Abstract
Ischaemic heart disease (IHD) remains the leading cause of morbidity and mortality among women and men yet women are more often underdiagnosed, have a delay in diagnosis, and/or receive suboptimal treatment. An implicit gender-bias with regard to lack of recognition of sex-related differences in presentation of IHD may, in part, explain these differences in women compared with men. Indeed, existing knowledge demonstrates that angina does not commonly relate to obstructive coronary artery disease (CAD). Emerging knowledge supports an inclusive approach to chest pain symptoms in women, as well as a more thoughtful consideration of percutaneous coronary intervention for angina in stable obstructive CAD, to avoid chasing our tails. Emerging knowledge regarding the cardiac autonomic nervous system and visceral pain pathways in patients with and without obstructive CAD offers explanatory mechanisms for angina. Interdisciplinary investigation approaches that involve cardiologists, biobehavioural specialists, and anaesthesia/pain specialists to improve angina treatment should be pursued.
Keywords: Angina, Central autonomic nervous system, Microvascular
Ischaemic heart disease (IHD) remains the leading cause of morbidity and mortality among women and men.1,2 Women, however, are relatively more underdiagnosed or have a delay in diagnosis,3,4 receive suboptimal treatment,5,6 and are underrepresented proportionately to prevalence in clinical trials.7 Women are more likely to have prehospital delay in presentation after symptom onset (by ∼30 min compared to men as reported by a recent study),3,8 as well as underdiagnosis of myocardial infarction (MI) and lower priority for emergency ambulance services, while less likely to be presented to a percutaneous coronary intervention (PCI)-capable facility and receive reperfusion therapy.9–11 In 846 ST-elevation myocardial infarction (STEMI) patients with a failed prehospital diagnosis, predictors of interhospital transfer to PCI-capable centre were female gender (odds ratio: 1.58), diabetes (1.98), and prior MI (2.86).9 Women do tend to be older with more comorbidities such as diabetes, which may contribute to delays in aggressive treatment (i.e. door-to-balloon time delay of ∼16 min compared to men), and lower statin use (21.1% vs. 23%).4,5 We may be narrowing the gender gap, as in a recent cohort of 2612 patients with acute coronary syndrome, there was no sex difference in short-term mortality.12 However, a large contemporary US STEMI registry demonstrates a delayed contact-to-reperfusion time in women compared to men, which was independently related to higher female mortality rates.13 What contributes to differences that result in outcome disparities for women?
A lack of recognition of sex differences in presentation of IHD may in part explain outcome disparities in women compared to men. One study reported that women with acute MI treated by male emergency physicians had a higher mortality compared to those treated by females.14 This difference was not observed in male physicians who had at least two female physician colleagues or had treated more women, suggesting the mortality difference could be improved by training. Furthermore, we have demonstrated that a network focused on improving emergency service recognition of STEMI resulted in improved female/equivalent gender in-hospital and long-term (5-year) age-adjusted mortality, suggesting that STEMI treatment disparities and mortality in women can be improved using protocols.15 Again, these results indicate that we can improve IHD outcomes for women by physician training and uniform deployment of evidence-based guideline protocols.
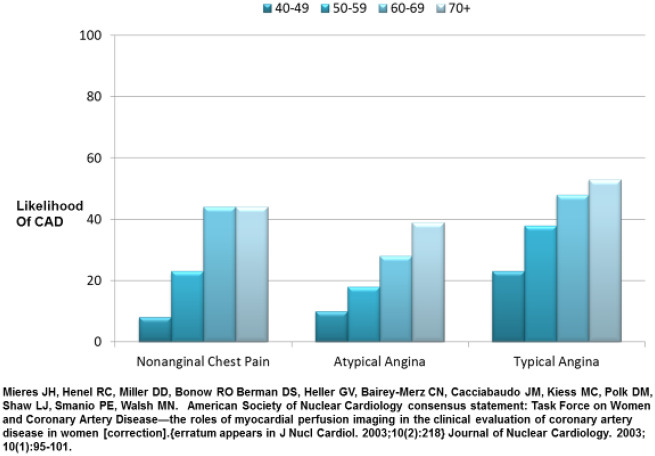
Why do these implicit IHD gender-biases exist? Historically, coronary heart disease (CHD) and coronary artery disease (CAD) were terms used to describe obstructive coronary stenosis, although over the past two decades it has become clear that IHD is a more appropriate term because not all patients with angina and ischaemia have obstructive epicardial stenoses. In particular, women are more likely to present with evidence of ischaemia, identified by objective evidence such as abnormal stress or biomarker testing, and no obstructive coronary arteries (INOCA) compared to men, although they paradoxically have more chest pain.16 Furthermore, this appears to have led to misperceptions regarding chest pain and the term angina. Early large angiographic studies17 determined that women with typical angina had less obstructive CAD compared to men, and contemporary study demonstrates that angina characterization is not diagnostic in women (Figure 1).18 While angina refers to symptoms and not the disease process itself and although angina may not be related to obstructive CAD also in men, the historical identification of IHD as either CHD or CAD has contributed to women being diagnosed with non-specific chest pain, and discharged from subspecialty care and treatment.19
Figure 1.
Angina classification and obstructive coronary artery disease in women. Most symptomatic women over 50 years are at intermediate risk (20–50%) (reprinted with permission).
How much do women and men differ with regard to chest pain symptoms relative to obstructive CAD? Prior analyses of gender-based differences of acute coronary syndrome have demonstrated varied results, but the majority of studies describe chest pain as the most frequent symptom in both genders.20,21 Angina is also the most frequently reported symptom in stable IHD in both men and women, but women more often present with atypical angina. While typical angina is characterized by retrosternal pain that is provoked by exertion and relieved by rest or nitroglycerine, atypical angina represents a more diverse symptom presentation with pain or discomfort not only in the chest but also in the arms, jaw, neck, and interscapular area.22 These symptoms do not necessarily occur at exertion, but can arise after exertion or be triggered by mental stress or even occur at rest. Symptoms may last intermittently over several hours, and atypical presentations include more vague symptoms such as fatigue, anxiety, dyspnoea, dyspepsia, and nausea.23,24
Why do relations between chest pain and obstructive CAD differ between women and men? It is now well established that myocardial ischaemia and infarction have a more diverse underlying pathophysiology in women compared to men which includes coronary microvascular dysfunction (CMD), coronary vasospasm, plaque erosions/microemboli, and spontaneous coronary artery dissection. The Women’s ischaemia Syndrome Evaluation (WISE) study demonstrated that over 50% of women with signs and symptoms suggestive of IHD lack epicardial coronary stenosis, and other studies worldwide have replicated this finding.25,26 Those with persistent angina experience higher rates of depression and anxiety with concurrent reduced functional capacity and impaired quality of life.27,28Hospitalizations and the use of recurrent diagnostic modalities to evaluate persistent and recurring angina is associated with significant healthcare costs.29
A number of findings from studies of PCI for treatment of stable angina in women and men with obstructive CAD30,31 further elaborate on this disconnect between angina and obstructive CAD. For example, in the ORBITA (Objective Randomized Blinded Investigation with optimal medical Therapy or Angioplasty in stable angina) trial,30 relief of epicardial stenosis with PCI in patients with obstructive CAD did not out-perform optimal medical therapy for relief of angina in obstructive CAD. In the FAME 2 (Fractional Flow Reserve vs. Angiography for Multivessel Evaluation) trial of open label randomization to fractional flow reserve (FFR)-guided PCI vs. medical therapy group, a large proportion of patients had an improvement in angina after being told that they had no significant obstructive lesion based on fractional flow reserve assessment.31,32 While these newer data suggest that relations between chest pain and obstructive CAD may be more nuanced that previously thought, inspection of other existing knowledge can assist with formulating investigation to address knowledge gaps in chest pain pathophysiology to improve IHD outcomes for women.
Existing Knowledge
Angina does not correlate with myocardial ischaemia
Lessons learned from silent ischaemia investigations in the ambulatory monitoring era inform us that 66% of ‘angina’ does not have evidence of myocardial ischaemia, while 85% of ambulatory ischaemia is symptomatically ‘silent’.33 Specifically, prior studies of ambulatory monitoring in women and men with angina and established obstructive CAD or a history of MI demonstrate that the majority of angina episodes do not have ST depressions on Holter monitoring.34–40 While transient ischaemia that lasts only seconds may be understandably asymptomatic, prolonged ST depressions is most often silent.41 Chest pain reports in the absence of ischaemia could not be attributed to ‘borderline’ ST-segment changes.33 Furthermore, the severity of ischaemia on stress testing does not correlate with angina severity.40,42 An important methodological limitation of the ambulatory studies is electrocardiographic detection of ischaemia, which has limited sensitivity and specificity. A recent small study which demonstrated correlation between immediate PCI-mediated improved coronary flow and angina in 21 subjects was unblinded, used an unvalidated angina measurement, as well as intra-arterial unfractionated heparin and intracoronary nitroglycerine before physiological measurement, and did not account for the well-established exercise training effect, limiting any conclusions.43
Myocardial ischaemia, including silent ischaemia, predicts an adverse cardiac prognosis,44 and silent ischaemia is considered a treatment target. There is a relationship between silent ischaemic burden and CAD status; e.g. those with multivessel obstructive CAD have more documented silent ischaemic episodes than single vessel CAD.45 Haemodynamic changes with elevations in heart rate and blood pressure preceding ST depression are documented, suggesting increased myocardial demand plays a role in silent ischaemia.46 A relevant knowledge gap is that there is no clear explanation of why silent and symptomatic ischaemia occurs in the same patient. Various mechanisms have been considered as an explanation for silent ischaemia, including (i) varying degrees of subclinical atherosclerosis in segmental myocardial territories which lead to a functional mismatch during hyperaemia (maximal vasodilator stress) or during mental stress (vasoconstrictive response), leading to perfusion defects; (ii) CMD or smooth muscle dysfunction; (iii) lack of stimulation of sub-epicardial pain fibres because ischaemic episodes are subendocardial; (iv) circadian variation in vasoconstriction; (v) high pain threshold or blunted pain perception; and (vi) increased adrenergic activity. Studies show that self-reported angina symptom severity may or may not be correlated with ischaemia burden.47,48
Angina does not correlate with obstructive coronary artery disease
Decades of research has shown that angina can occur in those with or without significant obstructive CAD, and angina also persists in many patients post-PCI. Data from multiple large studies indicate that approximately one-fifth to one-third of patients undergoing PCI have persistent or recurrent angina at follow-up.49,50 Invasive coronary reactivity testing can demonstrate coronary vasomotor abnormalities in patients with persistent angina and no obstructive CAD.51 A comprehensive coronary reactivity testing uses vasoactive agents such as adenosine, acetylcholine, and nitroglycerine to detect CMD, endothelial dysfunction, and vasospasm.15,51 In order to unify terminology and diagnostic criteria for vasomotor disorders, the Coronary Vasomotion Disorders International Study (COVADIS) Group has proposed standardized definitions and diagnostic criteria.52,53 Dipyridamole stress echo, cardiac positron emission tomography (PET), and cardiac magnetic resonance imaging (CMR) can also detect CMD. It is clear that a low coronary flow reserve (CFR) is associated with adverse prognosis in men and women.54,55 Among patients with no obstructive CAD, endothelial dysfunction measured by reduced brachial flow-mediated dilation is independently associated with ischaemia on stress imaging, but not with symptoms.56
There are sex differences in the impact of traditional risk factors on IHD risk (e.g. diabetes is associated with a higher IHD risk in women compared to men),57,58 thus potentially contributing to symptom pathophysiology. Women also have unique risk factors including higher rates of autoimmune/chronic inflammatory conditions that contribute to CMD.59,60 In women with typical and atypical angina who had systemic lupus erythematosus compared to asymptomatic controls, CMD was highly prevalent as detected by CMR.61
Angina is associated with psychological factors
Comorbid psychological factors such as anxiety and depression are highly prevalent in female and male patients with angina, and psychological stress can contribute to and exacerbate angina.62,63 Vaccarino et al.63,64 have shown that depression is associated with chest pain, regardless of obstructive CAD severity, and in women, but not in men, angina frequency (in the past month) was associated with more mental stress ischaemia (MSI).65 Although incidence studies have generally not found a gender difference in the link of psychological factors and CAD, the influence of various psychological stressors (such as anxiety, anger, hostility, social support) on triggering microvascular angina in women is understudied. While there is a poor relationship between angina and a positive conventional stress test for myocardial ischaemia, higher angina burden in everyday life is associated with myocardial ischaemia provoked by a mental stress test.65,66 Mental stress ischaemia is an important prognostic marker with an estimated two-fold increase in mortality, although it is not consistently related to obstructive CAD severity, and abnormal vascular reactivity to mental stress has been implicated.67,68 Vasoconstriction and microcirculatory dysfunction during mental stress in the peripheral circulation are also related to ischaemia with mental stress,69 a phenomenon that is more pronounced among women.70 In parallel, INOCA subjects have greater pulsatile arterial tonometry abnormality during a mental stress test.71
Angina and the autonomic nervous system
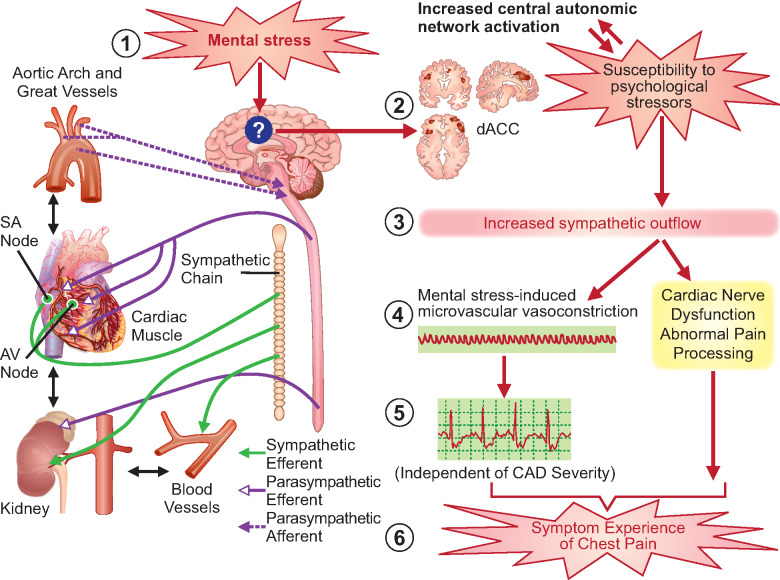
The central and peripheral autonomic nervous systems (ANS) play an important role in emotional regulation, pain processing, and cardiovascular sympathetic outflow to various organs (Take home figure). In patients previously referred to as cardiac syndrome X (CSX) in older literature, objective measures of CFR were not present, and measures of myocardial ischaemia inconsistent.72 It is now believed that a large portion of these patients who have documented ischaemia have CMD despite absence of obstructive CAD. Compared to those with obstructive CAD, abnormal cardiac nociception indicated by higher pain with contrast injection, right ventricle pacing, and adenosine infusion, as well as pain at a lower stimulus intensity was observed in patients with CSX. Whether the exaggerated pain sensitivity is due to abnormal sympathetic activation in the heart vs. abnormal ANS processing of visceral afferent signals is not known.73,74 Individual differences in pain sensitivity thresholds may also contribute to angina.75
Take home figure.
Autonomic vascular reactivity to mental stress and angina. In a patient susceptible to psychological risk factors, daily life mental stress (1) may induce a state of central autonomic network dysregulation with increased activity in the dorsal anterior cortical cingulate (2) and the associated limbic, subcortical, and medullary autonomic centres, leading to increased sympathetic outflow (3). The resultant vasoconstriction (4) has been demonstrated in coronary and peripheral circulation and leads to ischaemic changes detected by ST-segment changes on the electrocardiogram (regardless of coronary artery disease status) (5a). Increased sympathetic outflow may also disrupt anti-nociceptive pathways in some patients (5b), who are more likely to be women, leading to abnormal cardiac nociception. Both of these scenarios may lead to the symptom experience of chest pain (6).
Emerging Knowledge
Angina and visceral pain pathways
Myocardial pain signals converge with cutaneous afferent pain signals in the dorsal root ganglion, where slow conducting unmyelinated C-fibres convey dull pain, while fast conducting myelinated A-fibres conduct sharp pain.76 How these pain signals are generated and why they lead to varying degrees of often vague, visceral sensations is not understood, but in general it is thought that ischaemic myocardial pain mediators such as adenosine, substance P, and serotonin, trigger chemosensitive receptors that transmit pain via sympathetic afferents.74,77 The ANS integrates these signals with resultant sympathetic/parasympathetic modulation including, blood pressure, respiration, heart rate, and rhythm changes during ischaemia.
Increased pain sensitivity may be due to abnormal cortical processing of pain signals. Rosen et al.78,79 have reported brain activation in the hypothalamus, periaqueductal grey, thalami, the prefrontal cortex, and the left inferior anterior cingulate cortex (ACC) during angina in patients with ischaemia and obstructive CAD. In contrast, there was a failure of frontal cortex activation in silent ischaemia, although thalamic activation was similar to the angina group,78 suggesting that abnormal visceral pain processing of afferent pain signals could be contributing to silent ischaemia.79 Initial data indicate that those with MSI had greater activation in the dorsal ACC (dACC) detected by 15O-water PET compared to those with no MSI.80 The dACC has extensive connectivity to the insula, amygdala, and autonomic centres, and plays a role in emotional regulation, pain sensitivity, and autonomic cardiovascular responses. Information about relationships between dACC activation, autonomic outflow, and angina is needed.
Treating Angina—Are We Chasing Our Tails?
What do these prior and emerging findings contribute to our interpretation of contemporary understanding of chest pain and IHD in women, as well as studies of PCI for treatment of stable angina in obstructive CAD?1–3 Specifically, the lack of consistent relation between angina and myocardial ischaemia in obstructive CAD observed in prior decades helps us understand the lack of angina relief despite PCI-related improvement in myocardial ischaemia in the ORBITA trial.30 Prior research relating angina to the psychological factors of anxiety, depression, and mental stress help us interpret the FAME 2 trial findings that angina improved after patients were told that there was no significant lesion (FFR > 0.8), and angina also improved after patients received a stent to treat a significant lesion,31,32 due to anxiety-related angina perception and reporting. Thus, although current guidelines recommend PCI for persistent angina in patients with optimal anti-anginal treatment,81 it remains questionable whether PCI has a true therapeutic effect or merely a placebo effect. In the stable IHD setting, before a patient undergoes invasive coronary angiography, the possibility of recurrent symptoms post-PCI should be discussed with the patient to increase their awareness of the limitations of the procedure. We, respectively, suggest that the current controversy regarding treating angina with PCI is chasing our tails, as current data are consistent with prior knowledge, and therefore not productive. Furthermore, emerging knowledge regarding the cardiac ANS and visceral pain pathways in patients with and without obstructive CAD may offer explanatory mechanisms for these study findings.
Knowledge Gaps
While emerging data implicates mental stress, ANS, and visceral pain mechanisms for angina, a number of knowledge gaps exist. Specifically, why do women have more angina compared to men, despite paradoxically having less obstructive CAD? Are their sex differences in correlations of symptoms with objective evidence of perfusion abnormalities, including low CFR? The mechanistic pathways of how chronic stress may predispose to future development of angina, in the context of sex differences as well as social determinants of health are unclear. The contribution of ANS activation during acute mental stress with resultant haemodynamic and vascular reactivity to angina needs to be investigated with appropriate control groups. Investigation of whether visceral brain activation/deactivation patterns differ across angina and varying degrees of CAD severity, and in comparison, to asymptomatic groups with obstructive CAD, is needed. Standardized mental stress testing using mental arithmetic, anger recall, public speaking task, etc. have been used in the research realm, but have not been translated to clinical practice. Clarifying how physiologic responses to mental stress are influenced by underlying psychological risk factors (such as chronic anxiety and depression) and their contribution to angina burden will help guide novel angina treatment strategies. Blinded clinical trials that target ANS activation should be performed for angina to generate therapeutic guidelines. These mechanistic human studies would involve interdisciplinary investigation among cardiologists, biobehavioural specialists, and anaesthesia/pain specialists to improve angina treatment. An inclusive approach to management of persistent angina would include not only pharmacologic and revascularization therapies, but also lifestyle modification for risk factor control (i.e. smoking cessation, nutrition counselling, cardiac rehabilitation, stress management), along with psychological evaluation and support.
Conclusions
Ischaemic heart disease remains the leading cause of morbidity and mortality among women and men yet women are more often underdiagnosed, have a delay in diagnosis, or receive suboptimal treatment. A gender-bias with regard to lack of recognition of sex differences in presentation of IHD may in part explain these differences in women compared to men. Existing knowledge indicates that often angina does not correlate with myocardial ischaemia or obstructive CAD, and emerging knowledge supports an inclusive approach to chest pain symptoms in women, as well as a more thoughtful consideration of PCI for angina in stable obstructive CAD, to avoid chasing our tails. Emerging knowledge regarding the cardiac ANS and visceral pain pathways in patients with and without obstructive CAD offers explanatory mechanisms. Studies that targets ANS activation should be performed for the outcome of angina using interdisciplinary investigation among cardiologists, biobehavioural specialists, and anaesthesia/pain specialists to improve angina treatment.
Funding
This work was supported by K23HL105787, Emory Clinical Cardiovascular Research Institute (ECCRI), the Edythe L. Broad Women’s Heart Research Fellowship, the Constance Austin Women’s Heart Research Fellowship, the Erika J. Glazer Women’s Heart Research Initiative, and the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles.
Conflict of interest: C.N.B.M.: Sanofi, Abbott Diagnostics, iRhythm. All other authors declared no conflict of interest.
References
- 1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2. Timmis A, Townsend N, Gale C, Grobbee R, Maniadakis N, Flather M, Wilkins E, Wright L, Vos R, Bax J, Blum M, Pinto F, Vardas P, Goda A, Demiraj AF, Weidinger F, Metzler B, Ibrahimov F, Pasquet AA, Claeys M, Thorton Y, Kusljugic Z, Smajic E, Velchev V, Ivanov N, Antoniades L, Agathangelou P, Táborský M, Gerdes C, Viigima M, Juhani PM, Juilliere Y, Cattan S, Aladashvili A, Hamm C, Kuck K-H, Papoutsis K, Bestehorn K, Foussas S, Giannoulidou G, Varounis C, Kallikazaros I, Kiss RG, Czétényi T, Becker D, Gudnason T, Kearney P, McDonald K, Rozenman Y, Ziv B, Bolognese L, Luciolli P, Boriani G, Berkinbayev S, Rakisheva A, Mirrakhimov E, Erglis A, Jegere S, Marinskis G, Beissel J, Marchal N, Kedev S, Xuereb RG, Tilney T, Felice T, Popovici M, Bax J, Mulder B, Simoons M, Elsendoorn M, Steigen TK, Atar D, Kalarus Z, Tendera M, Cardoso JS, Ribeiro J, Mateus C, Tatu-Chitoiu G, Seferovic P, Beleslin B, Simkova I, Durcikova P, Belicova V, Fras Z, Radelj S, Gonzalez Juanatey JR, Legendre S, Braunschweig F, Kaufmann UP, Rudiger-Sturchler M, Tokgozoglu L, Unver A, Kovalenko V, Nesukay E, Naum A, de Courtelary PT, Martin S, Sebastiao D, Ghislain D, Bardinet I, Logstrup S; ESC Scientific Document Group. European Society of Cardiology: cardiovascular disease statistics 2017. Eur Heart J 2018;39:508–579. [DOI] [PubMed] [Google Scholar]
- 3. Bugiardini R, Ricci B, Cenko E, Vasiljevic Z, Kedev S, Davidovic G, Zdravkovic M, Miličić D, Dilic M, Manfrini O, Koller A, Badimon L. Delayed care and mortality among women and men with myocardial infarction. J Am Heart Assoc 2017;6:e005968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dreyer RP, Beltrame JF, Tavella R, Air T, Hoffmann B, Pati PK, Di Fiore D, Arstall M, Zeitz C. Evaluation of gender differences in door-to-balloon time in ST-elevation myocardial infarction. Heart Lung Circ 2013;22:861–869. [DOI] [PubMed] [Google Scholar]
- 5. Virani SS, Woodard LD, Ramsey DJ, Urech TH, Akeroyd JM, Shah T, Deswal A, Bozkurt B, Ballantyne CM, Petersen LA. Gender disparities in evidence-based statin therapy in patients with cardiovascular disease. Am J Cardiol 2015;115:21–26. [DOI] [PubMed] [Google Scholar]
- 6. Mosca L, Linfante AH, Benjamin EJ, Berra K, Hayes SN, Walsh BW, Fabunmi RP, Kwan J, Mills T, Simpson SL. National study of physician awareness and adherence to cardiovascular disease prevention guidelines. Circulation 2005;111:499–510. [DOI] [PubMed] [Google Scholar]
- 7. Scott PE, Unger EF, Jenkins MR, Southworth MR, McDowell TY, Geller RJ, Elahi M, Temple RJ, Woodcock J. Participation of women in clinical trials supporting FDA approval of cardiovascular drugs. J Am Coll Cardiol 2018;71:1960–1969. [DOI] [PubMed] [Google Scholar]
- 8. Shahin M, Obeid S, Hamed L, Templin C, Gamperli O, Nietlispach F, Maier W, Yousif N, Mach F, Roffi M, Windecker S, Raber L, Matter CM, Luscher TF. Occurrence and impact of time delay to primary percutaneous coronary intervention in patients with ST-Segment elevation myocardial infarction. Cardiol Res 2017;8:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mahmoud KD, Gu YL, Nijsten MW, de Vos R, Nieuwland W, Zijlstra F, Hillege HL, van der Horst IC, de Smet BJ. Interhospital transfer due to failed prehospital diagnosis for primary percutaneous coronary intervention: an observational study on incidence, predictors, and clinical impact. Eur Heart J Acute Cardiovasc Care 2013;2:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Melberg T, Kindervaag B, Rosland J. Gender-specific ambulance priority and delays to primary percutaneous coronary intervention: a consequence of the patients' presentation or the management at the emergency medical communications center? Am Heart J 2013;166:839–845. [DOI] [PubMed] [Google Scholar]
- 11. D'Onofrio G, Safdar B, Lichtman JH, Strait KM, Dreyer RP, Geda M, Spertus JA, Krumholz HM. Sex differences in reperfusion in young patients with ST-segment-elevation myocardial infarction: results from the VIRGO study. Circulation 2015;131:1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghadri JR, Sarcon A, Jaguszewski M, Diekmann J, Bataiosu RD, Hellermann J, Csordas A, Baumann L, Schoni AA, Luscher TF, Templin C. Gender disparities in acute coronary syndrome: a closing gap in the short-term outcome. J Cardiovasc Med (Hagerstown) 2015;16:355–362. [DOI] [PubMed] [Google Scholar]
- 13. Roswell RO, Kunkes J, Chen AY, Chiswell K, Iqbal S, Roe MT, Bangalore S. Impact of sex and contact-to-device time on clinical outcomes in acute ST-segment elevation myocardial infarction-findings from the national cardiovascular data registry. J Am Heart Assoc 2017;6:e004521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greenwood BN, Carnahan S, Huang L. Patient–physician gender concordance and increased mortality among female heart attack patients. Proc Natl Acad Sci USA 2018;115:8569.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wei J, Mehta PK, Grey E, Garberich RF, Hauser R, Bairey Merz CN, Henry TD. Sex-based differences in quality of care and outcomes in a health system using a standardized STEMI protocol. Am Heart J 2017;191:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Lerman A, Quyyumi AA, Sopko G. Insights from the NHLBI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol 2006;47(3 Suppl): S21–S29. [DOI] [PubMed] [Google Scholar]
- 17. Chaitman BR, Bourassa MG, Davis K, Rogers WJ, Tyras DH, Berger R, Kennedy JW, Fisher L, Judkins MP, Mock MB, Killip T. Angiographic prevalence of high-risk coronary artery disease in patient subsets (CASS). Circulation 1981;64:360–367. [DOI] [PubMed] [Google Scholar]
- 18. Diamond GA. A clinically relevant classification of chest discomfort. J Am Coll Cardiol 1983;1:574–575. [DOI] [PubMed] [Google Scholar]
- 19. Pacheco Claudio C, Quesada O, Pepine CJ, Noel Bairey Merz C. Why names matter for women: MINOCA/INOCA (myocardial infarction/ischemia and no obstructive coronary artery disease). Clin Cardiol 2018;41:185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Araújo C, Laszczyńska O, Viana M, Melão F, Henriques A, Borges A, Severo M, Maciel MJ, Moreira I, Azevedo A. Sex differences in presenting symptoms of acute coronary syndrome: the EPIHeart cohort study. BMJ Open 2018;8:e018798.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arslanian-Engoren C, Patel A, Fang J, Armstrong D, Kline-Rogers E, Duvernoy CS, Eagle KA. Symptoms of men and women presenting with acute coronary syndromes. Am J Cardiol 2006;98:1177–1181. [DOI] [PubMed] [Google Scholar]
- 22. Wenger NK. Clinical presentation of CAD and myocardial ischemia in women. J Nucl Cardiol 2016;23:976–985. [DOI] [PubMed] [Google Scholar]
- 23. Lichtman JH, Leifheit-Limson EC, Watanabe E, Allen NB, Garavalia B, Garavalia LS, Spertus JA, Krumholz HM, Curry LA. Symptom recognition and healthcare experiences of young women with acute myocardial infarction. Circ Cardiovasc Qual Outcomes 2015;8(2 Suppl 1): S31–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nugent L, Mehta PK, Bairey Merz CN. Gender and microvascular angina. J Thromb Thrombolysis 2011;31:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sharaf BL, Pepine CJ, Kerensky RA, Reis SE, Reichek N, Rogers WJ, Sopko G, Kelsey SF, Holubkov R, Olson M, Miele NJ, Williams DO, Merz CN, Group WS. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women's Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory). Am J Cardiol 2001;87:937–941; A933. [DOI] [PubMed] [Google Scholar]
- 26. Merz CN, Kelsey SF, Pepine CJ, Reichek N, Reis SE, Rogers WJ, Sharaf BL, Sopko G. The Women's Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol 1999;33:1453–1461. [DOI] [PubMed] [Google Scholar]
- 27. Handberg EM, Eastwood JA, Eteiba W, Johnson BD, Krantz DS, Thompson DV, Vaccarino V, Bittner V, Sopko G, Pepine CJ, Merz NB, Rutledge TR. Clinical implications of the Women's Ischemia Syndrome Evaluation: inter-relationships between symptoms, psychosocial factors and cardiovascular outcomes. Womens Health (Lond) 2013;9:479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Szpakowski N, Bennell M, Qiu F, Ko D, Tu J, Kurdyak P, Wijeysundera H. Clinical impact of subsequent depression in patients with a new diagnosis of stable angina: a population-based study. Circ Cardiovasc Qual Outcomes 2016;9:731–739. [DOI] [PubMed] [Google Scholar]
- 29. Shaw L, Merz N, Pepine C, Reis S, Bittner V, Kip K, Kelsey S, Olson M, Johnson D, Mankad S, Sharaf B, Rogers W, Pohost G, Sopko G. The economic burden of angina in women with suspected ischemic heart disease. Circulation 2006;114:894–904. [DOI] [PubMed] [Google Scholar]
- 30. Al-Lamee R, Thompson D, Dehbi HM, Sen S, Tang K, Davies J, Keeble T, Mielewczik M, Kaprielian R, Malik IS, Nijjer SS, Petraco R, Cook C, Ahmad Y, Howard J, Baker C, Sharp A, Gerber R, Talwar S, Assomull R, Mayet J, Wensel R, Collier D, Shun-Shin M, Thom SA, Davies JE, Francis DP; ORBITA Investigators. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet 2018;391:31–40. [DOI] [PubMed] [Google Scholar]
- 31. Rajkumar CA, Nijjer SS, Cole GD, Al-Lamee R, Francis DP. Moving the goalposts into unblinded territory: the larger lessons of DEFER and FAME 2 and their implications for shifting end points in ISCHEMIA. Circ Cardiovasc Qual Outcomes 2018;11:e004665.. [DOI] [PubMed] [Google Scholar]
- 32. De Bruyne B, Pijls NHJ, Kalesan B, Barbato E, Tonino PAL, Piroth Z, Jagic N, Möbius-Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Jüni P, Fearon WF; FAME 2 Trial Investigators. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012;367:991–1001. [DOI] [PubMed] [Google Scholar]
- 33. Krantz DS, Hedges SM, Gabbay FH, Klein J, Falconer JJ, Merz CN, Gottdiener JS, Lutz H, Rozanski A. Triggers of angina and ST-segment depression in ambulatory patients with coronary artery disease: evidence for an uncoupling of angina and ischemia. Am Heart J 1994;128:703–712. [DOI] [PubMed] [Google Scholar]
- 34. Pepine CJ. Does the brain know when the heart is ischemic? Ann Intern Med 1996;124:1006–1008. [DOI] [PubMed] [Google Scholar]
- 35. Schang SJ Jr, Pepine CJ. Transient asymptomatic S-T segment depression during daily activity. Am J Cardiol 1977;39:396–402. [DOI] [PubMed] [Google Scholar]
- 36. Gottlieb SO, Gottlieb SH, Achuff SC, Baumgardner R, Mellits ED, Weisfeldt ML, Gerstenblith G. Silent ischemia on Holter monitoring predicts mortality in high-risk postinfarction patients. JAMA 1988;259:1030–1035. [PubMed] [Google Scholar]
- 37. Aronow WS, Epstein S. Usefulness of silent myocardial ischemia detected by ambulatory electrocardiographic monitoring in predicting new coronary events in elderly patients. Am J Cardiol 1988;62:1295–1296. [DOI] [PubMed] [Google Scholar]
- 38. Langer A, Freeman MR, Armstrong PW. ST segment shift in unstable angina: pathophysiology and association with coronary anatomy and hospital outcome. J Am Coll Cardiol 1989;13:1495–1502. [DOI] [PubMed] [Google Scholar]
- 39. Bugiardini R, Borghi A, Pozzati A, Ruggeri A, Puddu P, Maseri A. Relation of severity of symptoms to transient myocardial ischemia and prognosis in unstable angina. J Am Coll Cardiol 1995;25:597–604. [DOI] [PubMed] [Google Scholar]
- 40. Quyyumi AA, Mockus L, Wright C, Fox KM. Morphology of ambulatory ST segment changes in patients with varying severity of coronary artery disease. Investigation of the frequency of nocturnal ischaemia and coronary spasm. Br Heart J 1985;53:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Deedwania PC, Carbajal EV. Silent ischemia during daily life is an independent predictor of mortality in stable angina. Circulation 1990;81:748–756. [DOI] [PubMed] [Google Scholar]
- 42. Quyyumi A. What causes silent myocardial ischemia? Postgrad Med 1989;86:62–75. [DOI] [PubMed] [Google Scholar]
- 43. Cook CM, Ahmad Y, Howard JP, Shun-Shin MJ, Sethi A, Clesham GJ, Tang KH, Nijjer SS, Kelly PA, Davies JR, Malik IS, Kaprielian R, Mikhail G, Petraco R, Al-Janabi F, Karamasis GV, Mohdnazri S, Gamma R, Al-Lamee R, Keeble TR, Mayet J, Sen S, Francis DP, Davies JE. Impact of percutaneous revascularization on exercise hemodynamics in patients with stable coronary disease. J Am Coll Cardiol 2018;72:970–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weiner DA, Ryan TJ, McCabe CH, Ng G, Chaitman BR, Sheffield LT, Tristani FE, Fisher LD. Risk of developing an acute myocardial infarction or sudden coronary death in patients with exercise-induced silent myocardial ischemia. A report from the Coronary Artery Surgery Study (CASS) registry. Am J Cardiol 1988;62:1155–1158. [DOI] [PubMed] [Google Scholar]
- 45. Mulcahy D, Keegan J, Crean P, Quyyumi A, Shapiro L, Wright C, Fox K. Silent myocardial ischaemia in chronic stable angina: a study of its frequency and characteristics in 150 patients. Br Heart J 1988;60:417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Deedwania PC, Nelson JR. Pathophysiology of silent myocardial ischemia during daily life. Hemodynamic evaluation by simultaneous electrocardiographic and blood pressure monitoring. Circulation 1990;82:1296–1304. [DOI] [PubMed] [Google Scholar]
- 47. Nakanishi R, Rana JS, Rozanski A, Cheng VY, Gransar H, Thomson LE, Miranda-Peats R, Hayes SW, Friedman JD, Berman DS, Min JK. Relationship of dyspnea vs. typical angina to coronary artery disease severity, burden, composition and location on coronary CT angiography. Atherosclerosis 2013;230:61–66. [DOI] [PubMed] [Google Scholar]
- 48. Steg PG, Greenlaw N, Tendera M, Tardif JC, Ferrari R, Al-Zaibag M, Dorian P, Hu D, Shalnova S, Sokn FJ, Ford I, Fox KM; Prospective Observational Longitudinal Registry of Patients With Stable Coronary Artery Disease Investigators. Prevalence of anginal symptoms and myocardial ischemia and their effect on clinical outcomes in outpatients with stable coronary artery disease: data from the International Observational CLARIFY Registry. JAMA Intern Med 2014;174:1651–1659. [DOI] [PubMed] [Google Scholar]
- 49. Marzilli M, Merz CN, Boden WE, Bonow RO, Capozza PG, Chilian WM, DeMaria AN, Guarini G, Huqi A, Morrone D, Patel MR, Weintraub WS. Obstructive coronary atherosclerosis and ischemic heart disease: an elusive link! J Am Coll Cardiol 2012;60:951–956. [DOI] [PubMed] [Google Scholar]
- 50. Venkitachalam L, Kip KE, Mulukutla SR, Selzer F, Laskey W, Slater J, Cohen HA, Wilensky RL, Williams DO, Marroquin OC, Sutton-Tyrrell K, Bunker CH, Kelsey SF. Investigators NH-SDR. Temporal trends in patient-reported angina at 1 year after percutaneous coronary revascularization in the stent era: a report from the National Heart, Lung, and Blood Institute-sponsored 1997-2006 dynamic registry. Circ Cardiovasc Qual Outcomes 2009;2:607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wei J, Mehta PK, Johnson BD, Samuels B, Kar S, Anderson RD, Azarbal B, Petersen J, Sharaf B, Handberg E, Shufelt C, Kothawade K, Sopko G, Lerman A, Shaw L, Kelsey SF, Pepine CJ, Merz CN. Safety of coronary reactivity testing in women with no obstructive coronary artery disease: results from the NHLBI-sponsored WISE (Women's Ischemia Syndrome Evaluation) study. JACC Cardiovasc Interv 2012;5:646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ong P, Camici PG, Beltrame JF, Crea F, Shimokawa H, Sechtem U, Kaski JC, Bairey Merz CN; Coronary Vasomotion Disorders International Study Group . International standardization of diagnostic criteria for microvascular angina. Int J Cardiol 2018;250:16–20. [DOI] [PubMed] [Google Scholar]
- 53. Beltrame JF, Crea F, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Bairey Merz CN; Coronary Vasomotion Disorders International Study Group . International standardization of diagnostic criteria for vasospastic angina. Eur Heart J 2017;38:2565–2568. [DOI] [PubMed] [Google Scholar]
- 54. Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014;129:2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol 2010;55:2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kulshreshtha A, Zheng Y, Quyyumi AA, Veledar E, Votaw J, Uphoff I, Bremner DJ, Goldberg J, Vaccarino V. Endothelial dysfunction is associated with occult coronary artery disease detected by positron emission tomography. IJC Metab Endocr 2014;4:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mehta PK, Wei J, Wenger NK. Ischemic heart disease in women: a focus on risk factors. Trends Cardiovasc Med 2015;25:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wenger NK. Coronary heart disease in women. The high prevalance of coronary risk factors and the importance of prevention. J Med Assoc Ga 1995;84:323–328. [PubMed] [Google Scholar]
- 59. Faccini A, Kaski JC, Camici PG. Coronary microvascular dysfunction in chronic inflammatory rheumatoid diseases. Eur Heart J 2016;37:1799–1806. [DOI] [PubMed] [Google Scholar]
- 60. Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J 2009;30:1837–1843. [DOI] [PubMed] [Google Scholar]
- 61. Ishimori ML, Martin R, Berman DS, Goykhman P, Shaw LJ, Shufelt C, Slomka PJ, Thomson LE, Schapira J, Yang Y, Wallace DJ, Weisman MH, Bairey Merz CN. Myocardial ischemia in the absence of obstructive coronary artery disease in systemic lupus erythematosus. JACC Cardiovasc Imaging 2011;4:27–33. [DOI] [PubMed] [Google Scholar]
- 62. Arnold SV, Spertus JA, Ciechanowski PS, Soine LA, Jordan-Keith K, Caldwell JH, Sullivan MD. Psychosocial modulators of angina response to myocardial ischemia. Circulation 2009;120:126–133. [DOI] [PubMed] [Google Scholar]
- 63. Hayek SS, Ko YA, Awad M, Del Mar Soto A, Ahmed H, Patel K, Yuan M, Maddox S, Gray B, Hajjari J, Sperling L, Shah A, Vaccarino V, Quyyumi AA. Depression and chest pain in patients with coronary artery disease. Int J Cardiol 2017;230:420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wei J, Pimple P, Shah AJ, Rooks C, Bremner JD, Nye JA, Ibeanu I, Murrah N, Shallenberger L, Raggi P, Vaccarino V. Depressive symptoms are associated with mental stress-induced myocardial ischemia after acute myocardial infarction. PLoS One 2014;9:e102986.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pimple P, Hammadah M, Wilmot K, Ramadan R, Al Mheid I, Levantsevych O, Sullivan S, Garcia EV, Nye J, Shah AJ, Ward L, Mehta P, Raggi P, Bremner JD, Quyyumi AA, Vaccarino V. Chest pain and mental stress-induced myocardial ischemia: sex differences. Am J Med 2018;131:540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Pimple P, Shah AJ, Rooks C, Douglas Bremner J, Nye J, Ibeanu I, Raggi P, Vaccarino V. Angina and mental stress-induced myocardial ischemia. J Psychosom Res 2015;78:433–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vaccarino V, Sullivan S, Hammadah M, Wilmot K, Al Mheid I, Ramadan R, Elon L, Pimple PM, Garcia EV, Nye J, Shah AJ, Alkhoder A, Levantsevych O, Gay H, Obideen M, Huang M, Lewis TT, Bremner JD, Quyyumi AA, Raggi P. Mental stress-induced-myocardial ischemia in young patients with recent myocardial infarction: sex differences and mechanisms. Circulation 2018;137:794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vaccarino V, Wilmot K, Al Mheid I, Ramadan R, Pimple P, Shah AJ, Garcia EV, Nye J, Ward L, Hammadah M, Kutner M, Long Q, Bremner JD, Esteves F, Raggi P, Quyyumi AA. Sex differences in mental stress-induced myocardial ischemia in patients with coronary heart disease. J Am Heart Assoc 2016;5:e003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hammadah M, Alkhoder A, Al Mheid I, Wilmot K, Isakadze N, Abdulhadi N, Chou D, Obideen M, O'Neal WT, Sullivan S, Tahhan AS, Kelli HM, Ramadan R, Pimple P, Sandesara P, Shah AJ, Ward L, Ko YA, Sun Y, Uphoff I, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Sheps DS, Raggi P, Vaccarino V, Quyyumi AA. Hemodynamic, catecholamine, vasomotor and vascular responses: determinants of myocardial ischemia during mental stress. Int J Cardiol 2017;243:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sullivan S, Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Alkhoder A, Isakadze N, Shah A, Levantsevych O, Pimple PM, Kutner M, Ward L, Garcia EV, Nye J, Mehta PK, Lewis TT, Bremner JD, Raggi P, Quyyumi AA, Vaccarino V. Sex differences in hemodynamic and microvascular mechanisms of myocardial ischemia induced by mental stress. Arterioscler Thromb Vasc Biol 2018;38:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mehta PK, Hermel M, Nelson MD, Cook-Wiens G, Martin EA, Alkhoder AA, Wei J, Minissian M, Shufelt CL, Marpuri S, Hermel D, Shah A, Irwin MR, Krantz DS, Lerman A, Noel Bairey Merz C. Mental stress peripheral vascular reactivity is elevated in women with coronary vascular dysfunction: results from the NHLBI-sponsored Cardiac Autonomic Nervous System (CANS) study. Int J Cardiol 2018;251:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cannon RO 3rd. Microvascular angina and the continuing dilemma of chest pain with normal coronary angiograms. J Am Coll Cardiol 2009;54:877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cannon RO 3rd, Quyyumi AA, Schenke WH, Fananapazir L, Tucker EE, Gaughan AM, Gracely RH, Cattau EL Jr, Epstein SE. Abnormal cardiac sensitivity in patients with chest pain and normal coronary arteries. J Am Coll Cardiol 1990;16:1359–1366. [DOI] [PubMed] [Google Scholar]
- 74. Leach A, Fisher M. Myocardial ischaemia and cardiac pain—a mysterious relationship. Br J Pain 2013;7:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bekkouche NS, Wawrzyniak AJ, Whittaker KS, Ketterer MW, Krantz DS. Psychological and physiological predictors of angina during exercise-induced ischemia in patients with coronary artery disease. Psychosom Med 2013;75:413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rosen SD. From heart to brain: the genesis and processing of cardiac pain. Can J Cardiol 2012;28(2 Suppl):S7–S19. [DOI] [PubMed] [Google Scholar]
- 77. Bertolet BD, Belardinelli L, Franco EA, Nichols WW, Kerensky RA, Hill JA. Selective attenuation by N-0861 (N6-endonorboran-2-yl-9-methyladenine) of cardiac A1 adenosine receptor-mediated effects in humans. Circulation 1996;93:1871–1876. [DOI] [PubMed] [Google Scholar]
- 78. Rosen SD, Camici PG. The brain-heart axis in the perception of cardiac pain: the elusive link between ischaemia and pain. Ann Med 2000;32:350–364. [DOI] [PubMed] [Google Scholar]
- 79. Rosen SD, Paulesu E, Nihoyannopoulos P, Tousoulis D, Frackowiak RS, Frith CD, Jones T, Camici PG. Silent ischemia as a central problem: regional brain activation compared in silent and painful myocardial ischemia. Ann Intern Med 1996;124:939–949. [DOI] [PubMed] [Google Scholar]
- 80. Bremner JD, Campanella C, Khan Z, Shah M, Hammadah M, Wilmot K, Al Mheid I, Lima BB, Garcia EV, Nye J, Ward L, Kutner MH, Raggi P, Pearce BD, Shah AJ, Quyyumi AA, Vaccarino V. Brain correlates of mental stress-induced myocardial ischemia. Psychosom Med 2018;80:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kolh P, Windecker S. ESC/EACTS myocardial revascularization guidelines 2014. Eur Heart J 2014;35:3235–3236. [DOI] [PubMed] [Google Scholar]