Abstract
Aims
Diastolic dysfunction (DD) is common among hypertrophic cardiomyopathy (HCM) patients, causing major morbidity and mortality. However, its cellular mechanisms are not fully understood, and presently there is no effective treatment. Patient-specific induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) hold great potential for investigating the mechanisms underlying DD in HCM and as a platform for drug discovery.
Methods and results
In the present study, beating iPSC-CMs were generated from healthy controls and HCM patients with DD. Micropatterned iPSC-CMs from HCM patients showed impaired diastolic function, as evidenced by prolonged relaxation time, decreased relaxation rate, and shortened diastolic sarcomere length. Ratiometric Ca2+ imaging indicated elevated diastolic [Ca2+]i and abnormal Ca2+ handling in HCM iPSC-CMs, which were exacerbated by β-adrenergic challenge. Combining Ca2+ imaging and traction force microscopy, we observed enhanced myofilament Ca2+ sensitivity (measured as dF/Δ[Ca2+]i) in HCM iPSC-CMs. These results were confirmed with genome-edited isogenic iPSC lines that carry HCM mutations, indicating that cytosolic diastolic Ca2+ overload, slowed [Ca2+]i recycling, and increased myofilament Ca2+ sensitivity, collectively impairing the relaxation of HCM iPSC-CMs. Treatment with partial blockade of Ca2+ or late Na+ current reset diastolic Ca2+ homeostasis, restored diastolic function, and improved long-term survival, suggesting that disturbed Ca2+ signalling is an important cellular pathological mechanism of DD. Further investigation showed increased expression of L-type Ca2+channel (LTCC) and transient receptor potential cation channels (TRPC) in HCM iPSC-CMs compared with control iPSC-CMs, which likely contributed to diastolic [Ca2+]i overload.
Conclusion
In summary, this study recapitulated DD in HCM at the single-cell level, and revealed novel cellular mechanisms and potential therapeutic targets of DD using iPSC-CMs.
Keywords: Induced pluripotent stem cells, Cardiomyocytes, Diastolic dysfunction, Hypertrophic cardiomyopathy, Calcium homeostasis, MYH7, MYBPC3, TNNT2
Translational perspective
Diastolic dysfunction (DD) is commonly associated with hypertrophic cardiomyopathy (HCM). DD can progress into diastolic heart failure, the major cause of morbidity and mortality due to HCM. However, the cellular pathogenic mechanisms of DD remain unclear, and thus an effective drug therapy has yet to be developed. The present study establishes human induced pluripotent stem cell-derived cardiomyocyte (iPSC-CM) model that recapitulates impaired diastolic function in HCM patients at the single cell level. Functional imaging analysis and drug testing based on this HCM iPSC-CM DD model provided new mechanistic insights into the cellular pathophysiology and potential therapeutic strategies of DD.
Introduction
Hypertrophic cardiomyopathy (HCM) is characterized by abnormal thickening of the ventricular wall and increased risk of arrhythmia, sudden death, and heart failure.1 Many familial HCM cases are caused by mutations in sarcomere genes.2 Diastolic dysfunction (DD), manifested by slowed or incomplete ventricular relaxation during diastole, is a prominent clinical feature in HCM patients.3 Without treatment, DD can progress into heart failure, which leads to significant morbidity and mortality.4–6 However, the underlying cellular mechanisms of DD in HCM are not well understood, which greatly impedes the development of specific and effective therapies.
To study the mechanisms of DD in HCM, various animal models (from rodents to large animals) and disease-induction methods have been used.7,8 However, significant interspecies differences make it difficult to extrapolate animal results to humans, and there has been no investigation of DD mechanism using human-origin cardiomyocytes. Human induced pluripotent stem cell (iPSC) technology has enabled patient-specific disease modelling of various cardiovascular diseases,9–13 which offered a unique opportunity to establish human cell modelling platforms of DD in HCM.
Here, we generated induced pluripotent stem cell-derived cardiomyocyte (iPSC-CM) models of DD that carry different mutations in three most commonly involved sarcomeric proteins in familial HCM [i.e. myosin heavy chain 7 (MYH7), myosin binding protein C3 (MYBPC3), and cardiac troponin T2 (TNNT2)]. These iPSC-CM models of DD recapitulated impaired diastolic function at the single-cell level. Based on various functional assays, we demonstrated that both elevated diastolic Ca2+ and increased myofilament Ca2+ sensitivity contribute to the cellular mechanisms of DD in HCM iPSC-CMs. To rescue impaired relaxation, we rebalanced Ca2+ homeostasis in HCM iPSC-CMs with optimized doses of Ca2+ blockers (verapamil, diltiazem) and late Na+ current blockers (ranolazine, eleclazine), all of which improved cellular diastolic performance. Our research provides a patient-specific iPSC-CM platform for modelling and elucidating cellular mechanisms of DD in HCM.
Methods
Peripheral blood mononuclear cell isolation and reprogramming
HCM patients with DD and healthy control individuals were recruited based on the most updated guidelines.14,15 All recruitment and consenting procedures were carried out according to Stanford Institutional Review Board (IRB) approved protocol. Patient-specific iPSC lines were generated through over-expression of 4-Yamanaka factors in peripheral blood mononuclear cells (Supplementary material online, Method).
Maintenance and differentiation of induced pluripotent stem cells
Human iPSCs were maintained in Essential 8™ Medium (Gibco®, Life Technology) and were differentiated into beating cardiomyocytes using monolayer differentiation protocol16 (Supplementary material online, Method).
Cell micropatterning on the hydrogel
The single iPSC-CMs were micropatterned according to the adult CM-like morphology on 10 kPa polyacrylamide hydrogel, which was pre-coated with Matrigel (Corning, 1:10 dilution in PBS) in a defined geometry relying on the stencil-based microfabrication techniques, as described previously.17
Statistical analysis
All data are presented as mean ± standard deviation. The statistical analysis was performed using SigmaPlot 12.5 and GraphPad Prism 8 (Supplementary material online, Method).
Results
Human induced pluripotent stem cell-derived cardiomyocytes carrying hypertrophic cardiomyopathy mutations exhibit diastolic dysfunction
We recruited six familial HCM mutation carriers who exhibited clinical records of diastolic dysfunction (DD) (Supplementary material online, Figure S1A). These patients carry MYH7 R663H (n = 2), MYBPC3 V321M (n = 1), MYBPC3 V219L (n = 1), and TNNT2 R92W (n = 2) mutations. We also recruited one HCM patient without the DD phenotype, and who carried a mutation in MYBPC3 (c.2905+1G>A). Healthy individuals (n = 2) without HCM mutations were used as wild type controls (Supplementary material online, Table S1). Peripheral blood mononuclear cells of all groups were isolated and reprogrammed to iPSCs. The quality of all derived iPSC lines was tested by immunostaining of pluripotent markers such as Sox2, Nanog, SSEA-4, and Oct4 (Supplementary material online, Figure S1B). Human iPSC-CMs were differentiated using standard monolayer methods.16 The purity of iPSC-CMs was >90% as evaluated by fluorescence-activated cell sorting (FACS) and immunostaining of cardiac sarcomere protein markers troponin T and α-actinin (Supplementary material online, Figure S1C and D).
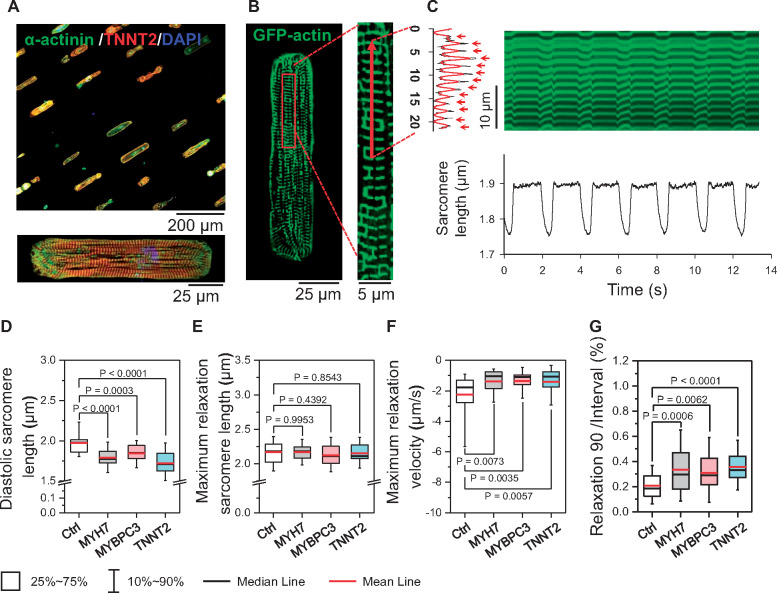
To measure the diastolic function, beating iPSC-CMs from each group were dissociated into single cells and seeded on a micropatterned matrix with a 1:7 width/length ratio (Figure 1A).17 After recovery, micropatterned iPSC-CMs exhibited well-aligned sarcomere structures along the longitudinal axis (Figure 1A, bottom panel). To mark the sarcomere structure in live cells, a GFP-human actin fusion protein was expressed in the iPSC-CMs (Figure 1B). Sarcomere shortening was recorded using confocal microscopy by line scanning along the myofilaments (Figure 1B and C). Our results show that the diastolic sarcomere length is significantly shorter in all three HCM iPSC-CM groups (1.788 ± 0.113, 1.844 ± 0.111, and 1.720 ± 0.130 µm for MYH7, MYBPC3, and TNNT2 groups, respectively) compared with the Ctrl cells (1.985 ± 0.138 µm) (Figure 1D). Interestingly, the maximum relaxed sarcomere lengths of Ctrl and HCM iPSC-CMs, measured in Ca2+-free Tyrode’s solution, were comparable (Figure 1E), indicating that the shorter diastolic sarcomere length in HCM iPSC-CMs was not due to structural remodelling of the sarcomere. Hypertrophic cardiomyopathy iPSC-CMs also exhibited a decreased maximum relaxation rate and prolonged normalized relaxation duration when compared with Ctrl iPSC-CMs (Figure 1F and G), whereas the beating rate and maximum contraction velocity remained unchanged (Supplementary material online, Figure S2A–D). These results suggest that iPSC-CM model exhibits a distinct DD phenotype at the single-cell level.
Figure 1.
Recapitulation of diastolic dysfunction in patient-specific hypertrophic cardiomyopathy (HCM) induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs). (A) Representative immunostaining image of micropatterned iPSC-CMs, showing aligned sarcomere structure. (B and C) Sarcomeres in single iPSC-CMs were indicated by GFP-actin over-expression, and the sarcomere shortening were captured by live-cell confocal line-scanning. (D–G) Results show shorter diastolic sarcomere length (D), unchanged maximum relaxation sarcomere length (E), slower relaxation velocity (F), and prolonged normalized relaxation duration (G) in all HCM iPSC-CM groups compared with iPSC-CMs by one-way ANOVA (Tukey method). N = 42, 36, 40, and 44 cells in Ctrl, MYH7, MYBPC3, and TNNT2 groups, respectively. For each group, data were generated from two different iPSC lines and three batches of differentiation.
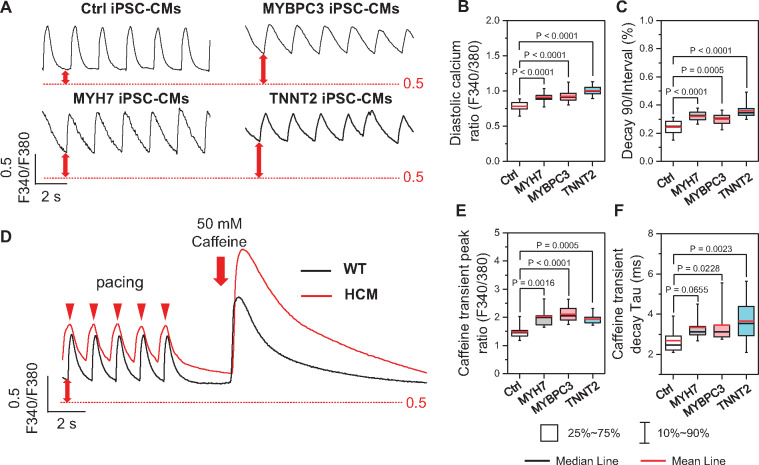
Abnormal Ca2+ handling properties in hypertrophic cardiomyopathy induced pluripotent stem cell-derived cardiomyocytes with diastolic dysfunction
Given the central role of Ca2+ in regulating cardiac excitation-contraction coupling, we sought to understand the mechanisms of DD by examining the Ca2+ handling in Ctrl and HCM iPSC-CMs with Fura-2 imaging (Figure 2A). Field stimulation was most stable at 0.5 HZ (Supplementary material online, Fig. S3A–D), which was used in the comparisons of all groups. Diastolic [Ca2+]i of all HCM groups was significantly higher than that in Ctrl iPSC-CMs (Figure 2B), while the normalized decay time of HCM iPSC-CMs was markedly prolonged compared with the Ctrl (Figure 2C). Moreover, caffeine-induced Ca2+ transients showed higher sarcoplasmic reticulum (SR) Ca2+ load and slightly increased caffeine transient decay Tau in HCM iPSC-CMs compared with Ctrl iPSC-CMs (Figure 2D–F), which suggests reduced Na+/Ca2+ exchange (NCX) function in HCM iPSC-CMs. We also characterized the iPSC-CMs from the HCM patient without clinical DD (Supplementary material online, Figure S4A), which showed normal diastolic Ca2+ and relaxation functions (Supplementary material online, Figure S4B–I).
Figure 2.
Ratiometric Fura-2 AM imaging reveals elevated diastolic Ca2+ in hypertrophic cardiomyopathy (HCM) induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs). (A) Representative traces of Fura-2 ratiometric Ca2+ imaging. (B and C) HCM iPSC-CMs showed elevated diastolic Ca2+ (B) and prolonged Ca2+ recycling (C) compared with Ctrl iPSC-CMs by one-way ANOVA (Tukey method). N = 51, 42, 43, and 72 cells in Ctrl, MYH7, MYBPC3, and TNNT2 groups, respectively. For each group, data were generated from two different iPSC lines and three batches of differentiation. (D) Representative traces of SR Ca2+ load measurement by caffeine-induced Ca2+ transient. (E and F) Caffeine-induced transient showed higher SR Ca2+ content (E) and slightly decreased caffeine-transient decay-Tau (F) in HCM iPSC-CMs compared with Ctrl iPSC-CMs by one-way ANOVA (Tukey method). N = 22, 28, 25, and 26 cells in Ctrl, MYH7, MYBPC3, and TNNT2 groups, respectively. For each group, data were generated from two different iPSC lines and two batches of differentiation.
Isogeneic induced pluripotent stem cell lines confirmed the pathogenicity of TNNT2 R92W mutation in diastolic dysfunction
To confirm the pathogenicity of the sarcomeric mutations in the observed DD phenotype, we next generated isogenic Ctrl and diseased iPSC lines of TNNT2 R92W mutation using CRISPR/Cas9 (Supplementary material online, Figure S5A–E).18 Functional assays showed that both heterozygous and homozygous mutation introduction led to elevated diastolic Ca2+ and prolonged Ca2+ transient decay time. Moreover, the mutation-carrying isogenic iPSC-CMs exhibited shorter diastolic sarcomere length, slower relaxation, and prolonged relaxation duration (Supplementary material online, Figure S5F–L). On the other hand, mutation-corrected patient iPSC-CMs showed improved diastolic Ca2+ homeostasis and diastolic function compared with the diseased cells (Supplementary material online, Figure S5F–L). Collectively, these results confirmed the TNNT2 R92W HCM mutation is pathogenic and causes DD in iPSC-CM models.
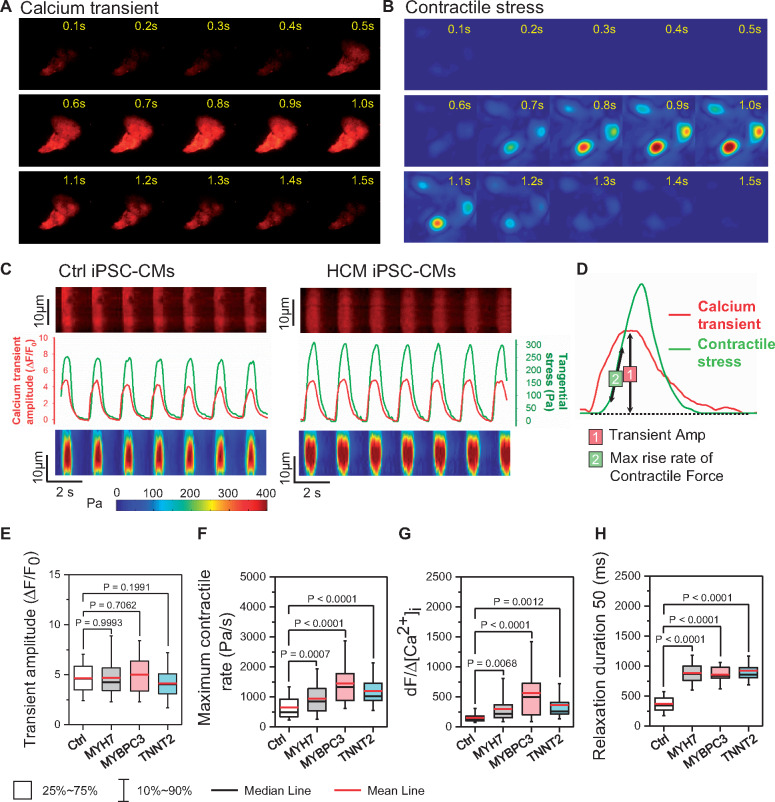
Functional imaging indicates enhanced risk of diastolic dysfunction in hypertrophic cardiomyopathy induced pluripotent stem cell-derived cardiomyocytes
To evaluate how elevated diastolic [Ca2+]i affects the relaxation in HCM iPSC-CMs, we next combined traction force microscopy (TFM) and [Ca2+]i recording to simultaneously assess Ca2+ handling and contractility (Figure 3A–D). While the peak Δ[Ca2+]i amplitude was unchanged, the maximum contractile velocity was significantly faster in HCM compared with Ctrl iPSC-CMs (Figure 3E and F). Based on previous study on the [Ca2+]i and contractile force relationship,19 we defined the ratio of the contraction rate to the Ca2+ transient amplitude (dF/Δ[Ca2+]i) as an index of myofilament Ca2+ sensitivity, which was significantly increased in all HCM groups compared with Ctrl (Figure 3G).
Figure 3.
Functional imaging indicates enhanced risk of diastolic dysfunction in hypertrophic cardiomyopathy (HCM) induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs). (A–C) Representative traces of simultaneous recording of Ca2+ transient and contractile stress. (D) Measurement of Ca2+ and contraction parameters from a single beating episode. (E–H) HCM iPSC-CMs showed unchanged Ca2+ transient amplitude (E), faster contractile rate (F), increased risk of DD as indicated by Ca2+ sensitivity index (G), and prolonged relaxation duration (H) compared with Ctrl iPSC-CMs by one-way ANOVA (Tukey method). N = 77, 190, 192, and 64 cells in Ctrl, MYH7, MYBPC3, and TNNT2 groups, respectively. For each group, data were generated from two different iPSC lines and three batches of differentiation.
To further validate the Ca2+ sensitivity index, we also measured dF/Δ[Ca2+]i in dilated cardiomyopathy (DCM) iPSC-CMs that carry a TNNT2 R173W mutation known to reduce the myofilament Ca2+ sensitivity.20 As expected, DCM iPSC-CMs showed unchanged diastolic [Ca2+]i, decreased transient amplitude, weaker contractile force, slower contractile rate, and reduced dF/Δ[Ca2+]i (Supplementary material online, Figure S6A–F). Taken together, HCM iPSC-CMs concomitantly exhibited elevated diastolic [Ca2+]i and enhanced myofilament Ca2+ sensitivity, both of which contribute to the increased diastolic tension and impaired relaxation, as reflected by prolonged relaxation duration of HCM iPSC-CMs in TFM measurement (Figure 3H).
Short-term β-adrenergic challenge exaggerates diastolic dysfunction in hypertrophic cardiomyopathy induced pluripotent stem cell-derived cardiomyocytes
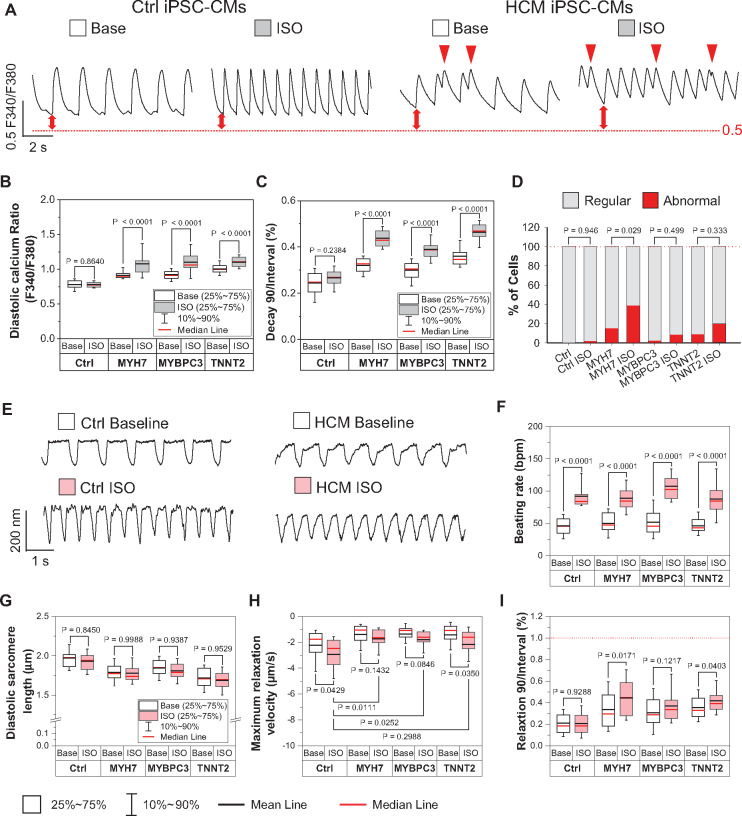
β-adrenergic signalling is the most important lusitropic regulation in the intact heart.21 To evaluate the effect of β-adrenergic activation in diastolic function regulation, we next measured the function of Ctrl and HCM iPSC-CMs in response to β-agonist isoproterenol (ISO, 1 μM) treatment (Figure 4A, E). Our results showed that ISO did not alter diastolic [Ca2+]i, but did increase the Ca2+ transient amplitude in Ctrl iPSC-CMs. However, in HCM iPSC-CMs, ISO significantly increased diastolic [Ca2+]i but not Ca2+ transient peak, which resulted in a reduction of transient amplitude (Figure 4B, Supplementary material online, Figure S7A). Additionally, the normalized Ca2+ transient decay times were significantly increased after ISO treatment in HCM but not Ctrl iPSC-CMs (Figure 4C). β-adrenergic stimulation increased the SR load in Ctrl iPSC-CMs by approximately 20%, but this increment was absent in HCM iPSC-CMs (Supplementary material online, Figure S7B). As diastolic [Ca2+]i was further elevated, the abnormal arrhythmia-like Ca2+ transients were also significantly increased in ISO-treated HCM iPSC-CMs (Figure 4D). In sarcomere shortening measurement, the beating rates of Ctrl and HCM iPSC-CMs were significantly accelerated by ISO (Figure 4F). However, HCM iPSC-CMs consistently showed shorter diastolic and peak sarcomere lengths, and slower relaxation velocity after ISO treatment compared with Ctrl iPSC-CMs (Figure 4G–H, Supplementary material online, Figure S7C). Moreover, both normalized relaxation and contraction duration 90 were further prolonged in ISO treated HCM iPSC-CMs compared with untreated HCM cells (Figure 4I, Supplementary material online, Figure S7D). Collectively, these results strongly suggest that short-term β-adrenergic challenge exaggerate DD in HCM iPSC-CMs.
Figure 4.
Short term β-adrenergic challenge exaggerates diastolic dysfunction in hypertrophic cardiomyopathy (HCM) induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs). (A) Representative traces of Fura-2 AM Ca2+ imaging of Ctrl and HCM iPSC-CMs with or without isoproterenol (ISO) treatment. Red arrow indicates abnormal Ca2+ handling events. (B and C) ISO challenge further enhanced diastolic Ca2+ concentrations and significantly prolonged the normalized transient decay times in HCM iPSC-CMs compared with non-ISO groups by two-way ANOVA (Holm–Sidak method). N > 31 cells in each group. (D) Percentage of abnormal Ca2+ handling events. N > 28 cells in each group. All groups were compared with non-ISO groups by z-test. (E) Representative traces of sarcomere shortening measurement in Ctrl and HCM iPSC-CMs with or without ISO treatment. (F–I) ISO increased beating rate in both Ctrl and HCM iPSC-CMs (F), yet HCM iPSC-CMs still showed shorter diastolic sarcomere length (G), slower relaxation velocity (H), and further prolonged relative relaxation duration (relaxation duration 90 normalized to the beating interval) in HCM iPSC-CMs after ISO treatment (I). All groups were compared with non-ISO group by two-way ANOVA (Holm–Sidak method). N > 28 cells in each group. For all the groups, data were generated from two different iPSC lines and three batches of differentiation.
Ca2+ and late Na+ current blockers rebalance Ca2+ homeostasis and restore diastolic function
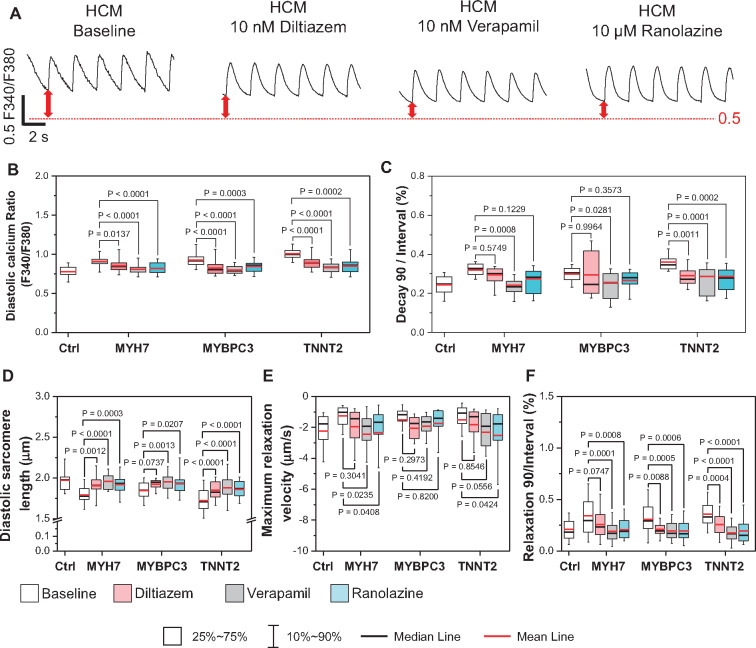
Ca2+ channel blockers have shown beneficial effects when prescribed to HCM patients with DD.22,23 In addition, late Na+ current (INaL) has been reported to play a role in the pathogenesis of DD, and late Na+ channel blockers improved diastolic function in both HF patient CMs and DD animal models.24,25 However, the therapeutic effects of Ca2+ current (ICa) and INaL inhibition in DD are not clear. To better understand the therapeutic mechanisms of these agents, we treated HCM iPSC-CMs with Ca2+ blockers (verapamil and diltiazem) and late Na+ channel blockers (ranolazine and eleclazine) (Figure 5A, Supplementary material online, Figure S8A). The drug doses (10–200 nM for verapamil, 10–500 nM for diltiazem, 1–10 µM for ranolazine, and 200 nM to 1 µM for eleclazine) were applied according to previous studies and clinical prescriptions (Supplementary material online, Figure S8A).11 Our results show that the treatment at an optimized dose of these drugs have restored diastolic [Ca2+]i and transient decay time in HCM iPSC-CMs (Figure 5B and C, Supplementary material online, Figure S8B–D). Sarcomere shortening measurements also confirmed that optimized dose of Ca2+ and late Na+ blockers partially recovered diastolic sarcomere lengths (Figure 5D, Supplementary material online, Figure S8E), increased relaxation rate, and reduced relaxation duration in HCM iPSC-CMs (Figure 5 and F, Supplementary material online, Figure S8F–G). Sacubitril/valsartan (neprilysin inhibitor and angiotensin receptor blockers) has been shown to be effective for treating HF patients with DD.26 However, when tested in our DD model, sacubtril/valsartan has no significant effect, probably because our single-cell iPSC-CM model does not reflect the signalling complexity that exists at the tissue/systemic level (Supplementary material online, Figure S8A–G).
Figure 5.
Ca2+ and late Na+ channel blockers rescued prolonged diastolic process in hypertrophic cardiomyopathy (HCM) induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) by re-balancing Ca2+ homeostasis in diseased cells. (A) Representative traces of Fura-2 AM Ca2+ imaging of Ctrl and HCM iPSC-CMs with and without Ca2+ and late Na+ blocker treatment. (B and C) Treatment by Ca2+ and late Na+ blocker significantly reduced diastolic Ca2+ concentration (B) and shortened Ca2+ transient decay duration (C). N > 27 cells in each groups. All groups were compared with untreated HCM iPSC-CMs by two-way ANOVA (Tukey method). (D–F) Using the sarcomere shortening assay, treatment by all three drugs retained the diastolic sarcomere length (D), increased relaxation rate (E), and reduced the normalized relaxation duration in HCM iPSC-CMs compared with untreated baseline (F). N > 28 cells for each groups. All groups were compared with untreated HCM iPSC-CMs by two-way ANOVA (Tukey method). For each group, data were generated from two different iPSC lines and three batches of differentiation.
Improve the viability of hypertrophic cardiomyopathy induced pluripotent stem cell-derived cardiomyocytes by targeting long-term Ca2+ overload
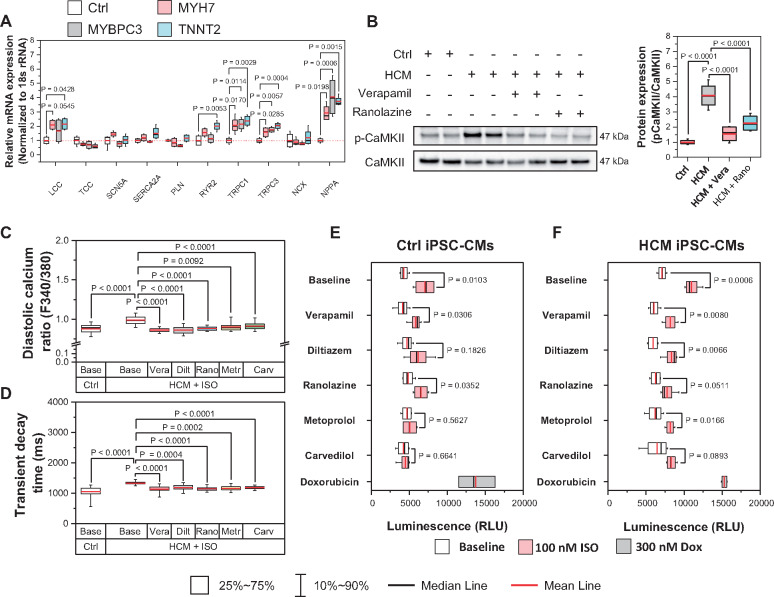
To understand the mechanisms of diastolic Ca2+ overload in HCM iPSC-CMs, we quantified the mRNA expression of key Ca2+ handling proteins. qPCR results show increased Cav1.2, MYH7, and stress marker (NPPA, NPPB) and decreased MYH6, Casq2, and Cav3 mRNA levels in HCM iPSC-CMs compared with Ctrl iPSC-CMs (Figure 6A, Supplementary material online, Figure S9A). Our results also showed that transient receptor potential cation channels (TRPCs) 1 and 3, the main cardiac TRPC subtypes, were both increased in HCM iPSC-CMs (Supplementary material online, Figure S9B, Figure 6E). As TRPCs mediate cation entry and contribute to cardiac hypertrophic remodelling,27,28 we next evaluated the TRPC-mediated Ca2+ influx via store-operated Ca2+ entry (SOCE) measurement (Supplementary material online, Figure S9C–E). Our results showed that Ctrl and HCM iPSC-CMs exhibited comparable basal [Ca2+]i in Ca2+-free buffer (Supplementary material online, Figure S9F), while HCM iPSC-CMs showed higher TRPC-dependent SOCE amplitude and rising rate compared with the Ctrl group (Supplementary material online, Figure S9G and H). To validate the role of TRPCs during the pathogenesis of DD, we also treated HCM iPSC-CMs with the newly developed TRPC3 specific blocker Pyr3, which improved Ca2+ handling and diastolic function in the DD model (Supplementary material online, Figure S8A–G).
Figure 6.
Viability of hypertrophic cardiomyopathy (HCM) induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) under long-term β-adrenergic activation is improved by rebalancing Ca2+ homeostasis. (A) Real time PCR expression profiles of key Ca2+ related proteins. All groups compared with Ctrl with Student’s t-test, data from two lines of Ctrl iPSC-CMs and two lines of HCM iPSC-CMs from at least two differentiation batches. (B) Western blot analysis shows increased CaMKIId activation (pThr286) in HCM iPSC-CMs compared with Ctrl iPSC-CMs, which was recovered by Ca2+ and late Na+ channel blocker treatment. All groups compared with HCM group with Student’s t-test. Data from two lines of each iPSC-CMs from at least two differentiation batches. (C and D) Long-term isoproterenol (ISO) treatment further enhanced the diastolic Ca2+ overload and prolonged Ca2+ transient decay time in HCM iPSC-CMs, which were partially rescued by Ca2+ and late Na+ channel blockers. N > 50 cells for each group. All groups compared with ISO treated HCM iPSC-CMs by one-way ANOVA (Tukey method). (E and F) Cell viability assay indicates that Ca2+ channel blockers partially restored viability of HCM iPSC-CMs after long-term ISO challenge. β-blockers were used as treatment control and doxorubicin was used as cell death control. All groups compared with baseline (white bar) by two-way ANOVA (Tukey method). For each group, data were generated from two different iPSC lines and two batches of differentiation.
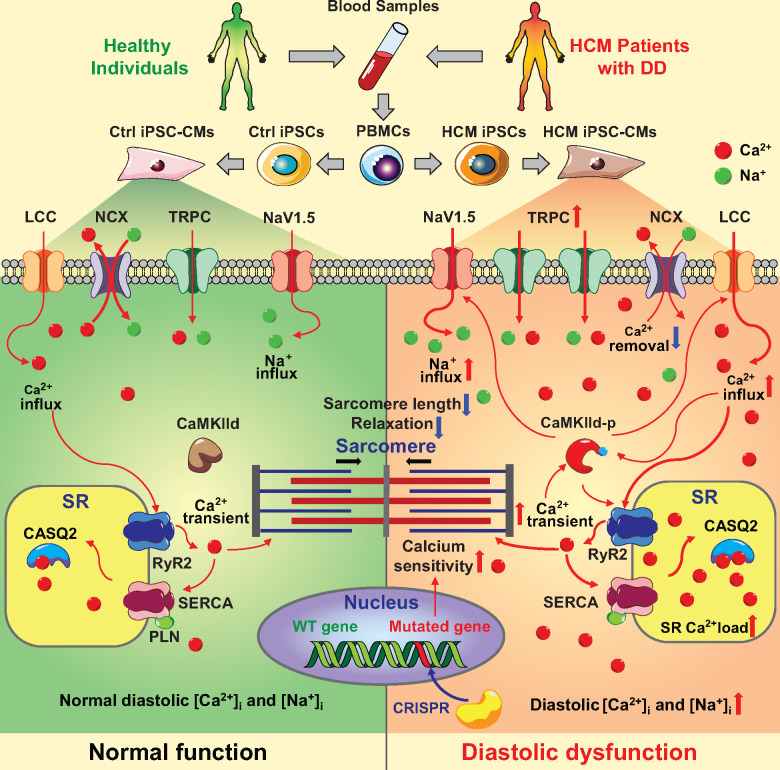
Take home figure.
iPSC-CM model reveals a novel cellular mechanism of diastolic dysfunction in hypertrophic cardiomyopathy.
A recent study reported that CaMKIId activation contributes to diastolic Na+ and Ca2+ overload and impaired diastolic function.29 Indeed, our western blot analysis showed that CaMKIId autophosphorylation was significantly up-regulated by 308 ± 42% in HCM iPSC-CMs compared with Ctrl iPSC-CMs, which was partially suppressed by ICa or INaL blockers (Figure 6B). To confirm the role of CaMKIId in DD, we sought to over-express (OE) the constitutively-active form of CaMKIId T287D in Ctrl iPSC-CMs, and to knock-down (KD) CaMKIId in HCM iPSC-CMs (Supplementary material online, Figure S10A–C). Our functional assays demonstrated CaMKIId gain-of-function lead to typical DD phenotype in Ctrl iPSC-CMs, while knock-down of CaMKIId in HCM iPSC-CMs partially recovered Ca2+ homeostasis, diastolic function, and long-term survival (Supplementary material online, Figure S10D–K). These results confirm that CaMKIId activation plays an importing role during the pathogenesis of DD in HCM iPSC-CMs.
To evaluate the long-term beneficial effects of ICa and INaL blockers in DD, we next compared the function and viability of HCM and Ctrl iPSC-CMs under chronic β-adrenergic challenge (100 nM ISO, 7 days) with and without treatments of β-blockers, ICa and INaL blockers, and doxorubicin (positive control for cell death). Our results showed long-term ISO treatment impaired diastolic function in HCM iPSC-CMs in terms of elevated diastolic [Ca2+]i and prolonged Ca2+ transient decay time, which were alleviated by ICa and INaL inhibition (Figure 6C and D). Long-term ISO treatment also activated apoptotic signalling, as both Ctrl and HCM iPSC-CMs exhibited increased caspase activity (Figure 6E and F), while β-blockers repressed the pro-apoptotic effect. Interestingly, ICa and INaL blockers elicited significant pro-survival effects only in HCM iPSC-CMs, presumably because diastolic Ca2+ handling was only altered in HCM iPSC-CMs.
Taken together, these results suggest that by rebalancing Ca2+ homeostasis, ICa and INaL blockers exert beneficial effects on HCM iPSC-CMs both in short-term functional performance and long-term signalling integrity.
Discussion
As a growing cause of congestive heart failure, DD has been widely modelled and investigated in various systems, including rodents, canine, swine, and newly isolated or cultured cardiomyocytes.7,8 However, few studies have been based in human cardiomyocytes, and none are patient-specific. Here, we established a novel platform to investigate DD using patient-specific HCM iPSC-CMs. Working with multiple functional imaging technologies, we systematically measured the relaxation function of diseased cells and demonstrated that diastolic Ca2+ overload and increased myofilament Ca2+ sensitivity both contribute to DD. This is the first demonstration that patient-specific iPSC-CM model can recapitulate DD disease phenotype at the single-cell level. Moreover, we showed that the diastolic function in HCM iPSC-CMs was improved by the treatment of commonly used drugs, such as ICa and INaL blockers, through rebalancing of Ca2+ homeostasis. These findings suggest that iPSC-CMs are a suitable platform for disease modelling, mechanism study, and drug testing of DD in HCM.
Hypertrophic cardiomyopathy mutations are known to sensitize myofilaments in cardiomyocytes.30 To measure the myofilament Ca2+ sensitivity, we established a novel iPSC-CM-based imaging method that combines [Ca2+]i imaging and TFM. Compared with traditional Ca2+ sensitivity assays in permeabilized muscle tissue or skinned cardiomyocytes, our method offers exceptional simplicity in usage, faster reading and analysis, and higher throughput potential.31 With this technology, we defined a novel parameter (dF/Δ[Ca2+]i) that reflects the myofilament Ca2+ sensitivity and indicates the risk of DD in our single-cell iPSC-CM model. Given our ability to recruit HCM patients with sarcomeric variants and generate their iPSCs, the functional imaging assays validated here show great promise as a potential diagnostic platform to predict the risk and severity of DD in carriers of known HCM mutations who lack clinical symptoms, as well as in individuals carrying variants of unknown significance.32
Ca2+ homeostasis is critical for the contractile function of cardiomyocytes,33 and Ca2+ mishandling has been implicated in the pathogenesis of various cardiac diseases.10,11,34 Our study showed that diastolic [Ca2+]i in HCM iPSC-CMs was significantly elevated, which contributes to increased basal tension and impaired diastolic function. Based on our observations, we believe that two mechanisms contribute to the diastolic [Ca2+]i overload in HCM iPSC-CMs:
First, Ca2+ removal via NCX is reduced in HCM iPSC-CMs, as evidenced by slower [Ca2+]i decline of caffeine-induced Ca2+ transients (Figure 2F). A possible reason for reduced NCX function with unaltered gene expression may be elevated [Na+]i, which limits the [Na+] gradient that fuels Ca2+ extrusion. Impaired Ca2+ extrusion through NCX loads Ca2+ in HCM iPSC-CMs and drives the observed higher SR Ca2+ content. Interestingly, ISO treatment greatly increased SR Ca2+ content in Ctrl iPSC-CMs but not in HCM iPSC-CMs, which indicates the SR Ca2+ load has reached a pump-leak balance in diseased cells. As SR Ca2+ leak is steeply dependent on SR Ca2+ content,35 the secondary rise in SR Ca2+ leak would be expected to promote the local activation of CaMKIId, which is concentrated at SR Ca2+ release sites. CaMKIId activation further increases Ca2+ influx via ICa, promotes arrhythmogenic SR Ca2+ leak, and increases INaL. All of these excess Ca2+ and Na+ fluxes further contribute to overload of diastolic [Ca2+]i.36 This positive inherent feedback cycle can be broken by partial blockade of ICa and INaL, which restored diastolic [Ca2+]i in HCM iPSC-CMs.
Second, HCM mutations increased myofilament Ca2+ sensitivity and elevate diastolic active force development and basal tension. As previous reports showed that pressure overload in heart leads to increased expression of TRPC channels that promote cardiac hypertrophy,27,28 TRPC up-regulation may contribute to DD pathogenesis in HCM iPSC-CM model. Indeed, qPCR profiling and SOCE measurements confirmed that the expression and activity of LTCC, and TRPC1/3 were up-regulated in HCM iPSC-CMs. Increased Ca2+ influx through these channels promoted diastolic Ca2+ overload, and impaired the relaxation of HCM iPSC-CMs, which was partially rescued by TRPC3 specific blocker Pyr3.
One important outcome of long-term diastolic Ca2+ overload in HCM iPSC-CMs is the activation of CaMKIId (Figure 6B). Chronic CaMKIId activation are commonly seen in heart failure. However, our gain- and loss-of-function study based on iPSC-CM models has clearly confirmed the critical role of CaMKIId activation during the pathogenesis of DD, which exacerbates the Ca2+ and Na+ disturbances, induces apoptosis, and influences transcriptional regulation.36 This finding indicate two possible working mechanisms through which ICa or INaL inhibition relieve symptoms in patient with DD:22–25 (i) ICa and INaL blockers limit overall Ca2+ and Na+ influx, which stabilize the diastolic and improve the ability of NCX to extrude Ca2+; (ii) ICa and INaL blockers maintain the Ca2+ homeostasis and restrict the CaMKIId activity. Indeed, re-balancing diastolic [Ca2+]i in HCM iPSC-CMs exhibited the beneficial effects in both short-term diastolic function and long-term cell viability. Therefore, all the key factors in this positive feedback loop during DD pathogenesis, including increased diastolic [Ca2+]i, CaMKIId activity, INaL, TRPCs, and SR leakage, could be potential therapeutic targets of DD at the cellular level (Supplementary material online, Figure S11).
One limitation of our study is that our single-cell iPSC-CM model cannot capture tissue or systemic level contributions of increased wall thickness and extracellular matrix changes,37 which may also contribute to DD in HCM. Other factors, such as changes in myocardium stiffness, conduction blockade, aging, and increased fibrosis cannot be recapitulated in our current model. The morphological and physiological differences of iPSC-CMs compared with adult ventricle cardiomyocytes also pose challenges to clinical applications.38 Future studies incorporating engineered heart tissues may enable further systematic investigations of DD at a more integrated level.
In summary, we report a human iPSC-based cellular model of HCM that recapitulates DD at the single-cell level. Functional imaging analysis highlighted diastolic Ca2+ overload and enhanced myofilament Ca2+ sensitivity, both of which contribute to the increased diastolic tension and impaired relaxation seen in HCM iPSC-CMs. Moreover, perturbed Ca2+ homeostasis in HCM iPSC-CMs exacerbates the DD, providing a window of potential therapeutic intervention by ICa, INaL, and CaMKII inhibitors which may restore diastolic function and prevent apoptosis during long-term adrenergic stress. Our findings here augment our knowledge of the pathogenesis in DD and the mechanisms underlying the beneficial effects of currently available drugs. Moreover, our work suggests that the iPSC-CM model provide a suitable platform for mutation-specific mechanistic studies and drug screening for DD in HCM.
Supplementary Material
Acknowledgements
We thank Andrew Olson from Stanford Neuroscience Microscopy Service (NMS), and Jon Mulholland and Cedric Espenel from Stanford Cell Sciences Imaging Facility (CSIF) for their help with confocal imaging (NIH NS069375). We thank Yan Zhuge from Stanford Cardiovascular Institute (SCVI) Biobank for her assistance with iPSC lines. We thank Vicky Y. Wang for her insightful suggestions on data processing. We thank Soah Lee for her help in cell patterning experiments. We thank Dr Mark Anderson and Dr Elizabeth Luczak for gifting us pCMV myc-CaMKIId WT and pCMV myc-CaMKIId T287D plasmids.
Funding
This work was supported by National Institutes of Health (NIH) K99 HL133473 (to H.W.), American Heart Association (AHA) 16POST31150011 (to H.W.), AHA 18POST34030106 (to H.Y.), NIH F32 HL134221 (to J.W.R.), AHA 18CDA34110411 (to A.C.Y.C.), NIH R01 HL113006, R01 HL126527, R01 HL130020, R01 HL128170, R01 HL141371 (to J.C.W.), R01 HL030077 (to D.M.B.), and AHA 17CSA33590101, R01 AR063963 (to H.M.B.).
Conflict of interest: J.C.W. is a co-founder of Khloris Biosciences but has no competing interests, as the work presented was performed independently. H.M.B. is a co-founder of Myoforte Therapeutics but has no competing interests, as the work presented was performed independently.
See page 3696 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz447)
References
- 1. Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA 2002;287:1308–1320. [DOI] [PubMed] [Google Scholar]
- 2. Seidman JG, Seidman C. The genetic basis for cardiomyopathy: from mutation identification to mechanistic paradigms. Cell 2001;104:557–567. [DOI] [PubMed] [Google Scholar]
- 3. Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: part I: diagnosis, prognosis, and measurements of diastolic function. Circulation 2002;105:1387–1393. [DOI] [PubMed] [Google Scholar]
- 4. Aurigemma GP, Gaasch WH. Clinical practice. Diastolic heart failure. N Engl J Med 2004;351:1097–1105. [DOI] [PubMed] [Google Scholar]
- 5. Gaasch WH, Zile MR. Left ventricular diastolic dysfunction and diastolic heart failure. Ann Rev Med 2004;55:373–394. [DOI] [PubMed] [Google Scholar]
- 6. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 7. Horgan S, Watson C, Glezeva N, Baugh J. Murine models of diastolic dysfunction and heart failure with preserved ejection fraction. J Card Fail 2014;20:984–995. [DOI] [PubMed] [Google Scholar]
- 8. Dubi S, Arbel Y. Large animal models for diastolic dysfunction and diastolic heart failure-a review of the literature. Cardiovasc Pathol 2010;19:147–152. [DOI] [PubMed] [Google Scholar]
- 9. Itzhaki I, Maizels L, Huber I, Zwi-Dantsis L, Caspi O, Winterstern A, Feldman O, Gepstein A, Arbel G, Hammerman H, Boulos M, Gepstein L. Modelling the long QT syndrome with induced pluripotent stem cells. Nature 2011;471:225–229. [DOI] [PubMed] [Google Scholar]
- 10. Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A, Pavlovic A, Lin S, Chen R, Hajjar RJ, Snyder MP, Dolmetsch RE, Butte MJ, Ashley EA, Longaker MT, Robbins RC, Wu JC. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med 2012;4:130ra47.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lan F, Lee AS, Liang P, Sanchez-Freire V, Nguyen PK, Wang L, Han L, Yen M, Wang Y, Sun N, Abilez OJ, Hu S, Ebert AD, Navarrete EG, Simmons CS, Wheeler M, Pruitt B, Lewis R, Yamaguchi Y, Ashley EA, Bers DM, Robbins RC, Longaker MT, Wu JC. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell 2013;12:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, Geva J, Roberts AE, Ma Q, Ding J, Chen J, Wang DZ, Li K, Wang J, Wanders RJ, Kulik W, Vaz FM, Laflamme MA, Murry CE, Chien KR, Kelley RI, Church GM, Parker KK, Pu WT. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med 2014;20:616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu H, Lee J, Vincent Ludovic G, Wang Q, Gu M, Lan F, Churko Jared M, Sallam Karim I, Matsa E, Sharma A, Gold Joseph D, Engler Adam J, Xiang Yang K, Bers DM, Wu Joseph C. Epigenetic regulation of phosphodiesterases 2A and 3A underlies compromised β-adrenergic signaling in an iPSC model of dilated cardiomyopathy. Cell Stem Cell 2015;17:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 15. Nagueh SF, Smiseth OA, Appleton CP, Byrd I, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD, Houston T, Oslo N, Phoenix A, Nashville T, Hamilton OC, Uppsala S, Ghent Liège B, Cleveland O, Novara I, Rochester M, Bucharest R, St. Louis M. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–1360. [DOI] [PubMed] [Google Scholar]
- 16. Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci USA 2012;109:E1848–E1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ribeiro AJ, Ang YS, Fu JD, Rivas RN, Mohamed TM, Higgs GC, Srivastava D, Pruitt BL. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci USA 2015;112:12705–12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc 2013;8:2281.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Backx PH, Gao WD, Azan-Backx MD, Marban E. The relationship between contractile force and intracellular [Ca2+] in intact rat cardiac trabeculae. J Gen Physiol 1995;105:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sommese RF, Nag S, Sutton S, Miller SM, Spudich JA, Ruppel KM. Effects of troponin T cardiomyopathy mutations on the calcium sensitivity of the regulated thin filament and the actomyosin cross-bridge kinetics of human β-cardiac myosin. PLoS One 2013;8:e83403.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiang Y, Kobilka BK. Myocyte adrenoceptor signaling pathways. Science 2003;300:1530–1532. [DOI] [PubMed] [Google Scholar]
- 22. Bonow RO, Rosing DR, Bacharach SL, Green MV, Kent KM, Lipson LC, Maron BJ, Leon MB, Epstein SE. Effects of verapamil on left ventricular systolic function and diastolic filling in patients with hypertrophic cardiomyopathy. Circulation 1981;64:787–796. [DOI] [PubMed] [Google Scholar]
- 23. Iwase M, Sotobata I, Takagi S, Miyaguchi K, Jing HX, Yokota M. Effects of diltiazem on left ventricular diastolic behavior in patients with hypertrophic cardiomyopathy: evaluation with exercise pulsed Doppler echocardiography. J Am Coll Cardiol 1987;9:1099–1105. [DOI] [PubMed] [Google Scholar]
- 24. Sossalla S, Wagner S, Rasenack EC, Ruff H, Weber SL, Schondube FA, Tirilomis T, Tenderich G, Hasenfuss G, Belardinelli L, Maier LS. Ranolazine improves diastolic dysfunction in isolated myocardium from failing human hearts—role of late sodium current and intracellular ion accumulation. J Mol Cell Cardiol 2008;45:32–43. [DOI] [PubMed] [Google Scholar]
- 25. Lovelock JD, Monasky MM, Jeong E-M, Lardin HA, Liu H, Patel BG, Taglieri DM, Gu L, Kumar P, Pokhrel N, Zeng D, Belardinelli L, Sorescu D, Solaro RJ, Dudley SC. Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circulation Research 2012;110:841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parikh KS, Sharma K, Fiuzat M, Surks HK, George JT, Honarpour N, Depre C, Desvigne-Nickens P, Nkulikiyinka R, Lewis GD, Gomberg-Maitland M, O’Connor CM, Stockbridge N, Califf RM, Konstam MA, Januzzi JL, Solomon SD, Borlaug BA, Shah SJ, Redfield MM, Felker GM. Heart failure with preserved ejection fraction expert panel report: current controversies and implications for clinical trials. JACC Heart Fail 2018;6:619–632. [DOI] [PubMed] [Google Scholar]
- 27. Wu X, Eder P, Chang B, Molkentin JD. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc Natl Acad Sci 2010;107:7000–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Camacho Londoño JE, Tian Q, Hammer K, Schröder L, Camacho Londoño J, Reil JC, He T, Oberhofer M, Mannebach S, Mathar I, Philipp SE, Tabellion W, Schweda F, Dietrich A, Kaestner L, Laufs U, Birnbaumer L, Flockerzi V, Freichel M, Lipp P. A background Ca2+ entry pathway mediated by TRPC1/TRPC4 is critical for development of pathological cardiac remodelling. Eur Heart J 2015;36:2257–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Howard T, Greer-Short A, Satroplus T, Patel N, Nassal D, Mohler PJ, Hund TJ. CaMKII-dependent late Na+ current increases electrical dispersion and arrhythmia in ischemia-reperfusion. Am J Physiol Heart Circ Physiol 2018;315:H794–H801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davis J, Davis LC, Correll RN, Makarewich CA, Schwanekamp JA, Moussavi-Harami F, Wang D, York AJ, Wu H, Houser SR, Seidman CE, Seidman JG, Regnier M, Metzger JM, Wu JC, Molkentin JD. A tension-based model distinguishes hypertrophic versus dilated cardiomyopathy. Cell 2016;165:1147–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fabiato A, Fabiato F. Dependence of the contractile activation of skinned cardiac cells on the sarcomere length. Nature 1975;256:54–56. [DOI] [PubMed] [Google Scholar]
- 32. Ma N, Zhang J, Itzhaki I, Zhang SL, Chen H, Haddad F, Kitani T, Wilson KD, Tian L, Shrestha R, Wu H, Lam CK, Sayed N, Wu JC. Determining the pathogenicity of a genomic variant of uncertain significance using CRISPR/Cas9 and human-induced pluripotent stem cells. Circulation 2018;138:2666–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bers DM. Cardiac excitation-contraction coupling. Nature 2002;415:198–205. [DOI] [PubMed] [Google Scholar]
- 34. Liang P, Sallam K, Wu H, Li Y, Itzhaki I, Garg P, Zhang Y, Termglichan V, Lan F, Gu M, Gong T, Zhuge Y, He C, Ebert AD, Sanchez-Freire V, Churko J, Hu S, Sharma A, Lam CK, Scheinman MM, Bers DM, Wu JC. Patient-specific and genome-edited induced pluripotent stem cell-derived cardiomyocytes elucidate single-cell phenotype of Brugada syndrome. J Am Coll Cardiol 2016;68:2086–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ Leak-Load relationship. Circ Res 2002;91:594–600. [DOI] [PubMed] [Google Scholar]
- 36. Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol 2011;51:468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu C, Oikonomopoulos A, Sayed N, Wu JC. Modeling human diseases with induced pluripotent stem cells: from 2D to 3D and beyond. Development 2018;145:dev156166.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tzatzalos E, Abilez OJ, Shukla P, Wu JC. Engineered heart tissues and induced pluripotent stem cells: macro- and microstructures for disease modeling, drug screening, and translational studies. Adv Drug Del Rev 2016;96:234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.