Abstract
Aims
Although group-level effectiveness of lipid, blood pressure, glucose, and aspirin treatment for prevention of cardiovascular disease (CVD) has been proven by trials, important differences in absolute effectiveness exist between individuals. We aim to develop and validate a prediction tool for individualizing lifelong CVD prevention in people with Type 2 diabetes mellitus (T2DM) predicting life-years gained without myocardial infarction or stroke.
Methods and results
We developed and validated the Diabetes Lifetime-perspective prediction (DIAL) model, consisting of two complementary competing risk adjusted Cox proportional hazards functions using data from people with T2DM registered in the Swedish National Diabetes Registry (n = 389 366). Competing outcomes were (i) CVD events (vascular mortality, myocardial infarction, or stroke) and (ii) non-vascular mortality. Predictors were age, sex, smoking, systolic blood pressure, body mass index, haemoglobin A1c, estimated glomerular filtration rate, non- high-density lipoprotein cholesterol, albuminuria, T2DM duration, insulin treatment, and history of CVD. External validation was performed using data from the ADVANCE, ACCORD, ASCOT and ALLHAT-LLT-trials, the SMART and EPIC-NL cohorts, and the Scottish diabetes register (total n = 197 785). Predicted and observed CVD-free survival showed good agreement in all validation sets. C-statistics for prediction of CVD were 0.83 (95% confidence interval: 0.83–0.84) and 0.64–0.65 for internal and external validation, respectively. We provide an interactive calculator at www.U-Prevent.com that combines model predictions with relative treatment effects from trials to predict individual benefit from preventive treatment.
Conclusion
Cardiovascular disease-free life expectancy and effects of lifelong prevention in terms of CVD-free life-years gained can be estimated for people with T2DM using readily available clinical characteristics. Predictions of individual-level treatment effects facilitate translation of trial results to individual patients.
Keywords: Cardiovascular, Type 2 diabetes mellitus, Lifetime prediction, Lifelong prevention
Introduction
People with Type 2 diabetes mellitus (T2DM) are at up to two-fold increased risk for cardiovascular disease (CVD) compared with people without T2DM independently from other risk factors.1 Estimated reductions in life expectancy and quality-adjusted life years due to CVD are substantial in people with T2DM especially in people diagnosed with T2DM at young ages.2,3 International guidelines on CVD prevention recommend lipid-lowering, blood pressure-lowering, and glucose-lowering treatment to achieve the respective targets and for some patients also aspirin use.4–6 More recently, new drugs have become available to further reduce the burden of CVD in patients with T2DM. These include PCSK9-inhibition, SGLT2-inhibibition, and GLP1-analogues.7 Guideline recommendations on the use of these preventive medications are based on the group-level effectiveness of such medication as shown in high-quality trials. Yet, important differences in absolute effectiveness are known to exist between individuals. Clinicians may struggle to identify individuals that benefit most from intensive and newer treatment options as the translation of group-level findings and recommendations to the individual patient level is extremely challenging. As individual effectiveness of preventive treatment is mainly determined by individual baseline CVD risk, risk estimation could help to individualize treatment.8,9 In general, people with higher individual cardiovascular risk will benefit more in absolute terms from lipid-lowering, glucose-lowering, or blood pressure-lowering than people with a lower cardiovascular risk.
Therefore, the use of CVD risk prediction models for people with T2DM, such as the UKPDS, ADVANCE, Fremantle, and New Zealand Diabetes risk scores have been recommended in various national guidelines.10–13 Yet, most existing prediction models predict 5-year risks of CVD.14
Medications for prevention of CVD, on the other hand, are usually continued life-long and for most patients this means much longer than 5 years. Therefore, estimates of long-term CVD risk and CVD-free life expectancy (i.e. expected number of remaining life-years without the occurrence of an incident or recurrent myocardial infarction or stroke) are usually more informative.15,16 Several lifetime-perspective models are already available for healthy individuals, but not for patients with T2DM.17,18
The objective of the present study is to develop and externally validate a prediction tool [i.e. the Diabetes Lifetime-perspective prediction (DIAL) model], for individualizing lifelong CVD prevention with lipid-lowering, antihypertensive, glucose-lowering, and aspirin treatment in people with T2DM by predicting treatment effects as gains in 10-year CVD risk, lifetime risk, and CVD-free life expectancy. Notably, CVD-free life expectancy for a person with a history of CVD should be interpreted as time without recurrent myocardial infarction or stroke.
Methods
Sources of data
The Swedish National Diabetes Registry (NDR) and the Scottish Care Information (SCI)—Diabetes database are population wide registers. The secondary Manifestation of ARTerial disease (SMART) study and European Prospective Investigation into Cancer-Netherlands (EPIC-NL) are prospective cohort studies and Action to Control Cardiovascular Risk in Diabetes (ACCORD), the Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation (ADVANCE), Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) and the Lipid-Lowering Trial component of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT) are randomized controlled trials, all including people with T2DM. Study details have been described elsewhere.19–27 The lifetime-perspective prediction model was developed in the Swedish NDR and externally validated in the remaining datasets. All use of data from registries, cohorts, and trials were approved by institutional review boards and all participants gave written informed consent before taking part in the cohorts and trials. All studies complied with the Declaration of Helsinki.
Participants
Participants were people aged >18 years with a diagnosis of T2DM with or without prevalent CVD. People with a previous diagnosis of cancer (ICD-10 codes C00–C97) or Stage 4 or 5 chronic kidney disease (estimated glomerular filtration rate, eGFR <30 mL/min) were excluded. A comprehensive overview of the eligibility criteria and definition of T2DM used for the original cohorts and trials are provided in Supplementary material online, Table S1.
Outcomes
Cardiovascular disease was defined as a non-fatal myocardial infarction, non-fatal stroke, or vascular mortality. In the Swedish NDR and the SCI—Diabetes database, this is based on linkage to cause of death registers and hospital discharge registers using ICD-10 codes. All endpoint definitions of all studies are described in Supplementary material online, Table S1. Non-vascular mortality was defined as all deaths other than those with an identified cardiovascular cause as described in Supplementary material online, Table S1.
Predictors
Based on existing diabetes risk scores and availability in routine clinical practice, 11 selected predictors were sex (female/male), current smoking (yes/no), systolic blood pressure (SBP in mmHg), body mass index (BMI in kg/m2), haemoglobin A1c (HbA1c) measured using the International Federation of Clinical Chemists (IFCC) reference method (in mmol/mol), eGFR estimated by the Chronic Kidney Disease Epidemiology Collaboration equation28 (CKD-EPI in mL/min/1.73 m2), non-high-density lipoprotein cholesterol (non-HDL-c in mmol/L), albuminuria (no/micro/macro), duration of T2DM (years since diagnosis), insulin treatment (yes/no), and history of CVD (yes/no).10–13,29 The number, proportion, and type of missing data, and methods dealing with missing data in each dataset are described in the Supplementary material online, Appendix. No multicollinearity was detected between predictors.
Statistical analysis
Development of the lifetime model
A random sample of 75% of people from the Swedish NDR (n = 292 024) was used as the development dataset. Continuous predictors were truncated at the 1st and 99th percentile to limit the effect of outliers. Using these data, we developed two complementary Fine and Gray competing risk adjusted Cox proportional hazard models with left truncation and right censoring: one for the prediction of CVD events using non-vascular mortality as the competing endpoint (i.e. model Part A), and another for the prediction of non-vascular mortality using CVD events as the competing endpoint (i.e. model Part B). Cumulative CVD-free survival was calculated using the complementary models making use of life-tables with 1-year time intervals. Cardiovascular disease-free life expectancy of an individual was defined as the median survival without myocardial infarction or stroke or death, which was the age where the estimated cumulative survival drops below 50%. Ten-year CVD risk was calculated by summation of the predicted cause-specific CVD risk in the first 10 years from a person’s current age onwards. Similarly, lifetime risk was calculated by the summation of the predicted cause-specific CVD risk from a person’s current age onwards until the maximum age of 95 years.15,30 A description of the statistical methods is described in the Supplementary material online, Appendix. The sample size was more than sufficient by conventional assessment for prediction models with >1000 endpoints per variable.31
Model validation
Goodness of fit was assessed for vascular events, non-vascular mortality, and the combined outcome of CVD-free survival separately using calibration plots for internal and external validation (see Supplementary material online, Appendix).32 The models were recalibrated based on the incidence of CVD and incidence of non-vascular mortality using the expected vs. observed ratio in data from all geographic regions. The logarithm of the expected vs. observed ratio was subtracted from the linear predictor. Discrimination was quantified using c-statistics.
Prediction of individual treatment effect
We combined competing risk adjusted Cox proportional hazard function A for prediction of CVD with hazard ratios (HRs) from randomized trials or meta-analyses to predict the individual treatment effect and lifetime benefit of treatment. The HR of smoking cessation on non-vascular mortality was added to competing risk adjusted Cox proportional hazard function B when predicting the effect of smoking cessation. All other predicted treatment effects were assumed to have no effect on non-vascular mortality (i.e. lipid, blood pressure, glucose, and aspirin treatment). This method of calculating projected individual treatment effects has previously been applied by the American Heart Association and American College of Cardiology in their ‘ASCVD risk estimator plus’ based on the Pooled Cohort Equations for primary prevention.18 By using life-tables, any gains in CVD-free survival is automatically adjusted for competing risks by increasing the time at risk for non-CVD mortality. In this study, we derived estimates of the effect of lipid-lowering, glucose-lowering, blood pressure-lowering, and aspirin treatment.9,33,34 The HRs for different medications used (statins, ezetimibe, PCSK9-inhibitors, antihypertensive medication, HbA1c-lowering, SGLT2-inhibitors, GLP1 analogues, and aspirin) to estimate treatment effects are described in the Supplementary material online, Appendix. We also derived estimates of the effect of smoking cessation. Smoking cessation was unlike drug therapy assumed to have an effect on both CVD and non-vascular mortality (i.e. cancer mortality).35,36
The lifetime benefit of treatment for an individual person was calculated as the difference between the predicted median CVD-free life expectancy with and without treatment. Similarly, lifetime and 10-year absolute CVD risk reduction for individual persons were estimated by calculating the difference between the predicted 10-year CVD risk with and without treatment. The 95% confidence interval (CI) was calculated for the estimates, representing uncertainty of the model development, and relative effects of trial results. This was performed using bootstrap techniques. However, due to computational issues, bags of little bootstraps were necessary. First, 100 random samples without replacement of n^0.8 = 292, 024^0.8 = 23 569 patients were computed. In each random sample, 400 bootstrap samples were taken to obtain boundaries of 95% CIs. The average of all 100 upper and lower 95% CI gave the 95% CI around the predicted estimates.37
Results
The selection of development and validation cohorts from the Swedish NDR is illustrated in Supplementary material online, Figure S1. Baseline characteristics of all study populations are described pooled by geographical origin in Table 1, and stratified by original study cohort in Supplementary material online, Table S2.
Table 1.
Baseline characteristics for study populations used in the Diabetes Lifetime-perspective prediction model pooled by geographical origin
| Derivation |
Validation |
||||||
|---|---|---|---|---|---|---|---|
| Group size | NDR derivation (n = 292 024) | NDR validation (n = 97 342) | Western- Europe (n = 7742) | Eastern- Europe (n = 2142) | North- America (n = 14 590) | Asia and Oceania (n = 5580) | Scotland (n = 167 731) |
| Age (year) | 65 (57–74) | 65 (57–74) | 65 (59–70) | 65 (59–71) | 63 (58–68) | 65 (60–69) | 60 (51–68) |
| Sex (male) | 164 672 (56%) | 54 584 (56%) | 5074 (66%) | 949 (44%) | 8488 (58%) | 3196 (57%) | 96 989 (58%) |
| Current smoking | 48 235 (17%) | 16 206 (17%) | 1419 (18%) | 377 (18%) | 1989 (14%) | 741 (13%) | 59 434 (35%) |
| Duration of diabetes mellitus (year) | 2 (0–7) | 2 (0–7) | 2 (2–5) | 7 (3–12) | 6 (2–12) | 7 (3–12) | 0 (0–0) |
| Insulin treatment | 49 388 (17%) | 16 639 (17%) | 606 (8%) | 43 (2%) | 3587 (25%) | 84 (2%) | 16 373 (10%) |
| Systolic blood pressure (mmHg) | 140 (128–150) | 140 (128–150) | 150 (137–164) | 148 (135–163) | 139 (127–150) | 141 (128–155) | 135 (125–145) |
| Body mass index (kg/m2) | 29 (26–33) | 29 (26–33) | 29 (26–32) | 30 (27–33) | 31 (28–35) | 26 (24–29) | 32 (28–36) |
| HbA1c (mmol/mol) | 50 (44–59) | 50 (44–59) | 53 (45–64) | 56 (46–69) | 63 (55–73) | 55 (48–67) | 53 (46–65) |
| Non-HDL-c (mmol/L) | 3.7 (3.0–4.5) | 3.7 (3.0–4.5) | 3.8 (3.1–4.6) | 4.3 (3.6–5.1) | 3.9 (3.1–4.6) | 3.8 (3.1–4.6) | 3.4 (2.7–4.3) |
| eGFR (mL/min/1.73 m2; CKD-EPI) | 84 (68–96) | 84 (68–96) | 72 (61–86) | 70 (59–83) | 81 (67–94) | 79 (65––92) | 83 (68–96) |
| Micro-albuminuria | 43 231 (15%) | 14 668 (15%) | 2707 (35%) | 560 (26%) | 2892 (20%) | 1731 (31%) | 24 631 (15%) |
| Macro-albuminuria | 20 526 (7%) | 6832 (7%) | 201 (3%) | 99 (5%) | 761 (5%) | 276 (5%) | 2318 (1 %) |
| History of CVD | 55 896 (19%) | 18 674 (19%) | 2618 (34%) | 771 (36%) | 4948 (34%) | 1784 (32%) | 24 853 (15%) |
Development of the Diabetes Lifetime-perspective prediction model
The calculation formulae including the coefficients of the Cox proportional hazard functions, age-specific baseline survivals, and HRs of intended treatment of the model are provided in Supplementary material online, Tables S3 and S4. The HRs for Cox proportional hazard functions A and B of the DIAL model are shown in Table 2. Quadratic transformation of continuous predictors was applied for BMI, SBP, HbA1c, non-HDL-c, and eGFR for Cox proportional hazard function A (CVD) and for BMI, SBP, and BMI for Cox proportional hazard function B (non-vascular mortality). Interactions between age and sex, smoking, history of CVD, and treatment with insulin, were added to Cox proportional hazard functions A and B. Due to the presence of competing risks, interactions with age and transformations of determinants the coefficients and HRs as presented in Table 2 should be interpreted with caution. For example, although the HR of history of CVD in model Part B (non-vascular mortality) is 0.25, this should not be interpreted as a protective effect from an aetiological perspective. More likely, from a prognostic perspective, increased incidence of vascular events in patients with a history of CVD simply results in a less frequent observation of non-vascular mortality. Also, since history of CVD interacts with age, the HRs are presented for a 65-year-old patients and change with changing age. Furthermore, HRs need to be seen in combination with the age-specific baseline hazards and are therefore difficult to interpret.
Table 2.
Hazard ratios derived from a multi-variable model used in the Diabetes Lifetime-perspective prediction model
| Cox proportional hazard function A (vascular events), HR (95% CI) | Cox proportional hazard function B (non-vascular mortality), HR (95% CI) | |
|---|---|---|
| Male sexa | 0.91 (0.88–0.94)a | 0.89 (0.87–0.91)a |
| Current smokinga | 1.04 (1.00–1.09)a | 1.46 (1.43–1.50)a |
| Duration of T2DM (years) | 1.02 (1.01–1.02) | 1.01 (1.01–1.01) |
| Insulin therapya | 1.02 (0.98–1.06)a | 1.04 (1.01–1.07)a |
| Systolic blood pressure (mmHg)b | 1.06 (0.95–1.17)b | 1.01 (0.93–1.10)b |
| Body mass index (kg/m²)b | 0.88 (0.81–0.97)b | 0.89 (0.84–0.95)b |
| HbA1c (mmol/L)b | 1.15 (1.05–1.26)b | 1.00 (1.00–1.00) |
| Non-HDL-c (mmol/L)b | 1.16 (1.10–1.23)b | 0.96 (0.92–1.00)b |
| eGFR (mL/min/1.73 m²)b | 0.64 (0.60–0.69)b | 0.99 (0.99–0.99) |
| Micro-albuminuria | 1.18 (1.14–1.22) | 1.17 (1.14–1.20) |
| Macro-albuminuria | 1.23 (1.18–1.28) | 1.24 (1.20–1.28) |
| History of cardiovascular disease | 9.99 (9.63–10.36)a | 0.25 (0.24–0.26)a |
Age-dependent variables. Hazard ratios are shown for the median age of 65 years.
Transformed variables. Hazard ratios are shown for the 75th percentile vs. the 25th percentile (systolic blood pressure: 150 mmHg vs. 128 mmHg; body mass index: 33 kg/m2 vs. 26 kg/m2; HbA1c: 59 mmol/L vs. 44 mmol/L; eGFR: 96 mL/min vs. 68 mL/min; and non-HDL-c: 4.5 mmol/L vs. 3.0 mmol/L).
Internal validation
Predicted 10-year risk for CVD and all-cause mortality (CVD risk and non-vascular mortality risk combined) showed good agreement with the 10-year observed risk in the development dataset (see Supplementary material online, Figure S2). The c-statistics were 0.83 (95% CI 0.83–0.84), 0.72 (0.72–0.73), and 0.77 (0.76–0.77) for 10-year CVD risk, 10-year non-vascular mortality risk, and 10-year CVD-free survival, respectively.
External validation
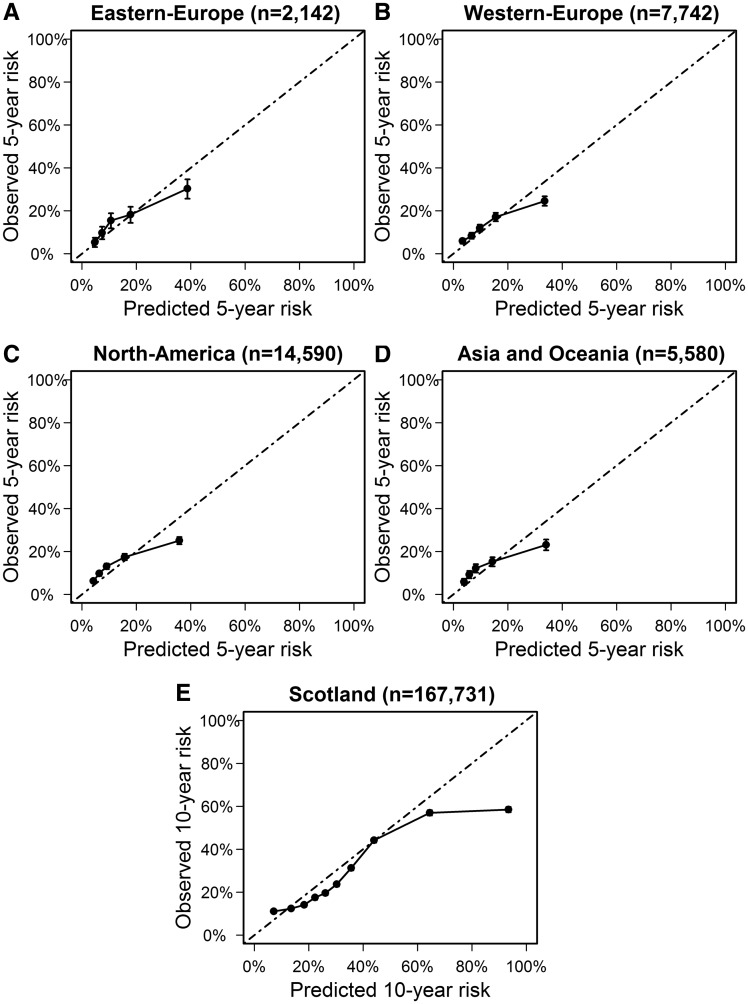
Predicted 5-year risk for CVD and all-cause mortality showed good agreement with the observed 5-year CVD-free survival in Western-Europe, Eastern-Europe, North-America, and Asia and Oceania (Figure 1). The c-statistic of the estimated 5-year CVD risk was between 0.64 and 0.65 in all geographically pooled datasets. C-statistics for 5-year non-vascular mortality risk (range 0.59–0.67) and 5-year risk for CVD and all-cause mortality (range 0.64–0.69) are shown in Supplementary material online, Table S5. Cardiovascular disease event rates were higher in the Scottish Care Information–Diabetes database (17 per 1000 people per year) compared with the Swedish NDR (11 per 1000 people per year). After recalibrating the model for differences in predicted vs. observed event rates, external validation in Scottish data showed good agreement between the predicted and observed 10-year risk for CVD and all-cause mortality (Figure 1). Discrimination of 10-year CVD risk was 0.64 (95% CI 0.64–0.65; see Supplementary material online, Table S5).
Figure 1.
Calibration plots in external dataset pooled by geographical region. Predicted vs. observed 5-year risk of cardiovascular disease and all-cause mortality according to the Diabetes Lifetime-perspective prediction model in quintiles of risk in (A) Eastern-Europe, (B) Western-Europe, (C) North-America, and (D) Asia and Oceania. (E) Predicted vs. observed 10-year risk of cardiovascular disease and all-cause mortality according to the Diabetes Lifetime-perspective prediction model in deciles of risk in Scotland. CVD, cardiovascular disease.
Individual lifetime estimates and treatment effects for people with Type 2 diabetes mellitus
An interactive calculator is provided at www.U-Prevent.com. Patient characteristics and current medication can be entered in this decision support tool to estimate individual risk and CVD-free survival. Also the individual effect from medication changes can be modelled in terms of CVD-free life-years gained, absolute risk reduction, and individual number needed to treat. Absolute treatment effects vary widely between individuals. As an example, we modelled that a combination therapy of simvastatin 40 mg, ezetimibe 10 mg, and SBP-lowering to 140 mmHg, conferred between 0.04 (95% CI 0.01–0.04) and 12.5 (95% CI 11.0–21.2) years gained without CVD events in people enrolled in the Swedish NDR. Treatment effect was lowest (<0.05 CVD-free years) in people who were 78 years or older, without a history of vascular disease, SBP of <140 mmHg, and low-density lipoprotein cholesterol (LDL-c) of <3.0 mmol/L at baseline. Treatment effect was highest (>10 CVD-free years) in people aged 55–70 years, with a history of vascular disease, SBP >160 mmHg and LDL-c >3.0 mmol/L at baseline. As another illustration example, Figure 2 shows the expected result of starting the same treatment (i.e. simvastatin 40 mg) in three different people with T2DM.
Figure 2.
Examples of treatment effects of simvastatin 40 mg vs. no treatment in people with different characteristics.
Discussion
In this study, we have developed and validated the DIAL model to predict CVD-free life expectancy, lifetime risk, and 10-year CVD risk in people with T2DM using widely available patient characteristics. The novelty of this tool compared with previous models is that it not only predicts 5-year of 10-year risk, but also long-term perspective outcomes. In addition, the DIAL model takes competing non-cardiovascular mortality into account and can, therefore, be used to estimate unbiased lifetime benefits of preventive treatment when combined with trial findings. Therefore, the DIAL model may help clinicians to translate group-level trial findings to the individual patient. The interactive calculator at www.U-Prevent.com facilitates the actual use of the DIAL model in clinical practice. We have validated the DIAL model in populations from different continents and have demonstrated that, after recalibration, it has the potential to support medical decision-making for CVD prevention in people with T2DM in diverse populations. The discriminative ability of the model was moderate in each external validation dataset consistent with external validation of previous risk scores. For example validation of the ADVANCE risk score in EPIC-NL and SMART gave c-statistics of 0.62 and 0.68, respectively.38 The cardiovascular event rate was higher in Scotland compared with Sweden, due to differences in multiple factors not taken into consideration in the model, including lifestyle differences. Recalibration of the DIAL model allows it to be adapted for use in populations with varying levels of CVD risk. Users can choose to apply either the low-risk CVD event rate (based on the Swedish cohort, i.e. 11/1000 people per year) or the high-risk event rate (based on the Scottish cohort, i.e. 17/1000 people per year), whichever is more appropriate for their population. Although calibration plots show an overestimation for patients at the highest risk, in clinical practice this is unlikely to lead to erroneous clinical decisions. Overestimation occurs in patients with 5-year risks of >20% (corresponding to 10-year risks of >40%). Even though overestimated, the true observed risk in these patients is still very high and should urge for intensive medical therapy anyway. Also, overestimation of risk in the high-risk patient category is not a specific limitation of the DIAL model, but in line with previous validation studies of CVD-risk models in diabetes patients.38
Several studies have convincingly demonstrated the advantage of lifetime prediction compared with traditional 5-year or 10-year risk predictions. A microsimulation model based on 5-year follow-up of the Rotterdam Study showed that the gain in total CVD-free life expectancy increased as risk factor levels increased. The gain in total CVD-free expectancy decreased with advancing age, whereas 10-year risk for CVD mortality, and therefore, 10-year risk reduction, increased with age.39 In other primary prevention settings for example, smoking cessation at age 60 years, 50 years, 40 years, or 30 years resulted in about 3 years, 6 years, 9 years, and 10 years of life-years gained, respectively. This indicates that the highest lifetime benefit can be gained by reducing risk factors early in life, ideally with lifestyle interventions but, if necessary, with drug treatment.40
In clinical practice, prediction of lifetime benefit in CVD-free life-years gained would enable patients (as well as clinicians) to better understand the potential benefits of treatment. Such information could help patients to participate in the decision-making process about treatment and may also motivate them to adhere to therapy. Clinicians and patients can balance the benefit and possible disadvantages of treatment, to decide whether preventive medication should be started or stopped. Also, the ability to estimate which preventive therapy is most effective (e.g. lipid-lowering, glucose-lowering, blood pressure-lowering, or aspirin treatment) can help to decide what treatment should be initiated first, and what treatment can be postponed or not prescribed to avoid excessive polypharmacy.
Using the concept of predicting lifetime benefit for making treatment decisions will result in changing characteristics of people eligible for treatment, towards higher proportions of younger people with higher risk factor levels. This group of people needs to be treated over a longer period of time resulting in higher treatment costs. Prediction based treatment using the DIAL model could theoretically also lead to higher cost-effectiveness of treatment on a group-level. This, of course, should be confirmed by future cost-effectiveness studies. Also, it is not clear whether stopping treatment in older people would offset these costs and health economic analyses are required to investigate and to establish appropriate thresholds of minimum gain in life-years free of CVD.
The strengths of this study include the use of a large number of people from diverse cohorts. Since the Swedish and SCI—Diabetes database are registries with information for over 90% of people with T2DM in both countries, there is limited selection of people with T2DM, in contrast to trial populations.41 Therefore, these registries are close to true representations of their populations and this increases the generalizability of the DIAL model to clinical practice. Extensive validation of the DIAL model in large and diverse populations supports the use of the DIAL model in people with T2DM without chronic kidney disease (eGFR <30) or metastatic cancer in different parts of the world. However, new external validation studies using data of other and new trials including T2DM patients could further enhance the validity of the DIAL model.
Some limitations of the DIAL model should be considered. Although our model can guide the decision to start treatment for the prevention of CVD, it must be emphasized that there are other reasons for people with T2DM to start preventive therapy (e.g. prevention of neuropathy, retinopathy, diabetic nephropathy, or foot ulcers). The DIAL model predictions do not incorporate these effects and may, therefore, underestimate the total treatment benefits. In addition, some people use preventive medication for other indications. For example, lipid-lowering drugs are also used for monogenetic dyslipidaemias, antihypertensive drugs are used to reduce progression of aneurysms, and diuretics are used for heart failure. The DIAL-score may not be applicable to people with such comorbidities. Additional risk factors such as socio-economic status, coronary calcium scores, and ethnicity have not been incorporated in the model and may have improved performance. However, addition of more risk factors to prediction models generally only leads to minor improvements model performance.42 Finally, the initial and most effective approaches to primary and secondary prevention of T2DM are lifestyle changes, such as sufficient physical activity, healthy diet and, where appropriate, weight loss. Clearly prediction of effects of lifestyle interventions would be valuable. However, it is currently not feasible to include lifestyle factors in prediction models given the lack of robust estimates of the effect size for lifestyle interventions from randomized controlled trials.
Other limitations of the methods used to develop and validate the DIAL model, and to estimate treatment effects should be acknowledged. Validation could only be performed for 10-year and 5-year predictions due to the limited follow-up in the included cohorts and trials. Lifetime estimates often go beyond 10-year predictions, and require the assumption that rates will be similar for a current 40-year-old in 40 years’ time to those of an 80-year-old today. This is a major assumption but previous studies have shown that lifetime estimates based on the methods we used appear to apply for a survival of up to 17 years.15 Nevertheless, longer-term validation would be preferable and will be possible as follow-up data accrue in Sweden and Scotland. Also, the lifetime benefits are calculated assuming immediate, lifelong, successful (i.e. targets reached) and uninterrupted treatment from their current age onwards. The estimated treatment effects are the maximum potential benefit with treatment (i.e. full adherence, usage as prescribed). In clinical practice treatment adherence to preventive medication is reported to be 50% primary and 66% in secondary cardiovascular prevention settings.43 Yet the DIAL model is intended to support medical decision-making by providing estimates about what a patient and healthcare professional can expect when adhering to a treatment as prescribed. The predicted treatment effects are based on the results of large randomized clinical trials in which adherence to treatment usually is about 80%. Furthermore, possible changes in risk factor levels over time were not taken into account. For example, blood pressure and cholesterol were assumed to remain stable over time. Therefore, re-evaluation of CVD-free survival and treatment effects after 5–10 years is advised based on our validation to ensure valid predictions to guide treatment decisions.
Conclusion
CVD-free life expectancy as well as the effect of lifelong lipid-lowering, glucose-lowering, blood pressure-lowering, and aspirin treatment in terms of CVD-free life-years gained can be reliably predicted for people with T2DM using readily available characteristics. The DIAL model may facilitate personalized treatment and support shared decision-making and patients’ motivation to adhere to prescribed drug-treatments to reduce CVD risk.
Supplementary Material
Acknowledgements
For the Swedish National Diabetes Registry, we thank all of the clinicians who were involved in the care of patients with diabetes for collecting data, and staff at the Swedish National Diabetes Registry. We acknowledge with gratitude the contributions of people and organizations involved in providing data, setting up, maintaining, and overseeing SCI-Diabetes, including the Scottish Diabetes Research Network that is supported by National Health Service (NHS) Research Scotland, a partnership involving Scottish NHS Boards and the Chief Scientist Office of the Scottish Government.
For the SMART study, we gratefully acknowledge the contribution of the SMART research nurses, Rutger van Petersen, MSc (data manager), Baukje G.F. van Dinther, MSc (manager Utrecht Cardiovascular Cohort), and the members of the SMART study group: Ale Algra, MD, PhD; Yolanda van der Graaf, MD, PhD; Diederick E. Grobbee, MD, PhD; Guy E.H.M. Rutten, MD, PhD, Julius Center for Health Sciences and Primary Care; Frank L.J. Visseren, MD, PhD, Department of Internal Medicine; Gert J. de Borst, MD, PhD, Department of Vascular Surgery; L. Jaap Kappelle, MD, PhD, Department of Neurology; Tim Leiner, MD, PhD, Department of Radiology; and Hendrik M. Nathoe, MD, PhD, Department of Cardiology.
Funding
This work was partly funded by Hartstichting [grant number 2016T026] (JAN Dorresteijn).
The EPIC-Netherlands study is supported by the ‘Europe Against Cancer’ Programme of the European Commission; Dutch Ministry of Health, Welfare and Sports; Netherlands Organisation for Health Research and Development; and World Cancer Research Fund.
The ASCOT study was supported by Pfizer, Servier Research Group (Paris, France), Leo Laboratories (Copenhagen, Denmark), and Solvay Pharmaceuticals (Brussels, Belgium).
The ALLHAT study was supported by contract NO1-HC-35130 with the National Heart, Lung, and Blood Institute and study medications were supplied by Bristol-Myers Squibb (pravastatin), and financial support was provided by Pfizer.
The Action in Diabetes and Vascular Disease: preterAx and diamicroN-MR Controlled Evaluation (ADVANCE) trial was funded by grants from the National Health and Medical Research Council of Australia and Servier International.
The ACCORD study was supported by grants from the National Heart, Lung, and Blood Institute; by other components of the National Institutes of Health, including the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Eye Institute; by the Centers for Disease Control and Prevention; and by General Clinical Research Centers.
For the present study, the above supporting sources had no involvement in study design, analysis, interpretation, and writing of the results and decision to submit the report for publication.
Conflict of interest: J.C. declares research funding from Servier. M.W. declares consultancy work for Amgen. And all other authors have nothing to disclose.
References
- 1. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J.. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF.. Lifetime risk for diabetes mellitus in the United States. JAMA 2003;290:1884–1890. [DOI] [PubMed] [Google Scholar]
- 3. Gu K, Cowie CC, Harris MI.. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care 1998;21:1138–1145. [DOI] [PubMed] [Google Scholar]
- 4. Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr, Sorlie P, Stone NJ, Wilson PW.. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(25 Suppl 2):S49–S73. [DOI] [PubMed] [Google Scholar]
- 5. Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H, Marre M, Marx N, Mellbin L, Ostergren J, Patrono C, Seferovic P, Uva MS, Taskinen MR, Tendera M, Tuomilehto J, Valensi P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Torbicki A, Wijns W, Windecker S, De Backer G, Ezquerra EA, Avogaro A, Badimon L, Baranova E, Betteridge J, Ceriello A, Funck-Brentano C, Gulba DC, Kjekshus JK, Lev E, Mueller C, Neyses L, Nilsson PM, Perk J, Reiner Z, Sattar N, Schachinger V, Scheen A, Schirmer H, Stromberg A, Sudzhaeva S, Viigimaa M, Vlachopoulos C, Xuereb RG.. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 2013;34:3035–3087. [DOI] [PubMed] [Google Scholar]
- 6. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FD, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WM; Authors/Task Force Members. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sattar N, Preiss D, Robinson JG, Djedjos CS, Elliott M, Somaratne R, Wasserman SM, Raal FJ.. Lipid-lowering efficacy of the PCSK9 inhibitor evolocumab (AMG 145) in patients with type 2 diabetes: a meta-analysis of individual patient data. Lancet Diabetes Endocrinol 2016;4:403–410. [DOI] [PubMed] [Google Scholar]
- 8. Rabar S, Harker M, O'Flynn N, Wierzbicki AS, Guideline Development G.. Lipid modification and cardiovascular risk assessment for the primary and secondary prevention of cardiovascular disease: summary of updated NICE guidance. BMJ 2014;349:g4356.. [DOI] [PubMed] [Google Scholar]
- 9. Zheng SL, Roddick AJ, Aghar-Jaffar R, Shun-Shin MJ, Francis D, Oliver N, Meeran K.. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA 2018;319:1580–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davis WA, Knuiman MW, Davis TM.. An Australian cardiovascular risk equat for type 2 diabetes: the Fremantle Diabetes Study. Intern Med J 2010;40:286–292. [DOI] [PubMed] [Google Scholar]
- 11. Elley CR, Robinson E, Kenealy T, Bramley D, Drury PL.. Derivation and validation of a new cardiovascular risk score for people with type 2 diabetes: the New Zealand diabetes cohort study. Diabetes Care 2010;33:1347–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kengne AP, Patel A, Marre M, Travert F, Lievre M, Zoungas S, Chalmers J, Colagiuri S, Grobbee DE, Hamet P, Heller S, Neal B, Woodward M, Group AC.. Contemporary model for cardiovascular risk prediction in people with type 2 diabetes. Eur J Cardiovasc Prev Rehabil 2011;18:393–398. [DOI] [PubMed] [Google Scholar]
- 13. Stevens RJ, Kothari V, Adler AI, Stratton IM.. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci (Lond) 2001;101:671–679. [PubMed] [Google Scholar]
- 14. van Dieren S, Beulens JW, Kengne AP, Peelen LM, Rutten GE, Woodward M, van der Schouw YT, Moons KG.. Prediction models for the risk of cardiovascular disease in patients with type 2 diabetes: a systematic review. Heart 2012;98:360–369. [DOI] [PubMed] [Google Scholar]
- 15. Dorresteijn JAN, Kaasenbrood L, Cook NR, van Kruijsdijk RC, van der Graaf Y, Visseren FLJ, Ridker PM.. How to translate clinical trial results into gain in healthy life expectancy for individual patients. BMJ 2016;352:i1548. [DOI] [PubMed] [Google Scholar]
- 16. Leening MJ, Berry JD, Allen NB.. Lifetime perspectives on primary prevention of atherosclerotic cardiovascular disease . JAMA 2016;315:1449–1450. [DOI] [PubMed] [Google Scholar]
- 17. Board JBS. Joint British Societies’ consensus recommendations for the prevention of cardiovascular disease (JBS3). Heart 2014;100(Suppl 2):ii1–ii67. [DOI] [PubMed] [Google Scholar]
- 18. Lloyd-Jones DM, Huffman MD, Karmali KN, Sanghavi DM, Wright JS, Pelser C, Gulati M, Masoudi FA, Goff DC Jr.. Estimating longitudinal risks and benefits from cardiovascular preventive therapies among Medicare patients: the million hearts longitudinal ASCVD risk assessment tool: a special report from the American Heart Association and American College of Cardiology. Circulation 2017;135:e793–e813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beulens JW, Monninkhof EM, Verschuren WM, van der Schouw YT, Smit J, Ocke MC, Jansen EH, van Dieren S, Grobbee DE, Peeters PH, Bueno-de-Mesquita HB.. Cohort profile: the EPIC-NL study. Int J Epidemiol 2010;39:1170–1178. [DOI] [PubMed] [Google Scholar]
- 20. Committee AM. Study rationale and design of ADVANCE: action in diabetes and vascular disease–preterax and diamicron MR controlled evaluation. Diabetologia 2001;44:1118–1120. [DOI] [PubMed] [Google Scholar]
- 21. Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J; ASCOT Investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005;366:895–906. [DOI] [PubMed] [Google Scholar]
- 22. Group AS, Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, Goff DC. Jr, Grimm RH Jr, Margolis KL, Probstfield JL, Simons-Morton DG, Sullivan MD. Action to control cardiovascular risk in diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007;99:21i–33i. [DOI] [PubMed] [Google Scholar]
- 23. Gudbjornsdottir S, Cederholm J, Nilsson PM, Eliasson B; Steering Committee of the Swedish National Diabetes Register. The National Diabetes Register in Sweden: an implementation of the St. Vincent Declaration for quality improvement in diabetes care. Diabetes Care 2003;26:1270–1276. [DOI] [PubMed] [Google Scholar]
- 24.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 25. Patel A; ADVANCE Collaborative Group. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007;370:829–840. [DOI] [PubMed] [Google Scholar]
- 26. Simons PC, Algra A, van de Laak MF, Grobbee DE, van der Graaf Y.. Second manifestations of ARTerial disease (SMART) study: rationale and design. Eur J Epidemiol 1999;15:773–781. [DOI] [PubMed] [Google Scholar]
- 27. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT.. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cederholm J, Eeg-Olofsson K, Eliasson B, Zethelius B, Nilsson PM, Gudbjornsdottir S.. Risk prediction of cardiovascular disease in type 2 diabetes: a risk equation from the Swedish National Diabetes Register. Diabetes Care 2008;31:2038–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Geskus RB. Cause-specific cumulative incidence estimation and the Fine and Gray model under both left truncation and right censoring. Biometrics 2011;67:39–49. [DOI] [PubMed] [Google Scholar]
- 31. Austin PC, Allignol A, Fine JP.. The number of primary events per variable affects estimation of the subdistribution hazard competing risks model. J Clin Epidemiol 2017;83:75–84. [DOI] [PubMed] [Google Scholar]
- 32. Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, Pencina MJ, Kattan MW.. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010;21:128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cholesterol Treatment Trialists’ Collaborators, Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, Baigent C.. Efficacy of cholesterol-lowering therapy in 18, 686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008;371:117–125. [DOI] [PubMed] [Google Scholar]
- 34. Thomopoulos C, Parati G, Zanchetti A.. Effects of blood-pressure-lowering treatment on outcome incidence in hypertension: 10—Should blood pressure management differ in hypertensive patients with and without diabetes mellitus? Overview and meta-analyses of randomized trials. J Hypertens 2017;35:922–944. [DOI] [PubMed] [Google Scholar]
- 35. Gellert C, Schottker B, Brenner H.. Smoking and all-cause mortality in older people: systematic review and meta-analysis. Arch Intern Med 2012;172:837–844. [DOI] [PubMed] [Google Scholar]
- 36. Mons U, Muezzinler A, Gellert C, Schottker B, Abnet CC, Bobak M, de Groot L, Freedman ND, Jansen E, Kee F, Kromhout D, Kuulasmaa K, Laatikainen T, O'Doherty MG, Bueno-de-Mesquita B, Orfanos P, Peters A, van der Schouw YT, Wilsgaard T, Wolk A, Trichopoulou A, Boffetta P, Brenner H. CHANCES Consortium. Impact of smoking and smoking cessation on cardiovascular events and mortality among older adults: meta-analysis of individual participant data from prospective cohort studies of the CHANCES consortium. BMJ 2015;350:h1551.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kleiner A, Talwalkar A, Sarkar P, Jordan MI.. A scalable bootstrap for massive data. J R Stat Soc B 2014;76:795–816. [Google Scholar]
- 38. van der Leeuw J, van Dieren S, Beulens JWJ, Boeing H, Spijkerman AMW, van der Graaf Y, van der A DL, Nöthlings U, Visseren FL, Rutten GE, Moons KG, van der Schouw YT, Peelen LM.. The validation of cardiovascular risk scores for patients with type 2 diabetes mellitus. Heart 2015;101:222–229. [DOI] [PubMed] [Google Scholar]
- 39. Ferket BS, van Kempen BJ, Heeringa J, Spronk S, Fleischmann KE, Nijhuis RL, Hofman A, Steyerberg EW, Hunink MG.. Personalized prediction of lifetime benefits with statin therapy for asymptomatic individuals: a modeling study. PLoS Med 2012;9:e1001361.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Doll R, Peto R, Boreham J, Sutherland I.. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328:1519.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saunders C, Byrne CD, Guthrie B, Lindsay RS, McKnight JA, Philip S, Sattar N, Walker JJ, Wild SH; Scottish Diabetes Research Network Epidemiology Group. External validity of randomized controlled trials of glycaemic control and vascular disease: how representative are participants? Diabet Med 2013;30:300–308. [DOI] [PubMed] [Google Scholar]
- 42. Austin PC, Pencinca MJ, Steyerberg EW.. Predictive accuracy of novel risk factors and markers: a simulation study of the sensitivity of different performance measures for the Cox proportional hazards regression model. Stat Methods Med Res 2017;26:1053–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Naderi SH, Bestwick JP, Wald DS.. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med 2012;125:882–887.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.