Abstract
Objective
To assess conditioned pain modulation efficiency in persons with and without migraine headaches.
Design
Cross-sectional assessment of experimental pain.
Setting
University campus and surrounding community in a large Midwestern US city.
Subjects
Twenty-three adults with and 32 without a history of migraine headaches participated in the study. Participants were mostly female (N = 40) with an average age of 23 years.
Methods
Four electrocutaneous stimulations of the supraorbital branch of the left trigeminal nerve were delivered at 150% of an individually determined pain threshold. Conditioned pain modulation was assessed by applying a noxious counterstimulus (forearm ischemia) and delivering four more electrocutaneous stimulations. After each stimulation, pain and the nociceptive blink reflex were assessed. Depression and pain catastrophizing were assessed to control for the potential influence of these variables on pain modulation.
Results
Participants with and without migraine headaches had similar baseline pain responsivity, without significant differences in pain report or nociceptive blink reflexes. Pain report was inhibited by conditioned pain modulation in both the migraine and control groups. However, unlike nonmigraine controls, participants with migraines did not exhibit an inhibition of nociceptive blink reflexes during the ischemia task. This pattern persisted after controlling for level of pain catastrophizing and depression.
Conclusions
Migraine sufferers exhibited impaired conditioned pain modulation of the nociceptive blink reflex, suggesting a deficiency in inhibition of trigeminal nociception, which may contribute to the development of migraine headaches.
Keywords: Migraine Headaches, Conditioned Pain Modulation, Nociceptive Blink Reflex, Pain Modulation
Introduction
Supraspinal mechanisms can both facilitate and inhibit pain and nociception and may contribute to development and/or maintenance of pain disorders [1–4]. A commonly studied mechanism of supraspinal pain modulation is conditioned pain modulation (CPM), in which a noxious stimulus at one body site (heterotopic counterstimulus or conditioning stimulus) leads to inhibition of concurrent pain and nociception (from a focal test stimulus) at a distant body site in healthy individuals. Animal models assessing a similar model of pain inhibition (diffuse noxious inhibitory controls [DNICs]) have implicated a descending pathway of pain inhibition emanating from the subnucleus reticularis dorsalis [5]; however, cognitive factors may also contribute to CPM effects in humans [6,7] and specific neurophysiological mechanisms of CPM in humans are yet to be fully elucidated. Most studies of CPM have looked at modulation of peripheral somatic pain, however, some studies have shown that, in healthy participants, CPM also modulates pain in the head and branches of the trigeminal nerve [8–12], thus making CPM a potential mechanism for studying the pathophysiology in headache disorders including migraine headaches.
Migraine headaches affect as much as 12% of the population of the United States [13]. Abnormalities in supraspinal processes related to pain/nociception have been implicated in the pathogenesis of migraine headaches, potentially through dysregulation of trigeminal nerve function. Research on CPM in persons with migraine headaches has found mixed results. Sandrini et al. [14] assessed CPM in migraine patients, chronic tension-type headache patients, and healthy controls by assessing both subjective pain and the nociceptive flexion reflex (a marker of spinal nociception assessed from the leg) during a cold pressor test of the contralateral hand. They found that migraine patients lacked the expected inhibition of pain and the nociceptive flexion reflex that was seen in healthy participants, and even showed facilitation of pain and nociception. Another study assessed the CPM of heat pain in the left leg during cold immersion of the right foot in migraine patients vs controls and found no group difference on the first CPM series [15]. However, they then delivered the CPM test three more times and found that migraine patients had less efficient CPM inhibition of pain on subsequent trials. de Tommaso et al. [16] also found impaired CPM inhibition of pain (both peripheral and supraorbital laser-evoked pain) and laser-evoked potentials in patients with migraine headaches compared with controls. In contrast, other studies have failed to find differences in CPM between patients with migraines and healthy controls [17–19]. Therefore, further research is needed to fully understand what, if any, alterations in CPM may be present in patients with migraine headaches and whether these impairments might contribute to the headache disorder. Further, only de Tommaso and colleagues [16] actually assessed CPM of trigeminal pain or nociception, which is of particular importance given the site specificity of migraine headaches.
The blink reflex, elicited by stimulation of the supraorbital branch of the trigeminal nerve, has been used to study trigeminal nociception. However, stimulation with a standard stimulating electrode results in blink reflexes that are not nociceptive specific, as evidenced by the fact that they are not eliminated following an anesthetic [20]. Laser stimulation does selectively activate nociceptors [21] but leads to sensitization, limiting its applicability in studies requiring repeated stimulation. Therefore, Kaube et al. [22] introduced a custom concentric electrode that is able to stimulate with a lower current to activate superficial nociceptors to the exclusion of deeper mechanical receptors. The blink reflex elicited by this electrode is nociceptive specific and has been shown to be inhibited by CPM in healthy persons [8,10]. As a result, they refer to it as the nociceptive blink reflex (nBR).
The present study extends previous research by assessing the CPM of trigeminal pain and nociception in participants with migraine headaches compared with a migraine-free control population. In addition to assessing subjective pain report, nBR was assessed by utilizing a custom concentric electrode to selectively activate supraorbital nociceptors via electrical stimulation.
Methods
Participants
Participants were recruited from the University of Tulsa and surrounding community via flyers. Participants were at least 18 years of age and were excluded if they had a self-reported history of cardiac, neurological, or neuromuscular disorder, recent psychological trauma, or chronic pain conditions other than migraine headaches. Due to the theoretical potential for some medications to impact endogenous pain modulation processes, participants were also excluded for recent use of over-the-counter (past 24 hours) or narcotic pain medication (past 48 hours) or current use of antidepressant or anxiolytic medications. Migraine and other headache diagnoses were obtained from a Structured Diagnostic Interview for Headache based on the Headache Classification Committee of the International Headache Society [23].
A total of 63 persons (23 with migraine headaches and 40 controls) participated in the study. Eight people were excluded due to missing data or equipment failure. Thus, data were included for 23 participants with migraine headaches and 32 controls (Table 1). Six of these participants (five migraine, one control) had missing nBR data (due to equipment failure) and were excluded from analyses looking at nBR data only. Final analyses for nBR included 18 participants with migraine headaches and 31 controls. The control group included persons who did not report problematic headaches: five had no history of headaches, and 27 met criteria for episodic tension-type headaches (ETTH). Participants were excluded for presence of chronic tension-type headaches (>14 days per month) and other headache disorders (i.e., cluster headaches, new daily persistent headaches, medication overuse headaches), identified via the Structured Diagnostic Interview for Headache. University students received course credit for participation, whereas community participants received a $20 gift card. Informed consent was obtained from all participants, and all procedures were approved by The University of Tulsa ethics review board.
Table 1.
Demographic and questionnaire data by group
| Migraine (N = 23) | Controls (N = 32) | t | P | d | |
|---|---|---|---|---|---|
| Gender, No. (% female) | 17 (74) | 23 (72) | 0.76 | ||
| Ethnicity, No. (%) | 0.43 | ||||
| Caucasian | 16 (70) | 28 (88) | |||
| Hispanic | 2 (9) | 0 (0) | |||
| African American | 1 (4) | 2 (6) | |||
| Asian | 1 (4) | 1 (3) | |||
| Native American | 1 (4) | 1 (3) | |||
| Other | 1 (4) | 0 (0) | |||
| Age, y | 24.32 (7.94) | 21.75 (8.71) | −1.10 | 0.28 | 0.36 |
| Depression, M (SD) | 13.87 (7.99) | 13.63 (8.66) | −0.11 | 0.92 | 0.03 |
| Pain catastrophizing, M (SD) | 14.86 (10.90) | 18.55 (15.22) | 1.03 | 0.31 | 0.28 |
| Baseline trigeminal stimulation pain, M (SD) | 58.79 (15.31) | 56.77 (13.65) | −0.52 | 0.61 | 0.15 |
| Baseline nBR magnitude, M(SD) | 1.12 (0.53) | 1.03 (0.40) | 0.66 | 0.51 | 0.19 |
| Pain due to ischemia, M (SD) | 52.35 (20.79) | 55.47 (26.07) | 0.48 | 0.64 | 0.13 |
| Hours slept the previous night, M (SD) | 7.09 (1.12) | 7.74 (1.79) | 1.51 | 0.14 | 0.44 |
| Current headache pain, M (SD) | 0.83 (1.34) | 1.21 (1.75) | 0.79 | 0.44 | 0.24 |
| Duration of headaches, M (SD), mo | 121.71 (98.41) | 42.80 (36.56) | −3.48 | <0.01 | 1.06 |
| Disability in the past 30 d, M (SD) | 1.44 (2.28) | 1.00 (1.61) | −0.80 | 0.43 | 0.22 |
| MIDAS total score, M (SD) | 14.00 (15.80) | 5.10 (12.14) | −2.21 | 0.03 | 0.63 |
| Headache-free d/wk, M (SD) | 5.91 (1.09) | 5.85 (1.45) | −0.15 | 0.88 | 0.05 |
Significance = P<.05, d = Cohen’s d values (small = 0.2, medium = 0.5, large = 0.8); MIDAS = Migraine Disability Assessment questionnaire.
Procedure
After an explanation of the experimental procedures, participants were consented and screened for eligibility. Eligible participants completed a demographics form to assess age, gender, ethnicity, and variables that could impact pain perception (i.e., medical diagnoses, current/recent medication use including pain medications, current pain, menstrual cycle phase), including an open-ended question about hours slept the previous night. Eligible participants were given instructions on use of the numerical rating scale (NRS) used to rate pain during the study (described below), and electrodes were applied.
The study consisted of two phases. During the first phase, pain threshold was assessed in response to electrocutaneous stimulations of the left supraorbital branch of the trigeminal nerve on the forehead. After each stimulation, the participant made a pain rating on the NRS scale, which was displayed on the computer screen positioned in front of them (a light on the computer screen came on, indicating when it was time to make a rating). Stimulations were delivered in two ascending/descending staircases beginning at 0 mA (current) and increasing in 0.5 mA steps until pain threshold (a rating ≥50 on a 0–100 NRS scale) was reached. There was a variable 15–17-second interval that began after a pain rating was made. After reaching the pain threshold, the intensity was decreased in 0.5-mA steps until a rating of ≤25 was achieved. The process was then repeated. Pain threshold was defined as the average intensity (in mA) of the four stimuli rated above and below 50 in the two ascending/descending staircases. A stimulus intensity of 150% of the pain threshold was used for all pain stimuli throughout the remainder of the experiment.
The second phase consisted of two counterbalanced procedures to assess supraspinal modulation of pain via two different mechanisms, CPM and emotional controls of nociception (ECON). Only the results from CPM are included in this paper. For a detailed description of the ECON paradigm, the reader is referred to Williams and Rhudy [8], and for a summary of ECON results from this study, the reader is referred to the Vincent et al. [24] abstract from the 2009 Society for Neuroscience annual conference. Questionnaires (described below) assessing headache variables and psychological constructs were completed during a break between the CPM and ECON procedures, unless noted otherwise. These variables were assessed to ensure the absence of group differences on variables that could impact study conclusions. To assess CPM, four stimulations were delivered at 150% of the pain threshold with a random interstimulus interval ranging between 15 and 25 seconds. Then, the noxious counterstimulus was applied by inducing forearm ischemia. To do so, participants completed hand exercises with the nondominant hand at 50% of their grip strength for two minutes, held their arm above their head for 15 seconds to promote desanguination, and then a blood pressure cuff was inflated to 220 mmHg for two minutes. Thirty seconds after cuff inflation, four more supraorbital nerve stimulations at 150% of the pain threshold were delivered with a random interstimulus interval of 10–20 seconds. Throughout ischemia, the NRS pain rating scale was displayed and participants provided a verbal rating of pain after each stimulation. The cuff was then deflated, and participants were asked to rate the pain due to the ischemia task on an NRS scale.
Apparatus
A computer running LabVIEW software (National Instruments, Austin, TX, USA) equipped with dual monitors and an analog to digital board (National Instruments, PCI-6036E) was used to control presentation of questionnaires, electrocutaneous stimulations, data collection, and data reduction. One 17” flat panel monitor was positioned 0.5 m in front of the participant to present rating scales, and the other monitor was in a separate room and was used by the experimenter to monitor the experiment.
Nociceptive Blink Reflex
The nBR was elicited by electrocutaneous stimulation of the left supraorbital branch of the trigeminal nerve using a concentric stimulating electrode applied 10 mm superior to the supraorbital foramen. As described above, stimulations were set at 150% of the participant’s pain threshold. The stimulating electrode was modeled after specifications outlined by Kaube et al. [22] and consisted of a 0.5-mm cathode in the center of a ring anode with a 10-mm internal diameter and 30-mm outer diameter. This concentric electrode has been shown to selectively activate A-δ nociceptors, to the exclusion of A-β fibers that are activated by mechanical (nonpainful) stimuli [22]. Thus the resulting nBR provides a physiological measure of trigeminal nociception. The nBR magnitude was defined as the mean response of the rectified and integrated electromyography response in the 27–87 milliseconds after the electrocutaneous stimulation minus the mean response of the 60-millisecond baseline before stimulation. This interval corresponds to the R2 component of the blink reflex, which is elicited by nociceptor activation [22,25–27].
Stimulations were delivered by a Grass Instruments Stimulator (Model S88, West Warwick, RI, USA), stimulus isolation unit (Model SIU8T), constant current unit (Model CCU1), and the custom concentric electrode mentioned above. Delivery of the stimulations was computer controlled, and the intensity (in mA) was regulated with a computer-controlled voltage regulator with a max of 40 mA.
The nBR was measured using orbicularis oculi EMG using one miniature electrode placed 1 cm inferior to the median of the left eye and another placed at the distal corner of the eye. A ground electrode was applied to the mastoid process behind the left ear. Before electrode application, the skin was cleaned with an alcohol swab and lightly abraded with NuPrep gel (Weaver, Aurora, CO, USA) to achieve impedance <5 KΩ. Electrodes were applied with conductive gel (EC60, Grass Instruments). EMG signals were sampled from one second before until six seconds after delivery of the supraorbital nerve stimulation during the baseline phase. Signals were recorded for the full two minutes during the noxious counterstimulus. EMG was sampled at 1000 Hz and collected/filtered using a Grass Instruments Model 15LT Bipolar Amplifier with a Quad AC (15A54) module. The raw EMG signal was rectified, amplified ×5000, and filtered for frequencies below 30 Hz and above 1000 Hz. A Chebychev filter (second order) was utilized during postprocessing to integrate the EMG signal.
Pain Report: Numerical Rating Scale
A numerical rating scale (oriented vertically) was presented to participants on the computer screen. After each electrocutaneous stimulation, a digital light next to the scale illuminated, indicating it was time to make a pain rating. The NRS was labeled, from bottom to top, 0 = no sensation, 1 = just noticeable, 25 = uncomfortable, 50 = painful, 75 = very painful, and 100 = maximum tolerable. Participants used a computer mouse to move an indicator to any point on the scale that corresponded with their pain experience and pressed a submit button when their answer was completed. As noted above, during the forearm ischemia task, the NRS was displayed; however, participants made verbal ratings by stating any number between 0 and 100 that corresponded with their pain, and this number was recorded by the experimenter. This method was chosen because pilot testing indicated that making computer ratings was too distracting during the forearm ischemia task. After completion of the CPM protocol, the NRS was presented again and participants were instructed to rate their pain due to the ischemia task.
Questionnaires
Headache Characteristics and Impact
A custom-built Headache Patient Information form was used to assess headache-related variables. Number of headache-free days per week was assessed with an open-ended question. Current headache pain was assessed with a 0–10 numerical rating scale with the following labels: 0 = no headache, 2 = slightly painful, 4 = mildly painful, 6 = painful, 8 = very painful, and 10 = extremely painful. Duration of headache disorder was assessed with an open-ended question (in years, months). Headache-related disability was assessed with two questions: “On average, how disabled have you been in the last seven days?” and “On average, how disabled have you been in the last 30 days?” The 0–10 response scale had the following labels: 0 = no impairment, 2 = minimal impairment, 4 = mildly impaired, 6 = moderately impaired, 8 = severely impaired, and 10 = completely impaired. This form was completed after consent and study explanation and before electrode application and experimental procedures.
Migraine Disability Assessment
The Migraine Disability Assessment (MIDAS) [28,29] is a self-report questionnaire assessing disability associated with headaches. The scale consists of seven items, with the first five inquiring about the number of days in the past three months on which headaches have impacted a particular area of functioning. The sixth item assesses the number of headache days in the past three months, and the seventh item rates pain intensity for headaches on a 0–10 scale. The total score is calculated by summing responses to the first five items, and scores are interpreted as follows; 0–5 = little or no disability, 6–10 = mild disability, 11–20 = moderate disability, and 21+ = severe disability. The MIDAS has been found to be a reliable (coefficient alpha = 0.83, test-retest correlation = 0.77) [29] and valid measure of headache-related disability [28]. The coefficient alpha in the present sample was 0.73.
Center for Epidemiological Studies Depression Scale
The Center for Epidemiological Studies Depression Scale (CES-D) [30] is a self-report questionnaire assessing symptoms of depression in the past week. The scale consists of 20 items answered on a four-point scale with responses of rarely or none of the time (less than one day), some or a little of the time (one to two days), occasionally or a moderate amount of time (three to four days), and most or all of the time (five to seven days), with scores from 0 to 3, respectively. Total scores can range from 0 to 60, with higher scores indicative of more depressive symptoms. A score of ≥16 is often used as an indicator of clinically significant depressive symptoms [31]. This cut-score was used to group participants into high and low depressive symptom groups. The measure has been shown to be reliable with a coefficient alpha of 0.85, with two-week test-retest reliability in the moderate range. The coefficient alpha for the present sample was 0.88.
Pain Catastrophizing Scale
The Pain Catastrophizing Scale (PCS) [32] is a self-report questionnaire that assesses the degree to which patients experienced certain pain catastrophizing cognitions or feelings during past painful experiences. The scale consists of 13 items, with patient responses ranging from 0 = not at all to 4 = all the time. The PCS total score was used in this study and ranges from 0 to 52, with higher scores reflective of greater pain catastrophizing. Participants completed the PCS at the end of the experimental procedures and were instructed to respond according to thoughts or feelings they had during painful stimuli during the study, thus assessing situation-specific catastrophizing. A score of ≥30 on the PCS can be interpreted as clinically meaningful [32]. This cut-score was used in the present study to group participants into high and low catastrophizing categories. The PCS has been shown to have good internal consistency with a coefficient alpha of 0.87 [33]. The coefficient alpha for the present sample was 0.96.
Data Analysis
To control for the influence of potential confounding variables, independent-samples t tests were completed to assess for differences in pain perception (pain report due to ischemia task and pain report and nBR during baseline supraorbital stimulation), depression, and catastrophizing between participants with and without migraine headaches. Chi-square tests were utilized to assess for differences in frequency of clinically significant depression and clinically significant pain catastrophizing in the control group vs the migraine group. Paired-samples t tests were completed independently for each group to assess for inhibition of pain report and the nBR due to conditioned pain modulation in participants with and without migraine headaches. For participants with migraine headaches, a change score representing inhibition of nBR due to CPM was computed by subtracting baseline nBR from ischemia nBR (negative number represents inhibition). Pearson’s correlations were computed between this CPM change score and participants’ report of headache-free days per week, current headache pain, duration of headache disorder, total score on the MIDAS, and disability due to headaches in the past week and past month. Similarly, a change score representing inhibition of pain due to CPM was computed by subtracting baseline electric pain from electric pain during ischemia (negative number represents inhibition), and correlations were computed between this pain inhibition and the above headache variables. To examine whether depression and pain catastrophizing explained any group differences found in CPM outcomes, repeated-measures analyses of covariance (ANCOVAs) were conducted (if necessary) with depression and pain catastrophizing entered as covariates. CPM phase (baseline vs conditioning) was the within-subjects factor, and group (migraine vs control) was the between-subjects factor. Significance was set at 0.05. Partial eta-squared (η2) was used as the effect size for F tests, and Cohen’s d was used for mean comparisons. Cohen [34] provides guidelines for interpreting η2 (small = 0.01, medium = 0.06, large = 0.14) and d (small = 0.20, medium = 0.50, large = 0.80). Analyses were completed in SPSS, and an a priori power analysis suggested that a sample size of 27 participants was required to achieve a power of 0.80. G*Power was utilized to calculate power with a compromise analysis for one-tailed effects. Based on the final sample sizes, assuming a small effect size of d = 0.30, power was estimated between 0.74 and 0.80 for primary analyses assessing CPM outcomes.
Results
Group Differences in Background Variables and Potential Confounds
Seventy-three percent of participants (N = 40) were female (72% of controls and 74% of participants with migraines) (Table 1). Age ranged from 18 to 69 years (M = 22.80, SD = 8.43) with no significant difference in age between groups. For participants without migraine headaches, 88% were Caucasian, 6% African American, 3% Asian, and 3% Native American (Table 1). For participants with migraine headaches, 70% were Caucasian, 9% Hispanic, and 4% each were African American, Asian, Native American, and other. Of participants with migraine headaches, 83% (N = 19) had migraine without aura, 13% (N = 3) had migraine with aura (data were missing for one subject regarding presence/absence of aura), and 4% (N = 1) had chronic migraines.
Participants with migraines and controls did not differ in CES-D depression scores, pain catastrophizing, pain due to forearm ischemia, baseline nerve stimulation pain, or baseline nBR magnitudes (Table 1). Moreover, chi-square tests indicated that cases of clinically significant depression (CES-D ≥ 16, χ2 = 0.001, P = 0.98) and clinically significant pain catastrophizing (PCS ≥ 30, χ2 = 2.41, P = 0.12) were not different between the two groups. Participants with migraine headaches had greater severity and interference with activities, as assessed by the MIDAS, due to headaches compared with the control participants (total score of 14.00 indicating “moderate disability” vs 5.10 indicating “little or no disability,” respectively) (Table 1). Participants in the migraine group reported a greater duration for their headache disorder compared with those in the control group with ETTH (approximately 10 years vs 3.5 years, respectively) (Table 1). Groups did not differ on current headache pain, headache-free days per week, self-reported disability in the past 30 days, or sleep quantity during the prior night (Table 1). Two participants (one control, one migraine) reported currently taking an anticonvulsant medication.
Conditioned Pain Modulation
Pain Report
For healthy controls, results indicated that there was a significant inhibition of pain report (t(31) = 2.55, P = 0.02, d = 0.36) during the counterstimulation task (M = 51.64, SD = 15.07) compared with baseline (M = 56.77, SD = 13.65). Similarly, for participants with migraine headaches, there was a significant inhibition of pain report (t(22) = 3.09, P = 0.01, d = 0.37) during the counterstimulation task (M = 52.55, SD = 18.24) compared with baseline (M = 58.79, SD = 15.31).
Nociceptive Blink Reflex
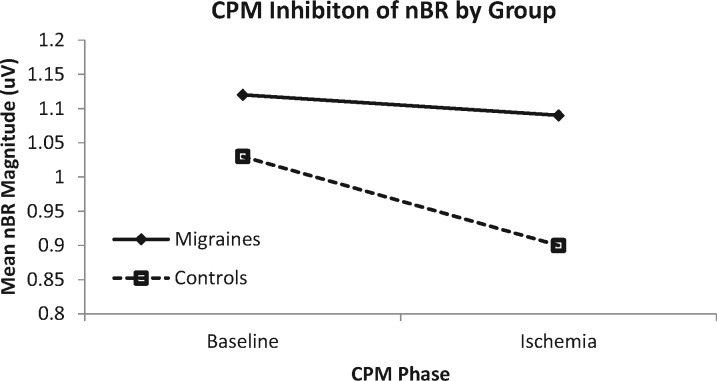
For healthy controls, results indicated that there was a significant inhibition of the nBR (t(30) = 3.12, P = 0.004, d = 0.32) during the counterstimulation task (M = 0.90, SD = 0.41) compared with baseline (M = 1.03, SD = 0.40) (Figure 1, Table 2). In contrast, for participants with migraine headaches, there was no significant difference (t(17) = 0.64, P = 0.53, d = 0.05) in nBR during the counterstimulation task (M = 1.09, SD = 0.60) compared with baseline (M = 1.12, SD = 0.53).
Figure 1.
Conditioned pain modulation (CPM) inhibition of the nociceptive blink reflex (nBR) in persons with migraine headaches and nonmigraine controls. The control group showed a significant inhibition of the nBR during the ischemia task compared with baseline. The migraine group did not evidence a significant change in nBR magnitude during the ischemia task, suggesting impaired CPM in this group.
Table 2.
Conditioned pain modulation of nociceptive blink reflex
| CPM Phase |
|||||
|---|---|---|---|---|---|
| Baseline M (SD) | Counterstimulus M (SD) | t | P | d | |
| Migraine | 1.12 (0.53) | 1.09 (0.60) | 0.64 | 0.53 | 0.05 |
| Controls | 1.03 (0.40) | 0.90 (0.41) | 3.12 | <0.01 | 0.32 |
Baseline nBR magnitude was not statistically different between the migraine and control groups (see Table 1 for corresponding statistics).
CPM = conditioned pain modulation; d=Cohen’s d values (small = 0.2, medium = 0.5, large = 0.8); nBR = nociceptive blink reflex.
Relationships Between CPM and Individual Difference Variables
Headache Variables
There were no significant correlations between headache variables (headache-free days per week, current headache pain, duration of headache disorder, MIDAS total score, and reported disability due to headaches) and CPM inhibition of nBR (r < 0.30, P > 0.20) or CPM inhibition of pain report (r < 0.37, P > 0.16).
Controlling for Psychological Variables
To examine whether depression and pain catastrophizing explained the lack of CPM inhibition of nBR in the migraine group, a repeated-measures ANCOVA was conducted with depression and pain catastrophizing entered as covariates. There was a significant main effect of CPM phase (F(1, 43) = 5.89, P = 0.02, η2 = 0.12), indicating significant pain inhibition during counterstimulation compared with baseline phases. However, the main effect was qualified by a significant CPM phase × group interaction (F(1, 43) = 5.32, P = 0.03, η2 = 0.11). Control participants had a significant inhibition of nBR (P < 0.01, d = 0.37) during counterstimulation (M = 0.88, SD = 0.40) compared with baseline (M = 1.03, SD = 0.41). Participants with migraine headaches did not have a significant difference in nBR (P = 0.74, d = 0.03) during counterstimulation (M = 1.08, SD = 0.62) vs baseline (M = 1.10, SD = 0.54). Thus, after controlling for depression and catastrophizing, the migraine group did not evidence a significant inhibition of nBR during counterstimulation, in contrast to the control group, which did have pain inhibition. This indicates that depression and pain catastrophizing did not account for the lack of CPM inhibition of nBR in the migraine group.
Discussion
Conditioned pain modulation assesses the extent of pain inhibition at one body site during a noxious counterstimulus and is often used as a measure of the efficiency of supraspinal pain modulation processes. Research has indicated that CPM may be impaired in persons with chronic or recurrent pain conditions, however, the evidence is limited and sometimes conflicting regarding CPM in persons with migraine headaches. The present study assessed CPM of both pain report and nBR in persons with and without migraine headaches.
Pain report was inhibited by CPM in both the migraine and healthy control groups. However, unlike controls, participants with migraines did not exhibit an inhibition of the nBR during the ischemic counterstimulation task. This pattern of modulation of nBR persisted after controlling for level of pain catastrophizing and depression.
These results suggest that the CPM of the nBR is impaired in individuals with a history of migraine headaches, suggesting a deficiency in endogenous inhibition of trigeminal nociception. Due to the cross-sectional nature of these data, it cannot be determined whether experiencing repeated migraines may lead to impaired CPM inhibition or if impaired CPM inhibition is a risk factor for experiencing migraines, though several studies support the latter [35–37]. Research indicates that impaired pain inhibitory responses (including DNIC/CPM) are predictive of increased severity of acute pain and poorer response to pain treatments and are associated with experiencing more frequent minor daily pains [35].
However, few have directly assessed the association between pain inhibition and the future development of chronic or recurrent pain conditions. Yarnitsky and colleagues [36] found that patients who had impaired CPM inhibition of pain before surgery were more likely to develop chronic postsurgical pain compared with those who had an efficient CPM response. In contrast, pain threshold and magnitude at baseline were not associated with the development of chronic postsurgical pain. Shahidi and colleagues [38] identified impaired CPM inhibition as a predictor of the development of chronic neck pain in a group of initially pain-free adults. In a group of children and adolescents, Holley and colleagues [37] found that impaired CPM inhibition was associated with progression of acute (one month or less) musculoskeletal pain to chronic pain (four months). As described by Yarnitsky [39], these studies suggest that a pronociceptive state, as evidenced by pain inhibitory deficits, likely predisposes the patient to development of future pain. Although much of this research has focused on chronic pain (as opposed to recurrent pain, as seen in episodic migraine), there is evidence that the pronociceptive state (as evidenced by deficient CPM) observed in chronic pain is also present in recurrent pain conditions [40,41], including episodic migraine [14]. This suggests that similar mechanisms likely contribute to the development and maintenance of chronic and recurrent/episodic pain conditions, and thus it can be hypothesized that impaired CPM as seen in the current study may be present before the onset of a migraine disorder and increase a person’s risk of developing migraines. However, to our knowledge, research has not yet assessed CPM as a predictor of migraine headache development. Future research should seek to refine hypotheses regarding the mechanisms involved in impaired CPM of nBR in persons with migraine disorders.
Despite evidence that CPM inhibition of the medullary mediated nBR is impaired in participants with migraines, there was no difference between groups in CPM inhibition of pain report. This is in contrast to the findings of Sandrini and colleagues [14], who assessed the CPM of the nociceptive flexion reflex and pain report during a cold pressor counterstimulation task in patients with migraines and chronic tension-type headaches compared with controls. Their results showed that the migraine and chronic tension-type headache groups had impaired inhibition of both the nociceptive flexion reflex and pain report.
Prior research has had mixed results regarding CPM inhibition of pain report in migraine patients with some studies finding impaired CPM [14–16] and others finding no deficits [17–19]. Differences in methodology could be a contributing factor to these seemingly conflicting results. Nahman-Averbuch and colleagues [15] found that compared with controls, migraine participants had waning efficacy of CPM over subsequent trials with no group differences on the initial test. This suggests persons with migraines may appear to have similar efficiency of CPM inhibition of pain if assessed only once or if assessed too soon after onset of the counterstimulus because testing may be occurring before significant waning of CPM efficacy. Indeed, the test stimulus in the present study began after just 30 seconds of the ischemia task and continued until only two minutes after initiation of ischemia. This is a significantly shorter period of time after onset of the counterstimulus compared with Sandrini and colleagues [14] and de Tommaso and colleagues [42] (up to five minutes and five or more minutes after counterstimulus onset, respectively); both studies found impaired CPM of pain report in migraine patients. Additionally, the pain due to forearm ischemia in the present study may be expected to increase throughout the course of the ischemia procedure, and thus the full effects of this counterstimulus may not be most visible early in the ischemia task. Future studies should assess CPM inhibition over longer time courses in migraine patients to better understand these differences.
Despite a significant CPM inhibition of pain report in both the migraine and control groups in the present study, migraine participants did not have CPM inhibition of nBR, whereas control participants did. Although it may seem logical that modulation of nociceptive reflexes would result in a similar modulation as pain report because these modulated signals travel to the brain, there is evidence that there are at least partially separate processes for modulation of nociception and supraspinal pain perception [43–46]. Several studies have found a divergence between reported pain and reflexive measures of nociception, including the nociceptive flexion reflex [43–45] and nBR [47,48]. Koh and Drummond [47] found that a serial subtraction task during electrical stimulation of the supraorbital region led to facilitation of the nBR but inhibition of reported pain. Studies assessing cognitive or emotional modulation of pain and nociception in patients with depression [49] or insomnia [50] have shown divergent effects on pain and nociception, such that nociceptive flexion reflexes were modulated similarly to control groups but pain report was not.
Another possibility for the divergence between modulation of pain report and the nBR is that pain report may be more susceptible to confound, bias, or other forms of cognitive modulation. Ischemia, the conditioning stimulus in our study, may have served as a distractor, which has previously been found to reduce perceived pain during experimental pain tasks [51]. Thus, despite an impaired CPM response in participants with migraine headaches, resulting in no inhibition of the nBR, the distracting effect of the ischemia task could lead to an inhibition of pain report. By contrast, inhibition of pain report in healthy controls may be due to an additive effect of distraction and CPM.
Given that impaired CPM in persons with chronic and recurrent pain disorders, including migraine disorders, has been hypothesized to play a role in the pathophysiology of idiopathic pain, it could also be hypothesized that the extent of CPM impairment may be associated with severity of the pain disorder. For example, a person with a greater CPM deficit may have developed the pain condition at a younger age and thus have a longer duration of illness, or may have more severe or impairing symptoms compared with a person with less impairment of CPM. This is supported by the research by Nahman-Averbach and colleagues [15], who found that a decreasing efficiency of CPM over repeated trials was associated with migraine severity. However, the present study did not find any significant correlations between migraine disorder variables (including frequency, current pain, duration of disorder, and disability) and CPM inhibition of pain. This is consistent with other research that has also failed to find a correlation between central sensitization and headache symptom variables [52]. This suggests that impaired CPM may confer an absolute risk (i.e., high risk vs low risk) rather than a progressive risk corresponding to increasing levels of impairment of CPM; however, future research should assess this further.
Previous research by Piche et al. [40] found that group differences in CPM showing impaired inhibition of pain report in persons with irritable bowel syndrome compared with controls were eliminated after controlling for psychological variables including depression and pain catastrophizing. In the present study, controlling for psychological variables did not alter the pattern of significant results for group differences in CPM of the nBR (i.e., significant inhibition of nBR in the control group, lack of inhibition of nBR in the migraine group). This could be explained by the lack of group differences in depression and catastrophizing scores in the present study, whereas in the Piche et al. [40] study, Irritable Bowel Syndrome participants had significantly higher scores on pain catastrophizing and depression scales. It is also possible that psychological variables have a larger impact on the modulation of pain report compared with modulation of nociceptive reflexes.
Our study has several strengths. CPM was assessed in both a nociceptive reflex and subjective pain report, which allowed mechanisms at medullary and supraspinal levels to be studied. Second, trigeminal nociception was assessed using the nBR in response to stimulation of the supraorbital branch of the trigeminal nerve, which, given the role of trigeminal pain pathways in migraine headaches, may be more specific to the pathophysiology of migraine disorders.
However, our study also had several limitations. We did not use a nonpainful counterstimulus as a comparison to the ischemia task, which would have allowed us to control for other factors, such as distraction, that could influence reported pain. Additionally, only one type of counterstimulus was used, which limits the generalizability of our findings, as different stimuli may elicit different subsystems of pain modulation [53,54]. Also, some members of the control group had nonmigraine headache disorders (specifically episodic tension-type headaches). Given that some research has found impairments in CPM in patients with chronic tension-type headaches [14], this may have decreased differences between the migraine and control groups in our study. Further, we did not have medical records to verify headache diagnoses or headache treatments. Participants in both the migraine and control groups were relatively young (mean age of about 23 years), and thus migraine participants may have a relatively short duration of their migraine disorder. Further, the severity of migraine disorders in the study population is likely on the low end of the spectrum (given the infrequent reported use of migraine prophylactic prescriptions and average reported disability in the moderate range), and almost all migraine participants had episodic migraines. This limits the generalizability of the results, necessitating replication in populations with chronic migraines and more severe episodic migraine disorders.
In replicating these data, future studies should include larger sample sizes to allow for more sophisticated statistical analyses, including Bonferroni corrections, and to improve the generalizability of findings. This could also allow for more thorough examination of potential confounds. In the present study, findings were maintained after controlling for depression and pain catastrophizing. However, there are other potential confounds (e.g., anxiety, migraine prophylactic medication use, method of pain report, etc.) that may influence the present findings. A larger sample would accommodate the inclusion of all these possible confounds. Another study limitation was the switch from a computer-entered to verbal rating for the NRS pain assessment. Research indicates that verbal NRS and electronic NRS scales are highly correlated [55], and the present results for the control group are consistent with the extensive research literature on CPM in healthy persons. This indicates that the current methodology likely provided a valid assessment of pain modulation; however, future studies would benefit from avoiding variations in the method of pain report to minimize potential confounds.
Due to the mixed findings of previous studies, future research should aim to replicate and extend our results. In particular, future studies are needed to elucidate whether impaired CPM inhibition is best conceptualized as a risk factor for or result of migraine pain and whether impaired CPM inhibition in healthy individuals is related to development of migraines over time. Clinically, impaired CPM may be a predictor of future development of migraine disorders. The identification of individuals at risk for migraine disorders would facilitate the development of strategies to prevent the onset of migraines. Additionally, future research should investigate how CPM may be altered with migraine treatment. Future studies should also investigate CPM across various headache disorders (tension-type headaches, cluster headaches, etc.) in addition to migraines, during headaches vs interictal periods, and across subgroups of migraine patients (e.g., mild vs severe, chronic vs episodic, or brief vs long duration).
In summary, migraine sufferers exhibited impaired CPM inhibition of the nBR, which suggests a deficiency in inhibition of trigeminal nociception. This deficiency may be closely tied to the development of migraine headaches and may represent a risk factor for development of migraines. Replication of these results is necessary to determine the role that impaired CPM plays in the development of migraine disorders. Identification of these mechanisms would enable individuals at risk for the development of migraines to be targeted to prevent the development of migraines.
Acknowledgments
The authors would like to thank the following individuals for their assistance with data collection for this project: Emily Main, Jennifer Russell, PhD, Mary Chandler, Ashley Vincent, MA, Jennifer DelVentura, PhD.
Disclosures and conflicts of interest: Dr. Emily Bartley is funded by the National Institute on Aging (K99AG052642). Dr. Rhudy is funded by the National Institute on Minority Health and Health Disparities (R01MD007807). No other relevant disclosures or conflicts of interest exist.
References
- 1. Gebhart GF. Descending modulation of pain. Neurosci Biobehav Rev 2004;27(8):729–37. [DOI] [PubMed] [Google Scholar]
- 2. Ossipov MH, Morimura K, Porreca F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care 2014;8(2):143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ren K, Dubner R. Descending modulation in persistent pain: An update. Pain 2002;100(1–2):1–6. [DOI] [PubMed] [Google Scholar]
- 4. Zambreanu L, Wise RG, Brooks JC, Iannetti GD, Tracey I. A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. Pain 2005;114(3):397–407. [DOI] [PubMed] [Google Scholar]
- 5. Bouhassira D, Villanueva L, Bing Z, le Bars D. Involvement of the subnucleus reticularis dorsalis in diffuse noxious inhibitory controls in the rat. Brain Res 1992;595(2):353–7. [DOI] [PubMed] [Google Scholar]
- 6. Goffaux P, de Souza JB, Potvin S, Marchand S. Pain relief through expectation supersedes descending inhibitory deficits in fibromyalgia patients . Pain 2009;145(1–2):18–23. [DOI] [PubMed] [Google Scholar]
- 7. Goffaux P, Redmond WJ, Rainville P, Marchand S. Descending analgesia—when the spine echoes what the brain expects. Pain 2007;130(1–2):137–43. [DOI] [PubMed] [Google Scholar]
- 8. Williams AE, Rhudy JL. Supraspinal modulation of trigeminal nociception and pain. Headache 2009;49(5):704–20. [DOI] [PubMed] [Google Scholar]
- 9. Drummond PD. The effect of trigeminal nociceptive stimulation on blink reflexes and pain evoked by stimulation of the supraorbital nerve. Cephalalgia 2003;23(7):534–40. [DOI] [PubMed] [Google Scholar]
- 10. Giffin NJ, Katsarava Z, Pfundstein A, Ellrich J, Kaube H. The effect of multiple stimuli on the modulation of the ‘nociceptive’ blink reflex. Pain 2004;108(1–2):124–8. [DOI] [PubMed] [Google Scholar]
- 11. Motohashi K, Umino M. Heterotopic painful stimulation decreases the late component of somatosensory evoked potentials induced by electrical tooth stimulation. Brain Res Cogn Brain Res 2001;11(1):39–46. [DOI] [PubMed] [Google Scholar]
- 12. Serrao M, Rossi P, Parisi L, et al. Trigemino-cervical-spinal reflexes in humans. Clin Neurophysiol 2003;114(9):1697–703. [DOI] [PubMed] [Google Scholar]
- 13. Mauser ED, Rosen NL. So many migraines, so few subspecialists: Analysis of the geographic location of United Council for Neurologic Subspecialties (UCNS) certified headache subspecialists compared to United States headache demographics. Headache 2014;54(8):1347–57. [DOI] [PubMed] [Google Scholar]
- 14. Sandrini G, Rossi P, Milanov I, et al. Abnormal modulatory influence of diffuse noxious inhibitory controls in migraine and chronic tension-type headache patients. Cephalalgia 2006;26(7):782–9. [DOI] [PubMed] [Google Scholar]
- 15. Nahman-Averbuch H, Granovsky Y, Coghill RC, et al. Waning of “conditioned pain modulation”: A novel expression of subtle pronociception in migraine. Headache 2013;53(7):1104–15. [DOI] [PubMed] [Google Scholar]
- 16. de Tommaso M, Difruscolo O, Sardaro M, et al. Effects of remote cutaneous pain on trigeminal laser-evoked potentials in migraine patients. J Headache Pain 2007;8(3):167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teepker M, Kunz M, Peters M, et al. Endogenous pain inhibition during menstrual cycle in migraine. Eur J Pain 2014;18(7):989–98. [DOI] [PubMed] [Google Scholar]
- 18. Perrotta A, Serrao M, Sandrini G, et al. Sensitisation of spinal cord pain processing in medication overuse headache involves supraspinal pain control. Cephalalgia 2010;30(3):272–84. [DOI] [PubMed] [Google Scholar]
- 19. Serrao M, Perrotta A, Bartolo M, et al. Enhanced trigemino-cervical-spinal reflex recovery cycle in pain-free migraineurs. Headache 2005;45(8):1061–8. [DOI] [PubMed] [Google Scholar]
- 20. Ellrich J, Katsarava Z, Przywara S, Kaube H. Is the R3 component of the human blink reflex nociceptive in origin? Pain 2001;91(3):389–95. [DOI] [PubMed] [Google Scholar]
- 21. Romaniello A, Valls-Sole J, Iannetti GD, et al. Nociceptive quality of the laser-evoked blink reflex in humans. J Neurophysiol 2002;87(3):1386–94. [DOI] [PubMed] [Google Scholar]
- 22. Kaube H, Katsarava Z, Kaufer T, Diener H, Ellrich J. A new method to increase nociception specificity of the human blink reflex. Clin Neurophysiol 2000;111(3):413–6. [DOI] [PubMed] [Google Scholar]
- 23. Headache Classification Committee of the International Classification of Headache Disorders, 2nd edition. Cephalgia 2004;24 suppl 1:1–160. 2004. [DOI] [PubMed]
- 24. Vincent A, Williams A, Bartley E, et al. Emotional Modulation of Trigeminal Pain and the Nociceptive Blink Reflex (nBR) in Persons with Migraine. Program No. 854.6/W32. Chicago, IL: Society for Neuroscience; 2009. [Google Scholar]
- 25. Ellrich J, Bromm B, Hopf HC. Pain-evoked blink reflex. Muscle Nerve 1997;20(3):265–70. [DOI] [PubMed] [Google Scholar]
- 26. Hopf HC. Topodiagnostic value of brain stem reflexes. Muscle Nerve 1994;17(5):475–84. [DOI] [PubMed] [Google Scholar]
- 27. Proietti Cecchini A, Sandrini G, Fokin IV, Moglia A, Nappi G. Trigeminofacial reflexes in primary headaches. Cephalalgia 2003;23(Suppl 1):33–41. [DOI] [PubMed] [Google Scholar]
- 28. Stewart WF, Lipton RB, Kolodner KB, et al. Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain 2000;88(1):41–52. [DOI] [PubMed] [Google Scholar]
- 29. Stewart WF, Lipton RB, Simon D, Von Korff M, Liberman J. Reliability of an illness severity measure for headache in a population osample of migraine sufferers. Cephalalgia 1998;18(1):44–51. [DOI] [PubMed] [Google Scholar]
- 30. Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1977;1(3):385–401. [Google Scholar]
- 31. Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychology Aging 1997;12(2):277–87. [DOI] [PubMed] [Google Scholar]
- 32. Crombez B, Bijttebier P, Eccleston C, et al. Pain Catastrophizing Scale (Child Version and Parent Version). Measurement Instrument Database for the Social Science; 2012. Retrieved from www.midss.ie [Google Scholar]
- 33. Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess 1995;7:524–32. [Google Scholar]
- 34. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1977. [Google Scholar]
- 35. Edwards RR. Individual differences in endogenous pain modulation as a risk factor for chronic pain. Neurology 2005;65(3):437–43. [DOI] [PubMed] [Google Scholar]
- 36. Yarnitsky D, Crispel Y, Eisenberg E, et al. Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. Pain 2008;138(1):22–8. [DOI] [PubMed] [Google Scholar]
- 37. Holley AL, Wilson AC, Palermo TM. Predictors of the transition from acute to persistent musculoskeletal pain in children and adolescents: A prospective study. Pain 2017;158(5):794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shahidi B, Curran-Everett D, Maluf KS. Psychosocial, physical, and neurophysiological risk factors for chronic neck pain: A prospective inception cohort study. J Pain 2015;16(12):1288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): Its relevance for acute and chronic pain states. Curr Opin Anaesthesiol 2010;23(5):611–5. [DOI] [PubMed] [Google Scholar]
- 40. Piche M, Arsenault M, Poitras P, Rainville P, Bouin M. Widespread hypersensitivity is related to altered pain inhibition processes in irritable bowel syndrome. Pain 2010;148(1):49–58. [DOI] [PubMed] [Google Scholar]
- 41. Williams AE, Heitkemper M, Self MM, Czyzewski DI, Shulman RJ. Endogenous inhibition of somatic pain is impaired in girls with irritable bowel syndrome compared with healthy girls. J Pain 2013;14(9):921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Tommaso M, Sardaro M, Pecoraro C, et al. Effects of the remote C fibres stimulation induced by capsaicin on the blink reflex in chronic migraine. Cephalalgia 2007;27(8):881–90. [DOI] [PubMed] [Google Scholar]
- 43. Terkelsen AJ, Andersen OK, Hansen PO, Jensen TS. Effects of heterotopic- and segmental counter-stimulation on the nociceptive withdrawal reflex in humans. Acta Physiol Scand 2001;172(3):211–7. [DOI] [PubMed] [Google Scholar]
- 44. Willer JC, Boureau F, Albe-Fessard D. Supraspinal influences on nociceptive flexion reflex and pain sensation in man. Brain Res 1979;179(1):61–8. [DOI] [PubMed] [Google Scholar]
- 45. Bouhassira D, Danziger N, Atta N, Guirimand F. Comparison of the pain suppressive effects of clinical and experimental painful conditioning stimuli. Brain 2003;126(5):1068–78. [DOI] [PubMed] [Google Scholar]
- 46. Yekta SS, Lamp S, Ellrich J. Heterosynaptic long-term depression of craniofacial nociception: Divergent effects on pain perception and blink reflex in man. Exp Brain Res 2006;170(3):414–22. [DOI] [PubMed] [Google Scholar]
- 47. Koh CW, Drummond PD. Dissociation between pain and the nociceptive blink reflex during psychological arousal. Clin Neurophysiol 2006;117(4):851–4.. [DOI] [PubMed] [Google Scholar]
- 48. Rehberg B, Baars JH, Kotsch J, Koppe P, von Dincklage F. Comparison of trigeminal and spinal modulation of pain and nociception. Int J Neurosci 2012;122(6):298–304. [DOI] [PubMed] [Google Scholar]
- 49. Terry EL, DelVentura JL, Bartley EJ, Vincent AL, Rhudy JL. Emotional modulation of pain and spinal nociception in persons with major depressive disorder (MDD). Pain 2013;154(12):2759–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. DelVentura JL, Terry EL, Bartley EJ, Rhudy JL. Emotional modulation of pain and spinal nociception in persons with severe insomnia symptoms. Ann Behav Med 2014;47(3):303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moont R, Pud D, Sprecher E, Sharvit G, Yarnitsky D. ‘Pain inhibits pain’ mechanisms: Is pain modulation simply due to distraction? Pain 2010;150(1):113–20. [DOI] [PubMed] [Google Scholar]
- 52. Filatova E, Latysheva N, Kurenkov A. Evidence of persistent central sensitization in chronic headaches: A multi-method study. J Headache Pain 2008;9(5):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pud D, Granovsky Y, Yarnitsky D. The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain 2009;144(1–2):16–9. [DOI] [PubMed] [Google Scholar]
- 54. Nir RR, Yarnitsky D. Conditioned pain modulation. Curr Opin Support Palliat Care 2015;9(2):131–7. [DOI] [PubMed] [Google Scholar]
- 55. Castarlenas E, Sánchez-Rodríguez E, Vega Rde L, Roset R, Miró J. Agreement between verbal and electronic versions of the numerical rating scale (NRS-11) when used to assess pain intensity in adolescents. Clin J Pain 2015;31(3):229–34. [DOI] [PubMed] [Google Scholar]