Discordant or concordant alcohol consumption between partners does not affect established pair bonds in female prairie voles. However, alcohol consumption does affect neural activity in several brain regions independently of the drinking status of the female’s partner.
Abstract
Aims
Discordant heavy alcohol use is a risk factor for disruption of intimate partner relationships. Modeling these relationships in prairie voles indicates that biological effects of alcohol can contribute to this risk. In particular, alcohol consumption disrupted an established preference for a female partner in male prairie voles if the partner was drinking water, but not if the partner was drinking alcohol. The current study investigated the effects of alcohol consumption on pair bonds in female prairie voles.
Methods
Female and male prairie voles established pair bonds during 1 week of cohabitation. Following cohabitation, females and their partners were put into mesh-divided cages where they were given access to 10% ethanol and water or only water for 1 week. Pair bonds in female prairie voles were tested using the partner preference test (PPT). Following the PPT, we examined oxytocin, vasopressin and FosB immunoreactivity across several brain regions.
Results
Female prairie voles consumed more alcohol if their male partner was also drinking alcohol, but not if their partner was drinking water. During PPT, females preferred their partner over a stranger, regardless of their partner’s drinking status. Alcohol consumption decreased oxytocin immunoreactivity in the paraventricular nucleus of the hypothalamus and increased FosB immunoreactivity in the centrally projecting Edinger–Westphal nucleus.
Conclusions
Established partner preference in female prairie voles is resistant to alcohol consumption. This finding suggests that the risk for disruption of intimate partner relationships in females is not mediated by a decreased motivation to be with their partners.
INTRODUCTION
Fictional literature and art, as well as human epidemiological research, abound with examples of interactions between excessive alcohol consumption and intimate partner relationships. The epidemiological research confirms a significant association between alcohol abuse and disruptions in these relationships. Alcohol and drug abuse has been shown to be the third most cited reason why couples get divorced in the United States (Amato and Previti, 2003). Heavy alcohol use specifically has been known to lead to higher rates of separation, relationship dissatisfaction and intimate partner violence (Leonard and Senchak, 1993; McLeod, 1993; Caces et al., 1999; Leonard and Quigley, 1999; Leonard and Rothbard, 1999; Collins et al., 2007)
The majority of studies on this subject have explored alcohol consumption in one partner without taking into account the amount of alcohol the other partner consumes. A very limited number of studies have explored the potential difference in effects of concordant and discordant heavy alcohol consumption on intimate relationships. Thus, couples in which one spouse drinks heavily, but the other one does not, are found to be unstable. On the other hand, couples with high alcohol drinking in both spouses are often as stable as abstinent couples and significantly more stable than couples in which only one spouse drinks (Marshal, 2003; Ostermann et al., 2005; Torvik et al., 2013; Leonard et al., 2014). Other studies have shown higher rates of separation and higher rates of marital dissatisfaction in heterosexual (Mudar et al., 2001; Homish and Leonard, 2007; Homish et al., 2009) and homosexual couples (Kelley et al., 2015) when there is a discrepancy in alcohol consumption between partners. Several factors could potentially contribute to these statistics, including neurobiological effects of alcohol, human-specific socioeconomic factors and psychological factors. Several studies indicate that while socioeconomic and psychological factors (i.e. depression and antisocial personality disorder) can impact marital stability, they do not modulate alcohol’s effects on this measure (Kenkel et al., 1994; Leonard et al., 2014). In addition, this idea contradicts greater stability of couples with two heavy drinking spouses versus couples with only one heavy drinker. Therefore, biological factors contributing to alcohol’s effects on stability of intimate partner relationships need to be examined.
It is difficult to establish causal relations between factors using only epidemiological studies. Human imaging studies have not yet been performed to evaluate effects of discordant drinking on neural activity. In contrast, animal models are invaluable in understanding neurobiological mechanisms underlying the causal effects of alcohol on physiology and behavior. Unfortunately, modeling human social relationships is difficult in traditional laboratory animals because mice and rats are not socially monogamous. Recently much progress in understanding the neurobiology of social relationships has been made by studying prairie voles (Microtus ochrogaster), a socially monogamous species. Prairie voles form life-long pair bonds with opposite-sex (Carter and Getz, 1993) and same-sex (DeVries et al., 1997) partners. In the laboratory, pair bonding can be investigated using the partner preference test (PPT). Studies testing partner preference in laboratory-housed prairie voles have identified several mechanisms regulating such bonds, including the causal contribution of central oxytocin and vasopressin systems (Insel and Hulihan, 1995; Pitkow et al., 2001). Subsequent studies confirmed the importance of these systems for human relationships thereby indicating the translational value of studies in prairie voles (Ebstein et al., 2009; Heinrichs et al., 2009; Meyer-Lindenberg et al., 2011).
Based on this translational perspective, previous studies have explored the effects of drugs of abuse on social relationships in prairie voles. In addition to being a translational model for life-long social attachments, prairie voles express preference for alcohol-containing solutions and can voluntarily consume high levels of alcohol without sucrose-fading procedures (Anacker et al., 2011a). Social factors have been shown to influence alcohol consumption in prairie voles, allowing to model social facilitation and inhibition of drinking, as well as effects of social hierarchies on drinking, and subsequently studying neural substrates of these effects (Anacker et al., 2011b, 2014b; Hostetler et al., 2012; Hostetler and Ryabinin, 2014).
More recent studies in prairie voles transitioned to investigating the effects of alcohol consumption on social attachments. Anacker et al. (2014a) explored how alcohol consumption affects the formation of pair bonds in male and female partners. Opposite-sex prairie voles received access to alcohol and water (or only water) during a 24-h cohabitation period. Pair bond formation was accessed using the PPT. Males exposed to alcohol displayed no preference for their partner over stranger females compared to control males who displayed a partner preference. Interestingly, females exposed to alcohol displayed a facilitation in partner preference compared to the female control group. Thus, alcohol consumption has sex-dependent effects on pair bond formation in prairie voles.
While Anacker et al. (2014a) confirmed the existence of biological mechanisms of alcohol’s effects on the formation of social bonds, they did not address effects of alcohol on maintenance of such bonds. Importantly, although less investigated, maintenance of social attachments in prairie voles includes additional mechanisms besides those involved in the formation of social bonds (Aragona et al., 2006; Resendez and Aragona, 2012). Therefore, a second study explored the effects of alcohol on established pair bonds in male prairie voles. Briefly, Walcott and Ryabinin (2017) introduced male and female prairie voles into a standard housing cage for one week. Following the 1 week of cohabitation, pairs were placed into semi-social housing cages with a mesh divider down the center of the cage. Within these mesh-divided cages, animals were introduced to 10% EtOH and water or continued to drink water for 1 week. In these experiments, when only the male, but not the female, consumed alcohol, male prairie voles showed a decrease in partner preference. On the other hand, when both the male and the female concordantly consumed alcohol, male prairie voles showed intact partner preference. This finding indicated that the association between discordant drinking in males observed in human epidemiological studies could be due to the existence of biological effects of alcohol on mechanisms regulating pair bond maintenance. Subsequent experiments identified that alcohol decreased oxytocin and increased FosB immunoreactivity in several brain regions irrespective of the partner’s drinking. However, there was an increase in FosB immunoreactivity in the periaqueductal gray only after discordant drinking in male prairie voles, suggesting involvement of this brain region in the effects of discordant alcohol drinking on pair bond maintenance.
Walcott and Ryabinin (2017) explored the effects of discordant alcohol consumption between partners on established pair bonds in male prairie voles. However, in humans the highest rate of separation is observed when a female partner is the heavy drinker and the male partner is an abstainer (Ostermann et al., 2005; Leonard et al., 2014). On the other hand, the occurrence of such couples is less frequent than those where the male is the heavy drinker and the female is an abstainer. To clarify the effects of alcohol on pair bond maintenance, it would be valuable to model heavy drinking in females and abstinence in males using animal models. Therefore, in the current study, we explored the effects of discordant alcohol consumption between partners on established pair bonds in female prairie voles. Following the behavioral analysis, we also analyzed levels of oxytocin, vasopressin and the neuronal activity marker FosB in our experimental subjects to assess the neurobiological effects of alcohol.
MATERIALS AND METHODS
Animals
Female adult prairie voles 66–109 days old at the beginning of the experiment were used from our breeding colony at the VA Portland Health Care System (VAPORHCS) Veterinary Medical Unit. The animals were weaned at 21 days of age and housed with same-sex siblings in cages (27×27×13 cm) on a 14:10 light/dark cycle with lights on at 6 a.m. All animals had access to cotton nestlets and ad libitum access to water and food. Prairie vole diet consist of a mixture of rabbit chow (LabDiet Hi-Fiber Rabbit; PMI Nutrition International, Richmond, IN), corn (Nutrena Cleaned Grains; Cargill, Inc., Minneapolis, MN) and oats (Grainland Select Grains; Grainland Cooperative, Eureka, IL) throughout the entire experiment. Prairie voles are inducible ovulators and do not cycle prior to a prolonged exposure to males. Therefore, virgin females were housed in a room separate from the rest of the colony. All experiments were performed under the approval of the Institutional Animal Care and Use Committees at VAPORHCS and Oregon Health & Science University (OHSU), Portland, Oregon, USA.
Housing conditions
Female subjects were housed with male partners in a standard housing cage for one week to establish a pair bond. Immediately following this week, female subjects were placed in a mesh-divided cage (27×27×13 cm) for 1 week with their opposite-sex partners, with each animal on each side of the divider. These cages have been described previously (Walcott and Ryabinin, 2017). These cages prevent animals from mating, but allow the transfer of visual, olfactory and tactile cues between animals and allow the experimenter to measure the individual amount of fluid consumed by each animal. Previous studies have shown that these cages do not disrupt established pair bonds (Curtis, 2010).
Two-bottle choice
Following the 1 week in the standard housing cages, pairs experienced a two-bottle choice paradigm in the mesh-divided cages. All animals received continuous access to two 25 mL glass tubes fitted with rubber stoppers with metal sippers attached. Fluid consumption was measured every 24 h and the location of the bottles was switched each day to prevent side bias. There were three drinking conditions in this study: (a) both female and male partners received access to one bottle of 10% EtOH and one bottle of H2O (both EtOH, n = 7); (b) female subject received access to one bottle of 10% and one bottle of H2O, while the male partner received access to two bottles of H2O (female EtOH only, n = 6); (c) both female subject and male partner received access to two bottles of H2O (Control, n = 7). Stranger males, who were later used in the PPT, received the same treatment as their male partner counterpart in a separate room.
Partner preference test
The partner preference test (PPT) is the standard way to measure pair bonds in voles. PPT has been described previously (Williams et al., 1992; Ahern et al., 2009). Briefly, it occurs in a three-chambered apparatus with the partner animal tethered to one chamber, while a stranger animal is tethered to the other. The subject animal is placed in the center (neutral) chamber and is allowed to move freely through the three chambers. The 3-h PPT occurred immediately following the two-bottle choice paradigm and was videotaped for later behavioral analysis. An experimenter who was blind to the group assignments and trained in detecting huddling behavior analyzed recorded videos. VLC Media Player (Boston, MA, USA) was used to view the recorded videos. Behavioral analysis software, JWatcher V1.0 (http://www.jwatcher.ucla.edu/), was used to measure the amount of time each animal spent huddling with the partner or stranger stimulus at a 5x playback speed.
For other Methods, see online Supplementary material.
RESULTS
Female alcohol consumption and preference are affected by the drinking status of male partners
To determine if a discrepancy in alcohol drinking between partners can lead to a decrease in partner preference in female prairie voles, we exposed prairie voles to three treatment conditions: (a) female and male partners both received 10% EtOH and water during the two-bottle choice paradigm (both EtOH); (b) females received 10% EtOH and water, while male partners received two bottles of water during the two-bottle choice paradigm (female only EtOH); (c) female and male partners both received two bottles of water during the two-bottle choice paradigm (Control).
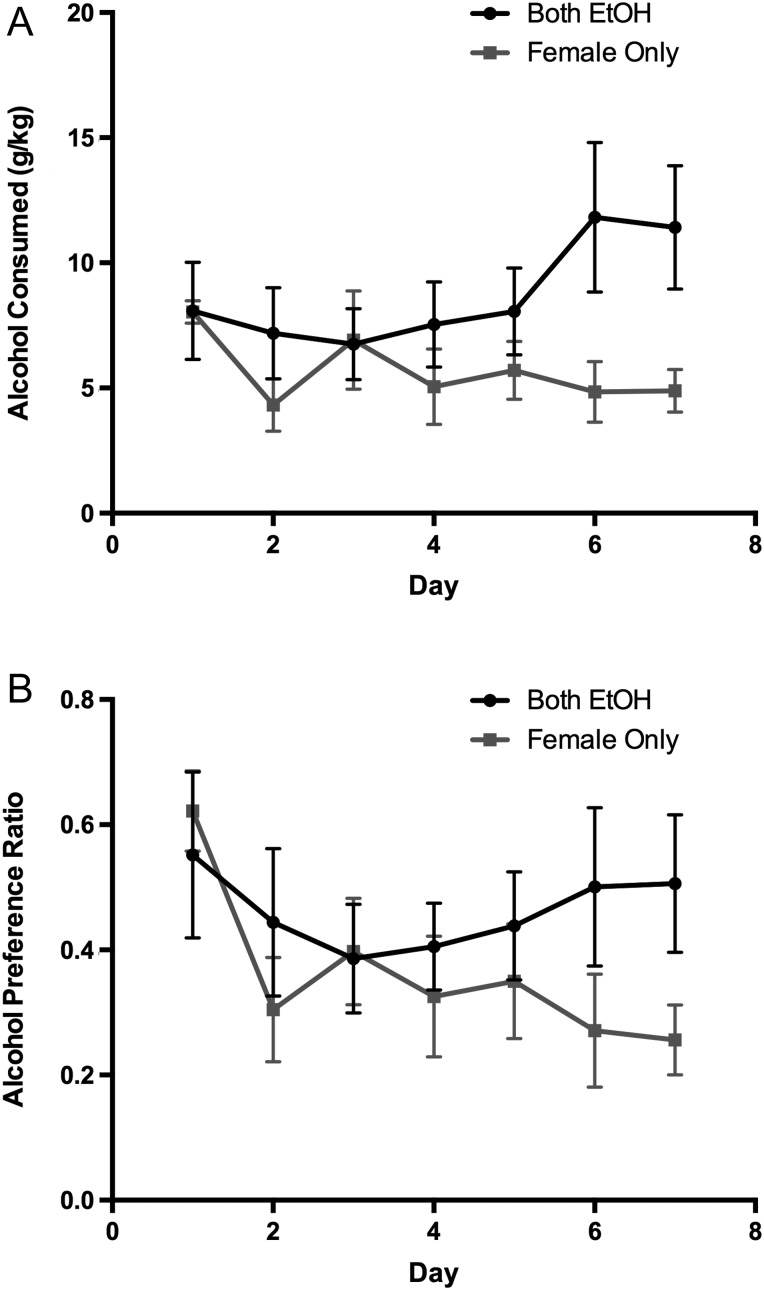
Females in the Both EtOH group displayed a mean intake of 8.7 ± 0.8 (mean ± SEM) grams of alcohol per kilogram of body weight (g/kg) over the seven-day two-bottle choice paradigm. Meanwhile, females in the female only EtOH group displayed a mean intake of 5.7 ± 0.5 g/kg over the seven-day two-bottle choice paradigm. A repeated-measures ANOVA analyzing alcohol consumption over the seven-day period revealed no significant main effect of time [F6,66 = 0.87, p = 0.52] or treatment [F1,11 = 2.68, p = 0.13]. However, there was a significant interaction between time and treatment [F6,66 = 2.21, p = 0.05]. Post hoc analysis revealed that alcohol consumption was significantly different between groups on Days 6 and 7 (Fig. 1A). To confirm that drinking was concordant in the both EtOH group, we also analyzed alcohol consumption between the female and male partners in the both EtOH group. Analysis revealed no significant main effect of time [F6,72 = 1.19, p = 0.32], sex [F1,12 = 0.46, p = 0.51] or interaction [F6,72 = 1.41, p = 0.22; data not shown] confirming that concordant drinking was indeed achieved in the both EtOH group.
Fig. 1.
Alcohol consumption and preference across the one week 10% ethanol drinking period. (A) Females in the both EtOH group had higher alcohol consumption during the last 2 days of the alcohol access period compared to females in the female Only group. (B) Alcohol preference for the females in the both EtOH group approached a significant difference on the last 2 days of alcohol access compared to the females in the female Only group. Error bars indicate mean ± SEM.
Females in the both EtOH group had a mean alcohol preference of 46.2 ± 2.2%, while females in the female only EtOH group had a mean preference of 36.1 ± 4.7% over the 7-day drinking period. A repeated-measures ANOVA revealed a significant main effect of time [F6,66 = 2.35, p = 0.04] and no effect of treatment [F1,11 = 0.92, p = 0.36]. There was a trend for a significant interaction between time and treatment [F6,66 = 1.89, p = 0.09]. Post hoc analysis revealed a trend for significant differences (P < 0.10) between treatment groups on Days 6 and 7 for alcohol preference (Fig. 1B) confirming that an opposite-sex partner’s drinking status can influence alcohol consumption and preference in female prairie voles.
Alcohol drinking does not alter female pair bond maintenance
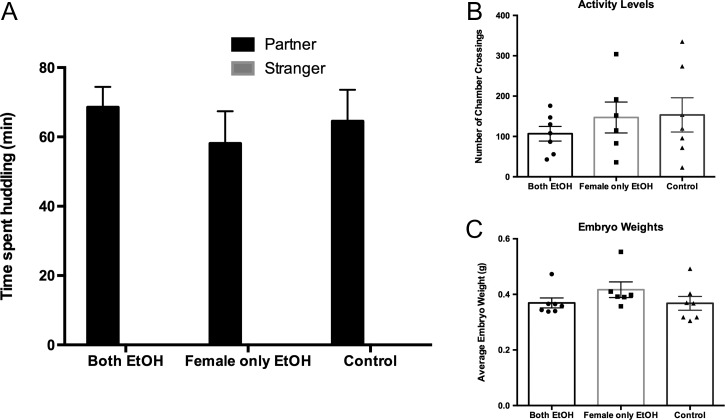
PPT was used to determine pair bond strength following alcohol consumption. During the test females did not huddle with stranger animals in any treatment group. Therefore, we analyzed the mean amount of time of partner huddling in each treatment group. One-way ANOVA revealed that there was no difference in the time spent huddling with partners between treatment groups [F2,17 = 1.14, p = 0.34; Fig. 2A]. Alcohol consumption can affect locomotor activity (Smoothy and Berry, 1985) and embryo weights (Ghimire et al., 2008) in rodent models; therefore, these factors might influence the expression of partner preference. Analyses of these measures in the present study did not identify any significant effects. Specifically, there were no significant differences between treatment groups for locomotor activity [F2,17 = 0.56, p = 0.58; Fig. 2B] during PPT or the average embryo weights [F2,17 = 1.32, p = 0.29; Fig. 2C] post the 2-week cohabitation period. These findings demonstrate that drinking alcohol or the drinking status of a male partner does not impact partner preference in female prairie voles.
Fig. 2.
The effects of concordant and discordant drinking on PPT, activity levels, and embryo weights. (A) Females in all three conditions displayed a partner preference during the PPT. However, there was no significant difference in the amount of time female prairie voles spent huddling with their male partner between the three conditions. (B) There was no difference in the number of cage crossings and (C) embryo weights between the three conditions. Error bars indicate mean ± SEM. Symbols in B and C show values in individual animals.
Effects of alcohol consumption on the brain during pair bond maintenance in female prairie voles
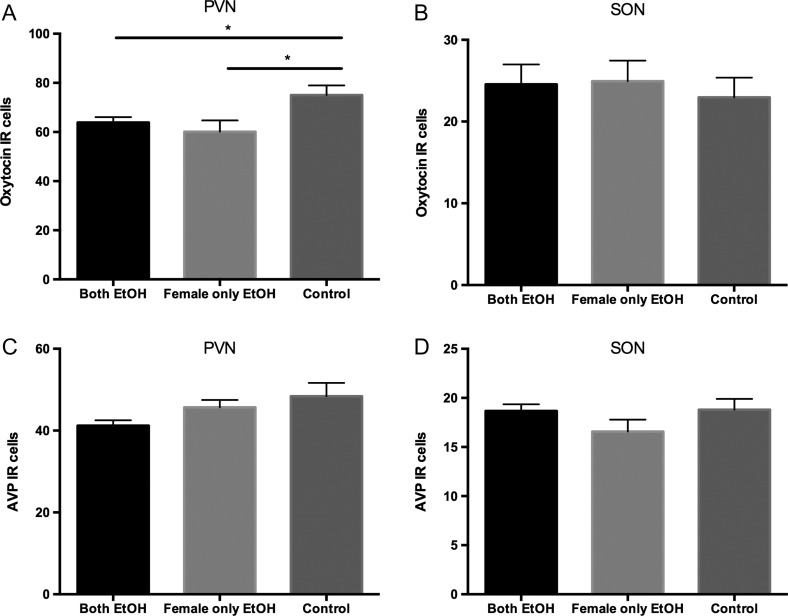
It has been shown that alcohol consumption decreases the number of oxytocin-immunoreactive (-ir) cells within the paraventricular nucleus of the hypothalamus (PVN) in male prairie voles (Stevenson et al., 2017; Walcott and Ryabinin, 2017). Therefore, we tested whether female prairie voles would show a decrease in oxytocin- and vasopressin-ir cells in the PVN and SON after one week of alcohol consumption. We found a significant effect of treatment on the number of oxytocin-ir cells within the PVN [F2,16 = 4.44, p = 0.03; Fig. 3A]. Post hoc analysis revealed that the females in both the both EtOH and female only EtOH groups had a significantly lower number of oxytocin-ir cells compared to the control group. To determine if this decrease could be caused by apoptosis, we ran IHC for cleaved Caspase 3. We found no colocalization of cleaved Caspase 3 with oxytocin-ir cells (Fig. S1, see online supplementary material for a color version of this figure). We saw no difference in the number of oxytocin-ir cells in the SON between treatment groups [F2,16 = 0.17, p = 0.84; Fig. 3B]. We saw a trend for a significant difference in the number of vasopressin-ir cells between treatment groups in the PVN [F2,16 = 2.80, p = 0.09; Fig. 3C] and no difference between groups in the SON [F2,16 = 1.55, p = 0.24; Fig. 3D]. Therefore, alcohol had similar effects on oxytocin-ir cells in the PVN of female prairie voles compared to previously observed effects in males. These effects were unlikely to be due to apoptosis through the cleaved Caspase 3 pathway.
Fig. 3.
Immunoreactivity for oxytocin and arginine vasopressin in the PVN and SON. (A) The number of oxytocin-immunoreactive (IR) cells in the paraventricular nucleus of the hypothalamus (PVN) was lower in female prairie voles who consumed alcohol compared to females who consumed only water. (B) The number of oxytocin-IR cells in the supraoptic nucleus (SON) did not differ between groups. (C) The number of arginine vasopressin (AVP) –IR cells in the PVN and (D) SON did not differ between the three groups. *p < 0.05. Error bars indicate mean ± SEM.
Since discordant alcohol consumption did not inhibit pair bond maintenance in female prairie voles, we needed to know if alcohol exhibited any effects on neuronal activity across the brain. For this purpose, we analyzed FosB-ir, a marker of both acute and long-term changes in neural activity in the brains of female animals from these experiments (Table 1). Across 18 brain regions, the only region with a significant difference between treatment groups was the centrally projecting Edinger–Westphal nucleus (EWcp) [F2,17 = 5.39, p = 0.01; Fig 4A]. Post hoc analysis revealed an increase in FosB-ir within the EWcp in females consuming alcohol compared to females consuming only water [Fig. 4A–D]. Combined with the data above, this finding indicates that alcohol consumption in female prairie voles affects neural activity independently from their male counterpart’s drinking status.
Table 1.
FosB immunoreactivity in analyzed brain regions.
| Brain region | Both EtOH | Female only EtOH | Control | p-value |
|---|---|---|---|---|
| Anterior Cingulate (CG1) | 1014 ± 63.7 | 903.8 ± 112.9 | 864.8 ± 61.7 | 0.5869 |
| Anterior Cingulate (CG2) | 1098 ± 89.8 | 982.3 ± 163.9 | 1071 ± 99.3 | 0.7783 |
| Agranular Insula (AI) | 549.8 ± 39.8 | 481 ± 30.3 | 523.6 ± 39.2 | 0.4924 |
| Granular Insula (GI) | 788.6 ± 40.7 | 608.3 ± 70.1 | 681.1 ± 59.7 | 0.1131 |
| Infralimbic Cortex | 622.1 ± 44.9 | 546.2 ± 96.0 | 547.1 ± 42.9 | 0.5705 |
| Retrosplenial Cortex | 525.9 ± 89.5 | 513.5 ± 99.2 | 486.1 ± 69.9 | 0.9401 |
| Dorsal Lateral Striatum | 732.9 ± 68.8 | 569.1 ± 99.8 | 763.5 ± 155.4 | 0.4759 |
| Dorsal Medial Striatum | 1672 ± 102.8 | 1642 ± 193.2 | 1658 ± 172.7 | 0.9912 |
| Nucleus Accumbens Core | 1925 ± 179.8 | 1931 ± 180.9 | 1918 ± 118.2 | 0.9983 |
| Nucleus Accumbens Shell | 1816 ± 114.0 | 1619 ± 238.4 | 1714 ± 110.6 | 0.6822 |
| Lateral Septum | 583.6 ± 76.7 | 624.8 ± 55.4 | 574.8 ± 47.2 | 0.8391 |
| Ventral Bed Nucleus of the Stria Terminalis | 224.9 ± 18.8 | 186.4 ± 24.2 | 193.0 ± 19.7 | 0.3918 |
| Dorsal Bed Nucleus of the Stria Terminalis | 167.8 ± 24.1 | 173.8 ± 40.5 | 167.1 ± 16.6 | 0.9828 |
| Paraventricular of the Hypothalamus | 33.4 ± 5.9 | 36.9 ± 5.6 | 38.1 ± 3.8 | 0.8090 |
| Hippocampus (CA1-3) | 403.4 ± 106.3 | 290.2 ± 29.5 | 376.5 ± 40.0 | 0.4108 |
| Dentate Gyrus | 280.4 ± 52.5 | 373.8 ± 22.6 | 356.7 ± 33.8 | 0.2242 |
| Periaqueductal Gray | 263.0 ± 27.5 | 250.7 ± 37.0 | 280.2 ± 35.6 | 0.8263 |
| Edinger–Westphal Nucleus | 14.9 ± 2.5 | 12.3 ± 1.9 | 6.4 ± 1.0 | 0.0169 |
Numbers represent mean ± standard error of the mean.
Fig. 4.
FosB immunoreactivity in the EWcp. (A) The number of FosB-IR cells in the centrally projecting Edinger–Westphal (EWcp) nucleus was significantly higher in female prairie voles in the both EtOH and female only EtOH groups compared to the water drinking control group. Representative photomicrographs of FosB immunoreactivity in the EWcp in the (B) both EtOH, (C) female only EtOH and (D) control groups. *p < 0.05. Error bars indicate mean ± SEM. Scale bars = 0.1 mm.
DISCUSSION
The present study investigated the effects of discordant and concordant alcohol consumption between partners on established pair bonds in female prairie voles. Consistent with what is expected in a socially monogamous species, and similar to male prairie voles (Walcott and Ryabinin, 2017), alcohol- and water-consuming females displayed a preference for their partner over the stranger male. However, in contrast to the previous study in male prairie voles, females showed no inhibition in partner preference after they consumed alcohol, regardless whether there was a discrepancy in alcohol consumption or not. Our finding is in agreement with the idea that maintenance of a pair bond is an evolutionary adaptive behavioral strategy that is not easily disrupted by the presence of alternative rewards. The lack of alcohol’s effects in the present study is unlikely due to an insufficient dose of consumed alcohol since withdrawal from alcohol consumption in a similar two-bottle choice procedure results in signs of hyperalgesia (Walcott et al., 2018). Therefore, the consumed doses of alcohol are high enough to produce at least some form of alcohol dependence. From an evolutionary perspective, it also appears that the maintenance of pair bonds in females should be more difficult to disrupt by an alternative reward than in males. However, the lack of effect of alcohol on maintenance of pair bonding does not concur with epidemiological observations that discordant heavy drinking in women is associated with disruption of human intimate relationships (Ostermann et al., 2005; Leonard et al., 2014).
The most likely reason for the apparent discrepancy of our finding from the existing epidemiological studies is in the nature of the PPT. In the PPT, the animal is given a choice to select the vicinity of its partner or a stranger. It is the tested female who is initiating the preference behavior in the current study. In contrast, the epidemiological studies do not assess who among the partners was the initiator of the separation. In other words, the likelihood of a couple’s separation also depended on the behavior of the abstinent partner. In contrast, the PPT is mostly targeted toward identifying the motivation of the tested individual. The lack of effect of alcohol on partner preference in the current study indicates that alcohol did not disrupt the motivation of the drinking females to spend their time in the vicinity with the partner.
While our current investigation focused on pair bond maintenance, a previous study from our laboratory found that alcohol inhibits the formation of pair bonds in male prairie voles and facilitates the formation of pair bonds in female prairie voles (Anacker et al., 2014a). Interestingly, an explanation has put forth that alcohol’s disruptive effects in married couples could be mediated by drinking that occurs when the spouses are not together (Roberts and Leonard, 1998; Homish and Leonard, 2005). Paradoxically, in this situation, not only the tendency of alcohol to disrupt formation and maintenance of bonds in males but also the tendency of alcohol to facilitate pair bond formation in females would promote the likelihood of an established couple’s separation. Unfortunately, this situation is even more difficult to model in animals. In the absence of epidemiological studies assessing the initiator of separation in humans, our findings add to the limited body of literature that shows that some drugs of abuse display sexual dimorphic effects in these socially monogamous species (Liu et al., 2010; Young et al., 2014; Anacker et al., 2014a).
In addition to the effects of alcohol on PPT, in the current study, we assessed if the drinking status of the male partner would influence the amount of alcohol consumed by the female subject. We observed that when female and male partners were both consuming alcohol, females increased alcohol consumption compared to females whose male partner had no access to alcohol. According to previous studies, same-sex prairie vole siblings can socially facilitate the amount of alcohol consumed by each partner (Anacker et al., 2011b). Meanwhile, there are mixed results on the ability of opposite-sex partners to socially facilitate the amount of alcohol consumed by each partner in prairie voles. Specifically, Walcott and Ryabinin (2017) showed that male prairie voles increased alcohol consumption and preference when an alcohol consuming female partner was present. In contrast, Hostetler et al. (2012), showed that male–female pairs do not socially facilitate the amount of alcohol consumed by each partner. Compared to the current experiments, the latter study had methodological differences that could play a role in the discrepancy in results. In particular, Hostetler et al. (2012) used gonadectomized prairie voles and shorter cohabitation prior to alcohol exposure, and it is not clear whether pair bonding occurred and was maintained during the mesh housing. In contrast, in the current study, animals were able to form and maintain pair bonds, which was confirmed by embryo weight. Together these studies suggest when prairie voles are able to form pair bonds with their partners, social facilitation of alcohol consumption does occur. Importantly, for the main aim of the study, it is unlikely that the lower alcohol consumption in the females exposed to discrepant drinking compared to the both EtOH group contributed to the lack of alcohol effects on pair bond maintenance as alcohol consumption in the female only EtOH group is identical to that in the analogous male only EtOH group in our previous study in which effects of alcohol on pair bond maintenance has been observed (Walcott and Ryabinin, 2017).
The present study showed that 1 week of alcohol consumption can result in a decreased number of oxytocin-IR cells in the PVN. Similar to the effects of alcohol on male prairie voles observed by us previously (Walcott and Ryabinin, 2017), this decrease in oxytocin-IR was independent of the drinking status of the female’s partner. This decrease in oxytocin-IR is also in agreement with previous studies in rats and in prairie voles that used longer exposures to alcohol (Silva et al., 2002; Stevenson et al., 2017). Our experiments expand these previous findings by showing that the effect occurs after a relatively short period (1 week) of voluntary alcohol self-administration. The mechanisms underlying alcohol’s effect on oxytocin neurons are currently unknown. Here we tested whether the decrease in oxytocin-IR could be due to apoptosis. One of the common early mechanisms of apoptosis involves cleaving the Caspase 3 protein (Porter and Jänicke, 1999). Examination of selected brain slices containing PVN did not show any colocalization between cleaved Caspase 3-ir and oxytocin-ir. This finding provides evidence against alcohol-induced apoptosis in oxytocin cells of PVN. It appears more likely that the decreased expression of oxytocin contributes to this effect of alcohol. Alternatively, the decreased oxytocin-ir could be due to another, more unusual, form of cell death or due to apoptosis occurring at earlier stages of alcohol consumption. Although a decreased number of oxytocin-containing cells in the PVN seemed not to contribute to any effects on pair bond maintenance, these cells regulate a number of social behaviors (Ross et al., 2009; Smith and Wang, 2014). Therefore, the mechanisms underlying the consistent effects of alcohol on these cells are worthy of further investigations.
Since no significant effects of alcohol on pair bond maintenance were observed in our study, it was important to confirm that alcohol consumption resulted in effects on neural activity. Similar to the Walcott and Ryabinin (2017) study, we did this using an antibody that detects the products of immediate early gene FosB. While acute neural activation leads to temporary expression of the full-length FosB protein, repeated neural activation results in accumulation of the delta-FosB protein (Kelz and Nestler, 2000; Nestler et al., 2001). Therefore, positive FosB-ir serves as a marker for both acute and prolonged effects of treatments on neural activity. Out of the 18 examined brain regions in our study, the only region that showed a difference between groups was the EWcp. Animals that were consuming alcohol, independently of the drinking status of their partner, showed an increase in FosB-IR within the EWcp. Immediate early gene expression in the EWcp is known to be strongly affected by voluntary and involuntary modes of alcohol exposure (Ryabinin and Wang, 1998; Bachtell et al., 1999; Ozburn et al., 2012). Interestingly, Walcott and Ryabinin (2017) showed that alcohol consumption in male prairie voles affected FosB-ir not only in the EWcp but also the nucleus accumbens, infralimbic cortex, and bed nucleus of the stria terminalis. One limitation to the current study was that females used in the PPT were also used to explore potential brain regions affected by alcohol exposure. Meanwhile, in the previous male prairie vole study a separate group of males was used to explore the brain regions. Since the antibody used in both studies detects both full-length FosB and delta-FosB, the PPT in the current study could have modified the FosB-ir levels. Importantly, even though there were no significant differences in FosB-ir in other brain regions, the finding is that FosB-IR was increased in the EWcp following alcohol consumption indicates that alcohol consumption resulted in physiological changes in the central nervous system.
Overall, we showed that discordant and concordant voluntary alcohol consumption does not affect established pair bonds in female prairie voles measured by the PPT. This effect does not mirror the effects of discordant heavy alcohol consumption on marital separation in humans. We showed that independently of a partner drinking status, alcohol affects several neuronal substrates in female prairie voles, including the PVN and EWcp. Future studies should further explore the relationship between these neuronal substrates and alcohol consumption. In addition, further research should be done to allow closer modeling of alcohol’s effects on marital dissolution in humans caused by heavy discordant alcohol consumption by female partners that would allow to determine the underlying biological mechanisms for this phenomenon.
Supplementary Material
CONFLICT OF INTEREST STATEMENT
None declared.
References
- Ahern TH, Modi ME, Burkett JP, et al. (2009) Evaluation of two automated metrics for analyzing partner preference tests. J Neurosci Meth 182:180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato PR, Previti D. (2003) People’s reasons for divorcing: gender, social class, the life course, and adjustment. J Fam Issues 24:602–26. [Google Scholar]
- Anacker AMJ, Ahern TH, Hostetler CM, et al. (2014. a) Drinking alcohol has sex-dependent effects on pair bond formation in prairie voles. Proc Natl Acad Sci USA 111:6052–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Loftis JM, Kaur S, et al. (2011. a) Prairie voles as a novel model of socially facilitated excessive drinking. Addict Behav 16:92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Loftis JM, Ryabinin AE. (2011. b) Alcohol intake in prairie voles is influenced by the drinking level of a peer. Alcohol Clin Exp Res 35:1884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Smith ML, Ryabinin AE. (2014. b) Establishment of stable dominance interactions in prairie vole peers: relationships with alcohol drinking and activation of the paraventricular nucleus of the hypothalamus. Soc Neuro 9:484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, et al. (2006) Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neuro 9:133–9. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Wang YM, Freeman P, et al. (1999) Alcohol drinking produces brain region-selective changes in expression of inducible transcription factors. Brain Res 847:157–65. [DOI] [PubMed] [Google Scholar]
- Caces MF, Harford TC, Williams GD, et al. (1999) Alcohol consumption and divorce rates in the United States. J Stud Alcohol 60:647–52. [DOI] [PubMed] [Google Scholar]
- Carter CS, Getz LL. (1993) Monogamy and the prairie vole. Sci Am 268:100–6. [DOI] [PubMed] [Google Scholar]
- Collins RL, Ellickson PL, Klein DJ. (2007) The role of substance use in young adult divorce. Addiction 102:786–94. [DOI] [PubMed] [Google Scholar]
- Curtis JT. (2010) Does fertility trump monogamy? Ani Behav 80:319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Johnson CL, Carter CS. (1997) Familiarity and gender influence social preferences in prairie voles (Microtus ochrogaster). Can J Zool 75:295–301. [Google Scholar]
- Ebstein RP, Israel S, Lerer E, et al. (2009) Arginine vasopressin and oxytocin modulate human social behavior. Ann N Y Acad Sci 1167:87–102. [DOI] [PubMed] [Google Scholar]
- Ghimire SR, Dhungel S, Rai D, et al. (2008) Effect of prenatal exposure of alcohol in the morphology of developing rat embryo. Nepal Med Coll J 10:38–40. [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. (2009) Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol 30:548–57. [DOI] [PubMed] [Google Scholar]
- Homish GG, Leonard KE. (2005) Marital quality and congruent drinking. J Stud Alcohol 66:488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homish GG, Leonard KE. (2007) The drinking partnership and marital satisfaction: the longitudinal influence of discrepant drinking. J Consult Clin Psychol 75:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homish GG, Leonard KE, Kozlowski LT, et al. (2009) The longitudinal association between multiple substance use discrepancies and marital satisfaction. Addiction 104:1201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler CM, Anacker AMJ, Loftis JM, et al. (2012) Social housing and alcohol drinking in male-female pairs of prairie voles (Microtus ochrogaster). Psychopharmacology (Berl) 224:121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler CM, Ryabinin AE. (2014) Social partners prevent alcohol relapse behavior in prairie voles. Psychoneuroendocrinology 39:152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. (1995) A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neuro 109:782–9. [DOI] [PubMed] [Google Scholar]
- Kelley ML, Lewis RJ, Mason TB. (2015) Discrepant alcohol use, intimate partner violence, and relationship adjustment among lesbian women and their relationship partners. J Fam Violence 30:977–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelz MB, Nestler EJ. (2000) DeltaFosB: a molecular switch underlying long-term neural plasticity. Curr Opin Neurol 13:715–20. [DOI] [PubMed] [Google Scholar]
- Kenkel DS, Ribar DC, Cook PJ, et al. (1994) Alcohol consumption and young adults’ socioeconomic status. Brookings Pap Econ Act Microecon 1994:119–75. [Google Scholar]
- Leonard KE, Quigley BM. (1999) Drinking and marital aggression in newlyweds: an event-based analysis of drinking and the occurrence of husband marital aggression. J Stud Alcohol 60:537–45. [DOI] [PubMed] [Google Scholar]
- Leonard KE, Rothbard JC. (1999) Alcohol and the marriage effect. J Stud Alcohol Suppl 13:139–46. [DOI] [PubMed] [Google Scholar]
- Leonard KE, Senchak M. (1993) Alcohol and premarital aggression among newlywed couples. J Stud Alcohol Suppl 11:96–108. [DOI] [PubMed] [Google Scholar]
- Leonard KE, Smith PH, Homish GG. (2014) Concordant and discordant alcohol, tobacco, and marijuana use as predictors of marital dissolution. Psychol Addict Behav 28:780–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Aragona BJ, Young KA, et al. (2010) Nucleus accumbens dopamine mediates amphetamine-induced impairment of social bonding in a monogamous rodent species. Proc Natl Acad Sci USA 107:1217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshal MP. (2003) For better or for worse? The effects of alcohol use on marital functioning. Clin Psychol Rev 23:959–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod JD. (1993) Spouse concordance for alcohol dependence and heavy drinking: evidence from a community sample. Alcohol Clin Exp Res 17:1146–55. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, et al. (2011) Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci 12:524–38. [DOI] [PubMed] [Google Scholar]
- Mudar P, Leonard KE, Soltysinski K. (2001) Discrepant substance use and marital functioning in newlywed couples. J Consult Clin Psychol 69:130–4. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW. (2001) DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci USA 98:11042–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann J, Sloan FA, Taylor DH. (2005) Heavy alcohol use and marital dissolution in the USA. Soc Sci Med 61:2304–16. [DOI] [PubMed] [Google Scholar]
- Ozburn AR, Mayfield RD, Ponomarev I, et al. (2012) Chronic self-administration of alcohol results in elevated ΔFosB: comparison of hybrid mice with distinct drinking patterns. BMC Neuro 13:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, et al. (2001) Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci 21:7392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter AG, Jänicke RU. (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Diff 6:99. [DOI] [PubMed] [Google Scholar]
- Resendez SL, Aragona BJ. (2012) Aversive motivation and the maintenance of monogamous pair bonding. Reviews Neuro 24:1–10. [DOI] [PubMed] [Google Scholar]
- Roberts LJ, Leonard KE. (1998) An empirical typology of drinking partnerships and their relationship to marital functioning and drinking consequences. J Marriage Fam 515–26. [Google Scholar]
- Ross HE, Cole CD, Smith Y, et al. (2009) Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuro 162:892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, Wang YM. (1998) Repeated alcohol administration differentially affects c-Fos and FosB protein immunoreactivity in DBA/2 J mice. Alcohol Clin Exp Res 22:1646–54. [PubMed] [Google Scholar]
- Silva SM, Madeira MD, Ruela C, et al. (2002) Prolonged alcohol intake leads to irreversible loss of vasopressin and oxytocin neurons in the paraventricular nucleus of the hypothalamus. Brain Res 925:76–88. [DOI] [PubMed] [Google Scholar]
- Smith AS, Wang Z. (2014) Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry 76:281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoothy R, Berry MS. (1985) Time course of the locomotor stimulant and depressant effects of a single low dose of ethanol in mice. Psychopharmacology (Berl) 85:57–61. [DOI] [PubMed] [Google Scholar]
- Stevenson JR, Young KA, Bohidar AE, et al. (2017) Alcohol consumption decreases oxytocin neurons in the anterior paraventricular nucleus of the hypothalamus in prairie voles. Alcohol Clin Exp Res 41:1444–51. [DOI] [PubMed] [Google Scholar]
- Torvik FA, Røysamb E, Gustavson K, et al. (2013) Discordant and concordant alcohol use in spouses as predictors of marital dissolution in the general population: results from the Hunt study. Alcohol Clin Exp Res 37:877–84. [DOI] [PubMed] [Google Scholar]
- Walcott AT, Ryabinin AE. (2017) Alcohol’s effects on pair-bond maintenance in male prairie voles. Front Psychi 8:37–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott AT, Smith ML, Loftis JM, et al. (2018) Social transfer of alcohol withdrawal-induced hyperalgesia in female prairie voles. Soc Neuro 105:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Carter CS, Insel T. (1992) Partner preference development in female prairie voles is facilitated by mating or the central infusion of oxytocin. Ann NY Acad Sci 652:487–9. [DOI] [PubMed] [Google Scholar]
- Young KA, Liu Y, Gobrogge KL, et al. (2014) Oxytocin reverses amphetamine-induced deficits in social bonding: evidence for an interaction with nucleus accumbens dopamine. J Neurosci 34:8499–8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.