the surface area |
| 12 | DLFC
|
100 μM | Blocks ATP production in glia | Decreases the number of active cells per visual field and the number of events in recordings |
Purinoreceptors and Glia
During the first postnatal week of mouse development (P0–P6), ATP contributes and modulates early electrical activity in hippocampal neuronal networks via P2X purinergic receptors (Safiulina et al. 2005). The supporting cells within Kölliker’s organ release ATP and excite neighboring hair cells, resulting in bursts of electrical activity in spiral ganglion neurons before the onset of hearing (Tritsch et al. 2007). Connexin hemichannel-mediated ATP release drives the propagation of spontaneous intercellular calcium waves through radial glial cells in the embryonic ventricular zone via activation of purinergic receptors (Weissman et al. 2004). In the present study, pulse application of exogenous ATP onto the SP neurons invariably caused plateau depolarizations which were sensitive to PPADS, indicating the presence of P2X receptors on SP neurons (Fig. 8A). Bath application of PPADS significantly decreases the duration and surface area of spontaneous depolarizing events consistent with a recent study (Beamer et al. 2017). However, while Beamer et al. (2017) attribute ATP to astrocytes, our histological analysis of mouse P2 cortex indicate that GFAP-positive astrocytes are largely absent at that age. Instead, vimentin-positive radial glia fibers occupy spaces between SP neurons (Fig. 7). Shutting down of ATP production in glial cells resulted in a significant reduction of the number of spontaneously active SP neurons and the number of calcium transients per visual field (Fig. 8E), strongly suggesting that similar to the retina and Kölliker’s organ, additionally in the SP zone, the supporting cells (radial glia) releases ATP to stimulate electrical activity of neurons (Stellwagen et al. 1999; Tritsch et al. 2007; Beamer et al. 2017). Via the connexin hemichannel-mediated release of ATP, the same radial glia fiber is stimulating proliferation in the SVZ and electrical activity in the SP (Stout et al. 2002; Goodenough and Paul 2003; Weissman et al. 2004).
Implications
Spontaneous firing of SP neurons is a heterogeneous neural substrate supported by a number of interacting pathways involving connexin-based hemichannels, gap-junctions, ATP-releasing glial cells, and to a lesser amount, cholinergic, glutamatergic, GABAergic, and glycinergic synaptic transmission involving horizontal and vertical axon afferents (Hanganu et al. 2009; Wess et al. 2017). So many cellular mechanisms converge onto the same goal, to render SP networks resistant to perturbation in the sense that environmental or genetic disruptions of crucial network components lead to the expression of alternative circuit mechanisms that generate activity similar to the endogenous pattern, suggesting that redundancy is built into the SP circuit to ensure that the spontaneous activity is maintained (Blankenship and Feller 2010). Understanding the mechanisms underlying precritical electrical activity in utero (Moore et al. 2014), as well as an increased awareness of its crucial role in brain development, could have some implications for the treatment of pregnant women. The present study indicates that blockers of hemichannels potentially can affect electrical activity in the developing cerebral cortex and the use of such agents in medical or commercial products (e.g., gadolinium in contrast agents used in neuroradiology, lindane in lotions and shampoos) during pregnancy should be carefully evaluated. When analyzing potential harmful effects of gadolinium-based contrast agents, it may be prudent to include pregnancy, especially in women with renal insufficiency.
Supplementary Material
Author Contributions
M.B.S. and S.D.A. designed research; M.B.S., E.J.M., M.M.M., and J.A.W. performed research; M.B.S., M.M.M., and J.A.W. analyzed data; M.B.S. and S.D.A. wrote the article.
Notes
NIH grants (Grant numbers MH104775 and U01MH109091) and Institutional HCRAC Grant to SDA. We are grateful to the UConn Health Department of Neuroscience for access to the shared equipment. Conflict of Interest: None declared.
References
- Allene C, Cattani A, Ackman JB, Bonifazi P, Aniksztejn L, Ben-Ari Y, Cossart R. 2008. Sequential generation of two distinct synapse-driven network patterns in developing neocortex. J Neurosci. 28:12851–12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antic SD. 2003. Action potentials in basal and oblique dendrites of rat neocortical pyramidal neurons. J Physiol. 550:35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer E, Kovacs G, Sperlagh B. 2017. ATP released from astrocytes modulates action potential threshold and spontaneous excitatory postsynaptic currents in the neonatal rat prefrontal cortex. Brain Res Bull. 135:129–142. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. 1989. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 416:303–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Tsien RW. 1995. Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40–5967). Mol Pharmacol. 48:540–549. [PubMed] [Google Scholar]
- Blankenship AG, Feller MB. 2010. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 11:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block BM, Stacey WC, Jones SW. 1998. Surface charge and lanthanum block of calcium current in bullfrog sympathetic neurons. Biophys J. 74:2278–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y, Gao K, Li K, Nakajima S, Kira S, Takeda M, Yao J. 2014. Purinergic control of AMPK activation by ATP released through connexin 43 hemichannels—pivotal roles in hemichannel-mediated cell injury. J Cell Sci. 127:1487–1499. [DOI] [PubMed] [Google Scholar]
- Cina C, Bechberger JF, Ozog MA, Naus CC. 2007. Expression of connexins in embryonic mouse neocortical development. J Comp Neurol. 504:298–313. [DOI] [PubMed] [Google Scholar]
- Clowry G, Molnar Z, Rakic P. 2010. Renewed focus on the developing human neocortex. J Anat. 217:276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JE, Sanchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Saez JC. 2002. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci USA. 99:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlew R, Bosma MM, Moody WJ. 2004. Spontaneous, synchronous electrical activity in neonatal mouse cortical neurones. J Physiol. 560:377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel V, Aronov D, Jorquera I, Represa A, Ben-Ari Y, Cossart R. 2007. A parturition-associated nonsynaptic coherent activity pattern in the developing hippocampus. Neuron. 54:105–120. [DOI] [PubMed] [Google Scholar]
- Deans MR, Gibson JR, Sellitto C, Connors BW, Paul DL. 2001. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron. 31:477–485. [DOI] [PubMed] [Google Scholar]
- Dupont E, Hanganu IL, Kilb W, Hirsch S, Luhmann HJ. 2006. Rapid developmental switch in the mechanisms driving early cortical columnar networks. Nature. 439:79–83. [DOI] [PubMed] [Google Scholar]
- Duque A, Krsnik Z, Kostovic I, Rakic P. 2016. Secondary expansion of the transient subplate zone in the developing cerebrum of human and nonhuman primates. Proc Natl Acad Sci USA. 113:9892–9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Gaspar P. 2012. Development and critical period plasticity of the barrel cortex. Eur J Neurosci. 35:1540–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JS, Stryker MP. 2012. Development and plasticity of the primary visual cortex. Neuron. 75:230–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O, Hanse E, Konnerth A. 1998. Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J Physiol. 507(Pt 1):219–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Antonini A, McConnell SK, Shatz CJ. 1990. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 347:179–181. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL. 2003. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 4:285–294. [DOI] [PubMed] [Google Scholar]
- Hanganu IL, Kilb W, Luhmann HJ. 2001. Spontaneous synaptic activity of subplate neurons in neonatal rat somatosensory cortex. Cereb Cortex. 11:400–410. [DOI] [PubMed] [Google Scholar]
- Hanganu IL, Kilb W, Luhmann HJ. 2002. Functional synaptic projections onto subplate neurons in neonatal rat somatosensory cortex. J Neurosci. 22:7165–7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu IL, Okabe A, Lessmann V, Luhmann HJ. 2009. Cellular mechanisms of subplate-driven and cholinergic input-dependent network activity in the neonatal rat somatosensory cortex. Cereb Cortex. 19:89–105. [DOI] [PubMed] [Google Scholar]
- Hassel B, Paulsen RE, Johnsen A, Fonnum F. 1992. Selective inhibition of glial cell metabolism in vivo by fluorocitrate. Brain Res. 576:120–124. [DOI] [PubMed] [Google Scholar]
- John SA, Kondo R, Wang SY, Goldhaber JI, Weiss JN. 1999. Connexin-43 hemichannels opened by metabolic inhibition. J Biol Chem. 274:236–240. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Ceriani F, Houston O, Polishchuk R, Polishchuk E, Crispino G, Zorzi V, Mammano F, Marcotti W. 2017. Connexin-mediated signaling in nonsensory cells is crucial for the development of sensory inner hair cells in the mouse cochlea. J Neurosci. 37:258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Kara P, Reid RC, Shatz CJ. 2003. Role of subplate neurons in functional maturation of visual cortical columns. Science. 301:521–525. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Luhmann HJ. 2010. The subplate and early cortical circuits. Annu Rev Neurosci. 33:23–48. [DOI] [PubMed] [Google Scholar]
- Kawamura M Jr., Ruskin DN, Masino SA. 2010. Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors, and KATP channels. J Neurosci. 30:3886–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo RP, Wang SY, John SA, Weiss JN, Goldhaber JI. 2000. Metabolic inhibition activates a non-selective current through connexin hemichannels in isolated ventricular myocytes. J Mol Cell Cardiol. 32:1859–1872. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. 1980. Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J Neurocytol. 9:219–242. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. 1990. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 297:441–470. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. 2002. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 25:409–432. [DOI] [PubMed] [Google Scholar]
- Linley JE. 2013. Perforated whole-cell patch-clamp recording. Methods Mol Biol. 998:149–157. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Sinning A, Yang JW, Reyes-Puerta V, Stuttgen MC, Kirischuk S, Kilb W. 2016. Spontaneous neuronal activity in developing neocortical networks: from single cells to large-scale interactions. Front Neural Circuits. 10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlinar B, Enyeart JJ. 1993. Block of current through T-type calcium channels by trivalent metal cations and nickel in neural rat and human cells. J Physiol. 469:639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody WJ, Bosma MM. 2005. Ion channel development, spontaneous activity, and activity-dependent development in nerve and muscle cells. Physiol Rev. 85:883–941. [DOI] [PubMed] [Google Scholar]
- Moore AR, Filipovic R, Mo Z, Rasband MN, Zecevic N, Antic SD. 2009. Electrical excitability of early neurons in the human cerebral cortex during the second trimester of gestation. Cereb Cortex. 19:1795–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AR, Zhou WL, Jakovcevski I, Zecevic N, Antic SD. 2011. Spontaneous electrical activity in the human fetal cortex in vitro. J Neurosci. 31:2391–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AR, Zhou WL, Jakovcevski I, Zecevic N, Antic SD. 2012. Physiological properties of human fetal cortex in vitro. In: Ballanyi K, editor. Isolated central nervous system circuits. Secaucus, NJ: Springer. p. 125–158. [Google Scholar]
- Moore AR, Zhou WL, Sirois CL, Belinsky GS, Zecevic N, Antic SD. 2014. Connexin hemichannels contribute to spontaneous electrical activity in the human fetal cortex. Proc Natl Acad Sci USA. 111:E3919–E3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajah B, Jones AM, Evans WH, Parnavelas JG. 1997. Differential expression of connexins during neocortical development and neuronal circuit formation. J Neurosci. 17:3096–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Saez PJ, Cortes-Campos C, Elizondo RJ, Shoji KF, Contreras-Duarte S, Figueroa V, Velarde V, Jiang JX, Nualart F, et al. 2011. Glucose increases intracellular free Ca(2+) in tanycytes via ATP released through connexin 43 hemichannels. Glia. 60:53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA, Luneborg NL, Becker DL, Mobbs P. 2005. Gap junctions modulate interkinetic nuclear movement in retinal progenitor cells. J Neurosci. 25:10803–10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado A, Yuste R, Katz LC. 1993. Gap junctional communication and the development of local circuits in neocortex. Cereb Cortex. 3:488–498. [DOI] [PubMed] [Google Scholar]
- Rakic P, Komuro H. 1995. The role of receptor/channel activity in neuronal cell migration. J Neurobiol. 26:299–315. [DOI] [PubMed] [Google Scholar]
- Reichling DB, MacDermott AB. 1991. Lanthanum actions on excitatory amino acid-gated currents and voltage-gated calcium currents in rat dorsal horn neurons. J Physiol. 441:199–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripps H, Qian H, Zakevicius J. 2002. Pharmacological enhancement of hemi-gap-junctional currents in Xenopus oocytes. J Neurosci Methods. 121:81–92. [DOI] [PubMed] [Google Scholar]
- Safiulina VF, Kasyanov AM, Sokolova E, Cherubini E, Giniatullin R. 2005. ATP contributes to the generation of network-driven giant depolarizing potentials in the neonatal rat hippocampus. J Physiol. 565:981–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter KR, Miller BH. 2015. Epigenetic mechanisms in schizophrenia. Prog Biophys Mol Biol. 118:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC, Lautermilch NJ, Smith RD, Gomez TM. 2000. Coding of neuronal differentiation by calcium transients. Bioessays. 22:811–817. [DOI] [PubMed] [Google Scholar]
- Spitzer NC, Root CM, Borodinsky LN. 2004. Orchestrating neuronal differentiation: patterns of Ca2+ spikes specify transmitter choice. Trends Neurosci. 27:415–421. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Shatz CJ, Feller MB. 1999. Dynamics of retinal waves are controlled by cyclic AMP. Neuron. 24:673–685. [DOI] [PubMed] [Google Scholar]
- Stout CE, Costantin JL, Naus CC, Charles AC. 2002. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 277:10482–10488. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Graham SH. 1994. Fluorocitrate and fluoroacetate effects on astrocyte metabolism in vitro. Brain Res. 664:94–100. [DOI] [PubMed] [Google Scholar]
- Syed MM, Lee S, He S, Zhou ZJ. 2004. Spontaneous waves in the ventricular zone of developing mammalian retina. J Neurophysiol. 91:1999–2009. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Macvicar BA. 2008. Connexin and pannexin hemichannels of neurons and astrocytes. Channels (Austin). 2:81–86. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. 2007. The origin of spontaneous activity in the developing auditory system. Nature. 450:50–55. [DOI] [PubMed] [Google Scholar]
- Viana F, Van den Bosch L, Missiaen L, Vandenberghe W, Droogmans G, Nilius B, Robberecht W. 1997. Mibefradil (Ro 40–5967) blocks multiple types of voltage-gated calcium channels in cultured rat spinal motoneurones. Cell Calcium. 22:299–311. [DOI] [PubMed] [Google Scholar]
- Vogt A, Hormuzdi SG, Monyer H. 2005. Pannexin1 and Pannexin2 expression in the developing and mature rat brain. Brain Res Mol Brain Res. 141:113–120. [DOI] [PubMed] [Google Scholar]
- Voloboueva LA, Suh SW, Swanson RA, Giffard RG. 2007. Inhibition of mitochondrial function in astrocytes: implications for neuroprotection. J Neurochem. 102:1383–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ. 1996. Subplate neurons—missing link in brain injury of the premature infant? Pediatrics. 97:112–113. [PubMed] [Google Scholar]
- Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. 2004. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 43:647–661. [DOI] [PubMed] [Google Scholar]
- Wenker IC, Kreneisz O, Nishiyama A, Mulkey DK. 2010. Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol. 104:3042–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess JM, Isaiah A, Watkins PV, Kanold PO. 2017. Subplate neurons are the first cortical neurons to respond to sensory stimuli. Proc Natl Acad Sci USA. 114:12602–12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AE, Griffith EC, Greenberg ME. 2002. Regulation of transcription factors by neuronal activity. Nat Rev Neurosci. 3:921–931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.