Abstract
Both short chain fatty acids (SCFAs) and long chain fatty acids (LCFAs) rely on free fatty acid receptors to signal their presence to the body, but their individual detection and putative reward systems are different. These separate, yet parallel, taste signaling pathways allow us to distinguish microbe-produced from triglyceride-based fatty acids. Free SCFAs indicate that the food has been fermented and may still contain living, probiotic microbes that can colonize the gut. Free LCFAs indicate the presence of calorie-rich triglycerides in foods. By contrast, LCFAs stimulate endocannabinoids, which reinforce overconsumption of triglycerides. Here we examine the separate oral detection and putative reward systems for both LCFA and SCFAs, and introduce a novel dietary LC:SC ratio as a guideline to improve metabolism and health.
Keywords: Taste, Fatty Acids, Short chain fatty acids, Long chain fatty acids, Triglycerides, Fermentation, Probiotic Microbes, Reward, Endocannabinoids, Nutrition, Nutrients, Health
Graphical Abstract
Introduction
Throughout the evolution of modern humans, ingestion of fatty and fermented foods as high energy and probiotic food sources has been beneficial. We propose here that there are two separate fat detection pathways in taste tissue: one for long chain fatty acids (LCFAs) and one for short chain fatty acids (SCFAs) that serve different nutritional needs. Interestingly, different taste receptors have been characterized that can identify both long and short chain fatty acids in the mouth [1]. Why do we need these receptors? LCFAs: The desire for and enjoyment of fat-rich foods has benefitted our species by providing necessary energy as well as lipid components that are crucial for cellular membranes, immune function, cell growth and repair, and brain expansion [2, 3]. Per gram, fats contain the most calories compared to other macronutrients and are an efficient way to ingest and store energy. Yet, there are few free LCFAs in food, as they are packaged as triglycerides (TGs). Recently, oral lipase was discovered to play a role in freeing fatty acids from TGs [4], thus providing ligands to stimulate LCFA taste receptors. The free LCFAs signal to the body that a high calorie meal is present, thus imparting an evolutionary advantage. The endocannabinoid system (ECS) is stimulated by the consumption of LCFAs, which activates reward circuits in the brain, suggesting a benefit of ingesting fatty foods [5]. SCFAs: Humans have also benefitted from synergistic relationships with gut-colonizing microbes. In the colon, microbes ferment fiber and other components of foods to provide humans with utilizable SCFAs. In foods, SCFAs are primarily derived from fermentation. Hence, SCFA receptors may be acting as indicators of fermentation, which conveys an evolutionary advantage of food safety and a possible source of “probiotic” microbes that can colonize the gut and promote health. Free fatty acid receptors (FFARs) and related signaling proteins (described in Table 1) provide a means of oral detection of fatty acids. The receptors and downstream effectors also act in the lower gastrointestinal system to activate satiety and reward. These detection and reward systems serve to increase intake of triglyceride-rich and fermented foods, each of which can have a selective evolutionary advantage for survival.
Table 1: Putative fatty acid receptors involved in taste signal transduction and regulation of energy metabolism.
This table represents a portion of the known free fatty acid receptors (FFARs), other G-protein coupled receptors (GPCRs), and related receptors and membrane signaling proteins involved in fatty acid signal transduction. We also indicate the receptive fields of these receptors and the functional consequences of their activations. This table is limited to the regulation of taste and energy homeostasis.
| Primary Fatty Acid Type |
Receptor/Protein | Primary Agonist | Expression | Functional Consequences |
|---|---|---|---|---|
| SCFA | FFAR2/GPR43 | SCFAs C2=C3>C4>other SCFAs (human) C2>C3>C4 (mouse) |
Taste bud cells Enteroendocrine cells Intestinal epithelial cells Pancreatic Beta-cells White adipocytes |
Transduces taste signal? [6] Promotes PYY, GLP-1 secretion? [7] Regulates cytokine production [1] Regulates insulin secretion [8] Suppresses lipolysis, promotes lipid oxidation [9] |
| FFAR3/GPR41 | SCFAs C3=C4=C5>C2>C1 |
Taste bud cells (mouse) Peripheral nerves Enteroendocrine cells Pancreatic Beta-cells White adipocytes |
Transduces taste signal? [10] Stimulates vagal afferents? [11] [12] Promotes PYY, GLP-1 secretion? [1] Suppresses GIP [1] Suppresses insulin secretion [1] Promotes leptin production [13] |
|
| GPR109A | Niacin, C4 | Colonic epithelial cells Adipose tissue |
Suppresses colonic inflammation and carcinogenesis [1] Regulates lipid metabolism by regulating adipose macrophages [1] |
|
| Olfr78 (mouse)/OR51E2 (human) | SCFAs C3>C2 |
Olfactory sensory neurons Enteroendocrine cells |
Transduces olfactory signals [1] Regulates gut hormone secretion? [14] |
|
| Olfr558 (mouse)/OR51E1 (human) | SCFAs C4>isovaleric>C5>C3[15] |
Olfactory sensory neurons Enteroendocrine cells |
Transduces olfactory signals [16] Promotes GLP-1, PYY secretion [17] |
|
| LCFAs | FFAR1/GPR40 | LCFAs | Taste bud cells (mouse) Enteroendocrine cells Pancreatic β cells |
Mediates taste sensitivity for fatty acids [1, 10] Promotes CCK, GIP, GLP-1 secretion [1] Enhances insulin secretion [1] |
| FFAR4/GPR120 | LCFAs | Taste bud cells Hypothalamus Enteroendocrine cells White adipocytes |
Promotes GLP-1 release, Regulates taste sensitivity [18] Mediates energy homeostasis [19] Promotes CCK, GIP and GLP-1 secretion [20] Promotes adipocyte differentiation [1] |
|
| CD36 | LCFAs | Taste bud cells Enteroendocrine cells |
Transduces taste signal [18] Regulates food intake through fat-induced satiety [18] |
|
| DRK Channel | LCFAs (PUFAs) | Taste cells buds | Enhances LCFA taste signaling [18] | |
| CB1R | Endocannabinoids (eCBs) | Taste bud cells Enteroendocrine cells White adipocytes |
Regulates taste sensitivity [21] Stimulates ghrelin release Inhibits CCK release [22] Promotes lipogenesis and adipogenesis [23] |
Long Chain Fatty Acids (LCFAs)
The detection of LCFAs in the oral cavity likely evolved for the necessary intake of energy and essential fatty acids which are crucial for growth and development [2]. Although LCFAs, specifically omega 6 and saturated FAs, are found in natural, unprocessed foods, they are present in overabundance in processed foods and snacks (doughnuts, ice cream, etc.), adding to the growing global obesity phenomenon [24]. In particular, the involvement of the endocannabinoid system (ECS), an inherently orexigenic system that drives consumption by strengthening food intake reinforcement mechanisms [25], suggests a physiological relevance in maximizing fat intake, likely required in case of a famine.
Taste Detection and Description
The oral sensation of free fatty acids (FFAs) has been described as “scratchy,” but only LCFAs elicit “fatty” [26]. Several G-Protein coupled receptors (GPCRs) and signaling proteins respond to LCFA in the mouth and may aid in transducing these “fatty” or “scratchy” sensations [18]. The taste receptors that initiate LCFA transduction are cluster-of-differentiation 36 (CD36) and free fatty acid receptor 4 (FFAR4), although other receptors and proteins are implicated in oral LCFA transduction [18, 27] (see Table 1). CD36 transduces the LCFA signal, whereas cannabinoid 1 receptor (CB1R) and FFAR4 regulate LCFA detection sensitivity by releasing glucagon-like peptide 1 (GLP-1) from taste cells, downregulating CD36 expression (Figure 1) [18] [28].
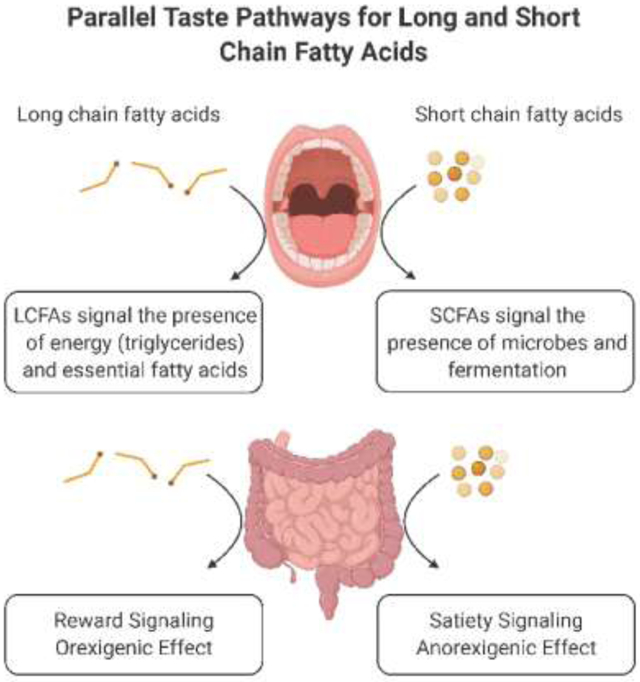
Figure 1. Summary of the parallels between Long Chain Fatty Acids (LCFA) and Short Chain Fatty Acids (SCFA) signaling versus the different functional consequences on appetite and satiety.
Key components involved in the fatty acid-induced taste signal described in the upper boxes for both LCFA and SCFA. ‘Cluster of differentiation’ (CD36) transduces the LCFA signal, whereas cannabinoid 1 receptor (CB1R) and free fatty acid receptor 4 (FFAR4) regulate LCFA detection sensitivity. The exact signaling mechanism for SCFA in taste bud cells is under investigation. The lower boxes depict the opposing effects LCFA versus SCFA have on satiety. Tasting LCFA stimulates the release of gut endocannabinoids (eCBs), which increases ghrelin secretion and decreases FFAR1-mediated cholecystokinin (CCK) release, thus overriding satiety signals and reinforcing fat intake and fat-induced reward through vagal nerve signaling. On the other hand, SCFAs act possibly via FFAR2, FFAR3 and/or olfactory receptor 51E1 (OR51E1), to increase glucagon-like peptide-1 (GLP-1) and peptide tyrosine tyrosine (PYY) release, decrease ghrelin release and increase satiety signals via the vagal nerve. Created with BioRender.com.
Single nucleotide polymorphisms (SNPs) in CD36 and FFAR4 in humans affect LCFA absolute detection thresholds and, consequently, total fat intake [29-31]. More specifically, obese patients with the common SNP rs1761667, which reduces CD36 gene expression, displayed a higher detection threshold (lower sensitivity) and greater liking for lipids than individuals with the common variant [32, 33]. This SNP is also associated with altered endocannabinoid (eCB) levels in both obese and normal weight individuals [34]. Obesity can also alter fat preference and intake by downregulating CD36 expression [35-37] and increasing eCB tone [38], e.g. salivary eCBs are higher in obese relative to non-obese individuals [39]. Perhaps the regulation of the LCFA detection components, CD36 and FFAR4, is mediated by the changes in peripheral eCB tone and CB1R signaling. This suggests that when ECS tone is increased by obesity, LCFA perception is decreased and fat liking is increased [40].
Liking and Reward
Although LCFA ingestion increases satiety signals through FFARs (see Table 1) [1], the ability of LCFAs to stimulate eCB signaling may override these satiety signals by reinforcing reward-driven intake [22, 25]. The endocannabinoid system influences a variety of factors controlling food intake and metabolism [41]. Components of the ECS are present in the hypothalamus and brainstem, areas that regulate food intake and energy expenditure, and in the nucleus accumbens, involved in the rewarding value of food. The ECS system has also been found in taste tissue where it modulates taste sensitivity [21, 27, 28]. The presence of LCFAs in the oral cavity activates a self-reinforcing feedback loop to eat more fat through the synthesis of eCBs in the gut and, subsequently, activates reward signaling and food-reinforcement mechanisms in the brain through vagal afferents and CB1R activation [42] (Figure 1). The effects of CB1 activation include: increasing olfactory and gustatory sensitivity, increasing appetitive and reward signaling in the brain, and promoting lipogenesis, among many others [40]. This supports the idea that the ECS evolved to ensure the ingestion of essential fatty acids. Since LCFAs are presented as triglycerides in foods, this system reinforces the continued intake of calorically dense meals as well.
The continuous availability of inexpensive, fatty, palatable foods, however, drives the ECS to reinforce preference for fatty foods when it is not necessary, as no impending food shortage will afflict developed countries. This reward system is beneficial evolutionarily, but is helping to drive the current obesity epidemic [40]. The continuous intake of fat results in a decrease in LCFA detection sensitivity, which can increase total fat intake in a positive feedback loop [36]. This is caused, in part, by dysregulation of CB1 receptors in the oral cavity which impairs LCFA sensitivity (see Taste Detection and Description). Once taste sensitivity has been impaired, reward signaling is favored over satiety signaling, further exacerbating the positive feedback cycle [25]. Decreased LCFA taste sensitivity results in greater total fat intake [37]. Over time, a sustained increase in fat intake could lead to sustained increases in gut production of eCBs and CB1 activation, resulting in a greater motivation to consume fatty foods as well as a propensity towards weight gain [22]. Lastly, obesity and elevated eCB tone all impact LCFA detection sensitivity [34], contributing to the positive cycle of continued high fat intake and a dysregulated appetitive circuitry.
Short Chain Fatty Acids
SCFAs are fatty acids with fewer than six carbons, such as acetic (C2), propionic (C3), butyric (C4), and valeric (C5) acids. They are generally found in foods as byproducts of microbial fermentation. Humans obtain SCFAs from fermented foods, such as: vinegar, cheese, kimchi, natto, and sourdough bread. They are also made in our gut by the fermentation of dietary fibers by microbes. Fermentation is an important process of preserving foods that has been used since ancient times. Not only does fermentation prevent food spoilage, but it also improves the bioavailability of nutrients and produces additional vitamins and antioxidants [43]. SCFAs are a principal way that microbial fermentation affects food aroma and flavor, although there are other end-products of fermentation that play a role in flavor [44], e.g. volatile compounds, free amino acids (such as glutamate), and free ribonucleosides (such as inosine- and guanosine monophosphate).
Taste Detection and Description
Recently, several GPCRs for taste and smell have been identified that are activated by SCFAs including: FFAR2 (GPR43), FFAR3 (GPR41), GPCR109a, and the olfactory receptors OR51E1 and OR51E2 (see Table 1). FFAR2 is activated more strongly by shorter-chain fatty acids (C2 = C3 > C4 > C5), whereas FFAR3 has the opposite sensitivity (C3 = C4 = C5 > C2) [1]. The transduction mechanisms of SCFA taste and preference for them are not yet well characterized. In humans, FFAR2 has been identified in fungiform papillae of taste bud cells and its expression level is associated with dietary fat intake [6]. Among short and long chain fatty acids, the SCFAs have the lowest detection threshold [45], which may explain, in part, the perceived intensity of SCFAs.
SCFAs are associated with rancid, cheesy, or pungent flavors, which are not palatable on their own. Cheese samples elevated in the acetic, propionic, butyric, and caproic acids were associated with flavors described as rancid, pungent, acrid, and "smelly feet" [46]. Despite their seemingly unpalatable flavors, fermented foods have long been enjoyed by every culture, which appears to be a paradox. Kefir, kimchi and tempeh are now sold at mainstream supermarkets, and kombucha is found at convenience stores and “on tap” at some restaurants. The most well-known SCFA, acetic acid (vinegar), is a very common food additive in dressings and condiments (e.g. ketchup).
The amounts and ratios of individual SCFAs and other microbial metabolites produced during fermentation can vary due to the species and strains of microbes present. The specific ratios of SCFAs, along with other metabolites, can thus confer a signature flavor that indicates the microbial community that produced them [47]. Preference for certain flavors in fermented foods may affect food choices, resulting in selective colonization of the gut microbiota with specific types of microbes. Thus, there may be an interaction between our SCFA chemosensory abilities and preferences and our resultant gut microbiome.
Liking and Reward
One reason people may be attracted to these pungent, fermented foods is they are metabolically satisfying. Satiety itself can be rewarding and our brains, either consciously or unconsciously, remember our contentment after such a meal [48]. Whether they are consumed as fermented foods directly or as a high fiber food to be fermented later within the gut, SCFAs increase satiety in three ways: i) direct action on the brain, ii) stimulation of gut hormones, and iii) neural activation. Acetate (the salt of acetic acid), crosses the blood brain barrier to act directly on the hypothalamus [49]. In the gut, SCFAs increase GLP-1 and peptide tyrosine-tyrosine (PYY) release by enteroendocrine cells affecting insulin secretion and satiety, possibly via activation of FFAR2, FFAR3, and/or OR51E1 [1, 7, 14, 17, 50] (Figure 1). SCFAs also have an anorexigenic or satiating effect by inhibiting ghrelin release [51] and stimulating leptin production via FFAR2 on adipocytes [13]. The third mechanism of satiety is carried via the vagus nerve [52]. Intraperitoneal injection of butyrate (the salt form of butyric acid), suppresses appetite for 1 hour in mice by activating afferent vagal neurons and the nucleus tractus solitarius (NTS) [12]. A possible mechanism is FFAR3, which has been identified on vagal afferent neurons [11]. SCFAs signaling can create an anorexigenic effect, in contrast to the orexigenic effect of LCFAs.
Both LCFAs and SCFAs can increase GLP-1 in the blood [1]. As described above, ingesting LCFAs increases “fatty” taste sensitivity by downregulating oral CD36 expression in mice, thereby increasing fat intake [18]. It is interesting to consider whether ingesting SCFAs has a parallel effect of increasing LCFA “fatty” taste sensitivity, resulting in decreased fat intake. Without the involvement of the strong reward signaling of the ECS, as seen with LCFAs, intake of SCFAs would provide satiation without promoting overconsumption. Increasing SCFAs may help overcome disruptions in GLP-1 release and promote “fatty” taste sensitivity and satiety.
Achieving an Optimal LCFA:SCFA Ratio
The current Westernized diet provides little SCFAs as it is heavily composed of ultra-processed foods (UPFs) that are low in fiber, compared to traditional diets that are high in fiber and fermented foods. UPFs, laden with rewarding LCFAs, make up nearly 60% of total energy intake in the US [53]. We propose a new ratio of LCFA:SCFA (LC:SC) be considered to improve health. For example, Mediterranean and Japanese diets are considered healthy for a variety of reasons, perhaps among them is the LC:SC ratio. Specifically, the Japanese diet is high in SCFAs from fermented foods, such as nattō, soy, rice vinegar, pickled vegetables and fruits, fish flakes (katsuobushi), and miso [54], and low in LCFAs, which are mainly from omega 3s in fish [55]. It is worth noting that Japan has the lowest obesity rate in the world, 4.3% with a BMI above 30 kg/m2 [56]. We propose that SCFAs have been under-appreciated as nutrients and should be considered when making dietary guidelines. Recommendations to increase dietary SCFAs through fermented foods and fiber would decrease the LC:SC ratio and likely improve health (see Figure 2). Furthermore, consumption of live probiotic microbes in fermented foods should also be considered under dietary guidelines.
Figure 2. LC:SC Balance in Western Diet.
The overabundance of long chain fatty acids, especially omega 6 fatty acids, in the Western Diet drives endocannabinoid system activation, which reinforces fat intake, promotes inflammation and reduces energy expenditure, ultimately contributing to diet-induced obesity. Increasing short chain fatty acid intake through fermented foods and dietary fiber would promote satiety, reduce inflammation and increase energy expenditure to protect against diet-induced obesity. Created with BioRender.com.
LC:SC=long chain fatty acid:short chain fatty acid
The LC:SC ratio is important for several reasons. SCFAs have a strong potential to benefit health by modulating metabolism [57], inflammation, and immunity via activation of FFAR2 and FFAR3 [1] (see Table 1). Once absorbed, SCFAs may activate FFAR2 and FFAR3 on pancreatic β-cells and regulate insulin secretion [8]. In adipocytes, SCFAs promote browning of white adipose tissue, increase fatty acid oxidation and energy expenditure, and protect against diet-induced obesity in mice in part through FFAR2 and FFAR3 activation [58]. Overall, SCFAs act to increase energy expenditure [9]. The beneficial effects of SCFAs can even pass through generations. In pregnant mice, SCFAs provide offspring with resistance to obesity via FFAR2 and FFAR3 [59].
Conclusion
There remains much to learn about the paradoxical taste and desirability of fermented foods and high fiber foods via the oral and post-ingestive activation of FFARs by SCFAs. Understanding the unique taste profile of SCFAs can have direct effects on food engineering. Capitalizing on the enjoyable flavor components of foods with SCFAs in food production can increase their desirability and may help re-incorporate them into current Western diets. Foods with SCFAs may play a pivotal role in preventing and treating several common metabolic and inflammatory disorders. We can also capitalize on our understanding of oral LCFA-induced reward and its dependency on the endocannabinoid system to inform dietary intake and consumption patterns. On one hand, this system reinforces the necessary intake of essential FAs, but on the other, plays a role in the overconsumption of dietary fat, especially in Western societies where ultra-processed foods are a substantial portion of the diet. Coupling the overabundance of ultra-processed foods with the endocannabinoid system is a recipe for disaster in population metabolic health. This self-reinforcing feedback loop further exacerbates the LC:SC ratio. Like the ratio for omega 6:omega 3 fatty acids, and more recently, the sodium:potassium ratio, optimal LC:SC has the potential to better guide dietary intake by shifting focus to dietary balance. We propose this intake ratio be investigated globally amongst different populations and correlated with health, obesity, and metabolic disease.
Acknowledgements
We would like to thank Linda Flammer for her helpful comments. P.A.S.B. was supported by NIH DC014286 and HATCH NJ14120
Footnotes
Conflict of Interest Statement
Nothing declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.**.Kimura I, et al. , Free Fatty Acid Receptors in Health and Disease. Physiol Rev, 2020. 100(1): p. 171–210.This review provides a current overview of free fatty acid receptors, their characterization and their role in metabolism and health.
- 2.Lichtenstein AH, et al. , Dietary Fat Consumption and Health. Nutrition Reviews, 2009. 56(5): p. 3–19. [DOI] [PubMed] [Google Scholar]
- 3.Kumar NG, et al. , Dietary Bioactive Fatty Acids as Modulators of Immune Function: Implications on Human Health. Nutrients, 2019. 11(12): p. 2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulkarni BV and Mattes RD, Lingual lipase activity in the orosensory detection of fat by humans. Am J Physiol Regul Integr Comp Physiol, 2014. 306(12): p. R879–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Marzo V, Ligresti A, and Cristino L, The endocannabinoid system as a link between homoeostatic and hedonic pathways involved in energy balance regulation. Int J Obes (Lond), 2009. 33 Suppl 2: p. S18–24. [DOI] [PubMed] [Google Scholar]
- 6.Liu D, et al. , Expression of the candidate fat taste receptors in human fungiform papillae and the association with fat taste function. Br J Nutr, 2018. 120(1): p. 64–73. [DOI] [PubMed] [Google Scholar]
- 7.Tolhurst G, et al. , Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein-Coupled Receptor FFAR2. Diabetes, 2012. 61(2): p. 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Priyadarshini M, et al. , An Acetate-Specific GPCR, FFAR2, Regulates Insulin Secretion. 2015. 29(7): p. 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sukkar AH, et al. , Regulation of energy expenditure and substrate oxidation by short-chain fatty acids. J Endocrinol, 2019. 242(2): p. R1–r8. [DOI] [PubMed] [Google Scholar]
- 10.Gilbertson TA and Khan NA, Cell signaling mechanisms of oro-gustatory detection of dietary fat: advances and challenges. Prog Lipid Res, 2014. 53: p. 82–92. [DOI] [PubMed] [Google Scholar]
- 11.Nøhr MK, et al. , Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience, 2015. 290: p. 126–37. [DOI] [PubMed] [Google Scholar]
- 12.Goswami C, Iwasaki Y, and Yada T, Short-chain fatty acids suppress food intake by activating vagal afferent neurons. J Nutr Biochem, 2018. 57: p. 130–135. [DOI] [PubMed] [Google Scholar]
- 13.Zaibi MS, et al. , Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Letters, 2010. 584(11): p. 2381–2386. [DOI] [PubMed] [Google Scholar]
- 14.Fleischer J, et al. , Expression of odorant receptor Olfr78 in enteroendocrine cells of the colon. Cell Tissue Res, 2015. 361(3): p. 697–710. [DOI] [PubMed] [Google Scholar]
- 15.Halperin Kuhns VL, et al. , Characterizing novel olfactory receptors expressed in the murine renal cortex. Am J Physiol Renal Physiol, 2019. 317(1): p. F172–F186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Audouze K, et al. , Identification of Odorant-Receptor Interactions by Global Mapping of the Human Odorome. 2014. 9(4): p. e93037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han YE, et al. , Olfactory Receptor OR51E1 Mediates GLP-1 Secretion in Human and Rodent Enteroendocrine L Cells. J Endocr Soc, 2018. 2(11): p. 1251–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Besnard P, Passilly-Degrace P, and Khan NA, Taste of Fat: A Sixth Taste Modality? Physiol Rev, 2016. 96(1): p. 151–76.This comprehensive review on the oral detection of fat characterizes all key players involved in transducing a 'fat-taste' signal.
- 19.Dragano NRV, et al. , Polyunsaturated fatty acid receptors, GPR40 and GPR120, are expressed in the hypothalamus and control energy homeostasis and inflammation. Journal of Neuroinflammation, 2017. 14(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sankoda A, et al. , Long-Chain Free Fatty Acid Receptor GPR120 Mediates Oil-Induced GIP Secretion Through CCK in Male Mice. Endocrinology, 2017. 158(5): p. 1172–1180. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida R, et al. , Endocannabinoids selectively enhance sweet taste. Proceedings of the National Academy of Sciences, 2010. 107(2): p. 935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.DiPatrizio NV, Endocannabinoids in the Gut. Cannabis Cannabinoid Res, 2016. 1(1): p. 67–77.This review gives a detailed look into the regulation and signaling of endocannabinoids in the gut and how this system drives fat intake.
- 23.Cota D, et al. , The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. Journal of Clinical Investigation, 2003. 112(3): p. 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erlanson-Albertsson C, How Palatable Food Disrupts Appetite Regulation. Basic & Clinical Pharmacology & Toxicology, 2005. 97(2): p. 61–73. [DOI] [PubMed] [Google Scholar]
- 25.Edwards A and Abizaid A, Driving the need to feed: Insight into the collaborative interaction between ghrelin and endocannabinoid systems in modulating brain reward systems. 2016. 66: p. 33–53. [DOI] [PubMed] [Google Scholar]
- 26.Galindo MM, et al. , G Protein-Coupled Receptors in Human Fat Taste Perception. Chemical Senses, 2012. 37(2): p. 123–139. [DOI] [PubMed] [Google Scholar]
- 27.Tarragon E and Moreno JJ, Role of Endocannabinoids on Sweet Taste Perception, Food Preference, and Obesity-related Disorders. Chem Senses, 2017. 43(1): p. 3–16. [DOI] [PubMed] [Google Scholar]
- 28**.Brissard L, et al. , Orosensory Detection of Dietary Fatty Acids Is Altered in CB(1)R(−/−) Mice. Nutrients, 2018. 10(10).This study implicates cannabinoid 1 receptor in the regulation of 'fat-taste' sensitivity, providing a novel role for the receptor in taste tissue.
- 29.Melis M, et al. , Associations between Orosensory Perception of Oleic Acid, the Common Single Nucleotide Polymorphisms (rs1761667 and rs1527483) in the CD36 Gene, and 6-n-Propylthiouracil (PROP) Tasting. Nutrients, 2015. 7(3): p. 2068–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vestmar MA, et al. , Functional and genetic epidemiological characterisation of the FFAR4 (GPR120) p.R270H variant in the Danish population. J Med Genet, 2016. 53(9): p. 616–23. [DOI] [PubMed] [Google Scholar]
- 31.Plesník J, et al. , The rs1527483, but not rs3212018, CD36 polymorphism associates with linoleic acid detection and obesity in Czech young adults. British Journal of Nutrition, 2018. 119(4): p. 472–478. [DOI] [PubMed] [Google Scholar]
- 32.Keller KL, et al. , Common Variants in the CD36 Gene Are Associated With Oral Fat Perception, Fat Preferences, and Obesity in African Americans. Obesity, 2012. 20(5): p. 1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pepino MY, et al. , The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J Lipid Res, 2012. 53(3): p. 561–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melis M, et al. , Polymorphism rs1761667 in the CD36 Gene Is Associated to Changes in Fatty Acid Metabolism and Circulating Endocannabinoid Levels Distinctively in Normal Weight and Obese Subjects. Frontiers in Physiology, 2017. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stewart JE, et al. , Marked differences in gustatory and gastrointestinal sensitivity to oleic acid between lean and obese men. 2011. 93(4): p. 703–711. [DOI] [PubMed] [Google Scholar]
- 36.Martínez-Ruiz NR, et al. , Oral fat perception is related with body mass index, preference and consumption of high-fat foods. Physiology & Behavior, 2014. 129: p. 36–42. [DOI] [PubMed] [Google Scholar]
- 37.Heinze JM, et al. , Detection thresholds for four different fatty stimuli are associated with increased dietary intake of processed high-caloric food. Appetite, 2018. 123: p. 7–13. [DOI] [PubMed] [Google Scholar]
- 38.Hillard CJ, Circulating Endocannabinoids: From Whence Do They Come and Where are They Going? Neuropsychopharmacology, 2018. 43(1): p. 155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matias I, et al. , Endocannabinoids measurement in human saliva as potential biomarker of obesity. PLoS One, 2012. 7(7): p. e42399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazier W, et al. , The Endocannabinoid System: Pivotal Orchestrator of Obesity and Metabolic Disease. Trends Endocrinol Metab, 2015. 26(10): p. 524–537. [DOI] [PubMed] [Google Scholar]
- 41.Simon V and Cota D, MECHANISMS IN ENDOCRINOLOGY: Endocannabinoids and metabolism: past, present and future. Eur J Endocrinol, 2017. 176(6): p. R309–R324. [DOI] [PubMed] [Google Scholar]
- 42.Dipatrizio NV, et al. , Endocannabinoid signaling in the gut mediates preference for dietary unsaturated fats. The FASEB Journal, 2013. 27(6): p. 2513–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Melini F, et al. , Health-Promoting Components in Fermented Foods: An Up-to-Date Systematic Review. Nutrients, 2019. 11(5).This review provides a current overview of the beneficial compounds of fermented foods and the microbes involved in their production.
- 44.Sanromán M.A.L.a.M.A., Production of Food Aroma Compounds: Microbial and Enzymatic Methodologies. Food Technol. Biotechnol, 2006. 44 (3): p. 335–353. [Google Scholar]
- 45.Running CA and Mattes RD, Different oral sensitivities to and sensations of short-, medium-, and long-chain fatty acids in humans. American Journal of Physiology-Gastrointestinal and Liver Physiology, 2014. 307(3): p. G381–G389. [DOI] [PubMed] [Google Scholar]
- 46.Faccia M, et al. , Short communication: Chemical-sensory and volatile compound characterization of ricotta forte, a traditional fermented whey cheese. J Dairy Sci, 2018. 101(7): p. 5751–5757. [DOI] [PubMed] [Google Scholar]
- 47.Yang X, et al. , Microbial Community Dynamics and Metabolome Changes During Spontaneous Fermentation of Northeast Sauerkraut From Different Households. Front Microbiol, 2020. 11: p. 1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Breslin PAS, The Sense of Taste Encompasses Two Roles: Conscious Taste Perception and Subconscious Metabolic Responses, in THINK TANK: Forty Neuroscientists Explore the Biological Roots of Human Experience, Linden DJ, Editor. 2018, Yale University Press: New Haven, p. 110–118. [Google Scholar]
- 49.Frost G, et al. , The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun, 2014. 5: p. 3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christiansen CB, et al. , The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. American Journal of Physiology-Gastrointestinal and Liver Physiology, 2018. 315(1): p. G53–G65. [DOI] [PubMed] [Google Scholar]
- 51.Engelstoft MS, et al. , Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metab, 2013. 2(4): p. 376–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sawchenko PE, Gold RM, and Leibowitz SF, Evidence for vagal involvement in the eating elicited by adrenergic stimulation of the paraventricular nucleus. Brain Res, 1981. 225(2): p. 249–69. [DOI] [PubMed] [Google Scholar]
- 53.Martinez Steele E, et al. , Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open, 2016. 6(3): p. e009892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murooka Y and Yamshita M, Traditional healthful fermented products of Japan. Journal of Industrial Microbiology & Biotechnology, 2008. 35(8): p. 791–798. [DOI] [PubMed] [Google Scholar]
- 55.Harris WS, Redefining target omega-3 index levels: The Japan Public Health Center Study. Atherosclerosis, 2018. 272: p. 216–218. [DOI] [PubMed] [Google Scholar]
- 56.Organization, W.H., 2020 World Health Statistics. 2020.
- 57*.Zhao L, et al. , Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science, 2018. 359(6380): p. 1151–1156.This study demonstrated that supplementation of short chain fatty acids regulated expression of free fatty acid receptor 2 and free fatty acid receptor 3 and prevented diet induced obesity in mice.
- 58.Lu Y, et al. , Short Chain Fatty Acids Prevent High-fat-diet-induced Obesity in Mice by Regulating G Protein-coupled Receptors and Gut Microbiota. Scientific Reports, 2016. 6(1): p. 37589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kimura I, et al. , Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science, 2020. 367(6481). [DOI] [PubMed] [Google Scholar]




