Abstract
Background:
Machine learning (ML), a subset of artificial intelligence (AI) that aims to teach machines to automatically learn tasks by inferring patterns from data, holds significant promise to aid psoriasis care. Applications include evaluation of skin images for screening and diagnosis as well as clinical management including treatment and complication prediction.
Objective:
To summarize literature on ML applications to psoriasis evaluation and management and to discuss challenges and opportunities for future advances.
Methods:
We searched MEDLINE, Google Scholar, ACM Digital Library, and IEEE Xplore for peer-reviewed publications published in English through December 1, 2019. Our search queries identified publications with any of the 10 computing-related keywords and “psoriasis” in the title and/or abstract.
Results:
Thirty-three studies were identified. Articles were organized by topic and synthesized as evaluation- or management-focused articles covering 5 content categories: (A) Evaluation using skin images: (1) identification and differential diagnosis of psoriasis lesions, (2) lesion segmentation, and (3) lesion severity and area scoring; (B) clinical management: (1) prediction of complications and (2) treatment.
Conclusion:
Machine learning has significant potential to aid psoriasis evaluation and management. Current topics popular in ML research on psoriasis are the evaluation of medical images, prediction of complications, and treatment discovery. For patients to derive the greatest benefit from ML advancements, it is helpful for dermatologists to have an understanding of ML and how it can effectively aid their assessments and decision-making.
Keywords: psoriasis, dermatology/trends, machine learning, artificial intelligence, systematic review
Introduction
Machine learning (ML) is a subset of artificial intelligence (AI) that aims to teach machines to automatically learn tasks by inferring patterns from data. With the advent of medical devices and electronic medical records, the amount of available medical data has grown exponentially, and with it, so has ML’s potential to learn medical tasks. It is helpful for clinicians to gain an understanding of what ML is, what its clinical applications are, and how they can work with machine-assisted diagnoses and decisions in the future to provide patients with the best possible care.
As a visual field with a large patient base, dermatology has seen perhaps some of the most advanced progress in ML research, especially in the automatic interpretation of medical images.1–8 Dermatological images are unique in which images of the skin can be taken in clinic or at home by a clinician, patient, or caregiver, providing relatively speedy access to valuable information on disease progression and patient outcomes. However, the unstandardized process of dermatological image capture also poses challenges, such as wide variability in quality metrics such as sharpness, exposure, color balance, and perspective.
Researchers have tackled medical image interpretation using a range of ML algorithms.1–12 Deep neural networks (DNNs) are one popular approach.1–8 Deep neural networks are powerful in which they are designed to learn patterns from large quantities of data without the need for user-provided domain knowledge of the task the DNN is trying to solve. Deep convolutional neural networks (DCNNs), a type of DNN especially well adapted for visual imagery, have been used to classify images of melanoma without being explicitly instructed to look for differences in asymmetry, borders, color, and diameter. Esteva et al used a DCNN trained on 129 450 clinical images consisting of 2032 different diseases to classify benign versus malignant skin lesions.1 Their ML model achieved sensitivity and specificity on par (area under the curve > 0.91) with 21 board-certified dermatologists at classifying malignant carcinomas versus benign seborrheic keratoses and malignant melanomas versus benign nevi.
The large patient base of dermatology also lends the field to big data analysis by ML. Electronic health records and online patient forums such as Reddit are examples of large databases that have been mined using natural language processing methods in order to identify population-level trends in dermatology patient experiences and therapeutics, such as the use of home therapies outside of standard clinical practice.13
Psoriasis is a skin disease with profound impacts on quality of life and significant morbidities such as increased susceptibility to inflammatory (psoriatic) arthritis and major cardiometabolic comorbidity.14–18 Given that the disease is largely evaluated and managed through visual inspection and that it has a significant prevalence estimated at 7.4 million adult Americans,19 psoriasis diagnosis and care lends itself well to ML tasks like those described above. We conducted a systematic review on studies applying ML to improve the clinical evaluation and management of psoriasis. We conclude with a discussion of ML’s challenges, opportunities, and future directions for dermatologists in psoriasis care.
Methods
We performed a literature search for peer-reviewed publications in 4 databases: MEDLINE, Google Scholar, ACM Digital Library, and IEEE Xplore. We chose these databases in order to cover medical (MEDLINE), computing (ACM Digital Library and IEEE Xplore), and general resources (Google Scholar). Peer-reviewed articles published in English up to December 1, 2019, were considered. We queried for studies with titles and/or abstracts containing any of the 10 ML-related keywords combined with “psoriasis” using the “AND” operator: “machine learning,” “artificial intelligence,” “segmentation,” “computer vision,” “neural networks,” “deep learning,” “supervised learning,” “unsupervised learning,” “natural language processing,” and “reinforcement learning.” An example query to demonstrate our search method is “psoriasis[Title/Abstract] AND machine learning[Title/Abstract].” The 10 keywords were chosen in order to cover a broad range of topics relevant to ML research on psoriasis.
Two reviewers (K.Y. and M.S.) independently evaluated citation titles and abstracts to assess study eligibility. Duplicates, non-peer-reviewed articles, non-English articles, and articles published only as an abstract were removed. Abstracts were assessed for relevance to ML research on psoriasis, and differences in opinion between the 2 reviewers were resolved through discussion. The remaining eligible publications were reviewed in full-text, summarized, grouped into topic categories, and qualitatively synthesized. Figure 1 reports our systematic review process using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses framework.20
Figure 1.
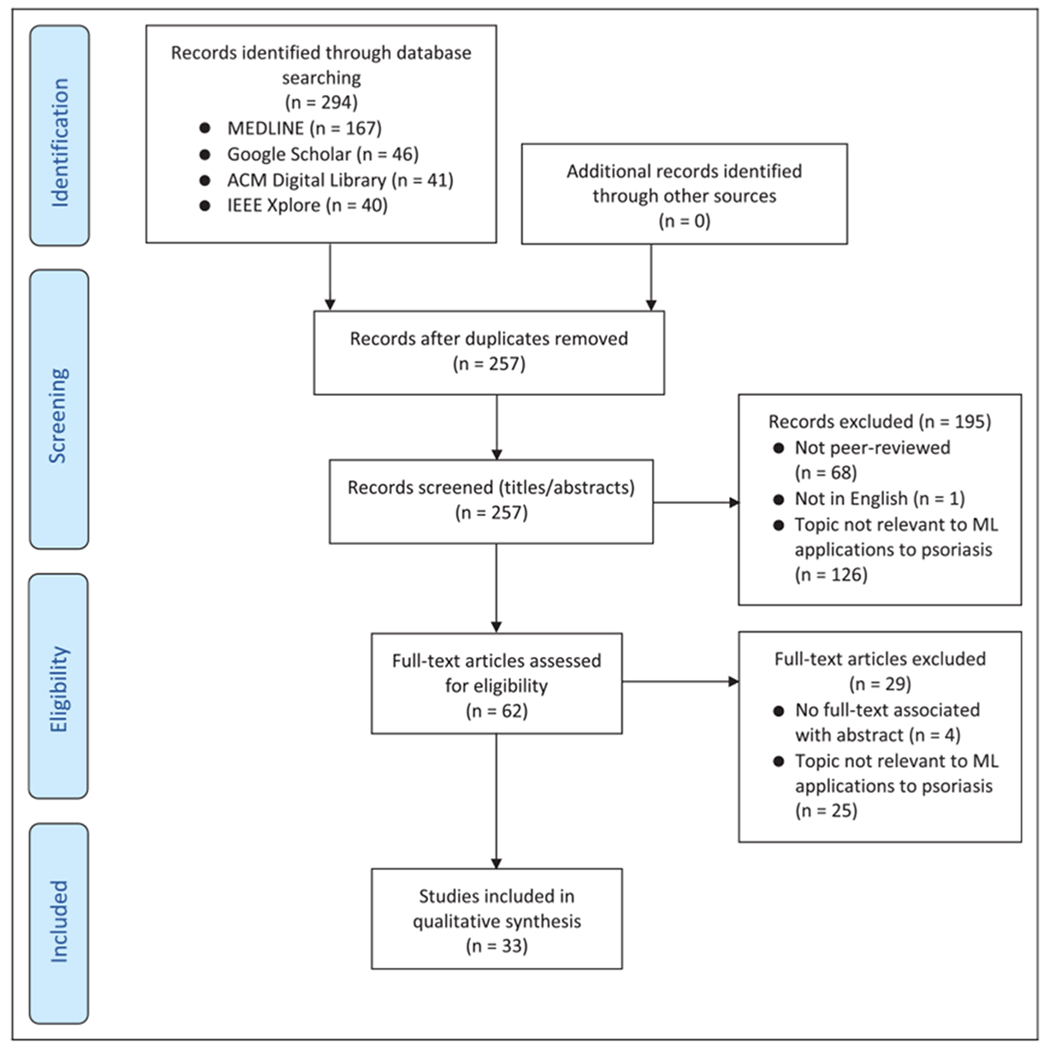
Systematic review flowchart according to the PRISMA framework. PRISMA indicates Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Results
Our search method identified 294 citations. After following the review protocol, 33 full-text articles were included for qualitative synthesis (Figure 1). These were divided into 2 broad categories each with 2 to 3 subcategories: (A) Evaluation using skin images: (1) identification and differential diagnosis of psoriasis lesions (8 articles), (2) lesion segmentation (8 articles), and (3) lesion severity and area scoring (12 articles); (B) clinical management: (1) prediction of complications (2 articles) and (2) treatment (3 articles). The 33 studies are summarized in Table 1 and are qualitatively synthesized in this review to describe the current state of advances in ML research on psoriasis, its limitations and challenges, and its future directions.
Table 1.
Summary of 33 Studies Reviewed on ML Applications to Psoriasis Evaluation and Management.
| Application category | Study | Objectives and results | Methods | Data set | Dataset source and annotations |
|---|---|---|---|---|---|
| A. Evaluation using skin images | |||||
| Identification and differential diagnosis of psoriasis lesions | Aggarwal (2019) | Demonstrated that data augmentation can improve machine learning image recognition of 5 dermatological diseases: acne, atopic dermatitis, impetigo, psoriasis, and rosacea. Each of the 5 diseases had an increase in AUC after data augmentation, with an average increase in AUC of 0.132 and SD of 0.033. The AUC with data augmentation was 0.87 for psoriasis | DCNNs with data augmentation | 332, 92, 138, 280, and 96 skin images for acne, atopic dermatitis, impetigo, psoriasis, and rosacea, respectively | Open-source dermatological images captured through DermNet, Dermatology Atlas, Hellenic Dermatological Atlas, and Google Images |
| Kim et al (2019) | Used smartphone-based multispectral imaging to discriminate between seborrheic dermatitis and psoriasis on the scalp. Demonstrated that machine learning-based methods for classification yielded better outcomes than conventional spectral classification methods, achieving a sensitivity of 65% to 75% and specificity of 70% to 80% | SVM, logistic regression (Logi), and multilayer perceptron (MLP) | 18 000 spectral signatures obtained from seborrheic dermatitis and psoriasis lesions on the scalp for training and 2000 spectral signatures for testing; followed by model validation with images from 60 patients in a clinical trial | Images captured via smartphone-based multispectral imaging system with an external CMOS camera; annotations confirmed by 3 different medical doctors | |
| Verma et al (2019) | Developed an ensemble data mining and machine learning method to classify 6 skin diseases: psoriasis, seborrheic dermatitis, lichen planus, pityriasis rosea, chronic dermatitis, and pityriasis rubra. The multi-model ensemble method achieved an accuracy of 98.64% | Classification and regression trees (CART), SVM, DT, RF, gradient boosted decision trees (GBDT) | 366 psoriasis histopathology images | Images from UC Irvine machine learning repository (Guvenir et al, 1998) | |
| Zhao et al (2019) | Developed a CNN to classify skin images of 9 common skin disorders (lichen planus, lupus erythematosus, basal cell carcinoma, squamous cell carcinoma, eczema, pemphigus, psoriasis, and seborrheic keratosis) as psoriasis vs. non-psoriasis. CNN classifier showed superior performance (missed diagnosis rate: 0.03, misdiagnosis rate: 0.04) than 25 Chinese dermatologists (missed diagnosis rate: 0.19, misdiagnosis rate: 0.10) in diagnosis of psoriasis on 100 clinical images | DCNN | 8021 skin images of 9 common disorders including 900 psoriasis images | Images collected by dermatologists at Xiangya hospital; Annotated by 3 dermatologists with > 10 years experience at Xiangya Hospital according to the corresponding medical record and pathology results | |
| Shrivastava et al (2016) | Developed a computer-aided detection system to classify skin images from psoriasis patients as healthy versus diseased using higher order spectra, texture, and color features, with a classification accuracy of more than 99% | Principal component analysis (PCA), SVM | 540 skin images (270 healthy and 270 diseased) from 30 psoriasis patients of Indian ethnic origin | Images captured and annotated by a dermatologist at the Psoriasis Clinic and Research Centre, Psoriatreat, Pune, Maharashtra, India | |
| Shrivastava et al (2016) | Assessed the reliability of a method developed in a previous study to classify skin images from psoriasis patients as healthy versus diseased, with a mean reliability index of 98.71% for 11 distinct data sizes | PCA, SVM | 540 skin images (270 healthy and 270 diseased) from 30 psoriasis patients of Indian ethnic origin | Images captured and annotated by a dermatologist at the Psoriasis Clinic and Research Centre, Psoriatreat, Pune, Maharashtra, India | |
| Shrivastava et al (2015) | Developed a computer-aided detection system to classify skin images from psoriasis patients as healthy vs. diseased using grayscale, color, redness, and chaoticness features, with a classification accuracy of more than 99% | PCA, SVM | 540 skin images (270 healthy and 270 diseased) from 30 psoriasis patients of Indian ethnic origin | Images captured and annotated by a dermatologist at the Psoriasis Clinic and Research Centre, Psoriatreat, Pune, Maharashtra, India | |
| Mashaly et al (2011) | Evaluated 4 techniques to classify skin images of 6 common papulosquamous skin diseases: psoriasis, lichen planus, atopic dermatitis, seborrheic dermatitis, pityriasis rosea, and pityriasis rubra pilaris. The rough sets method recorded the highest accuracy (78% to 94%) and sensitivity (825 to 96%) of segmentation compared with other techniques | Rough sets, Topological derivative, K-means clustering, watershed | 50 skin images from each of psoriasis, lichen planus, atopic dermatitis, seborrheic dermatitis, pityriasis rosea, and pityriasis rubra pilaris | Images from a dermatologic online image atlas, a dermatologic image database from the University of Iowa College of Medicine, and a dermatologic image atlas | |
| Lesion segmentation | Dash et al (2019) | Developed an automated psoriasis lesion segmentation method based on a modified U-Net architecture, referred as PsLSNet, achieving an accuracy of 94.8% with 89.6% sensitivity and 97.6% specificity | DCNN with a modified U-Net architecture (PsLSNet) | 5241 skin images of psoriasis lesions from 1026 psoriasis patients | Images captured and annotated by a dermatologist at the Psoriasis Clinic and Research Centre, Psoriatreat, Pune, Maharashtra, India |
| Pal et al (2018) | Developed an automated method to segment psoriasis skin biopsy images into dermis, epidermis and non-tissue regions, with the fully CNN method achieving an accuracy (defined as Ratio of Correct Pixel Classification) of 88% | DCNN | 90 psoriasis skin biopsy images | Psoriasis-affected tissue biopsies collected and clinically confirmed by a dermatologist of West Bengal, India, prior to imaging | |
| George et al (2017) | Developed an automated method for superpixel segmentation of psoriasis lesions in skin images, with a pixel accuracy of 86.99% | Multiscale superpixel clustering, K-means clustering | 676 psoriasis skin images from 44 psoriasis patients | Images obtained by clinical photographers at the Royal Melbourne Hospital, Australia, over 3 years; Automatically annotated using pixel-based skin segmentation method (George et al 2016) | |
| Jarad et al (2017) | Developed an automated method for psoriasis lesion segmentation in skin images using color spacing algorithms, with an accuracy of 95% | K-means clustering based on CIE Lab L*a*b color spaces | 80 psoriasis skin images (48 abnormal, 32 normal) | Images from database of psoriasis section of Ramadi Teaching Hospital, Ramadi, Anbar | |
| Shrivastava et al (2017) | Developed an automated method for segmentation of psoriasis lesions in skin images for accurate risk assessment using a Bayesian model, with a classification accuracy of 99.84% | SVM, DT, neural network | 670 cropped images from 110 psoriasis patient images (218 healthy, 29 mild, 138 moderate, 164 severe, 121 very severe) | Images captured and annotated by a dermatologist at the Psoriasis Clinic and Research Centre, Psoriatreat, Pune, Maharashtra, India | |
| Lu et al (2013) | Developed an automated method for segmentation of scaling in psoriasis skin images to evaluate disease severity | SVM | 103 psoriasis skin images | Images from University of Melbourne and St. Vincenťs Hospital Melbourne Department of Dermatology | |
| Taur et al (2006) | Developed an automated method for segmentation of psoriasis lesions in skin images using a MSSC | MSSC | 12 psoriasis skin images | Images from Taichung Veterans General Hospital, Taichung, Taiwan | |
| Taur (2003) | Developed an automated method for segmentation of psoriasis lesions in skin images into normal and affected regions | Neuro-fuzzy classifier | 3 psoriasis skin images | Images from Taichung Veterans General Hospital, Taichung, Taiwan | |
| Lesion severity and area scoring | George et al (2019) | Developed an automated method to score scale severity in psoriasis skin images using local descriptors, yielding a scale severity scoring accuracy of 80.81% | Bag-of-visual-words (BoVWs), SVM, RF | 96 psoriasis skin images | Images obtained by clinical photographers at the Royal Melbourne Hospital (RMH), Australia, over four years; Annotated by 3 dermatologists of RMH (correlation of 0.47, 0.51, and 0.34 between each dermatologist-pair) |
| Meienberger et al (2019) | Developed an automated algorithm to score psoriasis affected area, achieving an accuracy of more than 90% in 77% of the images and differed on average 5.9% from manually marked areas. The algorithm area estimates differed from physcians’ area estimates by 8.1% on average | DCNNs | 259 plaque psoriasis skin images from Caucasian patients (203 for training/validation, 56 for testing) | Frontal or dorsal photographs taken of Caucasian patients 18 to 80 years old with plaque psoriasis from University Hospital of Zurich Department of Dermatology in Switzerland; PASI assessed by physicians with more than 3 years of experience, supervised by a senior dermatologist | |
| Fink et al (2018) | Designed a total body imaging system that can also automate PASI measurements | Total body imaging system designed by authors | 10 psoriasis patients with 16 single body images per patient, captured using the authors’ total body imaging system | Total body imaging system created at University of Heidelberg Department of Dermatology, Germany; System’s PASI calculations compared to physicians’ assessments of 10 patients | |
| George et al (2018) | Developed a semi-supervised computer-aided system for automatic erythema severity scoring in psoriasis skin images, with a FI score of 0.71 for the random forest classifier | Patch-based dictionary learning, random forest, SVM, boosting | 676 psoriasis skin images from 44 psoriasis patients | Images obtained by clinical photographers at the Royal Melbourne Hospital, Australia, over 3 years | |
| Raina et al (2016) | Developed an automated method to score erythema severity in psoriasis plaque images, with good agreement with subjective assessment of erythema severity (kappa = 0.4203) | Linear discriminant analysis classifier | 80 psoriasis skin images from 20 psoriasis patients | Images taken from patients of Seton clinics including the University Medical Center Brackenridge Dermatology Clinic, Seton Family of Doctors at Hays, and Trinity Clinic; Labeled according to median rating by each of five dermatologists, including 1 attending and 4 experienced dermatology residents (intraclass correlation coefficient = 0.7306) | |
| Shrivastava et al. (2016) | Developed a psoriasis risk assessment system to risk stratify psoriasis severity from skin images | SVM, DT | 848 skin images (383 healthy, 47 mild, 245 moderate, 145 severe, 28 very severe) from Indian psoriasis patients | Images captured and annotated by a dermatologist at the Psoriasis Clinic and Research Centre, Psoriatreat, Pune, Maharashtra, India | |
| Shrivastava et al (2015) | Developed an automated method to risk stratify psoriasis severity from healthy and diseased skin images using color feature patterns, with an accuracy of ~99% | SVM, PCA | 540 skin images (270 healthy and 270 diseased) from 30 Indian psoriasis patients | Images captured and annotated by a dermatologist at the Psoriasis Clinic and Research Centre, Psoriatreat, Pune, Maharashtra, India | |
| Shrivastava et al (2015) | Reviewed technologies for psoriasis risk stratification in current and existing literature | Computer-aided diagnosis | N/A | N/A | |
| Fadzil et al (2013) | Developed an assessment method that incorporates 3D surface roughness with standard clustering techniques to objectively determine the PASI scaliness score for psoriasis lesions, with an accuracy of 94.12% | 3D surface roughness measurement, K-means clustering, fuzzy c-means clustering | 1999 psoriasis lesion images from 204 Malaysian psoriasis patients (1351 training, 648 test) | Images taken and annotated by dermatologists at Hospital Kuala Lumpar, Malaysia | |
| Savolainen et al (1997) | Developed a color segmentation method to assess involved surface area in psoriasis patients and compared performance to human eye assessments | CIA | 26 psoriasis patients with chronic plaque psoriasis (14 male, 7 female) | Images taken at Department of Dermatology and Venereology, Oulu University Hospital, Finland; Percent area of psoriasis involvement assessed simultaneously from projected slides of photographs by 2 dermatologists, 2 residents of dermatology, 2 nurses, and 2 medical students; Other PASI parameters assessed by a dermatologist | |
| Savolainen et al (1998) | Compared surface area estimates by human eye versus computer image analysis using color segmentation. Human eye estimates were higher than computer estimates, tending to overestimate in cases where the PASI was under 15 | CIA | 15 psoriasis patients (14 male, 1 female) | Images taken at Department of Dermatology and Venereology, Oulu University Hospital, Finland; PASI and skin area assessed by by dermatologist | |
| Gomez et al (2007) | Compared available change detection techniques in the visualization and quantification of bi-temporal psoriasis images | Simple image subtraction, PCA, post-classification comparison, MAD transform | 6 temporal series of psoriasis images (each consisting of 4 images collected in 1 week interval) | Images taken from Gentofte Hospital, Denmark; Lesion severity scored by dermatologists, and ground-truth change calculated by subtracting dermatologists’ scores at 2 different time instants | |
| B. Clinical management | |||||
| Prediction of complications | Munger et al (2019) | Used ML methods to determine top predictors of non-calcified coronary burden by Coronary Computed Tomography Angiography in psoriasis. These factors were related to obesity, dyslipidemia, and inflammation | RF | 263 consecutive patient records (January 2013-January 2018) with 62 available variables measured at baseline from the Psoriasis Atherosclerosis Cardiometabolic Initiative | Data from Psoriasis Atherosclerosis Cardiometabolic Initiative; Annotations obtained from patient records |
| Patrick et al (2018) | Used genetic data to assess the risk of psoriatic arthritis development in psoriasis patients. Identified 9 new loci for psoriasis or its subtypes and achieved 0.82 AUC in distinguishing PsA versus PsC when using 200 genetic markers | RF, conditional inference forest, shrinkage discriminant analysis, elastic net regression | Six cohorts with >7000 genotyped psoriatic arthritis and cutaneous-only psoriasis patients | 5 GWAS datasets: CASP, Exomechip with with GWAS content, Genizon, Kiel, PsA GWAS; 1 Immunochip dataset: PAGE | |
| Treatment | Patrick et al (2019) | Predicted drugs that can be repurposed to treat immune-mediated cutaneous diseases like psoriasis. The method confirmed drugs that are known to be effective for psoriasis and identified potential new drug candidates currently used to treat other diseases | NLP with word embeddings | 353 drugs indicated as not being used to treat psoriasis but used to treat some other immune-mediated disease, evaluated using a database of 20 million MEDLINE abstracts (3.3 billion words) | Database of 20 million MEDLINE abstracts (3.3 billion words) |
| Tomalin et al (2019) | Developed models to predict long-term treatment response to tofacitinib and etanercept though quantification of 157 inflammatory and cardiovascular proteins in the blood of psoriasis patients. Their models accurately predict the 12-week clinical endpoint for psoriasis following tofacitinib (auROC = 78%) or etanercept (auROC = 71%) treatment in a validation dataset | Prediction of Microarrays (PAM), Threshold Gradient Descent Regularization (TGDR), Generalized Linear Models (GLMnet), Partial Least Squares (PLS), Neural Networks (NNET), SVMs, RFs | 157 disease proteins measured from the blood of 266 patients with moderate-to-severe psoriasis | ClinicalTrials.gov: NCT01241591 (Bachelez et al, 2015) comparing efficacy and safety of tofacitinib and etanercept; PASI75 (outcome variable: patient labeled as a responder if PASI decreases by >75% after 12 weeks of treatment) determined by dermatologists | |
| Zhang et al (2014) | Used an NLP method to identify known and unknown drug-drug interactions from MEDLINE and EHR data | NLP with semantic predications | 224 unique drugs from 22 patients, evaluated using the SemMedDB database (21 million citations and 119 million sentences) | SemMedDB database (21 million citations and 119 million sentences); Salient predications validated by physicians | |
Abbreviations: AUC, area under the curve; CIA, computer image analysis; DT, decision tree; CNN, convolutional neural network; DCNN, deep convolutional neural network; MSSC, multiresolution-based signature subspace classifier; NLP, natural language processing; PCA, principal component analysis; RF, random forest; SVM, support vector machine.
Evaluation Using Skin Images
Similar to the previous example of skin cancer, ML can aid in the evaluation and diagnosis of skin diseases through the automatic interpretation of skin images. This includes the ability to identify an image of a psoriasis lesion as psoriasis and to differentiate it from other skin diseases, to trace the outlines of a psoriasis lesion in an image, and to score the severity and area of psoriasis from an image.
Identification and differential diagnosis of psoriasis lesions.
We identified 8 articles that applied ML to identify an image of a psoriasis lesion as psoriasis and to differentiate it from other skin diseases.21–28 Shrivastava et al have conducted a few studies to classify skin images from psoriasis patients as healthy versus diseased. After extracting feature information such as texture, color, and redness from images of psoriasis lesions, they used a support vector machine (SVM) model to classify 540 skin images from 30 psoriasis patients of Indian descent as healthy versus diseased, with a classification accuracy of approximately 99%.24–26 Other groups have focused on identifying psoriasis from images representing several common skin disorders, including diseases commonly mistaken for psoriasis like atopic dermatitis.21–23,27,28 For example, Zhao et al used convolutional neural networks to classify 8021 images of 9 common disorders—lichen planus, lupus erythematosus, basal cell carcinoma, squamous cell carcinoma, atopic dermatitis, pemphigus, psoriasis, and seborrheic keratosis—from patients at a Chinese hospital as psoriasis versus non-psoriasis.23 When tested on 100 new images, their algorithm showed superior performance to 25 Chinese dermatologists, with a misdiagnosis rate of 3% compared to 27% by dermatologists. Meanwhile, Kim et al focused specifically on the differential diagnosis of seborrheic dermatitis versus psoriasis on the scalp using smartphone-based multispectral imaging, achieving a sensitivity of 65% to 75% and specificity of 70% to 80%.22
Lesion segmentation.
After an image has been identified as containing psoriasis, it is useful to identify the outlines of a psoriasis lesion in the image, a task called “segmentation.” While this can be done manually, ML has the power to automate segmentation, enabling subsequent higher level tasks like automated body surface area (BSA) scoring. Fortunately, psoriasis plaques tend to be well-circumscribed, making it easier for a machine to segment a psoriasis lesion than for poorly circumscribed skin diseases.
We identified 8 studies tackling the task of psoriasis lesion segmentation.29–36 For example, Dash et al built a DCNN to automatically segment psoriasis lesions in RGB color images, trained on 5241 skin images from 1026 psoriasis patients.29 Their model achieved an accuracy of 94.8%, with 89.6% sensitivity and 97.6% specificity. Other groups have conducted similar work with a range of ML methods, including superpixel clustering,30 SVM,31,32 K-means clustering,33 and subspace classification.35,36 Besides skin images, ML is also being used to automatically segment psoriasis in skin biopsy images using DCNNs.34
Lesion severity and area scoring.
Segmentation of psoriasis lesions in a skin image from a psoriasis patient makes it possible to automate evaluation of psoriasis lesion severity and affected area. We identified 12 articles related to severity and area grading of psoriasis using skin images.37–48
Dermatologists grade psoriasis severity according to the Psoriasis Area and Severity Index (PASI) and Physician Global Assessment (PGA) systems.49 These severity grading systems involve clinical assessment of lesion erythema, scaliness, and induration by a dermatologist. Machine learning methods have been applied to automatically score erythema37,38 and scaliness39,40 from an image and to detect change in scaliness across time for a times series of images taken over a week.46 For erythema, automatic severity scoring method by George et al achieved an F1 score (weighted average of precision and recall) of 0.71.37 For scaliness, their method achieved an accuracy of 80.81%.39 Automatic scoring of induration from 2-dimensional images remains a bigger challenge due to its 3-dimensional nature.
Body surface area is another quantitative metric that a dermatologist will assess when evaluating a psoriasis patient, traditionally done in the clinic through a full body skin exam. Body surface area and a severity assessment averaged across all lesions (eg, PGA) are combined (eg, PGA × BSA or PASI) to evaluate psoriasis.49 Machine learning researchers are working to automate estimation of involved BSA.41,47,48 The DCNN of Meienberger et al achieved an accuracy of more than 90% in 77% of images, with automated area estimates differing from physicians’ area estimates by 8.1% on average.41 Additionally, total body imaging systems are being designed to generate more comprehensive images for automatic PASI and BSA measurements.42 Together, the information from ML-automated severity and area grading can be used to automatically risk stratify psoriasis lesions.43–45
Clinical Management
Prediction of complications.
Psoriasis is associated with a number of comorbidities,18 including psoriatic arthritis,14 cardiovascular disease,15 and diabetes.50 Machine learning can be used to identify characteristics that correlate with a psoriasis patient’s likelihood of developing complications. We identified 2 articles that used ML to assess the risk of complications of psoriasis. Patrick et al used genetic data to assess the risk of psoriatic arthritis.51 Munger et al used patient records to identify top predictors of noncalcified coronary plaque burden in psoriasis, which included obesity, dyslipidemia, and inflammation factors.52
Treatment.
We found 3 articles that used ML to advance research on psoriasis treatment, such as identifying new drugs and predicting patient response to approved therapies.53–55 Potential new drugs for psoriasis treatment can be identified using natural language processing (NLP) by mining medical literature databases such as MEDLINE in combination with clinical patient data. Patrick et al used NLP to predict drugs not currently prescribed for psoriasis that could be repurposed to treat psoriasis.53 This approach would be a cost-effective method to identify new psoriasis treatments, but the output may not be informative. For example, their highest scoring predictions for psoriasis treatment included budesonide—not a new finding as systemic steroids improve psoriasis but with an unacceptable safety profile—and hydroxychloroquine—which has no evidence of benefit in psoriasis and is reported to trigger psoriasis flares.56 Meanwhile, Zhang et al used NLP to uncover drug-drug interactions, such as potential for lisinopril (an antihypertensive) and sertraline (an antidepressant) to increase the likelihood and severity of psoriasis when used together.54 Finally, Tomalin et al used ensemble ML methods that predicted, with 71% accuracy, psoriasis patients’ long-term treatment response to tofacitinib and etanercept given blood quantification of 157 inflammatory and cardiovascular proteins.55 Their model represents 266 patients and must be validated in larger, independent patient cohorts before it can be clinically applied.
Discussion
Challenges
Bringing ML technologies into the dermatologist’s office faces challenges both common to dermatology and specific to psoriasis. Common challenges center on quality and quantity of data. A machine’s ability to learn is dependent on the quality of data it receives, an important limitation to emphasize for clinical researchers collecting the data that ML researchers use to train their algorithms. A big challenge is the standardization of data, especially for skin images that are oftentimes taken with no standardized protocol, leading to variation in color normalization, exposure, perspective, and other parameters that make it tough for ML algorithms to discriminate between true and artificial differences between captured lesions. The International Skin Imaging Collaboration has attempted to address this by producing a set of technique standards for skin lesion imaging,57 but ensuring adoption of these standards across dermatological practices is difficult, particularly when images are patient-generated. A data set that is too small also has the potential to introduce bias and inaccuracies, especially for computationally expensive systems like DNNs, which require a large training set to produce generalizable output. An unrepresentative and small data set is especially problematic for ML algorithms like DNNs, which already operate as opaque “black boxes” lacking explainable reasoning for decisions. If the rationale of the algorithm is hard to interpret and the input data are unrepresentative, we may inadvertently use biased ML algorithms that increase health inequities without knowing it. For dermatology, a data set unrepresentative of diverse skin types may exacerbate already existing health disparities by generating ML models that are erroneous for underrepresented groups.58,59 Finally, many algorithms developed for dermatology have not yet been tested in a clinical setting nor evaluated for important clinical metrics such as positive- and negative-predictive value. Thus, their clinical utility remains to be determined.
Other challenges are specific to features of psoriasis. For one, psoriasis can be present in many forms, including plaque, guttate, pustular, palmoplantar, and nail psoriasis. An ML algorithm trained on only the most common psoriasis manifestations would be unable to recognize rarer presentations as the same disease. Secondly, psoriasis can be present anywhere across the body and can vary in size and form within the same patient. Most photos capture only spots of psoriasis, precluding the calculation of an accurate BSA, and an ML algorithm may erroneously compare lesions on the extremities with those on the buttocks. Whole-body photography is one solution to this problem, but it is not available in most contexts including normal clinic visits and telemedicine. An ideal algorithm for psoriasis would be trained to sophisticatedly combine multiple images from a single patient to make a holistic assessment. Otherwise, an ML algorithm may inaccurately evaluate lesions in isolation, for example, deciding that lesions it sees as small are best spot-treated with topicals, while a dermatologist would be able to see that a patient with many small lesions dispersed across the body would be better treated with phototherapy. Savolainen et al attempted to build an algorithm that can conduct more sophisticated holistic assessments with a color segmentation method that estimated BSA in psoriasis patients across multiple images.48 Human eye estimates differed from their image analysis in almost one-third of cases, especially in cases with BSA <30%. Their algorithm had particular difficulties with cylindrical body parts like the limbs. They noted additionally that the process of photographing and processing multiple images was time-consuming and technically demanding. Fadzil et al also developed a method to assess the area of psoriasis lesions across images of multiple body regions (face, anterior and posterior trunk, and both left and right upper and lower limbs), with an accuracy of greater than 90% in 28 out of 30 cases.60 The scalp, buttocks, genitals, hands, and soles were not included, and 2 of the 30 cases demonstrated a significantly lower accuracy, showcasing the difficulty of accurate psoriasis area estimation using 2D images. These examples show the challenge of whole-body analysis; on the other hand, it may be possible for ML to detect information from individual plaques that are informative about the entire body.
Even if we zoom in on individual psoriasis lesions, there are still characteristic features of psoriasis that pose challenges. The 3 key measures to score psoriasis lesion severity are erythema, scale, and induration. An accurate assessment of erythema would benefit from color normalization and controlled illumination conditions; otherwise, a bright red lesion could appear dark brown.61,62 Interpreting scaliness requires complex texture analysis, and the silvery scale of psoriasis is complicated in how it reflects light. Finally, induration is challenging to assess accurately in 2 dimension.
Lastly, psoriasis sometimes causes post-inflammatory hyperpigmentation and hypopigmentation even after plaque clearance, especially in skin of color.59,63 This could be a source of confusion for an ML model attempting to, say, calculate erythema, if it has not also been trained to distinguish posttreatment pigmentation abnormalities from active lesions.
Future Directions and Relevance to Clinicians
Machine learning holds substantial promise to improve psoriasis care, from diagnostic evaluation to management and treatment. As a diagnostic aid, ML can automate tasks such as identifying areas of psoriasis in a photo, differentiating images of psoriasis from other common skin disorders, and scoring the severity of disease and area affected. Automation of these tasks can expedite the ability of dermatologists to make clinical assessments, which are significant given the high-volume nature of many dermatological practices.
As a therapy and management aid, ML can help prevent disease complications. For example, a psoriasis patient predicted by ML to have characteristics putting them at greater risk of cardiovascular complications, as was studied by Munger et al,52 could be targeted for preventative cardiology services.
Machine learning can also improve psoriasis treatment. Automated lesion-evaluation technologies could assist dermatologists in making treatment decisions and in monitoring patients. For example, a high PGA computed by ML could alert a dermatologist that a psoriasis patient may need more intensive systemic treatment or phototherapy over topicals. Long-term treatment response, drug-drug interactions, and potential new therapies for psoriasis can also be predicted using ML.53–55
Machine learning can not only provide information for dermatologist decision-making but also make joint decisions with dermatologists using an approach called reinforcement learning (RL). Reinforcement learning teaches a machine to make decisions in order to maximize some reward, with applications like using past decisions and their outcomes to inform future decisions. In sepsis management, RL has been used to identify optimal treatment decisions given the data on past decisions and outcomes from 17 083 hospital admissions.64 For psoriasis, RL could use a patient’s past positive and negative responses to particular treatments (eg, loss of treatment response to a particular biologic therapy) to systematically decide whether a patient should continue or switch treatment regimens. This could help dermatologists make care decisions earlier and with a more efficient and evidence-based approach.
Acknowledgments
Funding
The authors disclosed the receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Psoriasis Foundation [grant number NPF2019SSRG05], University of Pennsylvania Center for Clinical Epidemiology and Biostatistics, and funded in part through NIAMS 1P30AR069589-01.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017; 542(7639):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujisawa Y, Otomo Y, Ogata Y, et al. Deep learning-based, computer-aided classifier developed with a small dataset of clinical images surpasses board-certified dermatologists in skin tumour diagnosis. British J Dermatol. 2019;180(2):373–381. [DOI] [PubMed] [Google Scholar]
- 3.Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists. Ann Oncol. 2018;29(8): 1836–1842. [DOI] [PubMed] [Google Scholar]
- 4.Han SS, Kim MS, Lim W, Park GH, Park I, Chang SE. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J Investigat Dermatol. 2018;138(7):1529–1538. [DOI] [PubMed] [Google Scholar]
- 5.Han SS, Park GH, Lim W, et al. Deep neural networks show an equivalent and often superior performance to dermatologists in onychomycosis diagnosis: automatic construction of onychomycosis datasets by region-based convolutional deep neural network. PloS one. 2018;13(1):e0191493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tschandl P, Rosendahl C, Akay BN, et al. Expert-level diagnosis of nonpigmented skin cancer by combined convolutional neural networks. JAMA Dermatol. 2019;155(1):58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinker TJ, Hekler A, Enk AH, et al. Deep learning outperformed 136 of 157 dermatologists in a head-to-head dermoscopic melanoma image classification task. Euro J Cancer. 2019;113:47–54. [DOI] [PubMed] [Google Scholar]
- 8.Brinker TJ, Hekler A, Utikal JS, et al. Skin cancer classification using convolutional neural networks: systematic review. J Med Int Res. 2018;20(10):e11936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emre Celebi M, Alp Aslandogan Y, Stoecker WV, Iyatomi H, Oka H, Chen X. Unsupervised border detection in dermoscopy images. Skin Res Technol. 2007;13(4):454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maglogiannis I, Doukas CN. Overview of advanced computer vision systems for skin lesions characterization. IEEE Transact Inform Technol Biomed. 2009;13(5):721–733. [DOI] [PubMed] [Google Scholar]
- 11.Ferris LK, Harkes JA, Gilbert B, et al. Computer-aided classification of melanocytic lesions using dermoscopic images. J Ame Acad Dermatol. 2015;73(5):769–776. [DOI] [PubMed] [Google Scholar]
- 12.Zortea M, Schopf TR, Thon K, et al. Performance of a dermoscopy-based computer vision system for the diagnosis of pigmented skin lesions compared with visual evaluation by experienced dermatologists. Artif Intell Med. 2014;60(1):13–26. [DOI] [PubMed] [Google Scholar]
- 13.Okon E, Rachakonda V, Hong HJ, Callison-Burch C, Lipoff JB. Natural language processing of reddit data to evaluate dermatology patient experiences and therapeutics. J Am Acad Dermatol. 2019:S0190–9622(19)32371–0. [DOI] [PubMed] [Google Scholar]
- 14.Moll J, Wright V. Psoriatic arthritis. Sem Arthrit Rheumat. 1973; 3(1):55–78. [DOI] [PubMed] [Google Scholar]
- 15.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–1741. [DOI] [PubMed] [Google Scholar]
- 16.Dubreuil M, Rho YH, Man A, et al. Diabetes incidence in psoriatic arthritis, psoriasis and rheumatoid arthritis: a UK population-based cohort study. Rheumatology. 2013;53(2):346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Investigat Dermatol. 2009;129(10): 2411–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimball AB, Gladman D, Gelfand JM, et al. National Psoriasis Foundation clinical consensus on psoriasis comorbidities and recommendations for screening. J Ame Acad Dermatol. 2008;58(6): 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Ame Acad Dermatol. 2014;70(3):512–516. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aggarwal SLP. Data augmentation in dermatology image recognition using machine learning. Skin Res Technol. 2019;25(6): 815–820. [DOI] [PubMed] [Google Scholar]
- 22.Kim S, Kim J, Hwang M, et al. Smartphone-based multispectral imaging and machine-learning based analysis for discrimination between seborrheic dermatitis and psoriasis on the scalp. Biomed Optic Exp. 2019;10(2):879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao S, Xie B, Li Y, et al. Smart identification of psoriasis by images using convolutional neural networks: a case study in China. J Eur Acad Dermatol Venereol. 2020;34(3):518–524. [DOI] [PubMed] [Google Scholar]
- 24.Shrivastava VK, Londhe ND, Sonawane RS, Suri JS. Computer-aided diagnosis of psoriasis skin images with HOS, texture and color features: a first comparative study of its kind. Comp Meth Program Biomed. 2016;126:98–109. [DOI] [PubMed] [Google Scholar]
- 25.Shrivastava VK, Londhe ND, Sonawane RS, Suri JS. Reliability analysis of psoriasis decision support system in principal component analysis framework. Data Knowledge Engine. 2016;106: 1–17. [Google Scholar]
- 26.Shrivastava VK, Londhe ND, Sonawane RS, Suri JS. Reliable and accurate psoriasis disease classification in dermatology images using comprehensive feature space in machine learning paradigm. Exp Syst Appli. 2015;42(15–16):6184–6195. [Google Scholar]
- 27.Mashaly H, Masood N, Mohamed AS. Classification of papulosquamous skin diseases using image analysis. Skin Res Technol. 2012;18(1):36–44. [DOI] [PubMed] [Google Scholar]
- 28.Verma AK, Pal S, Kumar S. Classification of skin disease using ensemble data mining techniques. Asian Pacific J Can Prevent. 2019;20(6):1887–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dash M, Londhe ND, Ghosh S, Semwal A, Sonawane RS. PsLSNet: automated psoriasis skin lesion segmentation using modified U-net-based fully convolutional network. Biomed Sign Process Control. 2019;52:226–237. [Google Scholar]
- 30.George YM, Aldeen M, Garnavi R. Automatic psoriasis lesion segmentation in two-dimensional skin images using multiscale superpixel clustering. J Med Imag. 2017;4(4):044004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu J, Kazmierczak E, Manton JH, Sinclair R. Automatic segmentation of scaling in 2-d psoriasis skin images. IEEE Transact Med Imag. 2012;32(4):719–730. [DOI] [PubMed] [Google Scholar]
- 32.Shrivastava VK, Londhe ND, Sonawane RS, Suri JS. A novel and robust Bayesian approach for segmentation of psoriasis lesions and its risk stratification. Comp Meth Program Biomed. 2017; 150:9–22. [DOI] [PubMed] [Google Scholar]
- 33.Jarad TS, Dawood AJ. Accurate segmentation of psoriasis diseases images using k-means algorithm based on cielab (l* a* b) color space. J Theoret Appli Inform Technol. 2017;95(17):12. [Google Scholar]
- 34.Pal A, Garain U, Chandra A, Chatterjee R, Senapati S. Psoriasis skin biopsy image segmentation using deep convolutional neural network. Comp Meth Program Biomed. 2018;159:59–69. [DOI] [PubMed] [Google Scholar]
- 35.Taur JS, Lee GH, Tao CW, Chen CC, Yang CW. Segmentation of psoriasis vulgaris images using multiresolution-based orthogonal subspace techniques. IEEE Transact Syst Man Cybernet Part B (Cybernetics). 2006;36(2):390–402. [DOI] [PubMed] [Google Scholar]
- 36.Taur JS. Neuro-fuzzy approach to the segmentation of psoriasis images. J VLSI Sign Process Syst Sign Image Video Technol. 2003;35(1):19–27. [Google Scholar]
- 37.George Y, Aldeen M, Garnavi R. Psoriasis image representation using patch-based dictionary learning for erythema severity scoring. Comput Med Imaging Graph. 2018;66:44–55. [DOI] [PubMed] [Google Scholar]
- 38.Raina A, Hennessy R, Rains M, et al. Objective measurement of erythema in psoriasis using digital color photography with color calibration. Skin Res Technol. 2016;22(3):375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.George YM, Aldeen M, Garnavi R. Automatic scale severity assessment method in psoriasis skin images using local descriptors. IEEE J Biomed Health Inform. 2020;24(2):577–585. [DOI] [PubMed] [Google Scholar]
- 40.Fadzil MHA, Prakasa E, Asirvadam VS, Nugroho H, Affandi AM, Hussein SH. 3D surface roughness measurement for scaliness scoring of psoriasis lesions. Comp Biol Med. 2013;43(11): 1987–2000. [DOI] [PubMed] [Google Scholar]
- 41.Meienberger N, Anzengruber F, Amruthalingam L, et al. Observer-independent assessment of psoriasis affected area using machine learning. J Euro Acad Dermatol Venereol. 2020;34(6):1362–1368. [DOI] [PubMed] [Google Scholar]
- 42.Fink C, Fuchs T, Enk A, Haenssle HA. Design of an algorithm for automated, computer-guided PASI measurements by digital image analysis. J Med Syst. 2018;42(12):248. [DOI] [PubMed] [Google Scholar]
- 43.Shrivastava VK, Londhe ND, Sonawane RS, Suri JS. Exploring the color feature power for psoriasis risk stratification and classification: a data mining paradigm. Comp Biol Med. 2015;65:54–68. [DOI] [PubMed] [Google Scholar]
- 44.Shrivastava VK, Londhe ND, Sonawane RS, Suri JS. First review on psoriasis severity risk stratification: an engineering perspective. Comp Biol Med. 2015;63:52–63. [DOI] [PubMed] [Google Scholar]
- 45.Shrivastava VK, Londhe ND, Sonawane RS, Suri JS. A novel approach to multiclass psoriasis disease risk stratification: machine learning paradigm. Biomed Sign Process Control. 2016;28:27–40. [Google Scholar]
- 46.Gomez DD, Butakoff C, Ersbøll B, Carstensen JM. Automatic change detection and quantification of dermatological diseases with an application to psoriasis images. Patt Recogn Lett 2007; 28(9):1012–1018. [Google Scholar]
- 47.Savolainen L, Kontinen J, Alatalo E, Röning J, Oikarinen A. Comparison of actual psoriasis surface area and the psoriasis area and severity index by the human eye and machine vision methods in following the treatment of psoriasis. Acta Dermato-Venereol. 1998;78(6):466–467. [DOI] [PubMed] [Google Scholar]
- 48.Savolainen L, Kontinen J, Röning J, Oikarinen A. Application of machine vision to assess involved surface in patients with psoriasis. Brit J Dermatol. 1997;137(3):395–400. [PubMed] [Google Scholar]
- 49.Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 2005;64(Suppl 2):ii65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149(1):84–91. [DOI] [PubMed] [Google Scholar]
- 51.Patrick MT, Stuart PE, Raja K, et al. Genetic signature to provide robust risk assessment of psoriatic arthritis development in psoriasis patients. Nat Comm. 2018;9(1):4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munger E, Choi H, Dey AK, et al. Application of machine learning to determine top predictors of non-calcified coronary burden in psoriasis: an observational cohort study [published online ahead of print October 31, 2019]. J Am Acad Dermatol. 2019: S0190-9622(19)32983-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patrick MT, Raja K, Miller K, et al. Drug repurposing prediction for immune-mediated cutaneous diseases using a word-embedding-based machine learning approach. J Investigat Dermatol. 2019;139(3):683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang R, Cairelli MJ, Fiszman M, et al. Using semantic predications to uncover drug–drug interactions in clinical data. J Biomed Inform. 2014;49:134–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomalin LE, Kim J, da Rosa JC, et al. Early quantification of systemic inflammatory-proteins predicts long-term treatment response to Tofacitinib and Etanercept: Psoriasis response predictions using blood. J Investigat Dermatol. 2019;140(5):1026–1034. [DOI] [PubMed] [Google Scholar]
- 56.Abel EA, DiCicco LM, Orenberg EK, Fraki JE, Farber EM. Drugs in exacerbation of psoriasis. J Ame Acad Dermatol. 1986;15(5): 1007–1022. [DOI] [PubMed] [Google Scholar]
- 57.Katragadda C, Finnane A, Soyer HP, et al. Technique standards for skin lesion imaging: a Delphi consensus statement. JAMA Dermatol. 2017;153(2):207–213. [DOI] [PubMed] [Google Scholar]
- 58.Adamson AS, Smith A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 2018;154(11):1247–1248. [DOI] [PubMed] [Google Scholar]
- 59.Alexis AF, Paul B. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesth Dermatol. 2014;7(11):16. [PMC free article] [PubMed] [Google Scholar]
- 60.Fadzil MA, Ihtatho D, Affandi AM, Hussein S. Area assessment of psoriasis lesions for PASI scoring. J Med Engine Technol. 2009;33(6):426–436. [DOI] [PubMed] [Google Scholar]
- 61.Vezhnevets V, Sazonov V, Andreeva A. A survey on pixel-based skin color detection techniques. In: Proc. Graphicon, Moscow, Russia; 2003. September (Vol. 3, pp. 85–92). [Google Scholar]
- 62.Yang J, Lu W, Waibel A. Skin-color modeling and adaptation. In: Asian Conference on Computer Vision; 1998. January 8 (pp. 687–694). Springer, Berlin, Heidelberg. [Google Scholar]
- 63.Gläser R, Röwert J. Hyperpigmentation due to topical calcipotriol and photochemotherapy in two psoriatic patients. Brit J Dermatol. 1998;139(1):148–151. [DOI] [PubMed] [Google Scholar]
- 64.Komorowski M, Celi LA, Badawi O, Gordon AC, Aldo F. The Artificial Intelligence Clinician learns optimal treatment strategies for sepsis in intensive care. Nat Med. 2018;24(11):1716. [DOI] [PubMed] [Google Scholar]


