Abstract
Aims
Increases in extravascular lung water (EVLW) during exercise contribute to symptoms, morbidity, and mortality in patients with heart failure and preserved ejection fraction (HFpEF), but the mechanisms leading to pulmonary congestion during exercise are not well-understood.
Methods and results
Compensated, ambulatory patients with HFpEF (n = 61) underwent invasive haemodynamic exercise testing using high-fidelity micromanometers with simultaneous lung ultrasound, echocardiography, and expired gas analysis at rest and during submaximal exercise. The presence or absence of EVLW was determined by lung ultrasound to evaluate for sonographic B-line artefacts. An increase in EVLW during exercise was observed in 33 patients (HFpEFLW+, 54%), while 28 (46%) did not develop EVLW (HFpEFLW−). Resting left ventricular function was similar in the groups, but right ventricular (RV) dysfunction was two-fold more common in HFpEFLW+ (64 vs. 31%), with lower RV systolic velocity and RV fractional area change. As compared to HFpEFLW−, the HFpEFLW+ group displayed higher pulmonary capillary wedge pressure (PCWP), higher pulmonary artery (PA) pressures, worse RV-PA coupling, and higher right atrial (RA) pressures during exercise, with increased haemoconcentration indicating greater loss of water from the vascular space. The development of lung congestion during exercise was significantly associated with elevations in PCWP and RA pressure as well as impairments in RV-PA coupling (area under the curve values 0.76–0.84).
Conclusion
Over half of stable outpatients with HFpEF develop increases in interstitial lung water, even during submaximal exercise. The acute development of lung congestion is correlated with increases in pulmonary capillary hydrostatic pressure that favours fluid filtration, and systemic venous hypertension due to altered RV-PA coupling, which may interfere with fluid clearance.
Clinical trial registration
Keywords: Heart failure, Pulmonary oedema, Heart failure with preserved ejection fraction, Exercise haemodynamics
Introduction
The central pathognomonic feature in heart failure (HF) with preserved ejection fraction (HFpEF) is an elevation in cardiac filling pressures during exercise.1,2 These haemodynamic perturbations are correlated with dyspnoea severity, pulmonary limitations, impairments in aerobic capacity, and increased risk of death in HFpEF.3–6 The linkage between haemodynamics, symptoms, and clinical outcomes is believed to be related in large part to lung congestion. This develops when left atrial pressure increases beyond a critical threshold in animal models,7,8 but the invasive haemodynamic mechanisms underlying lung congestion during exercise in humans remain unclear.
An increase in extravascular lung water (EVLW) develops in HF when fluid filtration increases due to pulmonary capillary hypertension due to left HF. However, there also may be an important role for right HF. In experimental animal preparations, acute increases in systemic venous pressure cause increased lung congestion in animals with elevated left atrial pressure due to impaired pulmonary lymphatic drainage.9 Accordingly, we hypothesized that patients with HFpEF who develop lung congestion with exercise would display higher pulmonary capillary pressures causing excessive fluid filtration, but also higher systemic venous pressures due to impairments in right ventricular (RV) pulmonary artery (PA) coupling, as compared to HFpEF patients that do not develop increased EVLW.
To test this hypothesis, we performed a comprehensive, simultaneous assessment of invasive haemodynamics, lung ultrasound, echocardiography, blood sampling, and expired gas analysis to explore the mechanisms governing the development of EVLW during exercise in patients with HFpEF.
Methods
Patients referred to the Mayo Clinic cardiac catheterization laboratory for invasive haemodynamic exercise testing in the evaluation of unexplained dyspnoea were enrolled prospectively to two different studies employing the same protocol of simultaneous echocardiography, lung ultrasound, high-fidelity micromanometer invasive catheterization, blood gas sampling, and expired gas analysis at rest and during supine cycle ergometry at a matched workload of 20 W. Data from some of these patients have been published, but not as they relate to assessment of EVLW.10 Written informed consent was obtained from all patients and both studies were approved by the Mayo Clinic Institutional Review Board.
Study population
The diagnosis of HFpEF was defined by lifestyle-limiting symptoms of exertional dyspnoea and fatigue, left ventricular (LV) ejection fraction (EF) ≥50%, and pulmonary capillary wedge pressure (PCWP) of ≥15 mmHg at rest or ≥25 mmHg during exercise.1,2 Patients with decompensated HF, prior EF <50%, significant left-sided valvular heart disease (>mild stenosis, >moderate regurgitation), coronary disease requiring revascularization, infiltrative, restrictive or hypertrophic cardiomyopathies, constrictive pericarditis, obstructive or restrictive pulmonary disease, high-output HF, and primary RV myopathies were excluded.
Haemodynamic assessment
All studies were performed in the supine position on chronic medications in the fasting state as previously described.2,10,11 Following assessment of baseline (resting) haemodynamics, subjects underwent supine cycle ergometry with simultaneous expired gas analysis at a workload of 20 W (pedal speed 60 rpm) for 5 min. Right heart catheterization was performed through a 9-Fr sheath via the right internal jugular vein. Right atrial (RA) pressure, PA pressures, and PCWP were measured using high-fidelity micromanometers advanced through a balloon-tipped, end-hole catheter. PCWP position was confirmed by appearance on fluoroscopy, characteristic pressure waveforms, and oximetry (saturation ≥ 94%).
Expired gas analysis was measured continuously throughout each phase of the study (MedGraphics, St. Paul, MN, USA) to measure oxygen consumption (VO2). A 4–6-Fr radial arterial cannula was used to measure arterial blood pressure and allow sampling of arterial blood gases and measurement of haemoglobin concentration. Simultaneous PA blood samples were obtained to measure mixed venous O2 contents. Cardiac output or pulmonary blood flow (QP) was then calculated by the direct Fick method.
Haemodynamic pressure tracings were recorded, digitized (240 Hz), and stored for offline analysis. Pressures were taken at end-expiration as the average of three beats. To assess the total hydrostatic pressure favouring capillary filtration, PCWP was defined as the area under the curve including both the A and V waves at end-expiration, where intrathoracic pressure approximates atmospheric pressure. Because some authors have suggested that pressures be averaged over the entire respiratory cycle during exercise,12 we also determined mean pressures averaged over three respiratory cycles as a sensitivity analysis, were Presp indicates respiratory cycle averaged pressures.
Pulmonary vascular resistance was calculated as (mean PA-PCWP)/QP, PA compliance by the ratio of SV/PA pulse pressure, and pulmonary elastance by the ratio of PA systolic pressure/SV. The slope of increase in mean PA pressure, PCWP, and right atrial pressure (RAP) as a function of cardiac output (PAP/QP, PCWP/QP, RA/QP) were calculated from rest and exercise values.5,12 Left ventricular transmural pressure, which reflects the net distending pressure that favours LV filling, was calculated as PCWP − RA, given that RA pressure is an accurate estimate of pericardial pressure.13–15
Assessment of extravascular lung water
Lung ultrasound was performed simultaneously at rest and during exercise to detect the presence of EVLW. The sonographic signature of EVLW are ‘B-Lines’ which present as vertical, hyperechoic lines that originate from the pleural line and extend to the bottom of the ultrasound screen while moving synchronously with respirophasic motion of the visceral pleura.16–19 B-lines have been demonstrated to reflect both an increase in EVLW and to change dynamically with EVLW content.19–24
Lung ultrasound was performed using a phased array transducer in the left third intercostal space in two positions along the mid-axillary and mid-clavicular lines. The right chest was not imaged due to unavailability during cardiac catheterization. Imaging depth was optimized to ensure that B-lines were continuous from the pleural edge to the bottom of the lung window and moved with respiration. Because B-lines may also be caused by parenchymal lung disease (so-called ‘dry B-lines’), exercise EVLW was only considered to be present if B-lines appeared only during exercise, or if the number of B-lines increased during exercise as compared to rest.19
Assessment of right ventricular function and right ventricular pulmonary artery coupling
Prior to cardiac catheterization, comprehensive echocardiography was performed to assess left heart structure and function in accordance with current guidelines.25 Focused evaluation of right heart function was performed simultaneously with invasive measurements at rest and during exercise as previously described.11 Tricuspid annular plane systolic excursion (TAPSE), RV systolic tissue velocity at the lateral tricuspid annulus (RV s’), and fractional area change (FAC) were measured during rest and exercise.26 RV dysfunction was defined according to the American Society of Echocardiography recommendations as RV FAC < 35% or RV s’ <10 cm/s.26 Right ventricular-PA coupling was assessed by the ratio of TAPSE to PA pressure as in prior studies.27 Ratios of FAC and RV s’ to PA pressure were also examined as complementary indices of RV-PA coupling analogous to TAPSE/PA pressure ratio. Additionally, LA reservoir strain and LA compliance were measured as previously described.28
Assessment of oncotic pressure, haemoconcentration, and pulmonary ventilation
Plasma oncotic pressure was estimated by pre-exercise albumin levels. Acute changes in arterial haemoglobin during exercise were assessed as a measure of intravascular water content relative to baseline, whereby increases in haemoglobin identify an acute reduction in intravascular fluid content due to filtration of water out of the vascular space and into the interstitium during exercise in the setting of pulmonary and systemic venous congestion.29 Oxygen consumption (VO2), carbon dioxide production (VCO2), and minute ventilation (VE) were measured continuously using a portable metabolic cart (MedGraphics, St. Paul, MN, USA).3,30 Pulmonary dead-space fraction or the ratio of dead space ventilation to tidal volume (VD/VT) was determined using the modified alveolar gas equation.30 The VE/VCO2 slope was calculated from the slope of all VE and VCO2 data points during exercise.
Statistical analysis
Data are presented as mean (standard deviation), median (interquartile range), or number (%). Between-group differences were compared by unpaired t-test, Wilcoxon rank-sum test, χ2 or Fisher’s exact test as appropriate. Linear regression analyses and Pearson correlation coefficients were used to assess relationships between changes in variables of interest. Logistic regression was used to identify predictors for the development of EVLW during exercise. Analysis was performed using JMP 13.0.0 (SAS). A P-value of <0.05 was considered statistically significant. Standardized mean differences (SMD) were also calculated to provide estimates of effect size for differences between groups independent of traditional null hypothesis testing. Higher SMD indicates greater magnitude of differences between the two groups.
Results
Prevalence of lung water during exercise
Among 61 consecutive HFpEF patients without clinically apparent congestion prior to assessment, 54% (n = 33) either developed new B-lines (n = 23, 38%) or developed an increase in the number B-lines (n = 10, 16%) during exercise, indicating development of lung congestion (HFpEFLW+), while 46% (n = 28) did not develop B-lines (HFpEFLW−). As compared to HFpEFLW- subjects, the HFpEFLW+ group had slightly higher plasma NT-proBNP levels but other baseline characteristics, including atrial fibrillation and measures of oncotic pressure, were similar in the groups (Table 1).
Table 1.
Baseline characteristics
| HFpEFLW− (n = 28) | HFpEFLW+ (n = 33) | P-value | SMD (95% CI) | |
|---|---|---|---|---|
| Age (years) | 66 ± 10 | 66 ± 13 | 0.89 | 0.04 (-0.47 to 0.54) |
| Female (%) | 61 | 42 | 0.20 | — |
| Body mass index (kg/m2) | 33 ± 7 | 35 ± 8 | 0.32 | 0.26 (−0.25 to 0.76) |
| Comorbidities | ||||
| Coronary disease (%) | 26 | 27 | 1.00 | — |
| Diabetes mellitus (%) | 18 | 33 | 0.24 | — |
| Hypertension (%) | 100 | 91 | 0.24 | — |
| Atrial fibrillation (%) | 21 | 28 | 0.55 | — |
| Medications | ||||
| ACE or ARB (%) | 57 | 39 | 0.20 | — |
| Beta-blocker (%) | 32 | 55 | 0.12 | — |
| Loop diuretic (%) | 52 | 69 | 0.28 | — |
| Laboratories | ||||
| Creatinine (mg/dL) | 1.0 ± 0.3 | 1.3 ± 0.8 | 0.067 | −0.48 (−0.99 to 0.03) |
| Haemoglobin (g/dL) | 12.5 ± 1.3 | 12.0 ± 1.5 | 0.25 | −0.30 (−0.80 to 0.21) |
| Albumin (n = 39/61) (g/dL) | 4.0 ± 0.3 | 3.9 ± 0.4 | 0.21 | −0.40 (−1.03 to 0.24) |
| NT-proBNP (n = 46/61) (pg/mL) | 91 (30–423) | 336 (93–1058) | 0.017 | — |
| Echocardiography | ||||
| LV ejection fraction (%) | 62 ± 5 | 60 ± 5 | 0.17 | −0.37 (−0.90 to 0.17) |
| LV diastolic dimension (mm) | 51 ± 4 | 52 ± 5 | 0.64 | 0.14 (−0.43 to 0.70) |
| LV mass index (g/m2) | 94 ± 20 | 92 ± 19 | 0.81 | −0.07 (−0.66 to 0.51) |
| LA volume index (mL/m2) | 32 ± 10 | 37 ± 15 | 0.23 | 0.36 (−0.23 to 0.93) |
| LA reservoir strain (%) (n = 57/61) | 32 ± 14 | 24 ± 15 | 0.037 | −0.57 (−1.09 to −0.03) |
| LA compliance (%/mmHg) (n = 57/61) | 1.62 ± 0.86 | 1.00 ± 0.84 | 0.008 | −0.73 (−1.25 to −0.18) |
| LV e’ velocity (cm/s) | 7 ± 2 | 7 ± 2 | 0.88 | 0.05 (−0.49 to 0.58) |
| LV E/e’ ratio | 11.6 ± 4.9 | 12.9 ± 6.1 | 0.40 | 0.23 (−0.31 to 0.76) |
| MR (%) (no/mild/moderate) | 56/44/0 | 43/54/3 | 0.37 | — |
| TR (%) (no/mild/moderate) | 68/24/8 | 46/29/25 | 0.17 | — |
| RV dysfunctiona (%) | 31 | 64 | 0.011 | — |
Values are represented as mean ± standard deviation or median (interquartile range).
ACE, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; E/e’, ratio of early diastolic transmitral filling velocity (E) and early diastolic mitral annular tissue velocity (e’); LA, left atrial; LV, left ventricular; MR, mitral regurgitation; NT-proBNP, N-terminal pro brain natriuretic peptide; RV, right ventricular; SMD, standardized mean difference; TR, tricuspid regurgitation.
RV dysfunction was defined by American Society of Echocardiography recommendations as either RV FAC <35% or RV s’ <10 cm/s.
Baseline cardiac structure and function
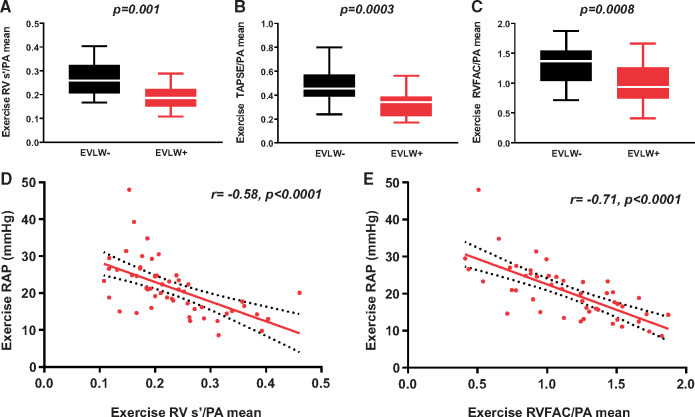
Left ventricular structure and function were similar in the HFpEFLW+ and HFpEFLW− groups, with similar LV chamber size, EF, and diastolic function indices (Table 1). In contrast, LA reservoir strain and LA compliance were lower in the HFpEFLW+ group. Despite similarities in LV function, there was a markedly higher prevalence of RV dysfunction in HFpEFLW+ (64% vs. 31%, P = 0.011; Table 1 and Figure 1), with lower RV s’ and FAC as compared to HFpEFLW− at rest (Table 2).
Figure 1.
The HFpEF group with extravascular lung water (EVLW+) had similar left ventricular function but worse right ventricular function compared with the HFpEF group without extravascular lung water (EVLW−) (A and B). Right atrial and pulmonary capillary wedge pressure were higher during exercise in the EVLW+ HFpEF group (C and D). *P=0.01, **P=0.001.
Table 2.
Resting haemodynamics, right heart function, and ventilatory measures
| HFpEFLW− (n = 28) | HFpEFLW+ (n = 33) | P-value | SMD (95% CI) | |
|---|---|---|---|---|
| Heart rate (b.p.m.) | 71 ± 12 | 72 ± 9 | 0.76 | 0.08 (−0.42 to 0.58) |
| Mean BP (mmHg) | 106 ± 13 | 104 ± 13 | 0.60 | −0.14 (−0.67 to 0.39) |
| Qp (L/min) | 5.5 ± 1.5 | 5.7 ± 2.2 | 0.69 | 0.11 (−0.40 to 0.61) |
| Ventricular filling pressures | ||||
| RA mean (mmHg) | 10 ± 4 | 13 ± 4 | 0.007 | 0.72 (0.19–1.24) |
| RAresp mean (mmHg) | 9 ± 5 | 12 ± 5 | 0.023 | 0.60 (0.08–1.11) |
| PCWP mean (mmHg) | 18 ± 5 | 23 ± 7 | 0.002 | 0.83 (0.30–1.35) |
| PCWPresp mean (mmHg) | 15 ± 5 | 19 ± 6 | 0.005 | 0.75 (0.22–1.26) |
| PCWP V wave (mmHg) | 22 ± 7 | 31 ± 12 | 0.001 | 0.88 (0.34–1.40) |
| LV transmural pressure (mmHg) | 7 ± 3 | 7 ± 3 | 0.46 | 0.19 (−0.31 to 0.70) |
| Pulmonary vascular function | ||||
| PA systolic pressure (mmHg) | 38 ± 7 | 49 ± 16 | 0.002 | 0.83 (0.30–1.34) |
| PA mean (mmHg) | 27 ± 5 | 34 ± 10 | 0.0003 | 1.24 (0.68–1.78) |
| PAresp mean (mmHg) | 24 ± 6 | 31 ± 10 | 0.002 | 0.83 (0.30–1.34) |
| PVR (Wood units) | 1.8 ± 1.0 | 2.4 ± 1.8 | 0.10 | 0.42 (−0.09 to 0.93) |
| PA compliance (mL/mmHg) | 4.5 ± 2.0 | 3.8 ± 1.9 | 0.17 | −0.36 (−0.86 to 0.16) |
| PA Ea (mmHg/mL) | 0.53 ± 0.18 | 0.68 ± 0.33 | 0.034 | 0.56 (0.04–1.06) |
| RV function | ||||
| RV s’ (cm/s) | 11 ± 2 | 9 ± 2 | 0.001 | −0.87 (−1.39 to −0.33) |
| TAPSE (mm) (n = 58/61) | 19 ± 4 | 17 ± 4 | 0.11 | −0.42 (−0.94 to 0.10) |
| RV FAC (%) (n = 55/61) | 52 ± 7 | 46 ± 9 | 0.006 | −0.77 (−1.31 to −0.21) |
| RV s’/PA mean (cm⋅s−1/mmHg) | 0.44 ± 0.16 | 0.29 ± 0.11 | <0.0001 | −1.11 (−1.64 to −0.55) |
| TAPSE/PA mean (mm/mmHg) (n = 58/61) | 0.72 ± 0.19 | 0.52 ± 0.17 | 0.0001 | −1.11 (−1.65 to −0.54) |
| FAC/PA mean (%/mmHg) (n = 55/61) | 2.05 ± 0.56 | 1.48 ± 0.58 | 0.0005 | −1.00 (−1.54 to −0.42) |
| Ventilation and gas exchange | ||||
| Arterial saturation (%) | 95 ± 2 | 95 ± 3 | 0.97 | 0.00 (−0.50 to 0.50) |
| VE (L/min) | 6.7 ± 1.5 | 7.5 ± 2.3 | 0.14 | 0.38 (−0.13 to 0.88) |
| VD/VT | 0.36 ± 0.05 | 0.37 ± 0.06 | 0.70 | 0.09 (−0.41 to 0.59) |
Values are represented as mean ± SD.
CaO2 – CvO2, arterial-venous O2 content difference; Ea, elastance; FAC, fractional area change; fb, respiratory rate; LV, left ventricular; PCWP, pulmonary capillary wedge pressure; PCWPresp, average pressure throughout respiration; PVR, pulmonary vascular resistance; Qp, pulmonary flow/cardiac output; RA, right atrial; RV, right ventricular; s’, systolic tissue Doppler velocity; SMD, standardized mean difference; TAPSE, tricuspid annular plane systolic excursion; VD/VT, dead space fraction; VE, minute ventilation; VE/VCO2, ventilatory efficiency; VT, tidal volume.
Baseline haemodynamics
As compared to HFpEFLW−, subjects with HFpEFLW+ displayed higher PCWP and greater V wave amplitude at rest (Table 2 and Figure 1). Similarly, RAP, PA systolic pressures, PA mean pressures, and PA Ea were higher in the HFpEFLW+ group as compared to HFpEFLW−. In contrast, LV transmural pressure was similar in the two groups. The HFpEFLW+ group displayed more deranged RV-PA coupling at rest, manifest by lower ratios of TAPSE, FAC, and RV s’ to PA mean pressure. Vital signs, cardiac output, arterial saturation, and ventilatory measures were similar in the groups at rest (Table 2).
Haemodynamics, ventricular function, and ventilation during exercise
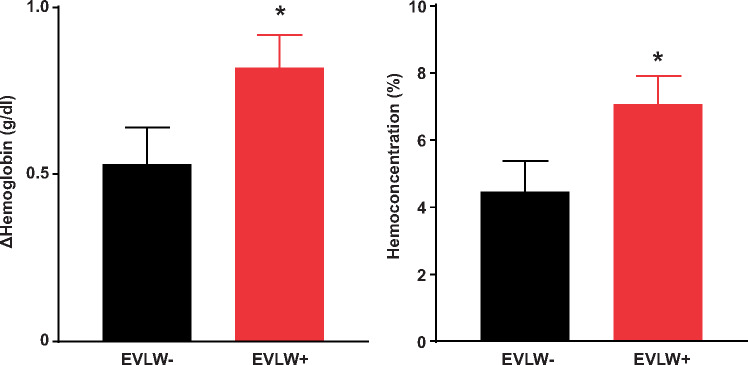
With exercise, patients in the HFpEFLW+ group developed higher RAP, PCWP, and greater increase in the amplitude of the PCWP V wave (Table 3 and Figure 1). Patients in the HFpEFLW+ group also displayed more severe exercise-induced pulmonary hypertension, worse RV-PA coupling, and greater increases in pulsatile RV load (higher PA Ea) compared to the HFpEFLW− group (Figure 2). The increase in RA pressure observed was correlated with the magnitude of RV-PA uncoupling during exercise (Figure 2). Even though the PCWP was higher in the HFpEFLW+ group this was associated with a lower transmural pressure during exercise, indicating that the PCWP elevation was in part driven by the right heart and relative pericardial restraint.15 There was no difference in heart rate or QP between the groups during exercise (Table 3).
Table 3.
Exercise haemodynamics, right heart function, and ventilatory measures
| HFpEFLW− (n = 28) | HFpEFLW+ (n = 33) | P-value | SMD (95% CI) | |
|---|---|---|---|---|
| Heart rate (b.p.m.) | 93 ± 16 | 91 ± 15 | 0.63 | −0.13 (−0.63 to 0.38) |
| Mean BP (mmHg) | 117 ± 13 | 118 ± 17 | 0.73 | 0.09 (−0.42 to 0.63) |
| Qp (L/min) | 8.4 ± 2.2 | 8.6 ± 3.3 | 0.73 | 0.09 (−0.42 to 0.60) |
| Ventricular filling pressures | ||||
| RA mean (mmHg) | 17 ± 5 | 24 ± 7 | <0.0001 | 1.12 (0.56–1.65) |
| RAresp mean (mmHg) | 16 ± 6 | 22 ± 6 | 0.0005 | 0.96 (0.42–1.48) |
| PCWP mean (mmHg) | 31 ± 5 | 38 ± 9 | 0.0004 | 0.97 (0.43–1.49) |
| PCWPresp mean (mmHg) | 26 ± 6 | 30 ± 7 | 0.008 | 0.70 (0.17–1.21) |
| PCWP V wave (mmHg) | 42 ± 9 | 53 ± 15 | 0.0007 | 0.92 (0.38–1.43) |
| LV transmural pressure (mmHg) | 12 ± 4 | 9 ± 6 | 0.008 | −0.71 (−1.22 to −0.17) |
| Pulmonary vascular function | ||||
| PA systolic pressure (mmHg) | 59 ± 10 | 73 ± 17 | 0.0002 | 1.01 (0.46–1.53) |
| PA mean (mmHg) | 44 ± 8 | 54 ± 11 | 0.0006 | 0.93 (0.39–1.46) |
| PAresp mean (mmHg) | 39 ± 7 | 48 ± 11 | 0.001 | 0.88 (0.34–1.40) |
| PVR (Wood units) | 1.8 ± 0.9 | 2.0 ± 1.3 | 0.38 | 0.24 (−0.28 to 0.75) |
| PA compliance (mL/mmHg) | 3.7 ± 1.4 | 3.2 ± 1.4 | 0.15 | −0.38 (−0.88 to 0.14) |
| PA Ea (mmHg/mL) | 0.68 ± 0.19 | 0.84 ± 0.32 | 0.028 | 0.60 (0.07–1.11) |
| R function | ||||
| RV s’ (cm/s) (n = 54/61) | 12 ± 2 | 10 ± 3 | 0.033 | −0.60 (−1.14 to −0.04) |
| TAPSE (mm) (n = 51/61) | 20 ± 4 | 18 ± 4 | 0.038 | −0.60 (−1.15 to −0.03) |
| RV FAC (%) (n = 50/61) | 55 ± 8 | 50 ± 10 | 0.035 | −0.62 (−1.17 to −0.04) |
| RV s’/PA mean (cm⋅s−1/mmHg) (n = 54/61) | 0.27 ± 0.07 | 0.20 ± 0.08 | 0.001 | −0.93 (−1.48 to −0.34) |
| TAPSE/PA mean (mm/mmHg) (n = 51/61) | 0.47 ± 0.12 | 0.34 ± 0.12 | 0.0003 | −1.08 (−1.66 to −0.47) |
| FAC/PA mean (%/mmHg) (n = 50/61) | 1.34 ± 0.33 | 0.99 ± 0.35 | 0.0008 | −1.03 (−1.61 to −0.41) |
| Integrated indices | ||||
| ΔPCWP/ΔQp (mmHg/L⋅min−1) | 3.7 (2.3–5.0) | 5.7 (4.2–8.3) | 0.007 | — |
| ΔRA/ΔQp (mmHg/L⋅min−1) | 2.1 (1.1–3.0) | 3.8 (2.8–7.5) | 0.0009 | — |
| ΔPA mean/ΔQp (mmHg/L⋅min−1) | 5.2 (3.9–8.9) | 6.8 (5.0–10.8) | 0.10 | — |
| Ventilation and gas exchange | ||||
| Arterial saturation (%) | 94 ± 2 | 95 ± 3 | 0.16 | 0.36 (−0.15 to 0.87) |
| VE (L/min) | 20 ± 6 | 24 ± 9 | 0.047 | 0.52 (0.00–1.03) |
| VD/VT | 0.28 ± 0.03 | 0.30 ± 0.05 | 0.040 | 0.53 (0.01–1.04) |
| VE/VCO2 | 33 ± 7 | 37 ± 7 | 0.060 | 0.49 (−0.03 to 1.00) |
| VE/VCO2 slope | 29 ± 6 | 27 ± 6 | 0.25 | −0.31 (−0.82 to 0.22) |
Values are represented as mean ± SD or median (interquartile range).
CaO2 – CvO2, arterial-venous O2 content difference; Ea, elastance; FAC, fractional area change; fb, respiratory rate; LV, left ventricular; PCWP, pulmonary capillary wedge pressure; PCWPresp, average pressure throughout respiration; PVR, pulmonary vascular resistance; Qp, pulmonary flow/cardiac output; RA, right atrial; RV, right ventricular; s’, systolic tissue Doppler velocity; SMD, standardized mean difference; TAPSE, tricuspid annular plane systolic excursion; VD/VT, dead space fraction; VE, minute ventilation; VE/VCO2, ventilatory efficiency; VT, tidal volume.
Figure 2.
Right ventricular pulmonary artery uncoupling during exercise was worse in the EVLW+ HFpEF group (A–C) and associated with higher right atrial pressure during exercise (D and E).
Patients in the HFpEFLW+ group displayed greater haemoconcentration with exercise as compared to HFpEFLW−, indicating greater translocation of fluid from the vascular space to the extravascular space (Figure 3). Patients in the HFpEFLW+ group also displayed greater increases in pulmonary dead space fraction and minute ventilation compared to the HFpEFLW− group. The VE/VCO2 ratio tended to be higher in the HFpEFLW+ group (P = 0.060) but the VE/VCO2 slope was not different between groups (Table 3).
Figure 3.
Heart failure and preserved ejection fraction patients that developed extravascular lung water had a greater rise in haemoglobin (A) and greater haemoconcentration (B) during exercise. *P<0.05.
Predictors of development of increased lung water
Elevation in PCWP and RA pressures during exercise distinguished patients developing EVLW from those who did not [area under the curve (AUC) 0.77 and 0.80, Table 4]. Discrimination was numerically better for pressures measured at end-expiration than for pressure averaged over the respiratory cycle, though both methods were predictive. There was no association between markers of LV function at rest and development of EVLW, but measures of LA reservoir strain, LA compliance, and PCWP V wave height were predictive. In contrast, all measures of abnormal RV-PA coupling at rest and during exercise were strongly predictive of developing EVLW during exercise (AUC 0.76–0.84, Table 4).
Table 4.
Predictors of increased extravascular lung water
| AUC | 95% CI | Optimal cut point | Sens | Spec | |
|---|---|---|---|---|---|
| Exercise haemodynamics | |||||
| RA mean (mmHg) | 0.800 | 0.665–0.890 | 19 | 79 | 67 |
| RAresp mean (mmHg) | 0.753 | 0.609–0.856 | 20 | 67 | 74 |
| PCWP mean (mmHg) | 0.771 | 0.635–0.867 | 32 | 82 | 57 |
| PCWPresp mean (mmHg) | 0.693 | 0.548–0.808 | 33 | 42 | 86 |
| PCWP V wave (mmHg) | 0.756 | 0.616–0.857 | 44 | 79 | 68 |
| PA mean (mmHg) | 0.749 | 0.604–0.853 | 48 | 69 | 75 |
| PA meanresp (mmHg) | 0.741 | 0.600–0.845 | 44 | 64 | 75 |
| Resting left heart function | |||||
| LV e’ velocity (cm/s) | 0.492 | 0.342–0.643 | — | — | — |
| LV s’ velocity (cm/s) | 0.558 | 0.371–0.729 | — | — | — |
| Left ventricular ejection fraction (%) | 0.590 | 0.434–0.730 | — | — | — |
| LA reservoir strain (%) (n = 57/61) | 0.664 | 0.512–0.789 | 15.4 | 42 | 92 |
| LA compliance (%/mmHg) (n = 57/61) | 0.713 | 0.563–0.828 | 0.72 | 52 | 96 |
| Resting right heart function and RV-PA coupling | |||||
| RV FAC (%) (n = 55/61) | 0.714 | 0.558–0.831 | 48 | 66 | 77 |
| TAPSE (mm) (n = 58/61) | 0.640 | 0.488–0.768 | — | — | — |
| RV s’ (cm/s) | 0.731 | 0.588–0.836 | 9 | 58 | 78 |
| RV FAC/mPAP (%/mmHg) (n = 55/61) | 0.767 | 0.620–0.869 | 1.75 | 76 | 69 |
| TAPSE/mPAP (mm/mmHg) (n = 58/61) | 0.791 | 0.648–0.886 | 0.61 | 80 | 75 |
| RV’s/mPAP (cm⋅s−1/mmHg) | 0.835 | 0.707–0.914 | 0.27 | 64 | 100 |
| Exercise right heart function and RV-PA coupling | |||||
| RV FAC (%) (n = 50/60) | 0.680 | 0.515–0.809 | 52 | 59 | 74 |
| TAPSE (mm) (n = 51/61) | 0.678 | 0.511–0.810 | 17 | 52 | 83 |
| RV s’ (cm/s) (n = 54/61) | 0.694 | 0.534–0.817 | 10.5 | 63 | 83 |
| RV FAC/mPAP (%/mmHg) (n = 50/61) | 0.763 | 0.606–0.870 | 0.95 | 54 | 87 |
| TAPSE/mPAP (mm/mmHg) (n = 51/61) | 0.805 | 0.651–0.902 | 0.37 | 73 | 88 |
| RV s’/mPAP (cm⋅s−1/mmHg) (n = 54/61) | 0.797 | 0.649–0.893 | 0.19 | 59 | 92 |
Optimal cut points are based on Youden’s index.
AUC, area under the receiver operating characteristic curve; CaO2 – CvO2, arterial-venous O2 content difference; Ea, elastance; FAC, fractional area change; fb, respiratory rate; LV, left ventricular; PCWP, pulmonary capillary wedge pressure; PCWPresp, average pressure throughout respiration; PVR, pulmonary vascular resistance; Qp, pulmonary flow/cardiac output; RA, right atrial; RV, right ventricular; s’, systolic tissue Doppler velocity; Sens, sensitivity; SMD, standardized mean difference; Spec, specificity; TAPSE, tricuspid annular plane systolic excursion; VD/VT, dead space fraction; VE, minute ventilation; VE/VCO2, ventilatory efficiency; VT, tidal volume.
Sensitivity analyses
Sensitivity analyses excluding the HFpEF patients with B-lines at rest, and using systolic PA pressure in place of mean PA pressure for RV-PA coupling assessments demonstrated similar results as in the overall population (Supplementary material online, Tables S1–S3).
Discussion
In this study, we directly evaluated for the development of increased EVLW during exercise and related it to simultaneously assessed invasive haemodynamics and measurements of ventricular function, ventricular-arterial coupling, and pulmonary mechanics, allowing for exploration of the relationships between haemodynamic perturbations and lung congestion during exercise in patients with HFpEF. We observed that development of increased EVLW during exercise is related not only to increases in pulmonary capillary pressures but also to increases in central venous pressures, which were observed to develop secondary to abnormalities in RV function and RV-PA coupling. These data provide new pathophysiologic insight on the complex interactions between haemodynamics and lung congestion in patients with HFpEF and identify an important and previously unappreciated role for abnormalities in RV-PA coupling in the pathogenesis of increased lung water.
Assessment of lung congestion
An emerging body of evidence has shown that increases in EVLW at rest and during exercise can be reliably demonstrated by lung ultrasound in patients with HF.16–19 The development of B-lines has been shown to reflect acute increases in lung congestion and to change dynamically with EVLW content.19–24 Increases in EVLW are associated with adverse outcomes, increased risk for HF hospitalization, and are currently being evaluated as novel treatment targets.
Normal and abnormal exercise responses
The normal lung can accommodate a 300% increase in blood flow during exercise without developing significant congestion. This is achieved by maintenance of low pulmonary capillary hydrostatic pressures through enhancement in left heart function and rapid, efficient clearance of any EVLW that is filtered across the alveolar-capillary membrane through pulmonary lymphatics.9,31,32 We observed that HFpEF patients developing lung congestion displayed similar increase in lung blood flow to those without congestion, but the HFpEFLW+ group displayed significantly greater increase in PCWP, shifting the balance in Starling forces favouring greater fluid filtration out of the capillaries.7,8 The greater loss of fluid from the vascular space in the HFpEFLW+ group was further evidenced by the greater degree of dynamic haemoconcentration during stress (Figure 3).
We also hypothesized the lung congestion would be greater in patients with higher systemic venous pressures due to abnormalities in right heart reserve. This idea was based upon studies in sheep where combined increases in left atrial and systemic venous pressure induced by balloon occlusion were shown to increase lung congestion far beyond that seen with isolated left atrial hypertension alone.9 Consistent with this hypothesis, we observed that the HFpEFLW+ group displayed higher RAP, which was associated with greater impairment in RV-PA coupling at rest and during exercise. We speculate that this relationship is caused by impaired lymphatic clearance of lung water in the setting of high central venous pressures from RV dysfunction, since the pulmonary lymphatics drain passively into the central veins,9,31,32 though the current data cannot address this potential mechanism directly.
Historically, patients developing right-sided HF have been conceptualized as having dry lungs, because the development of pulmonary congestion by definition requires RV output to exceed that of the LV.33 We show that even with the same rate of lung perfusion during exercise (similar increase in QP) and equivalent pulmonary transit time [similar heart rate (HR)], the combination of increased filtration pressure (elevated PCWP) and increased lymphatic ‘afterload’ (RA hypertension) caused by impaired RV-PA coupling was associated with an increase in lung congestion during stress (Take home figure). Patients with HFpEF, pulmonary hypertension (PH), and RV dysfunction are known to display worse functional capacity and clinical outcomes, and the current data may shed new light on the pathophysiology contributing to this observation.27,34–36
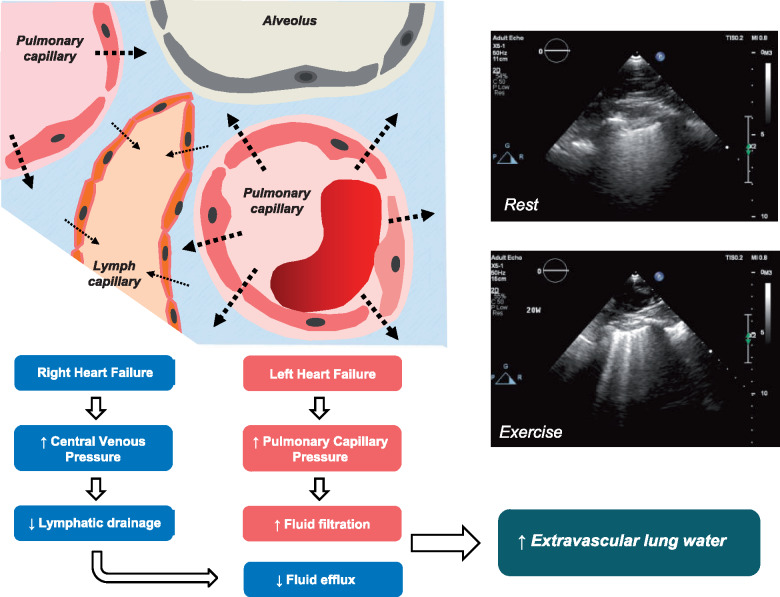
Take home figure .
Development of extravascular lung water associated with both increased pulmonary capillary wedge pressure leading to fluid filtration, as well as increased right atrial and central venous pressure potentially impeding lymphatic lung water drainage.
Chronic remodelling vs. acute congestion
In contrast to the current data, patients with advanced HF often display little evidence of lung congestion, despite high biventricular filling pressures. The discrepant findings may relate to presence or absence of adaptations that develop chronically in the lungs. While pulmonary congestion develops acutely with left atrial hypertension,7 with more sustained elevations there may be structural remodelling in the lung vasculature, which dampens the elevation in pulmonary capillary pressure even as left atrial pressures are high, while decreasing capillary permeability to reduce oedema formation.37,38 This remodelling effectively protects the alveoli from oedema but this occurs at the cost of impairments in lung diffusion in advanced HF patients, which are often not reversible even after aggressive decongestion.33–36
The patients with HFpEF enrolled in the current study displayed normal or mild elevation in PCWP at rest, but marked elevations in PCWP during exercise. With this ephemeral PCWP elevation, there may be less of a stimulus driving capillary and vascular structural remodelling.37–42 Earlier stage HFpEF patients have better lung diffusion than advanced HF but may be more vulnerable to the development of acute lung congestion during exercise as capillary pressures rise.
Greater pulmonary vascular disease in patients with lung congestion
Pulmonary vasoconstriction and remodelling are often conceptualized as a means to protect the lung capillaries from barotrauma in PH due to left heart disease, but the HFpEFLW+ group displayed more profound pulmonary vascular disease, with higher PA elastance at rest and during exercise. In tandem with worsening left atrial hypertension, these abnormalities combined to greatly increase PA pressures and impair RV-PA coupling reserve with stress.
The current data reveal how combined left and right heart limitations during exercise in HFpEF may induce a vicious cycle, whereby left atrial hypertension caused by reduced LA compliance worsens fluid filtration, but also leads to greater PH and impairments in RV-PA coupling, which increase systemic venous pressure to compromise lung lymphatic drainage, further increasing vascular oedema to exacerbate increases in RV afterload. Future study is required to determine the efficacy of novel therapies designed to reduce EVLW, either by directly targeting elevations in PCWP, through medical,43,44 or interventional approaches45,46 to enhance LA compliance or LV diastolic function, improving RV-PA coupling through pulmonary vasodilation10 or enhancement in RV inotropy (NCT03541603), reducing RA pressure through diuresis or alteration in venous tone,47 or possibly even altering capillary permeability. These data may also have implications for new experimental interventional procedures such as atrial septostomy.45 If right heart compliance is adequate, the volume load associated with this procedure may be well-tolerated, but in patients that develop RA hypertension, this could mitigate the benefit from LA decompression.
Limitations
The cross-sectional design of the study limits the ability to discern causality. All participants were referred for invasive exercise testing, introducing bias. Measurements were obtained at rest and during relatively low-level exercise, and we cannot exclude the possibility that some of the HFpEFLW− group would have gone on to develop pulmonary oedema with higher levels of exertion. However, most of the filling pressure elevation occurs at 20 W in HFpEF and studying patients at a common workload removes confounding differences due to differences in functional capacity.1,2 The HFpEFLW+ group had worse LA reservoir and RV function with higher NT-proBNP levels, and a trend to worse renal function. This raises the possibility that patients developing lung water represent a more advanced stage or longer natural history of disease compared to the HFpEFLW− group. Future natural history studies are required to further assess this hypothesis. Notably, even as patients were considered by their primary cardiologists to be clinically euvolaemic, there was evidence of subclinical congestion in the HFpEFLW+ group, with higher NT-proBNP and RV pressures, revealing that some may have been undertreated for HF. The focus of this study was on RV function and RV-PA coupling, and while LV function was assessed at rest, it was not evaluated during exercise. Although mitral and tricuspid regurgitation was similar between groups at rest, we did not assess exertional changes in valvular function. Cut points derived for prediction of lung water during exercise in this study require external validation in independent samples. Lung ultrasound may be performed in 1–28 chest regions.18,19 Because of the time constraints for imaging during exercise and the inability to scan the right thorax in the invasive laboratory, lung ultrasound was performed in two regions on the left chest. This may have reduced the sensitivity of our assessment for EVLW, and it is possible that some of the HFpEFLW− group might have displayed B-lines if more regions of the thorax were imaged. However, B-lines are 50% more likely to develop in the left lung during exercise as compared to the right, and the two regions imaged in this study (anterior and mid-axillary) represent the ‘wet spaces’ most likely to display B-lines during supine exercise.18,48 Scali et al.48 recently demonstrated that examinations during exercise including four regions (two per lung) provided equivalent information on lung congestion to the full 28 region assessment.
Conclusions
Over half of stable outpatients with HFpEF develop increases in interstitial lung water during exercise, even at low levels of exertion. The acute development of lung congestion in HFpEF is correlated with increases in pulmonary capillary hydrostatic pressures and systemic venous hypertension that is associated with impairments in RV-PA coupling. Further study is required to better delineate the treatment for and mechanisms causing lung congestion during exercise in HFpEF.
Supplementary Material
Acknowledgements
The authors thank the staff of the Earl Wood Catheterization Laboratory and patients who agreed to participate in research who allowed this study to be completed.
Funding
This work was supported by the National Institutes of Health [R01 HL128526, R01 HL 126638, U01 HL125205, and U10 HL110262 to B.A.B.], by T32 HL007111 from the NIH and a Fellow’s grant from the Heart Failure Society of America to Y.N.V.R., and a research fellowship from the Uehara Memorial Foundation, Japan to M.O.
Conflict of interest: none declared.
See page 3731 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz805)
References
- 1. Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010;3:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive-echocardiographic study. Circulation 2017;135:825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Obokata M, Olson TP, Reddy YN, Melenovsky V, Kane GC, Borlaug BA. Hemodynamics, dyspnea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J 2018;39:2810–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reddy YNV, Olson TP, Obokata M, Melenovsky V, Borlaug BA. Hemodynamic correlates and diagnostic role of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. JACC Heart Fail 2018;6:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eisman AS, Shah RV, Dhakal BP, Pappagianopoulos PP, Wooster L, Bailey C, Cunningham TF, Hardin KM, Baggish AL, Ho JE, Malhotra R, Lewis GD. Pulmonary capillary wedge pressure patterns during exercise predict exercise capacity and incident heart failure. Circ Heart Fail 2018;11:e004750.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dorfs S, Zeh W, Hochholzer W, Jander N, Kienzle RP, Pieske B, Neumann FJ. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J 2014;35:3103–3112. [DOI] [PubMed] [Google Scholar]
- 7. Guyton AC, Lindsey AW. Effect of elevated left atrial pressure and decreased plasma protein concentration on the development of pulmonary edema. Circ Res 1959;7:649–657. [DOI] [PubMed] [Google Scholar]
- 8. Levine OR, Mellins RB, Senior RM, Fishman AP. The application of Starling's law of capillary exchange to the lungs. J Clin Invest 1967;46:934–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laine GA, Allen SJ, Katz J, Gabel JC, Drake RE. Effect of systemic venous pressure elevation on lymph flow and lung edema formation. J Appl Physiol 1986;61:1634–1638. [DOI] [PubMed] [Google Scholar]
- 10. Reddy YNV, Obokata M, Koepp KE, Egbe AC, Wiley B, Borlaug BA. The beta-adrenergic agonist albuterol improves pulmonary vascular reserve in heart failure with preserved ejection fraction. Circ Res 2019;124:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J 2016;37:3293–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kovacs G, Herve P, Barbera JA, Chaouat A, Chemla D, Condliffe R, Garcia G, Grunig E, Howard L, Humbert M, Lau E, Laveneziana P, Lewis GD, Naeije R, Peacock A, Rosenkranz S, Saggar R, Ulrich S, Vizza D, Vonk Noordegraaf A, Olschewski H. An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J 2017;50:1700578.. [DOI] [PubMed] [Google Scholar]
- 13. Obokata M, Reddy YN, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gorter TM, Obokata M, Reddy YNV, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J 2018;39:2825–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borlaug BA, Reddy Y. The role of the pericardium in heart failure: implications for pathophysiology and treatment. JACC Heart Fail 2019;7:574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scali MC, Cortigiani L, Simionuc A, Gregori D, Marzilli M, Picano E. Exercise-induced B-lines identify worse functional and prognostic stage in heart failure patients with depressed left ventricular ejection fraction. Eur J Heart Fail 2017;19:1468–1478. [DOI] [PubMed] [Google Scholar]
- 17. Platz E, Merz AA, Jhund PS, Vazir A, Campbell R, McMurray JJ. Dynamic changes and prognostic value of pulmonary congestion by lung ultrasound in acute and chronic heart failure: a systematic review. Eur J Heart Fail 2017;19:1154–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Picano E, Scali MC, Ciampi Q, Lichtenstein D. Lung ultrasound for the cardiologist. JACC Cardiovasc Imaging 2018;11:1692–1705. [DOI] [PubMed] [Google Scholar]
- 19. Picano E, Pellikka PA. Ultrasound of extravascular lung water: a new standard for pulmonary congestion. Eur Heart J 2016;37:2097–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jambrik Z, Monti S, Coppola V, Agricola E, Mottola G, Miniati M, Picano E. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am J Cardiol 2004;93:1265–1270. [DOI] [PubMed] [Google Scholar]
- 21. Picano E, Frassi F, Agricola E, Gligorova S, Gargani L, Mottola G. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr 2006;19:356–363. [DOI] [PubMed] [Google Scholar]
- 22. Agricola E, Bove T, Oppizzi M, Marino G, Zangrillo A, Margonato A, Picano E. “Ultrasound comet-tail images”: a marker of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest 2005;127:1690.. [DOI] [PubMed] [Google Scholar]
- 23. Gargani L, Frassi F, Soldati G, Tesorio P, Gheorghiade M, Picano E. Ultrasound lung comets for the differential diagnosis of acute cardiogenic dyspnoea: a comparison with natriuretic peptides. Eur J Heart Fail 2008;10:70–77. [DOI] [PubMed] [Google Scholar]
- 24. Miglioranza MH, Gargani L, Sant'Anna RT, Rover MM, Martins VM, Mantovani A, Weber C, Moraes MA, Feldman CJ, Kalil RA, Sicari R, Picano E, Leiria TL. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging 2013;6:1141–1151. [DOI] [PubMed] [Google Scholar]
- 25. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39 e14. [DOI] [PubMed] [Google Scholar]
- 26. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713; quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 27. Guazzi M, Dixon D, Labate V, Beussink-Nelson L, Bandera F, Cuttica MJ, Shah SJ. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging 2017;10:1211–1221. [DOI] [PubMed] [Google Scholar]
- 28. Reddy YNV, Obokata M, Egbe A, Yang JH, Pislaru S, Lin G, Carter R, Borlaug BA. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur J Heart Fail 2019;21:891.. [DOI] [PubMed] [Google Scholar]
- 29. Rowell LB. Human Cardiovascular Control. New York: Oxford University Press; 1993. [Google Scholar]
- 30. Van Iterson EH, Johnson BD, Borlaug BA, Olson TP. Physiological dead space and arterial carbon dioxide contributions to exercise ventilatory inefficiency in patients with reduced or preserved ejection fraction heart failure. Eur J Heart Fail 2017;19:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Uhley HN, Leeds SE, Sampson JJ, Friedman M. Role of pulmonary lymphatics in chronic pulmonary edema. Circ Res 1962;11:966–970. [DOI] [PubMed] [Google Scholar]
- 32. Uhley HN, Leeds SE, Sampson JJ, Friedman M. Right duct lymph flow in experimental heart failure following acute elevation of left atrial pressure. Circ Res 1967;20:306–310. [DOI] [PubMed] [Google Scholar]
- 33. Wood P. An appreciation of mitral stenosis: ii. Investigations and results. Br Med J 1954;1:1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 2014;35:3452–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mohammed SF, Hussain I, AbouEzzeddine OF, Abou Ezzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation 2014;130:2310–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Obokata M, Reddy YNV, Melenovsky V, Pislaru S, Borlaug BA. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J 2019;40:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang W, Kingsbury MP, Turner MA, Donnelly JL, Flores NA, Sheridan DJ. Capillary filtration is reduced in lungs adapted to chronic heart failure: morphological and haemodynamic correlates. Cardiovasc Res 2001;49:207–217. [DOI] [PubMed] [Google Scholar]
- 38. Dixon DL, Mayne GC, Griggs KM, De Pasquale CG, Bersten AD. Chronic elevation of pulmonary microvascular pressure in chronic heart failure reduces bi-directional pulmonary fluid flux. Eur J Heart Fail 2013;15:368–375. [DOI] [PubMed] [Google Scholar]
- 39. West JB, Mathieu-Costello O. Vulnerability of pulmonary capillaries in heart disease. Circulation 1995;92:622–631. [DOI] [PubMed] [Google Scholar]
- 40. Townsley MI, Fu Z, Mathieu-Costello O, West JB. Pulmonary microvascular permeability. Responses to high vascular pressure after induction of pacing-induced heart failure in dogs. Circ Res 1995;77:317–325. [DOI] [PubMed] [Google Scholar]
- 41. Guazzi M, Agostoni P, Bussotti M, Guazzi MD. Impeded alveolar-capillary gas transfer with saline infusion in heart failure. Hypertension 1999;34:1202–1207. [DOI] [PubMed] [Google Scholar]
- 42. Agostoni PG, Guazzi M, Bussotti M, Grazi M, Palermo P, Marenzi G. Lack of improvement of lung diffusing capacity following fluid withdrawal by ultrafiltration in chronic heart failure. J Am Coll Cardiol 2000;36:1600–1604. [DOI] [PubMed] [Google Scholar]
- 43. Borlaug BA, Melenovsky V, Koepp KE. Inhaled sodium nitrite improves rest and exercise hemodynamics in heart failure with preserved ejection fraction. Circ Res 2016;119:880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Borlaug BA, Koepp KE, Melenovsky V. Sodium nitrite improves exercise hemodynamics and ventricular performance in heart failure with preserved ejection fraction. J Am Coll Cardiol 2015;66:1672–1682. [DOI] [PubMed] [Google Scholar]
- 45. Feldman T, Mauri L, Kahwash R, Litwin S, Ricciardi MJ, van der Harst P, Penicka M, Fail PS, Kaye DM, Petrie MC, Basuray A, Hummel SL, Forde-McLean R, Nielsen CD, Lilly S, Massaro JM, Burkhoff D, Shah SJ. Transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction (REDUCE LAP-HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]): a phase 2, randomized, sham-controlled trial. Circulation 2018;137:364–375. [DOI] [PubMed] [Google Scholar]
- 46. Borlaug BA, Carter RE, Melenovsky V, De Simone CV, Gaba P, Killu A, Naksuk N, Lerman L, Asirvatham SJ. Percutaneous pericardial resection: a novel potential treatment for heart failure with preserved ejection fraction. Circ Heart Fail 2017;10:e003612.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fudim M, Ganesh A, Green C, Jones WS, Blazing MA, DeVore AD, Felker GM, Kiefer TL, Kong DF, Boortz-Marx RL, Hernandez AF, Patel MR. Splanchnic nerve block for decompensated chronic heart failure: splanchnic-HF. Eur Heart J 2018;39:4255–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Scali MC, Zagatina A, Simova I, Zhuravskaya N, Ciampi Q, Paterni M, Marzilli M, Carpeggiani C, Picano E. B-lines with lung ultrasound: the optimal scan technique at rest and during stress. Ultrasound Med Biol 2017;43:2558–2566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.