Abstract
Aims
Prevalent right ventricular (RV) dysfunction (RVD) is associated with increased mortality in patients with heart failure with preserved ejection fraction (HFpEF), but no study has characterized long-term changes in RV structure and function within the same patient.
Methods and results
Patients with unequivocal HFpEF defined by either invasive haemodynamics or hospitalization for pulmonary oedema (n = 271) underwent serial echocardiographic evaluations >6 months apart. Clinical, structural, functional, and haemodynamic characteristics were examined. Over a median of 4.0 years (interquartile range 2.1–6.1), there was a 10% decline in RV fractional area change and 21% increase in RV diastolic area (both P < 0.0001). These changes greatly exceeded corresponding changes in the left ventricle. The prevalence of tricuspid regurgitation increased by 45%. Of 238 patients with normal RV function at Exam 1, 55 (23%) developed RVD during follow-up. Development of RVD was associated with both prevalent and incident atrial fibrillation (AF), higher body weight, coronary disease, higher pulmonary artery and left ventricular filling pressures, and RV dilation. Patients with HFpEF developing incident RVD had nearly two-fold increased risk of death (adjusted hazard ratio 1.89, 95% confidence interval 1.01–3.44; P = 0.04).
Conclusion
While previous attention has centred on the left ventricle in HFpEF, these data show that right ventricular structure and function deteriorate to greater extent over time when compared with changes in the left ventricle. Further study is required to evaluate whether interventions targeting modifiable risk factors identified for incident RVD, including abnormal haemodynamics, AF, coronary disease, and obesity, can prevent RVD and thus improve outcomes.
Keywords: Atrial fibrillation, Heart failure, HFpEF, Pulmonary hypertension, Right ventricle, Tricuspid regurgitation
See page 699 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy900)
Introduction
Approximately one-half of patients with heart failure (HF) have a preserved ejection fraction (HFpEF).1 Outcomes are poor in HFpEF, and there is no proven effective treatment. While disease paradigms have traditionally focused on pathological changes affecting the left ventricle in HFpEF,2 recent studies have shown that a significant number of patients also display right ventricular (RV) dysfunction (RVD), and patients with this phenotype suffer from high morbidity and mortality.3–10
The natural history, predictors and prognostic impact of incident RVD in HFpEF are undescribed, as current understandings are based exclusively on cross sectional data.3–10 Characterization of the natural history of RV structure and function in HFpEF, as well as the underlying mechanisms and risk factors causing RVD is necessary to improve pathophysiological understanding and inform the development of strategies for treatment and prevention. Accordingly, we examined chronic changes in RV structure and function in patients with invasively-proven HFpEF to characterize the incidence of RVD, identify its predictors, and determine whether development of RVD predicts outcome in HFpEF.
Methods
Study population
Subjects undergoing coronary angiography with or without exercise right heart catheterization at the Mayo Clinic were examined to identify patients with unequivocal evidence of HFpEF, defined by clinical symptoms of HF (exertional dyspnoea, fatigue), ejection fraction (EF) ≥50%, and either previous HF hospitalization for pulmonary oedema that resolved with diuretics, or directly measured elevation in left heart filling pressures (pulmonary capillary wedge pressure, PCWP) at rest (>15 mmHg) and/or with exercise (≥25 mmHg).11,12 To ensure the specificity of HFpEF diagnosis for the purposes of this analysis, patients with HFpEF diagnosed based upon echo-Doppler or natriuretic peptide-based criteria alone were not included. Patients with reduced EF (EF < 50%), significant valvular heart disease (>moderate left-sided regurgitation, >mild stenosis), significant pulmonary parenchymal disease, recent acute coronary syndrome [left ventricular (LV) and RV infarction] prior to Exam 1 or between Exams 1 and 2, constrictive pericarditis, high output HF, or cardiomyopathy were excluded.
From this group, patients with two or more echocardiographic evaluations in a compensated (outpatient) state that were performed minimum of >6 months from one another (Exams 1 and 2) were identified. When patients had multiple echocardiograms after Exam 1, the most recent study (distant from Exam 1) was used as Exam 2. Patients that developed acute coronary syndrome between Exams 1 and 2 were excluded.
In a secondary analysis, control subjects free of HF (n = 27) who were referred to exercise catheterization during the same period were included as a comparator group to provide perspective on longitudinal changes in RV structure and function in the absence of HF. The control subjects were required to display no cardiac abnormalities as determined by comprehensive clinical evaluation, imaging and invasive assessment, including normal rest and exercise PCWP (criteria above). The Mayo Clinic Institutional Review Board approved the study and the authors had full access to the data and take responsibility for its integrity.
Clinical assessment
Clinical history, laboratory data, and current medications were collected from detailed chart review. Atrial fibrillation (AF) was identified and classified as paroxysmal for episodes lasting <7 days and persistent for episodes of ≥7 days duration as verified by electrocardiography. Coronary artery disease (CAD) was defined by angiographic stenosis >50% in at least one epicardial coronary artery, previous myocardial infarction, or any previous revascularization.
Assessment of cardiac structure and function
Echocardiography was performed according to the American Society of Echocardiography guidelines.13 Left ventricular volumes and mass were determined by two-dimensional echocardiography. Left ventricular systolic function was assessed by EF and the systolic mitral annular tissue velocity at the septal annulus (mitral s′). Left ventricular diastolic function was assessed using the early diastolic mitral inflow velocity (E), early diastolic septal mitral annular tissue velocity (e′), and the ratio of E/e′. Left atrial (LA) volume was determined by the biplane method of disks and indexed to body surface area. Stroke volume (SV) was determined from the LV outflow dimension and pulse wave Doppler profile. Cardiac output (Qc) was calculated from the product of heart rate and SV.
Right heart measurements were performed in a blinded fashion by an experienced investigator (M.O.) in accordance with the current guidelines.14 Right ventricular basal dimension and area were measured at end-diastole using RV focused views. Right ventricular systolic function was assessed by fractional area change (FAC), which was obtained in all participants.8 As a sensitivity analysis to complement the primary study findings based upon FAC, RV deformation analyses were performed offline with commercially available software from RV focused views as previously described (Syngo, Siemens Medical Solutions, Munich, Germany).8,15 The average values of peak longitudinal systolic strain obtained from all segments of the free wall and septal wall of the right ventricle and only from the free wall were defined as RV global longitudinal strain (RV GLS) and RV free wall longitudinal strain (RV FWLS), respectively. Right ventricular measurements represent the mean of 3 beats. and are expressed as absolute values. Right ventricular dysfunction was defined by FAC <35%.3,8,14 Systolic tissue velocity at the lateral tricuspid annulus (tricuspid s′) and tricuspid annular plane systolic excursion were not systematically obtained in the majority of patients and were therefore not included.
Right atrial (RA) area was measured in the apical four-chamber view at end-systole. Right atrial pressure was estimated from the diameter of inferior vena cava (IVC) and its collapsibility during inspiration, scored as 5 [normal sized IVC (<1.7 cm) with normal inspiratory collapse (>50%)], 10 (borderline/dilated IVC with normal collapse), 15 (dilated IVC with >25% collapse), or 20 mmHg (dilated IVC with minimal or no collapse). Right ventricular systolic pressure (RVSP) was calculated as [4 × peak tricuspid regurgitation (TR) velocity] + estimated RA pressure.14 Tricuspid regurgitation severity was assessed based on qualitative estimation, as none/trivial, mild, moderate, or severe, respectively.
Invasive haemodynamics
Right heart catheterization was performed at the time of Exam 1 in a subset of patients as previously described.11,16,17 Pressures in the RA, pulmonary artery (PA), and PCWP were measured at end expiration (mean of ≥3 beats). Transpulmonary gradient (TPG) was calculated as PA mean pressure – PCWP and pulmonary vascular resistance (PVR) was calculated by the quotient of TPG and Qc, and PA compliance was determined as the ratio of SV to PA pulse pressure.18,19
Outcome assessment
Patient follow-up was initiated on the day of echocardiographic Exam 2. Vital status was determined from the Mayo Clinic registration database and the Rochester Epidemiology Project death database. Mortality data were ascertained from medical records, death certificates, obituaries, and notices of death in the local newspapers. Data on all Minnesota deaths were obtained from the State of Minnesota annually.
Statistical analysis
Data are reported as mean (standard deviation), median [interquartile range (IQR)], or number (%) unless otherwise specified. Between-group differences were compared by χ2 test, t test, or Mann–Whitney test, as appropriate. Within-group differences were compared by the McNemar test, paired t-test, or Wilcoxon signed rank test. Pearson’s or Spearman’s analyses were used to assess correlations, as appropriate. Linear regression models with an interaction term were performed to test the difference in the relationship between dependent and independent variables between two groups. Univariable and multivariable logistic regression analyses were used to examine independent association between baseline parameters and the development of RV dysfunction. Mortality rates were assessed using Kaplan–Meier curve analysis, and univariable and multivariable Cox proportional hazards models were used to assess the independent prognostic power.
Results
Baseline characteristics
A total of 271 HFpEF subjects met inclusion criteria for the study (Supplementary material online, Figure S1). Of this cohort, RV deformation analysis could be performed in 205 patients (76%). At Exam 1, subjects with HFpEF were older aged and obese and had typical comorbidities such as hypertension, AF, and CAD, representing a typical HFpEF population (Table 1). More than half of patients were treated with neurohormonal antagonists. Biventricular filling pressures and PA pressures were elevated in HFpEF (Table 1). Comparisons of clinical characteristics between HFpEF and controls are shown in Supplementary material online, Table S1.
Table 1.
Clinical characteristics at Exam 1
| HFpEF (n = 271) | |
|---|---|
| Age (years) | 71 ± 9 |
| Female, n (%) | 151 (56) |
| Body weight (kg) | 92 ± 22 |
| Body mass index (kg/m2) | 32 ± 7 |
| Comorbidities, n (%) | |
| Diabetes mellitus | 90 (33) |
| Hypertension | 227 (84) |
| AF (paroxysmal or persistent) | 114 (42) |
| Coronary artery disease | 155 (57) |
| Pacemaker | 37 (14) |
| Medications, n (%) | |
| ACEI or ARB | 161 (59) |
| Beta-blocker | 181 (67) |
| Calcium channel blocker | 84 (31) |
| Loop diuretic | 109 (40) |
| Aldosterone antagonist | 28 (10) |
| Laboratories | |
| Haemoglobin (g/dL) | 12.4 ± 1.7 |
| Creatinine (mg/dL) | 1.2 ± 0.4 |
| Invasive haemodynamics (n = 147) | |
| RA pressure (mmHg) | 10 ± 5 |
| PA systolic pressure (mmHg) | 45 ± 15 |
| PA mean pressure (mmHg) | 29 ± 9 |
| PCWP (mmHg) | 17 ± 6 |
| Cardiac output (L/min) | 5.0 ± 1.4 |
| PVR (WU) | 2.6 ± 1.8 |
| PA compliance (mL/mmHg) | 3.4 ± 1.6 |
| TPG (mmHg) | 12 ± 6 |
ACEI, angiotensin-converting enzyme inhibitors; AF, atrial fibrillation; ARB, angiotensin-receptor blockers; HFpEF, heart failure with preserved ejection fraction; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RA, right atrial; TPG, transpulmonary gradient.
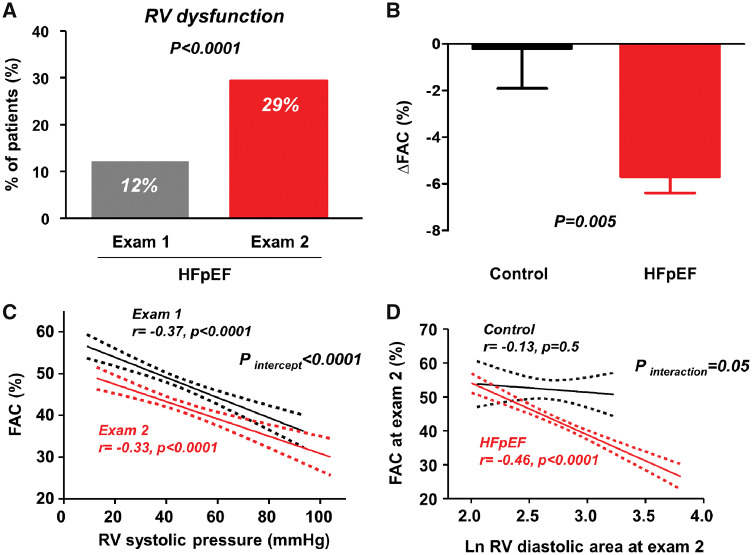
As expected, subjects with HFpEF displayed impaired LV systolic and diastolic function, evidenced by decreased mitral tissue Doppler velocities, elevated E/e′ ratio, and LA enlargement (Table 2). Mean FAC was preserved but 33 patients (12%) displayed RVD based on FAC at Exam 1 (Figure 1A). Right ventricular systolic pressure was slightly elevated and moderate or severe TR was present in 20% of patients.
Table 2.
Longitudinal changes in left and right heart structure and function
| HFpEF (n = 271) |
P-value Exams 1 vs. 2 | ||
|---|---|---|---|
| Exam 1 | Exam 2 | ||
| Vital signs | |||
| Systolic BP (mmHg) | 132 ± 21 | 126 ± 20 | 0.0006 |
| Diastolic BP (mmHg) | 70 ± 12 | 66 ± 12 | 0.0002 |
| Heart rate (b.p.m.) | 67 ± 13 | 69 ± 13 | 0.1 |
| Left heart structure and function | |||
| LV diastolic dimension (mm) | 49 ± 6 | 49 ± 6 | 0.5 |
| LV mass index (g/m2) | 102 ± 29 | 103 ± 30 | 0.8 |
| LV ejection fraction (%) | 62 ± 7 | 59 ± 10 | 0.0002 |
| Cardiac output (L/min) | 5.9 ± 1.5 | 5.8 ± 1.4 | 0.2 |
| LA volume index (mL/m2) | 44 ± 15 | 46 ± 20 | 0.002 |
| Mitral E-wave (cm/s) | 98 ± 30 | 99 ± 34 | 0.6 |
| Mitral annular e′ (cm/s) | 6.6 ± 2.2 | 6.0 ± 2.2 | 0.0003 |
| E/e′ ratio | 16 ± 8 | 19 ± 11 | 0.001 |
| Medial mitral annular s′ (cm/s) | 6.6 ± 2.1 | 5.9 ± 1.8 | <0.0001 |
| Right heart structure and function | |||
| RV basal dimension (mm) | 34 ± 7 | 37 ± 8 | <0.0001 |
| RV diastolic area (cm2) | 15 ± 5 | 17 ± 6 | <0.0001 |
| RV fractional area change (%) | 48 ± 10 | 42 ± 11 | <0.0001 |
| RV free wall strain (%, n = 205) | 20.4 ± 6.2 | 18.6 ± 6.9 | 0.003 |
| RV global longitudinal strain (%, n = 205) | 17.7 ± 5.2 | 16.0 ± 5.7 | 0.0006 |
| RVSP (mmHg) | 44 ± 15 | 45 ± 17 | 0.3 |
| FAC/RVSP (%/mmHg) | 1.26 ± 0.63 | 1.09 ± 0.52 | <0.0001 |
| Right atrial area (cm2) | 16 ± 7 | 17 ± 8 | 0.03 |
| Moderate or severe TR (%) | 55 (20%) | 78 (29%) | 0.003 |
BP, blood pressure; E/e′, the ratio of early mitral diastolic inflow velocity to early diastolic mitral annular velocity; FAC, fractional area change; HFpEF, heart failure with preserved ejection fraction; LA, left atrial; LV, left ventricular; RV, right ventricular; RVSP, right ventricular systolic pressure; TR, tricuspid regurgitation.
Figure 1.
(A) Right ventricular dysfunction, defined by fractional area change <35% was markedly increased from Exam 1 to Exam 2 in patients with heart failure with preserved ejection fraction. (B) Compared with controls, patients with heart failure with preserved ejection fraction displayed greater decline in fractional area change. (C) The relationship between right ventricular fractional area change and estimated right ventricular systolic pressure shifted downward from Exams 1 to 2, indicating that the depression in right ventricular function was due to worsening myocardial dysfunction rather than greater afterload mismatch. (D) Right ventricular dilation was associated with right ventricular dysfunction in heart failure with preserved ejection fraction but not in controls. FAC, fractional area change; HFpEF, heart failure with preserved ejection fraction; RV, right ventricular.
Longitudinal changes in biventricular structure and function in heart failure with preserved ejection fraction
The median time between Exams 1 and 2 was 4.0 years (IQR 2.1–6.1). Systolic and diastolic blood pressures were decreased from Exam 1 to Exam 2 in HFpEF (Table 2), possibly related to an increase in use of beta blockers, loop diuretics, and aldosterone antagonists (Supplementary material online, Table S2). Despite favourable reductions in blood pressure in HFpEF patients, there were mild decreases in LV EF and mitral annular s′ velocities, and worsening of LV diastolic function evidenced by increases in LA volume and E/e′ ratio and decrease in mitral annular e′ velocity. Left ventricular dimensions, volumes, mass, and cardiac output did not change between Exams 1 and 2 in subjects with HFpEF (Table 2, Figure 2).
Figure 2.
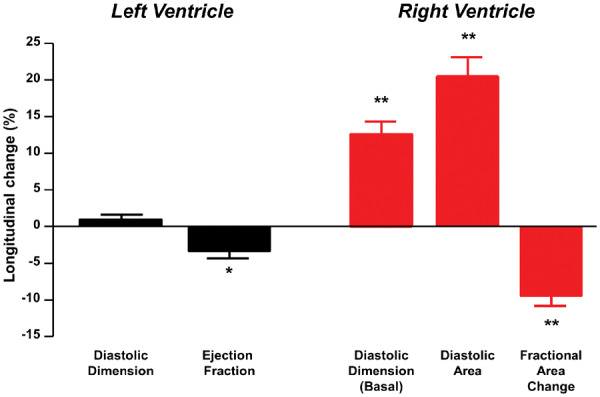
Changes in structure and function in right ventricle in heart failure with preserved ejection fraction greatly exceeded those observed in the left ventricle. *P = 0.0001 between Exams 1 and 2; **P < 0.0001 between Exams 1 and 2.
In contrast to the minor changes in LV structure and EF, RV structure and function worsened significantly in patients with HFpEF over time, with 20% increases in RV diastolic area and 10% decreases in RV FAC, resulting in a 2.5-fold increase in the prevalence of RVD in HFpEF (Table 2, Figures1 and 2). The RV FWLS and RV GLS also decreased significantly in the subset of HFpEF patients with analysable data. Proportional (percent) changes in RV FAC and RV basal diameter were significantly greater than proportional changes in LV EF and diastolic dimension, respectively (both P < 0.0001).
Right atrial area increased and the severity of tricuspid insufficiency worsened between Exams 1 and 2 in HFpEF, but there was no increase in RVSP in the HFpEF (Table 2). Accordingly, the relationship between RV function and RVSP shifted downward over time (Figure 1C, Supplementary material online, Figure S2), identifying that the depression in RV function was due to increasing myocardial dysfunction rather than greater afterload mismatch from worsening pulmonary hypertension. Sensitivity analysis performed separately among patients with and without invasive assessment showed similar results (Supplementary material online, Table S3).
Comparison with longitudinal changes in controls
In the control group, there were no chronic changes in blood pressure, heart rate, LV structure, LV systolic or diastolic function, RV size, RV systolic function, RA size, RVSP, or RV-PA coupling over this interval (Supplementary material online, Table S4), and no control subject developed RVD during follow-up. Compared with controls, subjects with HFpEF displayed greater decrease in RV FAC (Figure 1B and Supplementary material online, Table S4), greater RV and RA dilation, and greater increases in E/e′ ratio. Worsening RV function in HFpEF was intimately associated with greater RV remodelling, with no corresponding changes in controls (Figure 1D).
Association with the development of right ventricular dysfunction in heart failure with preserved ejection fraction
Of HFpEF patients with normal RV function at the Exam 1 (n = 238), 55 (23%) developed RVD by Exam 2, with a marked reduction in FAC in this group (48 ± 8% to 29 ± 5%, P < 0.0001). The duration of time that elapsed between Exams 1 and 2 was similar in HFpEF patients with vs. without development of incident RVD [4.4 (2.4–6.4) years vs. 4.0 (2.1–6.1) years, P = 0.7]. When compared with HFpEF patients who maintained RV function over time, those who developed RVD displayed greater body weight and higher prevalence of diabetes, AF, and CAD at Exam 1, with lower heart rate, larger LV size and mass, higher biatrial volumes, more RV dilation, lower FAC, and higher RVSP (Table 3). Treatment with loop diuretics at Exam 1 was associated with increased risk for developing RVD.
Table 3.
Correlates of the development of right ventricular dysfunction
| Development of RV dysfunction |
P-value | OR (95% CI) | P-value | ||
|---|---|---|---|---|---|
| No (n = 183) | Yes (n = 55) | ||||
| Age (years) | 71 ± 10 | 71 ± 9 | 0.9 | 1.01 (0.74–1.36) | 0.9 |
| Female, n (%) | 112 (61) | 26 (47) | 0.1 | 0.57 (0.31–1.05) | 0.1 |
| Body mass index (kg/m2) | 32 ± 7 | 34 ± 7 | 0.1 | 1.27 (0.95–1.71) | 0.1 |
| Body weight (kg) | 89 ± 21 | 98 ± 25 | 0.02 | 1.46 (1.07–1.93) | 0.01 |
| Comorbidities, n (%) | |||||
| Diabetes mellitus | 53 (29) | 24 (44) | 0.04 | 1.88 (1.01–3.51) | 0.04 |
| Hypertension | 157 (86) | 45 (82) | 0.5 | 0.75 (0.33–1.66) | 0.5 |
| Prevalent AF at Exam 1 | 57 (31) | 31 (56) | 0.0007 | 2.86 (1.54–5.30) | 0.0009 |
| Incident persistent AF (n = 155/37)a | 33 (21) | 16 (43) | 0.006 | 2.82 (1.32–6.00) | 0.007 |
| Coronary artery disease | 96 (52) | 38 (69) | 0.03 | 2.03 (1.07–3.85) | 0.03 |
| Pacemaker | 23 (13) | 9 (16) | 0.5 | 1.36 (0.59–3.14) | 0.5 |
| Medications, n (%) | |||||
| ACEI or ARB | 103 (56) | 37 (67) | 0.1 | 1.60 (0.85–3.01) | 0.1 |
| Beta-blocker | 118 (64) | 40 (73) | 0.3 | 1.47 (0.75–2.86) | 0.3 |
| Calcium channel blocker | 57 (31) | 21 (38) | 0.3 | 1.37 (0.73–2.56) | 0.3 |
| Loop diuretic | 63 (34) | 28 (51) | 0.03 | 1.98 (1.07–3.64) | 0.03 |
| Aldosterone antagonist | 19 (10) | 4 (7) | 0.5 | 0.68 (0.22–2.08) | 0.5 |
| Laboratories | |||||
| Haemoglobin (g/dL) | 12.3 ± 1.7 | 12.5 ± 1.5 | 0.5 | 1.12 (0.82–1.52) | 0.5 |
| Creatinine (mg/dL) | 1.2 ± 0.4 | 1.2 ± 0.4 | 0.4 | 1.13 (0.85–1.50) | 0.4 |
| Haemodynamics | |||||
| Systolic BP (mmHg) | 133 ± 21 | 134 ± 22 | 0.8 | 1.04 (0.76–1.40) | 0.8 |
| Diastolic BP (mmHg) | 71 ± 13 | 70 ± 11 | 0.9 | 0.98 (0.73–1.33) | 0.9 |
| Heart rate (b.p.m.) | 67 ± 12 | 63 ± 11 | 0.03 | 0.69 (0.49–0.98) | 0.04 |
| LV structure and function | |||||
| LV diastolic dimension (mm) | 49 ± 6 | 50 ± 5 | 0.03 | 1.39 (1.02–1.91) | 0.04 |
| LV mass index (g/m2) | 100 ± 29 | 111 ± 27 | 0.01 | 1.45 (1.06–1.92) | 0.01 |
| LV ejection fraction (%) | 62 ± 6 | 61 ± 7 | 0.4 | 0.87 (0.64–1.18) | 0.4 |
| Cardiac output (L/min) | 5.9 ± 1.5 | 5.8 ± 1.7 | 0.6 | 0.91 (0.67–1.24) | 0.6 |
| LA volume index (mL/m2) | 41 ± 14 | 46 ± 12 | 0.01 | 1.38 (1.03–1.88) | 0.03 |
| Mitral E-wave (cm/s) | 96 ± 31 | 103 ± 27 | 0.1 | 1.28 (0.95–1.72) | 0.1 |
| Mitral annular e′ (cm/s) | 6.4 ± 2.1 | 6.8 ± 2.5 | 0.3 | 1.18 (0.87–1.61) | 0.3 |
| E/e′ ratio | 16 ± 7 | 17 ± 8 | 0.4 | 1.14 (0.84–1.53) | 0.4 |
| Medial mitral annular s′ (cm/s) | 6.8 ± 2.2 | 6.4 ± 2.0 | 0.2 | 0.80 (0.57–1.13) | 0.2 |
| RV structure and function | |||||
| Right atrial area (cm2) | 15 ± 5 | 17 ± 6 | 0.003 | 1.55 (1.15–2.08) | 0.004 |
| RV basal dimension (mm) | 32 ± 7 | 36 ± 6 | <0.0001 | 2.10 (1.48–2.97) | <0.0001 |
| RV diastolic area (cm2) | 13 ± 4 | 16 ± 4 | <0.0001 | 2.01 (1.46–2.77) | <0.0001 |
| RV fractional area change (%) | 52 ± 6 | 48 ± 8 | 0.01 | 0.64 (0.46–0.87) | 0.005 |
| RVSP (mmHg) | 41 ± 13 | 48 ± 17 | 0.006 | 1.53 (1.15–2.13) | 0.002 |
| Moderate or severe TR (%) | 27 (15%) | 10 (18%) | 0.5 | 1.28 (0.58–2.85) | 0.5 |
| Invasive haemodynamics (n = 126) | |||||
| RA pressure (mmHg) | 9 ± 4 | 11 ± 4 | 0.03 | 1.57 (1.01–2.52) | 0.04 |
| PA systolic pressure (mmHg) | 42 ± 13 | 50 ± 20 | 0.01 | 1.68 (1.07–2.58) | 0.02 |
| PA mean pressure (mmHg) | 27 ± 8 | 32 ± 10 | 0.03 | 1.81 (1.15–2.80) | 0.01 |
| PCWP (mmHg) | 16 ± 5 | 20 ± 5 | 0.002 | 1.98 (1.25–3.29) | 0.004 |
| PVR (WU) | 2.3 ± 1.1 | 3.5 ± 3.9 | 0.3 | 1.54 (0.91–2.61) | 0.1 |
| PA compliance (mL/mmHg) | 3.5 ± 1.6 | 3.0 ± 1.4 | 0.2 | 0.68 (0.34–1.36) | 0.3 |
| TPG (mmHg) | 11 ± 5 | 13 ± 9 | 0.5 | 1.25 (0.82–1.91) | 0.3 |
ORs are expressed as per 1 SD increment.
ACEI, angiotensin-converting enzyme inhibitors; AF, atrial fibrillation; ARB, angiotensin-receptor blockers; BP, blood pressure; CI, confidential interval; E/e′, the ratio of early mitral diastolic inflow velocity to early diastolic mitral annular velocity; HFpEF, heart failure with preserved ejection fraction; LA, left atrial; LV, left ventricular; OR, odds ratio; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RA, right atrial; RV, right ventricular; RVSP, right ventricular systolic pressure; SD, standard deviation; TPG, transpulmonary gradient; TR, tricuspid regurgitation.
Analysed in HFpEF patients with sinus rhythm or paroxysmal atrial fibrillation at Exam 1.
There were no differences in LV systolic and diastolic function between HFpEF patients who developed RVD and those who did not at Exam 1 (Table 3). However, the development of RVD was associated with greater deterioration in both LV systolic and diastolic function during the follow-up period (Supplementary material online, Figure S3).
Among the subgroup of patients undergoing invasive haemodynamic evaluation, development of incident RVD was associated with higher RA pressure, PA pressures, and PCWP at Exam 1 (Table 3). Pulmonary capillary wedge pressure at the time of Exam 1 [odds ratio (OR) 1.98 per 1 SD, 95% confidence interval (CI) 1.25–3.29; P = 0.004] and PA mean pressure at the time of Exam 1 (OR 1.81 per 1 SD, 95% CI 1.15–2.80; P = 0.01) were each associated with the development of RVD in HFpEF. Elevated PVR at Exam 1 tended to increase risk of incident RVD, but this did not reach statistical significance.
Multivariable logistic regression analyses incorporating the strongest univariate variables associated with RVD revealed that higher body weight, CAD, prevalent AF, higher echocardiographic RVSP, and greater RV dilation were associated with development of incident RVD in patients with HFpEF (Table 4).
Table 4.
Multivariable logistic regression models for association with incident RV dysfunction
| Multivariable model 1 |
Multivariable model 2 |
Multivariable model 3a |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| FAC (per 1 SD) | 0.65 (0.47–0.92) | 0.02 | 0.75 (0.51–1.07) | 0.1 | 0.78 (0.50–1.21) | 0.3 |
| Body weight (per 1 SD) | 1.49 (1.07–2.11) | 0.01 | 1.25 (0.94–1.93) | 0.09 | 1.19 (0.78–1.85) | 0.4 |
| CAD at Exam 1 | 2.29 (1.14–4.62) | 0.02 | 1.98 (0.95–4.14) | 0.07 | — | — |
| Loop diuretics | 1.29 (0.66–2.51) | 0.5 | — | — | — | — |
| AF at Exam 1 | 2.89 (1.4–5.68) | 0.002 | 2.57 (1.26–5.24) | 0.009 | — | — |
| Development of persistent AF | — | — | — | — | 2.70 (1.16–6.25) | 0.02 |
| RVSP (per 1 SD) | — | — | 1.39 (1.00–1.94) | 0.04 | 1.51 (1.04–2.19) | 0.03 |
| RV diastolic area (per 1 SD) | — | — | 1.57 (1.06–2.33) | 0.02 | 1.69 (1.04–2.77) | 0.03 |
AF, atrial fibrillation; CAD, coronary artery disease; CI, confidential interval; FAC, fractional area change; HFpEF, heart failure with preserved ejection fraction; RV, right ventricular; RVSP, right ventricular systolic pressure; SD, standard deviation.
Model 3 was analysed in HFpEF patients with sinus rhythm or paroxysmal atrial fibrillation at Exam 1 (n = 192).
Incident atrial fibrillation and the development of right ventricular dysfunction in heart failure with preserved ejection fraction
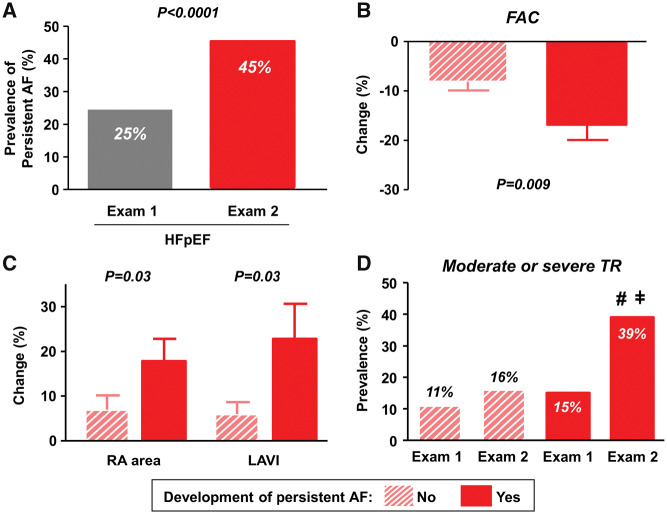
The prevalence of persistent AF in HFpEF patients nearly doubled between Exams 1 and 2 (Figure 3A). Development of persistent AF was strongly and independently associated with incident RVD (Tables3 and 4). Of the 204 HFpEF patients who were not in persistent AF at Exam 1, 54 (26%) developed new persistent AF. Heart rates were adequately controlled in this group (mean 68 ± 14 b.p.m. vs. 70 ± 14 in HFpEF with vs. without persistent AF, P = 0.6), and there were no differences in baseline RV systolic function in HFpEF patients who did and did not develop persistent AF (FAC, 49 ± 10 vs. 51 ± 8%, P = 0.2). However, HFpEF patients who developed persistent AF displayed greater reduction in RV function and more biatrial dilation than those who did not (Figure3B and C). Development of new persistent AF was also associated with an increase in the prevalence of moderate-severe TR from 15% to 39% (Figure 3D).
Figure 3.
(A) Prevalence of persistent atrial fibrillation was markedly increased in heart failure with preserved ejection fraction from Exams 1 to 2. (B and C) Heart failure with preserved ejection fraction patients who developed persistent atrial fibrillation displayed greater reduction in right ventricular fractional area change and more biatrial dilation than those who did not. (D) Development of incident persistent atrial fibrillation was associated with an increase in the prevalence of moderate-severe tricuspid regurgitation from 15% to 39%. #P < 0.001 vs. heart failure with preserved ejection fraction who did not develop persistent atrial fibrillation; ǂP = 0.03 vs. Exam 1. AF, atrial fibrillation; HFpEF, heart failure with preserved ejection fraction; LAVI, left atrial volume index; RA, right atrial; TR, tricuspid regurgitation.
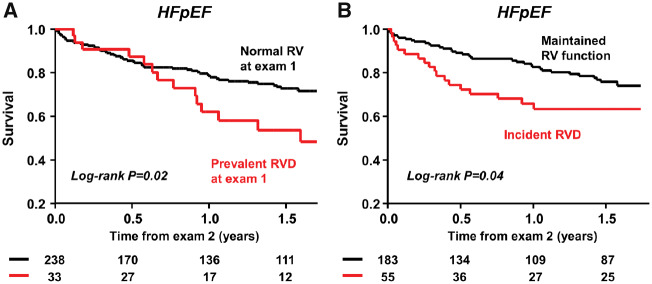
Impact of development of right ventricular dysfunction on mortality in heart failure with preserved ejection fraction
Over a median follow-up of 15 months (IQR 5.1–21), there were 71 deaths (26%) in the HFpEF patient cohort. Patients with RVD at Exam 1 displayed increased mortality compared with those with normal RV function (Figure 4A). Of HFpEF subjects with normal RV function at baseline (n = 238), development of incident RVD was associated with increased mortality when compared with patients that maintained RV function (Figure 4B).
Figure 4.
(A) Compared with heart failure with preserved ejection fraction patients with normal right ventricular function at Exam 1, those with right ventricular dysfunction displayed increased mortality. (B) Of heart failure with preserved ejection fraction subjects with normal right ventricular function at baseline (n = 238), patients who developed right ventricular dysfunction had higher mortality rates than those who maintained right ventricular function overtime. HFpEF, heart failure with preserved ejection fraction; RVD, right ventricular dysfunction.
In an unadjusted Cox model, the development of RVD was associated with an 80% increased risk of death [hazard ratio (HR) 1.82, 95% CI 1.01–3.19; P = 0.04]. Development of RVD remained significantly associated with mortality after adjustment for other established risk factors associated with mortality in HFpEF, including age, body mass index, AF, ejection fraction, and E/e′ ratio (adjusted HR 1.89, 95% CI 1.01–3.44; P = 0.04).
Discussion
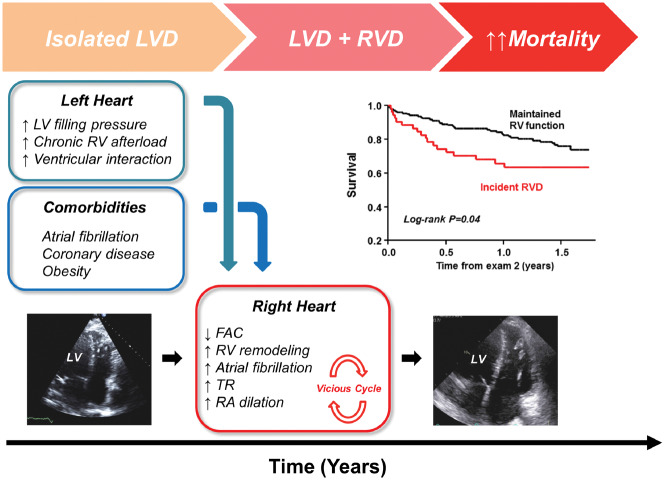
This is the first study to examine how RV structure and function change over time in HFpEF. Over a median duration of just 4 years, patients with HFpEF displayed significant declines in RV systolic function that was coupled with adverse RV remodelling and dilatation. Importantly, these longitudinal changes in right heart structure and function greatly exceeded corresponding changes in the left ventricle. The presence of AF, coronary disease, greater body weight, higher PA and PCWP, and RV dilation at the index examination were identified as risk factors that associate with incident RVD. Interval development of persistent AF during the follow-up period was also associated with incident RVD, possibly mediated in part by greater biatrial dilation and worsening of tricuspid insufficiency. The development of RVD was associated with a nearly two-fold increased risk of death in HFpEF, even after adjusting for other established risk markers. While traditional paradigms in HFpEF have emphasized the central role of left heart disease, the current longitudinal data reveal that proportionally greater changes in the right heart play a key role in the advanced stages of HFpEF, contributing to adverse outcomes (Take home figure). Further study is required to test whether interventions targeting the risk factors identified for incident RVD, including unfavourable haemodynamics, heart rhythm, and comorbidities, can reduce the burden of RVD and improve outcomes in this patient population.
Take home figure.
Right ventricular dysfunction develops over time in patients with heart failure with preserved ejection fraction that initially present with isolated left ventricular dysfunction, and changes in right ventricular structure and function greatly exceed changes observed in the left side of the heart in these more advanced phases of disease. Development of right ventricular dysfunction is related to elevated pulmonary artery and left heart filling pressures, as well as common comorbidities in heart failure with preserved ejection fraction including atrial fibrillation, coronary disease, and obesity. FAC, fractional area change; LVD, left ventricular dysfunction; RA, right atrial; RVD, right ventricular dysfunction; TR, tricuspid regurgitation.
Development of right ventricular dysfunction in heart failure with preserved ejection fraction
Right ventricular dysfunction is common in patients with HFpEF when assessed in cross sectional studies.3,4,9,10 However, no study has yet evaluated longitudinal changes in RV function or structure in HFpEF. We observed that HFpEF patients displayed a substantial decline in RV function and even greater degree of RV dilation over a median interval of 4.0 years. Notably, these changes in the right heart structure and systolic function greatly exceeding corresponding changes in the left side of the heart.
Previous studies have established prevalent RVD as a predictor of adverse outcomes in people with HFpEF,3,4,9,10 but no study has yet evaluated the prognostic implications of the development of incident RVD, or determined which patients go on to develop RVD in longitudinal evaluation. In the current study, we demonstrate that incident RVD is associated with increased death in HFpEF, even among patients with normal RV function at baseline (Figure 4B). These data confirm the prognostic significance of RVD and suggest that therapies targeting the risk factors for development of RVD may prevent its genesis and thus improve clinical outcomes in HFpEF patients.
Mechanisms of right ventricular dysfunction in heart failure with preserved ejection fraction
Previous studies have reported that RVD in HFpEF was associated with the severity of PH, LV function, male gender, AF, CAD, obesity, and renal dysfunction.3,4,7,20–22 Although these risk factors are mechanistically plausible, clear cause-effect relationships remain unclear because all studies to date have been cross sectional analyses without repeat, within-subject assessments.
In the current study, numerous features present at Exam 1, including AF, higher body weight, CAD, adverse haemodynamics including higher LV filling pressures and PA pressures, and RV dilation were independently associated with development of RVD. These data support the notion that HFpEF patients with more advanced disease are more likely to develop incident RVD over time, and as such, RVD can be considered to be a reflection of greater disease progression in the HFpEF syndrome. PH is highly prevalent in HFpEF and associated with worse symptoms, reduced exercise capacity, and increased mortality.23,24 Given that the right ventricle is highly sensitive to afterload, PH is likely the most important mechanism of RVD in HFpEF.3
The data from the current study shows that each 1 SD increment in PA systolic pressure was associated with 39–51% increased risk of development of RVD, and each 1 SD increment in PCWP was associated with a 98% increased risk for incident RVD. The reason for the somewhat greater association between incident RVD and higher PCWP is unclear, but may relate to the fact that PCWP increases RV afterload directly by increases in PA pressure due to passive PH and also indirectly by elevating RV pulsatile loading.18
Elevated PCWP may also promote LA remodelling, increasing risk for AF, which we identified as a strong risk factor for incident RVD, and also lead to chronic LA dysfunction, which has been associated with RVD.25 While the use of diuretics increased during follow-up period (40% at Exam 1 to 63% at Exam 2), decongestion still may have been suboptimal, as suggested by the persistent elevation in E/e′ ratio (a surrogate for filling pressures) at Exam 2. Recent data have highlighted the importance of targeting high filling pressures with diuretics for symptom relief and to reduce hospitalizations in HFpEF.26–28 The current data provide compelling support for this dictum by identifying a potentially important role of filling pressure reduction to reduce the risk of progression to chronic RV remodelling and dysfunction.
Right ventricular remodelling in heart failure with preserved ejection fraction
Eccentric cardiac remodelling is not believed to be a major player in patients with HFpEF, at least in the left ventricle.1 We confirm the absence of significant LV remodelling over time in the current study, though there was mild progression in LA dilatation (Table 2). In contrast, we observed significant chamber remodelling in the right heart, evidenced by marked increases in RV diameter and diastolic area, and substantial increases in RA area. The deleterious changes in RV wall stress and chamber geometry associated with remodelling further worsen RV function, as observed in the HFpEF patients in the current study (Figure 1D). Right ventricular and RA dilatation increase tricuspid annular diameter to worsen tricuspid insufficiency (Figure 3), which may further promote systemic venous congestion and impair left heart filling, particularly during exercise.29 We also observed concomitant but significant lesser decline in LV systolic function in HFpEF patients. These data suggest that RV remodelling, as well as PH, may adversely impact left heart function through ventricular interdependence, as observed in patients with right heart failure due to acute pulmonary embolism, HFpEF patients with the pulmonary vascular disease, or obese-related HFpEF.16,30,31
While afterload mismatch is clearly an important contributor to RVD, the development of RV failure is not simply explained by pulmonary hypertension alone. Development of RV dysfunction, myocyte apoptosis, fibrosis, and microvascular rarefaction are greater in animals with pulmonary vascular disease than in animals with isolated PA banding alone, even at the same degree of elevation in PA pressure load.32 We observed that RVSP remained unchanged on average over time in patients with HFpEF, but the relationship between RV FAC and RVSP shifted downward at Exam 2 (Figure 1C). This relationship was also confirmed by RV FWLS (Supplementary material online, Figure S2). These findings suggest that RV dysfunction was caused by worsening myocardial dysfunction and not simply afterload mismatch, although our data cannot exclude the possibility that increases in RV afterload between the two examinations might have caused deterioration in RV function that was not evident from the single time point assessments.
Comorbidities and right ventricular dysfunction
Obesity is very common in HFpEF. When compared with non-obese HFpEF, obese HFpEF patients display greater burden of RV systolic dysfunction.7,16 The current study extends these data by showing that greater body mass is also associated with incident RVD in HFpEF. Obesity and increased adiposity may worsen RV function, possibly through plasma volume expansion, RV remodelling, inflammation, or by enhancing ventricular interdependence.16 Further study is required to determine whether weight loss can reverse, mitigate or protect against the development of RV remodelling and dysfunction in HFpEF.33
Coronary disease is also common and associated with poor outcomes in HFpEF.34 Like obesity, CAD is associated with prevalent RVD in cross sectional HFpEF studies.3,4,35 We observed that CAD predicts development of RVD in HFpEF, even in the absence of clinically-evident myocardial infarction. A recent study has demonstrated that cardiac injury in HFpEF is correlated with limitations in LV systolic and diastolic reserve in HFpEF, even in the absence of epicardial disease.36 Taken together, these data suggest that ischaemia, whether due to epicardial or microvascular coronary disease, might also contribute to RVD. Revascularization may preserve LV function in HFpEF.34 Further study is required to determine if management of epicardial or microvascular coronary disease can improve or prevent adverse changes in RV structure and function.
Atrial fibrillation is associated with high left filling pressure, exercise intolerance, and increased mortality in HFpEF.37–39 We found that prevalent AF at baseline was associated with incident RVD in HFpEF, confirming and extending upon data from cross-sectional studies.3,4,6,21,22 Elevation in left heart filling pressure in AF may adversely affect RV structure and function by increasing pulsatile load to the right ventricle,18 inducing pulmonary vascular disease,25 or both. Similar to a previous study of prevalent RVD,3 AF was associated with incident RVD independent of RVSP in the current study, suggesting that effects of AF on RV function are in part via a load-independent mechanism.
An important finding from the current study was that the development of new persistent AF was strongly associated with incident RVD in patients with HFpEF. This was also coupled with biatrial dilation and worsening TR, perhaps due to annular dilation (Figure 3). In addition to the rhythm irregularity, tachycardia, neurohormonal activation, and microvascular dysfunction, annular dilation secondary to AF might decrease basal RV contractile performance.40 These data support implementation of clinical trials to evaluate whether restoring and maintaining sinus rhythm can prevent RVD or improve RV function in people with HFpEF.
Limitations
This is a retrospective study conducted in a tertiary referral centre. All patients were referred for cardiac catheterization, introducing referral bias, but this was deliberately chosen to ensure high specificity of diagnosis based upon either invasive confirmation or prior hospitalization for pulmonary oedema. RV function was assessed by FAC based alone based upon image availability, and further study is warranted to examine serial changes in RV function using other indices, in particular measures of longitudinal function such as systolic annular velocity or tricuspid annular plane systolic excursion, which were not available. Because echocardiograms were not obtained at prespecified intervals, we cannot determine the time to development of incident RVD. Future prospective studies with more frequent assessments at prespecified intervals would be useful to address this limitation. Invasive haemodynamics were not required for patients that had a history of HF hospitalization, and this reduced the sample size with invasive data, which may have limited power to determine whether invasive haemodynamic indicators of pulmonary vascular function are predictive of incident RVD. Patients with HFrEF were excluded from this analysis, so we cannot determine if the long-term changes in RV structure and function are specific to HFpEF or common to all patients with HF. Further study comparing HFpEF and HFrEF patients would provide greater insight into this question.
Conclusion
While HFpEF is often considered as a primary disorder of the left ventricle, we found that patients with this phenotype display relatively greater remodelling and deterioration in ventricular function over time in the right heart. Development of RVD is associated with adverse outcome and is linked to potentially modifiable risk factors, including AF, coronary disease, increased body weight, and abnormal cardiac haemodynamics. Clinical trials targeting these risk factors are indicated to determine whether RV structure and function can be maintained in order to improve clinical outcomes in patients with HFpEF.
Funding
B.A.B. is supported by U10 HL110262 and RO1 HL128526, Y.N.R. is supported by T32 HL007111, V.M. is supported by Czech Heath Research Council (AZV 17-28784A) and M.O. is supported by a scholarship from the Banyu Life Science Foundation International, Japan.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Reddy YN, Borlaug BA. Heart failure with preserved ejection fraction. Curr Probl Cardiol 2016;41:145–188. [DOI] [PubMed] [Google Scholar]
- 2. Chatterjee NA, Steiner J, Lewis GD. It is time to look at heart failure with preserved ejection fraction from the right side. Circulation 2014;130:2272–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 2014;35:3452–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mohammed SF, Hussain I, AbouEzzeddine OF, Abou Ezzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation 2014;130:2310–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burke MA, Katz DH, Beussink L, Selvaraj S, Gupta DK, Fox J, Chakrabarti S, Sauer AJ, Rich JD, Freed BH, Shah SJ. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail 2014;7:288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hussain I, Mohammed SF, Forfia PR, Lewis GD, Borlaug BA, Gallup DS, Redfield MM. Impaired right ventricular-pulmonary arterial coupling and effect of sildenafil in heart failure with preserved ejection fraction: an ancillary analysis from the phosphodiesterase-5 inhibition to improve clinical status and exercise capacity in diastolic heart failure (RELAX) trial. Circ Heart Fail 2016;9:e002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gorter TM, Hoendermis ES, van Veldhuisen DJ, Voors AA, Lam CS, Geelhoed B, Willems TP, van Melle JP. Right ventricular dysfunction in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Heart Fail 2016;18:1472–1487. [DOI] [PubMed] [Google Scholar]
- 8. Gorter TM, van Veldhuisen DJ, Bauersachs J, Borlaug BA, Celutkiene J, Coats AJS, Crespo-Leiro MG, Guazzi M, Harjola V-P, Heymans S, Hill L, Lainscak M, Lam CSP, Lund LH, Lyon AR, Mebazaa A, Mueller C, Paulus WJ, Pieske B, Piepoli MF, Ruschitzka F, Rutten FH, Seferovic PM, Solomon SD, Shah SJ, Triposkiadis F, Wachter R, Tschöpe C, de Boer RA. Right heart dysfunction and failure in heart failure with preserved ejection fraction: mechanisms and management. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018;20:16–37. [DOI] [PubMed] [Google Scholar]
- 9. Hoeper MM, Lam CSP, Vachiery JL, Bauersachs J, Gerges C, Lang IM, Bonderman D, Olsson KM, Gibbs JSR, Dorfmuller P, Guazzi M, Galie N, Manes A, Handoko ML, Vonk Noordegraaf A, Lankeit M, Konstantinides S, Wachter R, Opitz C, Rosenkranz S. Pulmonary hypertension in heart failure with preserved ejection fraction: a plea for proper phenotyping and further research. Eur Heart J 2017;38:2869–2873. [DOI] [PubMed] [Google Scholar]
- 10. Borlaug BA, Obokata M. Is it time to recognize a new phenotype? Heart failure with preserved ejection fraction with pulmonary vascular disease. Eur Heart J 2017;38:2874–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010;3:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 13. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 14. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713; quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 15. Longobardo L, Suma V, Jain R, Carerj S, Zito C, Zwicke DL, Khandheria BK. Role of two-dimensional speckle-tracking echocardiography strain in the assessment of right ventricular systolic function and comparison with conventional parameters. J Am Soc Echocardiogr 2017;30:937–946.e6. [DOI] [PubMed] [Google Scholar]
- 16. Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J 2016;37:3293–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, Cingolani OH, Mudd JO, Borlaug BA, Redfield MM, Lederer DJ, Kass DA. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation 2012;125:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M,. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 20. Bosch L, Lam CSP, Gong L, Chan SP, Sim D, Yeo D, Jaufeerally F, Leong KTG, Ong HY, Ng TP, Richards AM, Arslan F, Ling LH. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail 2017;19:1664–1671. [DOI] [PubMed] [Google Scholar]
- 21. Aschauer S, Kammerlander AA, Zotter-Tufaro C, Ristl R, Pfaffenberger S, Bachmann A, Duca F, Marzluf BA, Bonderman D, Mascherbauer J. The right heart in heart failure with preserved ejection fraction: insights from cardiac magnetic resonance imaging and invasive haemodynamics. Eur J Heart Fail 2016;18:71–80. [DOI] [PubMed] [Google Scholar]
- 22. Gorter TM, van Melle JP, Rienstra M, Borlaug BA, Hummel YM, van Gelder IC, Hoendermis ES, Voors AA, van Veldhuisen DJ, Lam CSP. Right heart dysfunction in heart failure with preserved ejection fraction: the impact of atrial fibrillation. J Card Fail 2018;24:177–185. [DOI] [PubMed] [Google Scholar]
- 23. Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol 2009;53:1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gorter TM, van Veldhuisen DJ, Voors AA, Hummel YM, Lam CSP, Berger RMF, van Melle JP, Hoendermis ES. Right ventricular-vascular coupling in heart failure with preserved ejection fraction and pre- vs. post-capillary pulmonary hypertension. Eur Heart J Cardiovasc Imaging 2018;19:425–432. [DOI] [PubMed] [Google Scholar]
- 25. Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail 2015;8:295–303. [DOI] [PubMed] [Google Scholar]
- 26. Zile MR, Bennett TD, El Hajj S, Kueffer FJ, Baicu CF, Abraham WT, Bourge RC, Warner Stevenson L. Intracardiac pressures measured using an implantable hemodynamic monitor: relationship to mortality in patients with chronic heart failure. Circ Heart Fail 2017;10:e003594. [DOI] [PubMed] [Google Scholar]
- 27. Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, Henderson J, Cowart P, Stevenson LW. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail 2014;7:935–944. [DOI] [PubMed] [Google Scholar]
- 28. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011;377:658–666. [DOI] [PubMed] [Google Scholar]
- 29. Andersen MJ, Nishimura RA, Borlaug BA. The hemodynamic basis of exercise intolerance in tricuspid regurgitation. Circ Heart Fail 2014;7:911–917. [DOI] [PubMed] [Google Scholar]
- 30. Gorter TM, Obokata M, Reddy YN, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J 2018; doi: 10.1093/eurheartj/ehy331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Belenkie I, Dani R, Smith ER, Tyberg JV. Effects of volume loading during experimental acute pulmonary embolism. Circulation 1989;80:178–188. [DOI] [PubMed] [Google Scholar]
- 32. Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation 2009;120:1951–1960. [DOI] [PubMed] [Google Scholar]
- 33. Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol 2014;63:2817–2827. [DOI] [PubMed] [Google Scholar]
- 35. Ghio S, Guazzi M, Scardovi AB, Klersy C, Clemenza F, Carluccio E, Temporelli PL, Rossi A, Faggiano P, Traversi E, Vriz O, Dini FL. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail 2017;19:873–879. [DOI] [PubMed] [Google Scholar]
- 36. Obokata M, Reddy YNV, Melenovsky V, Kane GC, Olson TP, Jarolim P, Borlaug BA. Myocardial injury and cardiac reserve in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 2018;72:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lam CS, Rienstra M, Tay WT, Liu LC, Hummel YM, van der Meer P, de Boer RA, Van Gelder IC, van Veldhuisen DJ, Voors AA, Hoendermis ES. Atrial fibrillation in heart failure with preserved ejection fraction: association with exercise capacity, left ventricular filling pressures, natriuretic peptides, and left atrial volume. JACC Heart Fail 2017;5:92–98. [DOI] [PubMed] [Google Scholar]
- 38. Zakeri R, Borlaug BA, McNulty SE, Mohammed SF, Lewis GD, Semigran MJ, Deswal A, LeWinter M, Hernandez AF, Braunwald E, Redfield MM. Impact of atrial fibrillation on exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ Heart Fail 2014;7:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation 2013;128:1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seo HS, Ha JW, Moon JY, Choi EY, Rim SJ, Jang Y, Chung N, Shim WH, Cho SY, Kim SS. Right ventricular remodeling and dysfunction with subsequent annular dilatation and tethering as a mechanism of isolated tricuspid regurgitation. Circ J 2008;72:1645–1649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.