SUMMARY:
We describe the case of a 63-year-old woman who developed a coronavirus disease 2019–associated acute encephalopathy with perivascular gadolinium enhancement.
A 63-year-old woman was admitted to our hospital on day 7 (day 1 is the first day of symptoms) with fever, dry cough, fatigue, and subjective dyspnea. A nasopharyngeal swab for Severe Acute Respiratory Syndrome coronavirus 2 (SARS-Cov-2) was positive. Arterial blood gas analysis in room air showed mild hypocapnia (partial pressure of carbon dioxide, 31), causing mild respiratory alkalosis (pH = 7.48) and a low level of oxygen (partial pressure of carbon dioxide, 67). A chest x-ray was performed, showing bilateral ground-glass opacities. The patient had hypertension, but no other comorbidities were identified.
She was first admitted to the emergency ward and treated with a continuous positive airway pressure helmet; lopinavir/ritonavir was started as compassionate use approved by the ethics committee, as well as empirical piperacillin-tazobactam. Her respiratory parameters progressively worsened during the next days until she showed dyspnea, and her PaO2/FiO2 ratio was 110 with a continuous positive airway pressure helmet at 15 cm of water and an inspiratory fraction of oxygen of 70%. On day 11, she was intubated and transferred to the intensive care unit (ICU). During her ICU stay, she initially received mechanical ventilation at a medium level of positive end-expiratory pressure (up to 14) and inhaled nitric oxide and underwent two 20-hour prone-position cycles. She also developed a moderately acute kidney injury (measured creatinine clearance of 28 mL/minute), which never required replacement therapy, and high levels of hepatic enzymes and bilirubin, which caused us to stop lopinavir/ritonavir. An angiotensin-converting-enzyme inhibitor was introduced to reduce blood pressure in the ventilator weaning phase after 15 days on sedative drugs and opioids.
Regarding the treatment given in the ICU that was different from the usual care, she received anakinra (an interleukin 1 antagonist) due to a persistent high level of C-reactive Protein and ferritin and a high dose of subcutaneous heparin to target an activated partial thromboplastin time ratio of 2. On day 24, antibiotics were suspended until she developed ventilator-associated pneumonia by methicillin-sensitive Staphylococcus aureus, and cefazolin was given. Gradually, her clinical conditions improved, and she was weaned from the ventilator on day 28, after 24 hours of spontaneous breathing. On day 29, she was transferred back to medium intensity care in relatively good condition: afebrile, low-flow oxygen therapy via nasal cannula with normal peripheral capillary oxygen saturation and respiratory mechanics, normal blood pressure, and diuresis with acute kidney and hepatic injuries improving.
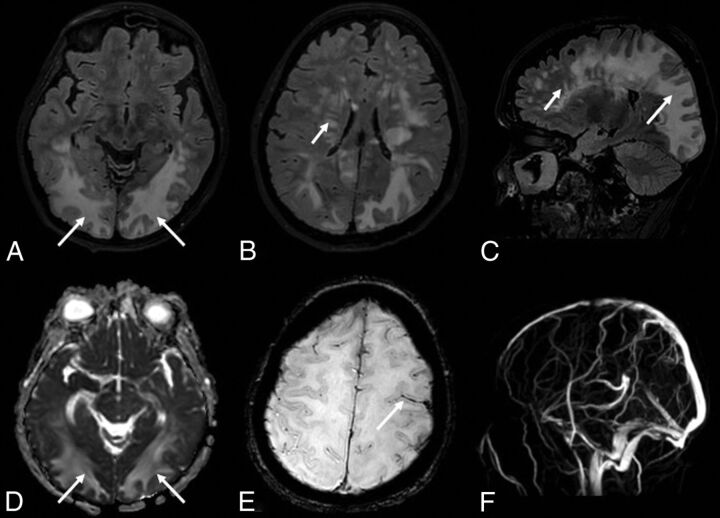
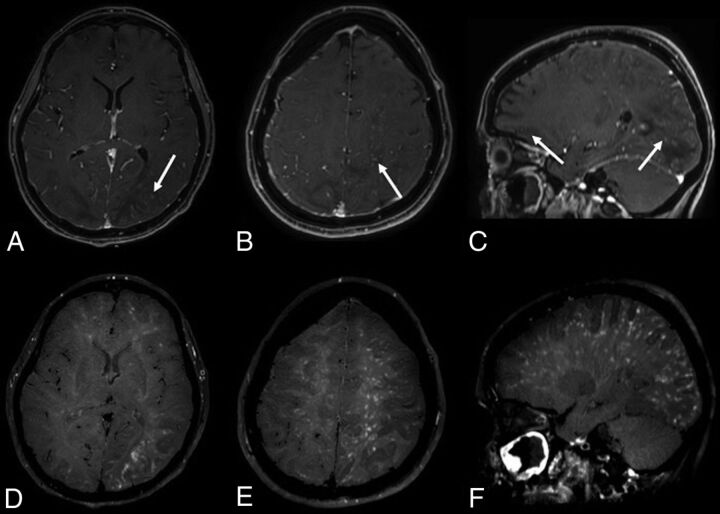
On day 30, she presented with left head and eye deviation and continuous tonic seizures on the left side of the body. After ineffective treatment with diazepam and lacosamide, the patient was sedated, intubated, and transferred again to the ICU. A phenytoin IV load was started, followed by enteral maintenance dosages, associated with diazepam infusion with clinical resolution. An electroencephalogram showed periodic lateralized epileptiform discharges in the right posterior regions, without clinical correlation. A lumbar puncture revealed only a slightly elevated protein level (70 mg/dL). A polymerase chain reaction test for virus detection in the CSF, including SARS-Cov-2, was negative. MR imaging showed cortical swelling and restricted diffusivity in the posterior regions of the right hemisphere, corresponding to the epileptic focus (On-line Figure). We observed multiple, partially confluent, tumefactive lesions of the sub- and supratentorial white matter hemispheres, with prevalence in the posterior hemisphere regions, which appeared as increased signal intensity on T2-weighted images, consistent with brain edema. The susceptibility-weighted images showed a subarachnoid blood effusion along the left precentral sulcus and excluded intraparenchymal hemorrhagic petecchiae. MR venography excluded intracranial thrombosis (Fig 1). The contrast-enhanced T1-weighted black-blood sequence showed a miliariform perivascular gadolinium enhancement in the supra- and subtentorial white matter. Neither wall enhancement of the large intracranial arterial vessels nor leptomeningeal enhancement was observed on postcontrast sequences (Fig 2).
FIG 1.
The FLAIR sequence shows multiple lesions of the white matter of both hemispheres (A–C), more striking in the posterior regions with a tumefactive appearance (long arrows) and with a multifocal perivascular pattern in the deep white matter regions (short arrows). The lesions (arrows) show high values on the apparent diffusion coefficient map (D), suggesting increased water content in the parenchyma. The SWI sequence (E) shows a subarachnoid blood effusion along the left precentral sulcus (arrow). MR venography (F) excludes intracranial thrombosis.
FIG 2.
The contrast-enhanced 3D conventional T1-weighted sequence (A–C) shows multiple spotlike gadolinium enhancement (arrows) in the posterior white matter and left hemisphere. The postcontrast 3D-T1-weighted black-blood sequence (D–F) shows a stratiform perivascular gadolinium enhancement in the white matter of both cerebral hemispheres. Neither wall enhancement of the large intracranial arterial vessels nor leptomeningeal enhancement are observed on postcontrast sequences.
The patient gradually improved. On day 41, she was extubated and was discharged to the ward 2 days later with residual mild left hemiparesis and left lateral hemianopsia. A control electroencephalogram showed only sporadic sharp waves and spikes in the right posterior regions. At follow-up, on MR imaging on day 49, the cerebellar lesions disappeared, cerebral lesions were markedly reduced, perivascular enhancement was not detected, and diffusion-weighted imaging findings were negative. On day 61, the patient was discharged from the hospital and referred to rehabilitation. At 180-day follow-up, the patient was at home, without motor deficits but with partial visual loss in the left eye (extended Glasgow Outcome Scale, 4; mRS score, 3).
We made a diagnosis of posterior reversible encephalopathy syndrome (PRES)-like encephalopathy with peculiar MR imaging findings. The miliariform perivascular postgadolinium enhancement has not previously been reported among typical and atypical MR imaging findings in PRES.1,2 The perivascular enhancement might be a sign of immune-mediated small-vessel damage, leading to altered integrity of the blood-brain barrier and brain edema, as supposed in PRES.3
Supplementary Material
ABBREVIATIONS:
- ICU
intensive care unit
- PRES
posterior reversible encephalopathy syndrome
- SARS-Cov-2
Severe Acute Respiratory Syndrome coronavirus 2
Footnotes
Disclosures: Marco Carbonara—UNRELATED: Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: European Union, Comments: In the context of CENTER-TB1–related activities.
References
- 1.Bartynski WS. Posterior reversible encephalopathy syndrome, Part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol 2008;29:1036–42 10.3174/ajnr.A0928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saad AF, Chaudhari R, Wintermark M. Imaging of atypical and complicated posterior reversible encephalopathy syndrome. Front Neurol 2019;10:964 10.3389/fneur.2019.00964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol 2015;14:914–25 10.1016/S1474-4422(15)00111-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.