Abstract
Aims
Trimethyllysine (TML) serves as a nutrient precursor of the gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) and is associated with incident cardiovascular (CV) events in stable subjects. We examined the relationship between plasma TML levels and incident CV events in patients presenting with acute coronary syndromes (ACS).
Methods and results
Plasma levels of TML were quantified in two independent cohorts using mass spectrometry, and its relationship with CV events was investigated. In a Cleveland Cohort (N = 530), comprised of patients presenting to the emergency department with chest pain and suspected ACS, TML was associated with major adverse cardiac events (MACE, myocardial infarction, stroke, need for revascularization, or all-cause mortality) over both 30 days [3rd tertile (T3), adjusted odds ratio (OR) 1.77, 95% confidence interval (CI) 1.04–3.01; P < 0.05] and 6 months (T3, adjusted OR 1.95, 95% CI 1.15–3.32; P < 0.05) of follow-up independent of traditional CV risk factors and indices of renal function. Elevated TML levels were also associated with incident long-term (7-year) all-cause mortality [T3, adjusted hazard ratio (HR) 2.52, 95% CI 1.50–4.24; P < 0.001], and MACE even amongst patients persistently negative for cardiac Troponin T at presentation (e.g. 30-day MACE, T3, adjusted OR 4.49, 95% CI 2.06–9.79; P < 0.001). Trimethyllysine in combination with TMAO showed additive significance for near- and long-term CV events, including patients with ‘negative’ high-sensitivity Troponin T levels. In a multicentre Swiss Cohort (N = 1683) comprised of ACS patients, similar associations between TML and incident 1-year adverse cardiac risks were observed (e.g. mortality, adjusted T3 HR 2.74, 95% CI 1.28–5.85; P < 0.05; and MACE, adjusted T3 HR 1.55, 95% CI 1.04–2.31; P < 0.05).
Conclusion
Plasma TML levels, alone and together with TMAO, are associated with both near- and long-term CV events in patients with chest pain and ACS.
Keywords: Trimethyllysine, Trimethylamine N-oxide, Incident major adverse cardiac events, Myocardial infarction, Acute coronary syndromes, Gut microbiota
See page 2710 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz377)
Introduction
Each year, about 20 million patients present with acute coronary syndromes (ACS) to emergency departments (EDs) in North America and Europe.1 Current risk stratification markers for ACS identify proteins released into the circulation as a result of myocardial necrosis, and focus predominantly on ruling out near-term adverse risks, as well as to identify those for whom more invasive diagnostic testing and revascularization may be warranted.2–4 In contrast, traditional cardiovascular (CV) risk factors used for long-term risk prediction (e.g. cholesterol, triglycerides, high-sensitivity C-reactive protein) typically are not utilized in the ACS setting because they do not provide significant prognostic value for near-term risks.5 Identification of circulating biomarkers that provide both near- and long-term prognostic value in both ACS subjects and stable subjects alike is of interest, as this may help to both identify pathways relevant to cardiovascular disease (CVD) pathogenesis, as well as improve potential preventive CV risk reduction efforts.
Nearly a decade ago, employing untargeted metabolomics as a discovery platform, we identified circulating analytes in plasma whose levels are associated with incident CVD risks.6 These studies led to the discovery of a mechanistic link between the gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) and both development of atherosclerosis,6 and phenotypes associated with vulnerable plaque, including arterial endothelial cell activation,7 and enhanced platelet reactivity and thrombosis potential.8 Following the initial clinical studies showing circulating TMAO levels predict incident risks for major adverse cardiovascular events (MACE, myocardial infarction (MI), stroke, or all-cause mortality),9 and enhanced thrombotic event risks,8 multiple subsequent studies and meta-analyses have confirmed the association between TMAO and both near- and long-term adverse risks in multiple cohorts.10–13
In more recent untargeted metabolomics studies, we similarly aimed to discover circulating metabolites associated with incident MACE in stable subjects undergoing elective cardiac evaluations. The amino acid N6, N6, N6-trimethyl-L-lysine (trimethyllysine, TML) was identified to be reproducibly associated with CVD risks in stable subjects undergoing elective diagnostic cardiac risk assessments,14 and multiple mechanistic studies revealed TML is a relatively abundant post-translational modification of protein lysine residues in both plants and animals alike, and can serve as a dietary precursor for gut microbiota-dependent generation of TMAO.14 Trimethyllysine is also a known intermediate in carnitine biosynthesis.15,16 Few investigations have examined the relationship between TML and phenotypes relevant to CVD risks. In one small study of subjects with evidence of carotid artery atherosclerosis, TML was associated with increased mortality risks.17 In another study of stable patients with coronary artery disease (CAD), TML levels were associated with incident (∼8-year follow-up) risk (20% increase) for development of Type 2 diabetes.18 Herein, we sought to examine the relationship between systemic levels of TML and both near- and long-term CVD and mortality risks among patients presenting to the ED with the complaint of chest pain, and amongst patients presenting with adjudicated ACS.
Methods
Full descriptions of methods are provided in the Supplementary material online.
Study populations
Two independent cohorts of patients with suspected vs. adjudicated ACS were examined. Patients from both the Cleveland Cohort and the Swiss ACS Cohort gave informed consent, and study protocols were approved by respective institutional review boards. All studies were conducted according to the declaration of Helsinki.
The Cleveland Cohort (N = 530) was a single-centre, prospective cohort study designed to examine cardiac Troponin T (cTnT) in the diagnosis of MI, as described previously,19,20 with samples reanalysed with the newer (5th generation) high-sensitivity cTnT assay (Roche Diagnostics). Adult patients 18 years old and above who presented with chest pain of suspected cardiac origin to the ED within 24 h of initial onset were eligible. Blood was collected at presentation (baseline), 4, 8, and 16 h later and plasma frozen at −70°C until analysis. Of the initial 530 patients, 112 were cardiac Troponin T (cTnT)-positive (cTnT ≥0.1 µg/L) at initial evaluation period, and 418 patients were persistently negative for serial testing of cTnT. All events were adjudicated with medical record checks.
The Swiss ACS Cohort (N = 1683) was a multicentre (Bern, Zurich, Geneva, and Lausanne), prospective study, performed as part of the Special Program University Medicine (SPUM-ACS) study (ClinicalTrials.gov number: NCT01000701, https://clinicaltrials.gov/ct2/show/NCT01000701) between December 2009 and October 2012. It involved consecutive and prospective recruitment of subjects presenting with adjudicated ACS, as previously described.10,21 More extensive descriptions of the Cleveland Cohort, the Swiss ACS Cohort, their treatment at presentation for suspected ACS, clinical diagnoses definitions, and outcome adjudication methods are provided in the Supplementary material online.
Biochemical analyses
Cardiac Troponin T (reference value ≤0.1 µg/L) was measured by commercial ELISA on an ES300 analyzer (Enzymun Troponin T; Roche Diagnostics, Boehringer Mannheim, Indianapolis, IN, USA).22 The lower limit of detection (LOD) of this assay is 0.02 µg/L. The Roche assay was performed with the use of the Elecsys system (05092744190 Troponin T hs Elecsy, Roche Diagnostics, Indianapolis, IN, USA) for cTnT (5th generation), with a LOD is 0.005 µg/L, the 99th percentile 0.014 µg/L, and the 10% CV is 0.013 µg/L.23 Estimated glomerular filtration rate (eGFR; in mL/min per 1.73 m2) was calculated for each cohort using the modification of Diet in Renal Disease study equation.24 Trimethyllysine and TMAO were quantified using stable isotope dilution LC/MS/MS analyses as previously described using a Shimadzu Nexera Ultra High Performance Liquid Chromatograph (UHPLC) system interfaced with Shimadzu 8050 triple quadrupole mass spectrometer.14 More extensive descriptions of analytical methods are provided in the Supplementary material online.
Statistical analysis
The Wilcoxon–rank sum test or Student’s t-test for continuous variables and the χ2 test or Fisher’s exact test for categorical variables were used to determine significant difference between groups. The statistical analysis of MACE is different between the Cleveland Cohort and Swiss ACS cohorts. For the Cleveland cohort, due to the lack of time to event information excepting for mortality, MACE is a binary composite—‘yes’ or ‘no’ to non-fatal MI, non-fatal stroke, the need for revascularization, or all-cause mortality. Odds ratio (OR) for binary MACE and corresponding 95% confidence interval (CI) were calculated using both univariable (unadjusted) and multivariable (adjusted) logistic regression models. For the Swiss cohort, MACE is a time-to-event outcome, and is defined as time to first occurrence of non-fatal MI, non-fatal stroke, need for revascularization, or all-cause mortality. Kaplan–Meier curves (unadjusted) along with Cox proportional hazards regression models were used for time-to-event analysis. Hazard ratio (HR) with 95% CI for incident MACE and all-cause mortality were calculated for both univariable (unadjusted) and multivariable (adjusted) Cox models. Both logistic regression models and Cox models were adjusted for traditional cardiac risk factors including age, sex, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), smoking, presence or absence of diabetes mellitus and hypertension, and indices of renal function (eGFR). Area under the receiver operating characteristic curves (AUC) and Brier score were calculated to evaluate the incremental ability of TML for predicting an adverse event, and adjusted with the same covariates from the logistic regression model (binary endpoint) or Cox model (time to event data) (i.e. with traditional CV risk factors and eGFR).25,26 All analysis was performed with R 3.5.1. (Vienna, Austria), and P-value <0.05 was considered statistically significant.
Results
Baseline characteristics of patients in both Cleveland and Swiss ACS Cohorts
Baseline characteristics of both the Cleveland Cohort and the Swiss ACS Cohort, both cumulative and stratified by tertiles of plasma TML, are shown in Tables 1and2, respectively. Elevated TML levels at presentation in each cohort were observed amongst subjects who were older, had a history of diabetes mellitus or hypertension, and were more likely to have lower HDL-C. The Cleveland Cohort consisted of 530 sequential patients presenting to the ED with complaint of chest pain, 88 patients (16.6%) had unstable angina (UA) and 199 patients (37.6%) had adjudicated ACS at presentation. One hundred and twelve patients (21.1%) were noted to be cTnT positive (cTnT ≥0.1 µg/L) during the initial evaluation period (baseline, 4, 8, or 16 h post-presentation sample) and 418 patients remained persistently negative for cTnT testing. Furthermore, 5th generation high-sensitivity TnT (hs-TnT) was used to re-measure samples for all patients (N = 530) using either baseline (N = 415) or 4 h (N = 115) samples for those without remaining baseline sample. Of these, 247 patients (N = 192/415 baseline, N = 55/115 4 h) had an hs-TnT level below the universal 99th percentile cut-off derived from healthy patients (hs-TnT ≤0.014 µg/L).27,28 Follow-up was completed for 30-day and 6-month periods. Thirty day outcomes included MI in 115 patients, stroke in one patient, revascularization in 160 patients, all-cause death in seven patients, and the composite MACE in 202 patients (38.1%). Cumulative outcomes at 6 months included MI in 117 patients, stroke in six patients, revascularization in 163 patients, all-cause death in 29 patients, and the composite MACE in 220 patients (41.5%). All-cause mortality at 7 years was available in 89% (N = 474) of the Cleveland Cohort patients. The Swiss ACS population included 1683 patients with adjudicated ACS and onset of symptoms within 72 h. In the Swiss ACS Cohort, 881 patients (52.3%) had ST-elevation myocardial infarction, 731 patients (43.4%) had non-ST-elevation myocardial infarction, and 71 patients (4.2%) had UA at presentation. Outcomes at 1 year included MI in 52 patients, stroke in 21 patients, revascularization in 99 patients, all-cause death in 71 patients including cardiac death 56 patients (78.9%), and the composite MACE in 190 patients (11.3%).
Table 1.
Baseline characteristics of patients in the Cleveland Cohort stratified according to trimethyllysine tertiles
| Characteristics | All patients (N = 530) | Tertile 1 (N = 174) <0.74 µM | Tertile 2 (N = 181) 0.74–1.11 µM | Tertile 3 (N = 175) >1.11 µM | P-value | |
|---|---|---|---|---|---|---|
| Age (years) | 62.4 ± 13.9 | 59.2 ± 13.7 | 63.6 ± 13.1 | 64.5 ± 14.3 | <0.001 | |
| Male (%) | 57.5 | 46.0 | 64.1 | 62.3 | 0.001 | |
| C-reactive protein (mg/dL) | 0.56 (0.19–1.19) | 0.57 (0.16–1.13) | 0.48 (0.17–1.04) | 0.61 (0.24–1.37) | 0.18 | |
| History of hyperlipidaemia (%) | 50.0 | 44.0 | 49.1 | 56.6 | 0.063 | |
| History of diabetes (%) | 27.2 | 19.9 | 27.3 | 34.1 | 0.013 | |
| History of hypertension (%) | 65.6 | 58.2 | 65.3 | 73.0 | 0.016 | |
| History of revascularization (%) | 33.9 | 22.8 | 35.3 | 42.9 | 0.001 | |
| History of CAD (%) | 48.9 | 52.8 | 46.2 | 47.9 | 0.46 | |
| History of smoking (%) | 61.1 | 55.3 | 60.7 | 67.1 | 0.089 | |
| HDL-C (mg/dL) | 39.0 (32.0–47.0) | 42.0 (34.8–48.0) | 38.0 (32.0–48.0) | 36.0 (30.0–45.0) | 0.001 | |
| LDL-C (mg/dL) | 104.5 (80.0–133.2) | 106 (80.8–132.2) | 102.0 (77.0–126.0) | 108.0 (81.0–141.0) | 0.25 | |
| Adjudicated ACS (%) | 37.6 | 26.0 | 37.6 | 49.1 | <0.001 | |
| TMAO (µM) | 4.28 (2.55–7.91) | 2.9 (1.89–4.61) | 3.98 (2.53–6.87) | 7.14 (4.38–13.54) | <0.001 | |
| Baseline hs-TnT (µg/L) | 0.018 (0.009–0.045) | 0.014 (0.007–0.032) | 0.017 (0.009–0.036) | 0.029 (0.011–0.067) | 0.002 | |
| TML (µM) | 0.89 (0.68–1.33) | 0.6 (0.49–0.68) | 0.89 (0.81–1.00) | 1.58 (1.35–2.03) | <0.001 | |
| eGFR (mL/min/1.73 m2) | 75.8 (54.7–91.2) | 85.3 (69.6–101.3) | 76.3 (61.5–89.7) | 59.4 (34.6–81.2) | <0.001 | |
| Baseline medications (%) | ||||||
| Aspirin (%) | 36.5 | 30.4 | 38.4 | 40.5 | 0.14 | |
| ACE inhibitors (%) | 25.4 | 21.5 | 20.7 | 33.7 | 0.01 | |
| Statin (%) | 13.0 | 10.1 | 11.0 | 17.8 | 0.08 | |
| Beta-blockers (%) | 23.1 | 18.4 | 23.8 | 27.0 | 0.18 | |
Continuous data are presented as mean ± standard deviation or median (interquartile range), and categorical variables are presented as %. One-way ANOVA or Kruskal–Wallis test were used for numeric data comparison. The χ2 test was used for categorical data.
Table 2.
Baseline characteristics of patients in Swiss ACS Cohort stratified according to trimethyllysine tertiles
| Characteristics | All patients (N = 1683) | Tertile 1 (N = 556) <0.56 µM | Tertile 2 (N = 567) 0.56–0.73 µM | Tertile 3 (N = 560) >0.73 µM | P-value |
|---|---|---|---|---|---|
| Age (years) | 63.9 ± 12.4 | 61.5 ± 11.6 | 62.4 ± 12.3 | 68.0 ± 12.4 | <0.001 |
| Male (%) | 77.8 | 65.3 | 83.4 | 84.5 | <0.001 |
| C-reactive protein (mg/dL) | 2.8 (1.1–8.0) | 2.5 (1.08–6.03) | 2.4 (1.08–6.93) | 3.6 (1.4–12.5) | <0.001 |
| History of hyperlipidaemia (%) | 62.6 | 62.9 | 59.6 | 65.4 | 0.14 |
| History of diabetes (%) | 17.5 | 13.8 | 15.3 | 23.2 | <0.001 |
| History of hypertension (%) | 59.3 | 52.7 | 56.3 | 68.9 | <0.001 |
| History of revascularization (%) | 17.4 | 14.7 | 15.2 | 22.3 | 0.001 |
| History of CAD (%) | 36.8 | 37.2 | 37.6 | 35.6 | 0.77 |
| History of smoking (%) | 67.8 | 69.1 | 69.3 | 65.0 | 0.22 |
| HDL-C (mg/dL) | 43.3 (36.3–53.4) | 46.4 (37.9–55.7) | 43.3 (35.6–53.0) | 42.2 (35.6–51.0) | <0.001 |
| LDL-C (mg/dL) | 118.7 (90.1–148.5) | 125.3 (94.7–154.7) | 121.4 (95.9–150.8) | 108.7 (80.8–136.9) | <0.001 |
| TMAO (µM) | 2.87 (1.94–4.85) | 2.29 (1.59–3.26) | 2.60 (1.90–3.94) | 4.35 (2.82–7.17) | <0.001 |
| Hs-TnT (µg/L) | 0.20 (0.06–0.71) | 0.18 (0.05–0.54) | 0.20 (0.06–0.7) | 0.24 (0.06–0.93) | 0.005 |
| TML (µM) | 0.64 (0.52–0.81) | 0.48 (0.42–0.52) | 0.64 (0.60–0.69) | 0.90 (0.81–1.09) | <0.001 |
| eGFR (mL/min/1.73 m2) | 100.2 (74.2–126.9) | 112.7 (90.3–137.9) | 103.8 (83.7–129.1) | 77.4 (54.5–105.9) | <0.001 |
| Baseline medications (%) | |||||
| Aspirin (%) | 33.3 | 26.8 | 30.9 | 42.4 | <0.001 |
| ACE inhibitors (%) | 18.3 | 16.1 | 14.8 | 24.0 | <0.001 |
| Statin (%) | 31.4 | 28.4 | 30.0 | 35.8 | 0.02 |
| Beta-blockers (%) | 26.3 | 21.4 | 23.9 | 33.8 | <0.001 |
Continuous data are presented as mean ± standard deviation or median (interquartile range), and categorical variables are presented as %. One-way ANOVA or Kruskal–Wallis test were used for numeric data comparison. The χ2 test was used for categorical data.
Trimethyllysine association with major adverse cardiac events and long-term mortality in the Cleveland Cohort
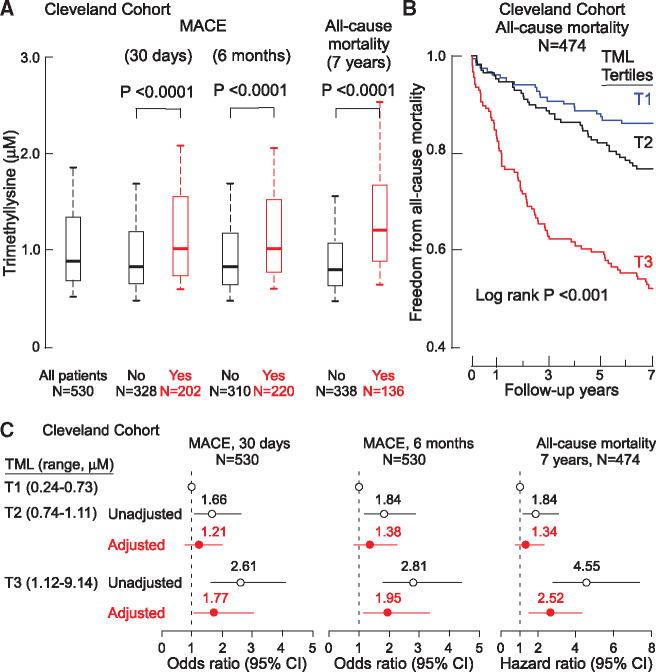
We first examined the relationship between the levels of TML and incident CV events and long-term mortality within the Cleveland Cohort. Individuals with higher plasma TML levels at presentation were more likely to experience MACE over the initial 30-day and 6-month periods following enrolment (P < 0.0001 each; Figure 1A). Over 7-year follow-up, 136 deaths occurred in the study cohort. Trimethyllysine levels were also higher in individuals who died in the ensuing long-term (7-year) period (P < 0.0001; Figure 1A). Kaplan–Meier survival estimates showed enhanced mortality with increasing TML (log rank P < 0.0001; Figure 1B). Elevated TML levels were also associated with significant increased odds for MACE at 30 days (3rd vs. 1st TML tertile: OR 2.61, 95% CI 1.67–4.08; P < 0.001) and 6 months of follow-up (3rd vs. 1st TML tertile: OR 2.81, 95% CI 1.81–4.38; P < 0.001) (Figure 1C); and TML was also associated with increased risk of 7-year mortality (3rd vs. 1st TML tertile: HR 4.55, 95% CI 2.84–7.30; P < 0.001) (Figure 1C). Following multivariable logistic regression or Cox models adjusting for traditional risk factors (age, sex, smoking, hypertension and diabetes mellitus, LDL-C, and HDL-C) and indices of renal function, elevated (3rd vs. 1st tertile) plasma TML remained independently associated with MACE over the ensuing 30-day (OR 1.77, 95% CI 1.04–3.01; P < 0.05) and 6-month (OR 1.95, 95% CI 1.15–3.32; P < 0.05) periods, as well as increased risk of all-cause mortality over the ensuing 7-year period (HR 2.52, 95% CI 1.50–4.24; P < 0.001) (Figure 1C). The inclusion of TML as a covariate did not result in a significant improvement in risk estimation to the fully adjusted model for MACE at 30 days as monitored by either AUC or Brier score. Similar results were observed for MACE at 6 months and 7-year all-cause mortality (Supplementary material online, Table S1).
Figure 1.
Systemic levels of trimethyllysine are associated with incident cardiovascular events in the Cleveland Cohort. (A) Box–Whisker plots of trimethyllysine levels among patients presenting with chest pain indicating the relationship of trimethyllysine with (yes) and without (no) incident major adverse cardiac events (myocardial infarction, stroke, the need for revascularization, or all-cause mortality) and mortality over follow-up periods. (B) Kaplan–Meier plot depicting 7-year risk for all-cause mortality stratified by the tertiles of trimethyllysine levels. (C) Forest plots illustrating the odds of major adverse cardiac events at 30 days and 6 months and the risk of all-cause mortality by 7 years according to the tertiles of trimethyllysine levels. Symbols represent odds ratios or hazard ratios, and the 5–95% confidence interval is indicated by line length. Odds ratios were calculated using multivariable logistic regression modelling and hazard ratios by Cox modelling using adjustments for age, sex, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, smoking, history of diabetes mellitus and hypertension, and indices of renal function (estimated glomerular filtration rate).
We also explored sex-specific association of TML levels with MACE at 30 days and 6 months (male, N = 305; female N = 225), and all-cause mortality at 7 years (male, N = 278; female, N = 196). Elevated TML levels (Tertile 3 vs. Tertile 1) were associated with increased odds of MACE at 30 days and 6 months and increased risk of 7-year all-cause mortality in males and females alike. Moreover, the association remained significant for females independent of traditional risk factors and indices of renal function (Supplementary material online, Figure S1) for both MACE (6 months) and mortality (7 years). Similar sub-cohort analyses showed elevated TML levels were associated with MACE and 7-year all-cause mortality amongst patients with vs. without history of CAD (Supplementary material online, Figure S1).
Association of trimethyllysine levels with adverse clinical outcomes in patients persistently negative for Troponin T at presentation with chest pain
We next examined whether trimethyllysine levels may provide clinical utility among patients without evidence of myocardial necrosis (i.e. remain negative for Troponin T throughout the monitoring period of baseline, 4, 8, and 16 h after admission). Of the patients that remained persistently negative for Troponin T (cTnT <0.1 µg/L) during the initial evaluation period (N = 418), TML was higher among those who subsequently experienced a MACE compared to those who did not over the ensuing 30 days {median [interquartile range (IQR)] 1.10 µM (0.85–1.60) vs. 0.83 µM (0.65–1.18); P < 0.0001} and 6 months [median (IQR) 1.07 µM (0.85–1.59) vs. 0.82 µM (0.64–1.17); P < 0.0001]. Amongst patients initially negative for Troponin T, the frequency of experiencing a MACE over the ensuing 30 days and 6 months increased with increasing TML tertiles [e.g. 8.9% and 11.1% in tertile (T) 1 (<0.72 µM), 22.5% and 28.2% in T2 (0.73–1.08 µM), and 32.6% and 37.6% in T3 (≥1.09 µM), (P < 0.05 for trend)]. Trimethyllysine levels in relation to MACE among patients persistently negative for cTnT testing are shown in Figure 2. As compared to patients in the first tertile of TML levels, patients in the highest tertile (T3) revealed a significantly increased (4.96-fold) odds of MACE at 30 days (OR 4.96, 95% CI 2.49–9.89; P < 0.001). Among patients persistently negative for cTnT, plasma TML levels remained significantly associated with MACE at both 30 days (T3 OR 4.49, 95% CI 2.06–9.79; P < 0.001) and 6 months (T3 OR 4.41, 95% CI 2.11–9.23; P < 0.001), even following adjustments for traditional risk factors and indices of renal function (P < 0.001 each, Figure 2). The Brier scores are relatively low, which correspond to greater prediction importance; however, the inclusion of TML as a covariate did not indicate a significant improvement in risk estimation over traditional CV risk factors and indices of renal function in either Brier score or AUC for MACE at 30 days and 6 months (Supplementary material online, Table S1).
Figure 2.
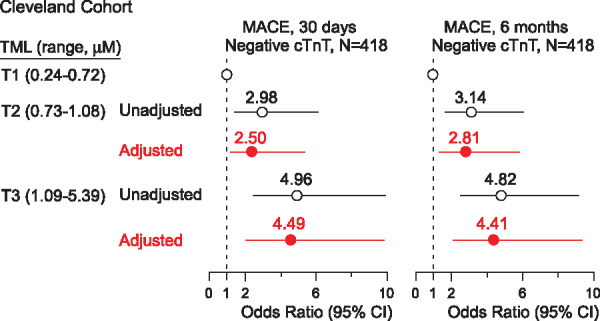
Association of plasma trimethyllysine levels with incident major adverse cardiac events for patients persistently negative for Troponin T. Forest plots indicating the odds of major adverse cardiac events at 30 days and 6 months according to trimethyllysine levels ranked by tertiles among patients persistently negative for Troponin T test (cTnT <0.1 µg/L) in the Cleveland Cohort. Symbols represent odds ratios and the 5–95% confidence interval is indicated by line length. Odds ratios were calculated using multivariable logistic regression modelling using adjustments for age, sex, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, smoking, history of diabetes mellitus hypertension, and indices of renal function (estimated glomerular filtration rate).
Value of simultaneous trimethyllysine and trimethylamine N-oxide analyses for incident cardiovascular events, the Cleveland Cohort
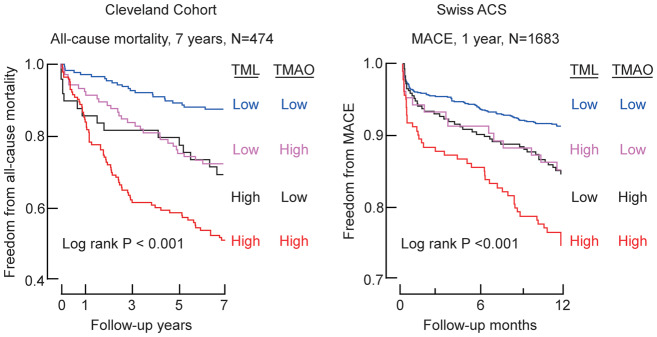
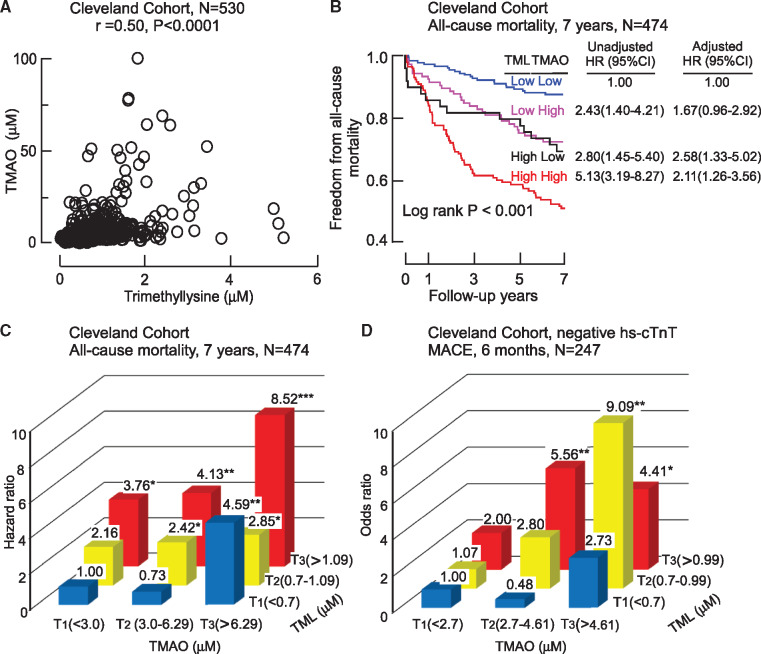
Isotope tracer studies reveal dietary TML can serve as a TMAO-producing nutrient precursor via gut microbiota, and in clinical studies, combining TML with TMAO provides long-term prognostic value among stable subjects undergoing cardiac evaluations.14 We, therefore, hypothesized that concurrent assessment of TML and TMAO could enhance risk stratification among patients presenting with suspected ACS. Among subjects in the Cleveland Cohort at initial presentation (baseline), Spearman’s correlation analyses showed a significant association between plasma TML and TMAO levels (r = 0.50, P < 0.0001; Figure 3A). Kaplan–Meier survival plots stratifying TML and TMAO into low vs. high levels (below vs. above median value) shows that elevated TML and TMAO levels were each associated with a graded increase (log rank P < 0.001) in incident mortality risk, with highest cumulative mortality rate noted among those with both high TML and TMAO levels (Figure 3B). When TMAO levels were included in the Cox regression models, elevated levels of both TML and TMAO retained significant prognostic value even after adjustments for multiple risk factors and indices of renal function (Figure 3B). To further examine both the independence and potential additive significance of TML and TMAO, we stratified the whole cohort into tertile groups of both TML and TMAO. Patients having the lowest tertile of both TML and TMAO levels (reference group) experienced an absolute annual mortality rate of only 1.4%/year. In contrast, patients with combined highest tertiles of TML and TMAO levels experienced an 8.6%/year annual event rate, and a significant 8.52-fold relative risk of incident mortality in 7 years (unadjusted HR 8.52, 95% CI 4.04–17.98; P < 0.001) (Figure 3C, Supplementary material online, Table S2). Moreover, even among patients with low TMAO levels (T1) at presentation, increasing levels of TML were associated with significant increase in mortality risk (3.76-fold risk when TML is the highest tertile yet TMAO remains low; P < 0.05) (Figure 3C, Supplementary material online, Table S2). Similarly, among patients with the low TML levels, increasing TMAO levels dose-dependently were associated with increased mortality risk [e.g. 4.59-fold risk when TMAO is high (T3) yet TML is low (T1); P < 0.01. Figure 3C, Supplementary material online, Table S2].
Figure 3.
Plasma trimethyllysine in relation to both incident cardiovascular events and trimethylamine N-oxide in the Cleveland Cohort. (A) Correlation between plasma levels of trimethyllysine and trimethylamine N-oxide in the entire Cleveland Cohort. (B) Kaplan–Meier plot illustrating the relationship between plasma trimethyllysine and risk of incident 7-year risk for mortality according to trimethyllysine and trimethylamine N-oxide levels where each marker is categorized above vs. below the median level in the cohort, median plasma concentration of trimethyllysine (0.89 µM) and trimethylamine N-oxide (4.28 µM) within the cohort was used to stratify patients as ‘high’ (≥median) or ‘low’ (<median) values, also shown are hazard ratio (95% confidence interval) for the indicated trimethyllysine and trimethylamine N-oxide grouping using either an unadjusted model, or following adjustments for traditional cardiovascular risk factors (age, sex, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, smoking, diabetes mellitus, and hypertension) and indices of renal function (estimated glomerular filtration rate). (C) Plot of unadjusted hazard ratio for incident 7-year mortality risk stratified by tertiles (low, intermediate, and high) of trimethylamine N-oxide (cut-off values 3.0 and 6.29 µM) and trimethyllysine (cut-off values of 0.7 and 1.09 µM). *P < 0.05, **P < 0.01, ***P < 0.001 relative to low/low TMAO/TML group. (D) Plot of unadjusted odds ratio for 6-month major adverse cardiac events stratified by the tertiles which indicated low, intermediate and high levels of trimethylamine N-oxide (cut-off values 2.7 and 4.61 µM) and trimethyllysine (cut-off values of 0.7 and 0.99 µM) among patients who were below the 99th percentile cut-off on hs-cTnT (N = 247). *P < 0.05, **P < 0.01 relative to low/low TMAO/TML group.
High-sensitivity troponin assays are enabling earlier diagnosis of MI and when negative, help identify subjects at reduced risk over the ensuing 30 days.27,29–31 We, therefore, explored the potential clinical value of TML and TMAO levels for incident CV events in patients who were below the 99th percentile cut-off on the 5th generation (Roche) high-sensitivity Troponin T testing at baseline and 4 h (≤0.014 µg/L, threshold used globally,27,28 and lower than the 0.019 µg/L cut-off the 99th percentile in the USA31). Subjects were again stratified based on tertiles of both TML and TMAO levels. Among these 247 patients (hs-cTnT ≤0.014 µg/L), the overall MACE rate over the ensuing 6-month period was 21.9% (n = 54/247). Elevated TML and TMAO levels remained significantly associated with increased CV events (T3/T3, TML/TMAO vs. T1/T1, TML/TMAO, unadjusted OR 4.41, 95% CI 1.34–14.56; P < 0.05) (Figure 3D, Supplementary material online, Table S3), and TML provided prognostic value in combination with TMAO for MACE odds, even in patients with ‘negative’ hs-TnT levels (i.e. <99th % cut-off on Roche 5th generation assay).
Elevated plasma trimethyllysine levels are associated with incident cardiovascular events in an independent cohort of acute coronary syndrome patients: the multicentre Swiss ACS Cohort
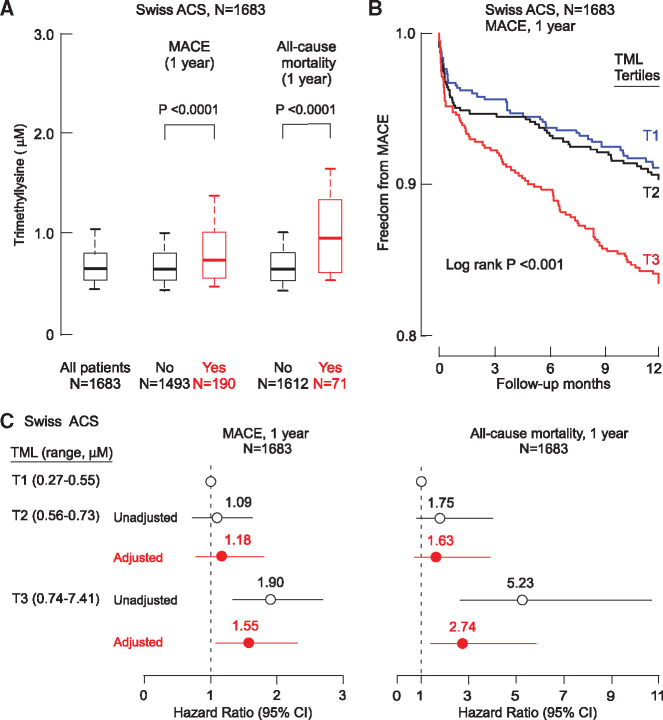
We next examined the clinical prognostic value of TML in an independent cohort, the multicentre Swiss ACS Cohort, a prospective study of 1683 patients with adjudicated ACS and 1-year longitudinal follow-up.10,21 Trimethyllysine levels were higher among patients who experienced a MACE (MI, stroke, need for revascularization, or all-cause mortality) in the 1-year follow-up period compared to those who did not experience MACE (median 0.72 vs. 0.64 μM, P < 0.001; Figure 4A). Trimethyllysine levels were also higher among subjects who died within 1 year after presentation compared with those who did not (median 0.94 vs. 0.64 μM, P < 0.0001; Figure 4A). Kaplan–Meier survival plots illustrate the dose-dependent association observed between increasing TML and reduced freedom from MACE (log rank P < 0.001; Figure 4B). Within the multisite Swiss ACS Cohort, the 1-year risk of incident MACE or mortality increased in consecutive TML tertiles, with individuals in the top tertile (relative to bottom tertile) showing a 1.90-fold risk of MACE (HR 1.90, 95% CI 1.34–2.70; P < 0.001) and a 5.23-fold risk of all-cause mortality (HR 5.23, 95% CI 2.56–10.67; P < 0.001; Figure 4C). After adjustment for traditional risk factors and indices of renal function, elevated TML levels were independently associated with 1-year MACE (HR 1.55, 95% CI 1.04–2.31; P < 0.05) and mortality (HR 2.74, 95% CI 1.28–5.85; P < 0.01) risks (Figure 4C). The inclusion of TML as a covariate showed a trend towards an improved risk estimation in the fully adjusted model for predicting incident 1-year MACE, albeit not at a statistically significant level [AUC 0.78 (0.72–0.84) (with TML) vs. 0.76 (0.71–0.82) (without TML), P = 0.084]. Addition of TML to the fully adjusted model for predicting 1-year all-cause mortality did not show significant improvement in AUC. The Brier scores are very low, which indicate close to prefect prediction, and the inclusion of TML did not indicate a significant improvement in risk estimation of MACE and all-cause mortality by Brier score (Supplementary material online, Table S1). We also calculated cumulative risk with death not associated with MACE as a competing risk and comparison was made by Fine–Gray test.32 For this analysis, MACE was redefined as time to first occurrence of non-fatal MI, non-fatal stroke, need for revascularization, or CVD mortality. Essentially similar results were observed, with 3rd tertile TML levels significantly associated with cumulative incidence of MACE (HR 1.77, 95% CI 1.24–2.55; P = 0.002). Following adjustments with a fully loaded model (traditional risk factors and indices of renal function), a trend was observed that failed to reach significance (adjusted HR 1.44, 95% CI 0.96–2.16; P = 0.081).
Figure 4.
Association of systemic levels of trimethyllysine with incident cardiovascular events in the multicentre Swiss ACS Cohort. (A) Box–Whisker plots of trimethyllysine levels among acute coronary syndrome patients depicting the relation of trimethyllysine with (yes) and without (no) incident major adverse cardiac events and mortality in 1 year. (B) Kaplan–Meier plots illustrating 1-year major adverse cardiac events risk stratified by the tertiles of trimethyllysine levels. (C) Forest plots indicating the risks of incident major adverse cardiac events and mortality according to the tertiles of trimethyllysine levels in 1 year. Symbols represent hazard ratios and the 5–95% confidence interval is indicated by line length. Hazard ratios by Cox modelling using adjustments for age, sex, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, smoking, history of diabetes mellitus and hypertension, and indices of renal function (estimated glomerular filtration rate).
Similar to results observed with the Cleveland Cohort, a significant positive association was observed between plasma TML and TMAO levels in the multicentre Swiss ACS Cohort (Spearman r = 0.42, P < 0.001, N = 1683; Figure 5A). Moreover, in Kaplan–Meier plots stratifying both TML and TMAO levels above vs. below median TML and TMAO levels, the highest cumulative MACE rate was observed in patients with high levels of both TML and TMAO, and lowest cumulative event rate in subjects possessing both low TML and TMAO levels. In Cox survival analyses including both TMAO and multivariate risk factor adjustment for traditional risks factors and indices of renal function, an elevated TML level was associated with a 2.1-fold risk for MACE (1 year) among those with concurrently high TMAO level (adjusted HR 2.10, 95% CI 1.41–3.12; P < 0.001; Figure 5B). Trimethyllysine and TMAO also showed prognostic value when ranked as tertiles (Figure 5C, Supplementary material online, Table S4).
Figure 5.
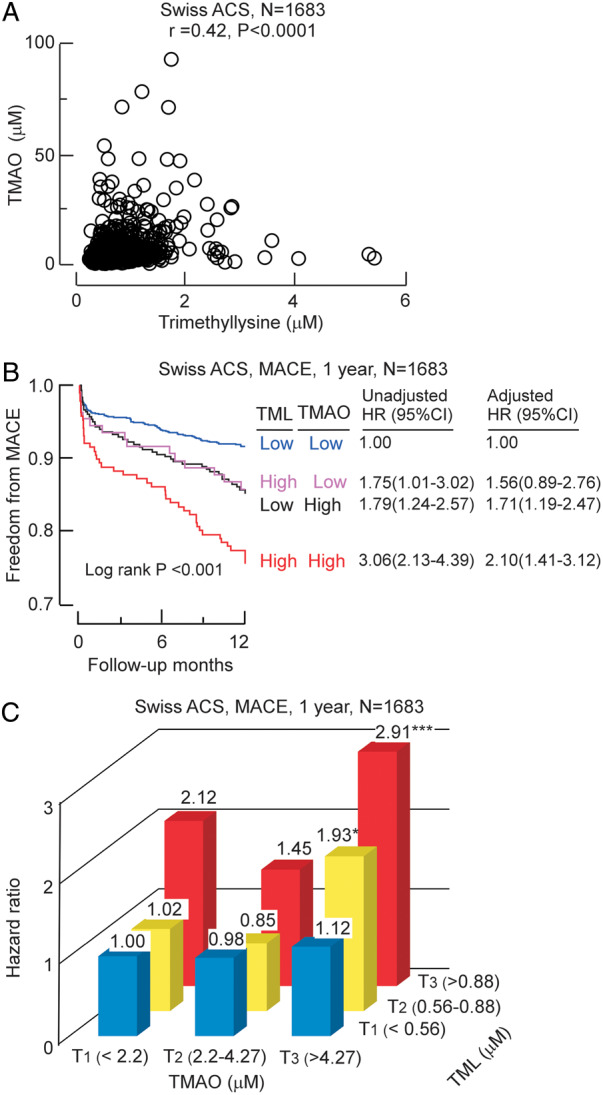
Relationship between plasma trimethyllysine and both incident cardiovascular events and trimethylamine N-oxide in Swiss ACS cohort. (A) Correlation between plasma levels of trimethyllysine and trimethylamine N-oxide in the entire Swiss ACS Cohort (N = 1683). (B) Kaplan–Meier estimates indicating the relationship between plasma trimethyllysine and incident 1-year major adverse cardiac events according to trimethyllysine and trimethylamine N-oxide levels where each marker is categorized above vs. below indicated cut-off in the cohort, cut-off plasma concentration of trimethyllysine (0.88 µM) and trimethylamine N-oxide (4.27 µM) within the cohort was used to stratify patients as ‘high’(≥cut-off) or ‘low’(<cut-off) values, also shown are hazard ratio (95% confidence interval) for the indicated trimethyllysine and trimethylamine N-oxide grouping using either an unadjusted model, or following adjustments for traditional cardiovascular risk factors (age, sex, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, smoking, diabetes mellitus, and hypertension) and indices of renal function (estimated glomerular filtration rate). (C) Plot of hazard ratio for incident 1-year major adverse cardiac events risk stratified by indicated low, intermediate, and high levels of trimethylamine N-oxide (cut-off values 2.2 and 4.27 µM) and trimethyllysine (cut-off values of 0.56 and 0.88 µM). *P < 0.05, **P < 0.01, ***P < 0.001 relative to low/low TMAO/TML group.
Discussion
Patients with ACS experience a wide range of adverse cardiac events. Risk stratification, based on clinical characteristics is challenging, yet risk evaluation is needed to guide and optimize triage, management decisions and preventive efforts.33,34 We report that, in patients who are presenting with either suspected or adjudicated ACS, TML is independently and additively (with TMAO, traditional risk factors and indices of renal function) associated with adverse outcomes in patients at risk for either near- or long-term CV events (Take home figure). Plasma TML levels were also associated with MACE even in patients who were consistently negative for Troponin T. Moreover, amongst patients presenting with chest pain and yet were initially ‘negative’ with hs-TnT levels (i.e. <99% cut-off on Roche 5th generation assay), elevated TML levels (both alone and in combination with TMAO) were associated with near-term adverse outcomes. The present results suggest that rapid quantification of TML and TMAO at presentation may provide added value for identifying patients at risk for either near-term or long-term adverse events. Thus, TML may help to identify those who might benefit from early intervention, and escalation in preventive risk reducing efforts who might not otherwise be recognized using traditional electrocardiographic or laboratory diagnostic testing.
Take home figure.
Plasma trimethyllysine levels, alone and in combination with trimethylamine N-oxide, are associated with both near- and long-term cardiovascular risks in patients who present with chest pain and acute coronary syndromes in two independent cohorts: the Cleveland Cohort and the Swiss ACS Cohort.
The mechanism through which elevated TML levels are associated with incident CVD risks remains unclear. Trimethyllysine is a precursor of gut microbiota-dependent TMAO14; however, unlike other TMAO-producing dietary sources such as choline and carnitine, whose prognostic value is attenuated upon addition of TMAO to multivariable regression models,35,36 the addition of TMAO to models failed to significantly attenuate the potential clinical utility of TML in two cohorts examined. Similar results were recently observed with stable subjects and long-term MACE risks.14 This result suggests that the association between TML and CV risks is independent of TMAO. Trimethyllysine originates in the human body from both endogenous and exogenous sources. Dietary sources of TML are easily absorbable from both plant and animal based foods including vegetables, seafood, and meat, where protein-bound TML is widely present.14,37–40 Trimethyllysine is also generated endogenously as part of post-translational modification of proteins, and has classically been studied in the context of histone modification during chromatin remodelling and regulation of gene expression, processes widely observed in both Animalia and Plantae kingdoms.41–46 Methylation of protein lysine residues is also recognized to occur beyond nuclear protein targets, including cytosolic and secreted proteins.37,44–48 In addition, the free amino acid TML is an intermediate in carnitine biosynthesis.16 Whether any of these processes account for the significant association observed between circulating TML levels and adverse CV events remains unclear. The present studies further confirm that untargeted metabolomics, the discovery platform that identified the link between TML and MACE, is a powerful approach for identifying novel pathways reproducibly associated with CV events. Further studies are warranted to explore the underlying mechanisms accounting for the strong association between circulating TML levels and subsequent MACE.
Limitations
The present studies monitored TML levels at a single time point and did not examine changes in TML over time. Moreover, samples analysed were not collected under fasting conditions, and concentration of TML may dependent on diet. Cause of death was not available for the Cleveland cohort, so all-cause death was used as the mortality endpoint. As with all associative studies, some uncontrolled confounding effect may not have been taken into account. Nevertheless, the association between TML and ACS was reproducible in both the US (single-site) and Swiss (multi-site) cohorts.
Conclusions
In summary, plasma levels of TML, both alone and in combination with TMAO provide independent and reproducible clinical value for incident MACE and mortality amongst patients presenting with chest pain and ACS.
Funding
This work was supported by the National Institute of Health and the Office of Dietary Supplements (HL113452, HL103866, DK106000, and HL126827). This work was also supported in part by a grant from the Leducq Foundation. Mass spectrometry studies were performed on instruments housed in a facility supported in part by a Center of Excellence Award by Shimadzu Scientific Instruments. T.F.L. received support from the Swiss National Science Foundation (SPUM 33CM30-124112 and 32473B_163271); the Swiss Heart Foundation; and the Foundation for Cardiovascular Research – Zurich Heart House, Zurich. The SPUM consortium was also supported by Roche Diagnostics, Rotkreuz, Switzerland (providing the kits for high-sensitivity Troponin T), Eli Lilly, Indianapolis (USA), AstraZeneca, Zug; Medtronic, Münchenbuchsee; Merck Sharpe and Dome (MSD), Lucerne; Sanofi-Aventis, Vernier; and St. Jude Medical, Zurich (all Switzerland). S.L.H. was partially supported by a gift from the Leonard Krieger endowment.
Conflict of interest: S.L.H., B.S.L., and Z.W. are named as co-inventors on patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. S.L.H., B.S.L., and Z.W. report being eligible to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Lab, Quest Diagnostics, and P&G. S.L.H. is a paid consultant for P&G; and has received research funds from P&G, Pfizer Inc., and Roche Diagnostics. All other authors no conflict of interest to declare.
Supplementary Material
This paper was guest edited by Prof. Filippo Crea, Rome.
References
- 1. Hollander JE, Than M, Mueller C. State-of-the-art evaluation of emergency department patients presenting with potential acute coronary syndromes. Circulation 2016;134:547–564. [DOI] [PubMed] [Google Scholar]
- 2. Zhelev Z, Hyde C, Youngman E, Rogers M, Fleming S, Slade T, Coelho H, Jones-Hughes T, Nikolaou V. Diagnostic accuracy of single baseline measurement of Elecsys troponin T high-sensitive assay for diagnosis of acute myocardial infarction in emergency department: systematic review and meta-analysis. BMJ 2015;350:h15.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chapman AR, Anand A, Boeddinghaus J, Ferry AV, Sandeman D, Adamson PD, Andrews J, Tan S, Cheng SF, D’Souza M, Orme K, Strachan FE, Nestelberger T, Twerenbold R, Badertscher P, Reichlin T, Gray A, Shah ASV, Mueller C, Newby DE, Mills NL. Comparison of the efficacy and safety of early rule-out pathways for acute myocardial infarction. Circulation 2017;135:1586–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chapman AR, Lee KK, McAllister DA, Cullen L, Greenslade JH, Parsonage W, Worster A, Kavsak PA, Blankenberg S, Neumann J, Sorensen NA, Westermann D, Buijs MM, Verdel GJE, Pickering JW, Than MP, Twerenbold R, Badertscher P, Sabti Z, Mueller C, Anand A, Adamson P, Strachan FE, Ferry A, Sandeman D, Gray A, Body R, Keevil B, Carlton E, Greaves K, Korley FK, Metkus TS, Sandoval Y, Apple FS, Newby DE, Shah ASV, Mills NL. Association of high-sensitivity cardiac troponin I concentration with cardiac outcomes in patients with suspected acute coronary syndrome. JAMA 2017;318:1913–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eagle KA, Ginsburg GS, Musunuru K, Aird WC, Balaban RS, Bennett SK, Blumenthal RS, Coughlin SR, Davidson KW, Frohlich ED, Greenland P, Jarvik GP, Libby P, Pepine CJ, Ruskin JN, Stillman AE, Van Eyk JE, Tolunay HE, McDonald CL, Smith SC Jr. Identifying patients at high risk of a cardiovascular event in the near future: current status and future directions: report of a national heart, lung, and blood institute working group. Circulation 2010;121:1447–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, Lusis AJ, Shih DM. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J Am Heart Assoc 2016;5:e002767.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WH, DiDonato JA, Brown JM, Lusis AJ, Hazen SL. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 2016;165:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Raber L, Windecker S, Rodondi N, Nanchen D, Muller O, Miranda MX, Matter CM, Wu Y, Li L, Wang Z, Alamri HS, Gogonea V, Chung YM, Tang WH, Hazen SL, Luscher TF. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J 2017;38:814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suzuki T, Heaney LM, Jones DJ, Ng LL. Trimethylamine N-oxide and risk stratification after acute myocardial infarction. Clin Chem 2017;63:420–428. [DOI] [PubMed] [Google Scholar]
- 12. Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, Trimarco B, Esposito G, Perrino C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J 2017;38:2948–2956. [DOI] [PubMed] [Google Scholar]
- 13. Qi J, You T, Li J, Pan T, Xiang L, Han Y, Zhu L. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: a systematic review and meta-analysis of 11 prospective cohort studies. J Cell Mol Med 2018;22:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li XS, Wang Z, Cajka T, Buffa JA, Nemet I, Hurd AG, Gu X, Skye SM, Roberts AB, Wu Y, Li L, Shahen CJ, Wagner MA, Hartiala JA, Kerby RL, Romano KA, Han Y, Obeid S, Luscher TF, Allayee H, Rey FE, DiDonato JA, Fiehn O, Tang WHW, Hazen SL. Untargeted metabolomics identifies trimethyllysine, a TMAO-producing nutrient precursor, as a predictor of incident cardiovascular disease risk. JCI Insight 2018;3:e99096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hulse JD, Ellis SR, Henderson LM. Carnitine biosynthesis. beta-hydroxylation of trimethyllysine by an alpha-ketoglutarate-dependent mitochondrial dioxygenase. J Biol Chem 1978;253:1654–1659. [PubMed] [Google Scholar]
- 16. Hoppel CL, Cox RA, Novak RF. N6-trimethyl-lysine metabolism. 3-hydroxy-N6-trimethyl-lysine and carnitine biosynthesis. Biochem J 1980;188:509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skagen K, Troseid M, Ueland T, Holm S, Abbas A, Gregersen I, Kummen M, Bjerkeli V, Reier-Nilsen F, Russell D, Svardal A, Karlsen TH, Aukrust P, Berge RK, Hov JE, Halvorsen B, Skjelland M. The Carnitine-butyrobetaine-trimethylamine-N-oxide pathway and its association with cardiovascular mortality in patients with carotid atherosclerosis. Atherosclerosis 2016;247:64–69. [DOI] [PubMed] [Google Scholar]
- 18. Strand E, Rebnord EW, Flygel MR, Lysne V, Svingen GFT, Tell GS, Loland KH, Berge RK, Svardal A, Nygard O, Pedersen ER. Serum carnitine metabolites and incident type 2 diabetes mellitus in patients with suspected stable angina pectoris. J Clin Endocrinol Metab 2018;103:1033–1041. [DOI] [PubMed] [Google Scholar]
- 19. McErlean ES, Deluca SA, van Lente F, Peacock F, Rao JS, Balog CA, Nissen SE. Comparison of troponin T versus creatine kinase-MB in suspected acute coronary syndromes. Am J Cardiol 2000;85:421–426. [DOI] [PubMed] [Google Scholar]
- 20. Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, Goormastic M, Pepoy ML, McErlean ES, Topol EJ, Nissen SE, Hazen SL. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med 2003;349:1595–1604. [DOI] [PubMed] [Google Scholar]
- 21. Klingenberg R, Aghlmandi S, Räber L, Gencer B, Nanchen D, Heg D, Carballo S, Rodondi N, Mach F, Windecker S, Jüni P, von Eckardstein A, Matter CM, Lüscher TF. Improved risk stratification of patients with acute coronary syndromes using a combination of hsTnT, NT-proBNP and hsCRP with the GRACE score. Eur Heart J Acute Cardiovasc Care 2018;7:129–138. [DOI] [PubMed] [Google Scholar]
- 22. Muller-Bardorff M, Hallermayer K, Schroder A, Ebert C, Borgya A, Gerhardt W, Remppis A, Zehelein J, Katus HA. Improved troponin T ELISA specific for cardiac troponin T isoform: assay development and analytical and clinical validation. Clin Chem 1997;43:458–466. [PubMed] [Google Scholar]
- 23. Aldous SJ, Florkowski CM, Crozier IG, Elliott J, George P, Lainchbury JG, Mackay RJ, Than M, Flaws DF, Borowsky J. Comparison of high sensitivity and contemporary troponin assays for the early detection of acute myocardial infarction in the emergency department. Ann Clin Biochem 2011;48:241–248. [DOI] [PubMed] [Google Scholar]
- 24. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gerds TA. Package ‘riskRegression’. https://cran.r-project.org/web/packages/riskRegression/riskRegression.pdf (29 January 2019).
- 26. Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, Pencina MJ, Kattan MW. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010;21:128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem 2010;56:254–261. [DOI] [PubMed] [Google Scholar]
- 28. Body R, Reynard C. One shot to rule out: does the limit of detection of a high-sensitivity troponin assay hit the mark? Clin Chem 2017;63:21–23. [DOI] [PubMed] [Google Scholar]
- 29. Korley FK, Jaffe AS. Preparing the United States for high-sensitivity cardiac troponin assays. J Am Coll Cardiol 2013;61:1753–1758. [DOI] [PubMed] [Google Scholar]
- 30. Thelin J, Melander O, Ohlin B. Early rule-out of acute coronary syndrome using undetectable levels of high sensitivity troponin T. Eur Heart J Acute Cardiovasc Care 2015;4:403–409. [DOI] [PubMed] [Google Scholar]
- 31. Peacock WF, Baumann BM, Bruton D, Davis TE, Handy B, Jones CW, Hollander JE, Limkakeng AT, Mehrotra A, Than M, Ziegler A, Dinkel C. Efficacy of high-sensitivity troponin T in identifying very-low-risk patients with possible acute coronary syndrome. JAMA Cardiol 2018;3:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S; ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 34. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimsky P; Group ESCSD. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 35. Wang Z, Tang WH, Buffa JA, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Hazen SL. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J 2014;35:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Servillo L, Giovane A, Cautela D, Castaldo D, Balestrieri ML. Where does N(epsilon)-trimethyllysine for the carnitine biosynthesis in mammals come from? PLoS One 2014;9:e84589.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Porras-Yakushi TR, Whitelegge JP, Miranda TB, Clarke S. A novel SET domain methyltransferase modifies ribosomal protein Rpl23ab in yeast. J Biol Chem 2005;280:34590–34598. [DOI] [PubMed] [Google Scholar]
- 39. Davis AT, Kruggel EM, Randall S. Excess dietary lysine increases skeletal muscle and plasma trimethyllysine in rats. J Nutr 1993;123:1109–1116. [DOI] [PubMed] [Google Scholar]
- 40. Fischer M, Hirche F, Kluge H, Eder K. A moderate excess of dietary lysine lowers plasma and tissue carnitine concentrations in pigs. Br J Nutr 2009;101:190–196. [DOI] [PubMed] [Google Scholar]
- 41. Handy DE, Castro R, Loscalzo J. Epigenetic modifications: basic mechanisms and role in cardiovascular disease. Circulation 2011;123:2145–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Greer EL, Maures TJ, Hauswirth AG, Green EM, Leeman DS, Maro GS, Han S, Banko MR, Gozani O, Brunet A. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature 2010;466:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rhein VF, Carroll J, He J, Ding S, Fearnley IM, Walker JE. Human METTL20 methylates lysine residues adjacent to the recognition loop of the electron transfer flavoprotein in mitochondria. J Biol Chem 2014;289:24640–24651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. LaBadie J, Dunn WA, Aronson NN Jr. Hepatic synthesis of carnitine from protein-bound trimethyl-lysine. Lysosomal digestion of methyl-lysine-labelled asialo-fetuin. Biochem J 1976;160:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dunn WA, Englard S. Carnitine biosynthesis by the perfused rat liver from exogenous protein-bound trimethyllysine. Metabolism of methylated lysine derivatives arising from the degradation of 6-N-[methyl-3H]lysine-labeled glycoproteins. J Biol Chem 1981;256:12437–12444. [PubMed] [Google Scholar]
- 46. Dunn WA, Rettura G, Seifter E, Englard S. Carnitine biosynthesis from gamma-butyrobetaine and from exogenous protein-bound 6-N-trimethyl-L-lysine by the perfused guinea pig liver. Effect of ascorbate deficiency on the in situ activity of gamma-butyrobetaine hydroxylase. J Biol Chem 1984;259:10764–10770. [PubMed] [Google Scholar]
- 47. Huszar G. Tissue-specific biosynthesis of epsilon-N-monomethyllysine and epsilon-N-trimethyllysine in skeletal and cardiac muscle myosin: a model for the cell-free study of post-translational amino acid modifications in proteins. J Mol Biol 1975;94:311–326. [DOI] [PubMed] [Google Scholar]
- 48. Morse RK, Vergnes JP, Malloy J, McManus IR. Sites of biological methylation of proteins in cultured chick muscle cells. Biochemistry 1975;14:4316–4325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.