The results of our study indicate that delivering a guided imagery smoking cessation intervention over the telephone is feasible and has the potential to help smokers quit.
Keywords: Tobacco, Smoking, Cessation, Intervention, Guided Imagery, Telephone, Quitline
Abstract
Background
Evidence supports the use of guided imagery for smoking cessation; however, scalable delivery methods are needed to make it a viable approach. Telephone-based tobacco quitlines are a standard of care, but reach is limited. Adding guided imagery to quitline services might increase reach by offering an alternative approach.
Purpose
To develop and test the feasibility and potential impact of a guided imagery-based tobacco cessation intervention delivered using a quitline model.
Methods
Participants for this randomized feasibility trial were recruited statewide through a quitline or community-based methods. Participants were randomized to guided imagery Intervention Condition (IC) or active behavioral Control Condition (CC). After withdrawals, there were 105 participants (IC = 56; CC = 49). The IC consisted of six sessions in which participants created guided imagery audio files. The CC used a standard six-session behavioral protocol. Feasibility measures included recruitment rate, retention, and adherence to treatment. We also assessed 6-month quit rates and consumer satisfaction.
Results
Both the IC and CC protocols were feasible to deliver. We finalized protocols and materials for participants, coaches and study staff, and delivered the protocols with fidelity. We developed successful recruitment methods, and experienced high retention (6 months = 81.9%) and adherence (all sessions = 66.7%). Long-term quit rates (IC = 27.9%; CC = 38.1%) compared favorably to those of quitlines, and program satisfaction was high, suggesting that the protocols are acceptable to smokers and may contribute to smoking abstinence.
Conclusions
The guided imagery intervention is feasible and promising, suggesting that a fully powered RCT to test the efficacy of the intervention is warranted.
Trial registration number
Implications.
Practice: Delivering a guided imagery tobacco cessation intervention using a quitline model is feasible and produced quit rates comparable to standard cognitive behavioral telephone counseling.
Policy: Incorporating guided imagery tobacco cessation interventions might increase the reach of quitlines by offering an alternative approach to standard quitline services.
Research: Future research should be aimed at establishing the efficacy of the guided imagery intervention versus and active standard cognitive behavioral control.
Background
Tobacco use remains the leading cause of preventable disease and death in the USA [1]. The vast majority (68%) of adult smokers want to quit, and more than 55% make a quit attempt in any given year [2]. However, most smokers (66%) attempt to quit on their own and only 10% quit successfully [2]. Novel approaches are needed to reach and assist tobacco users to quit. One such approach is the use of guided imagery. Guided imagery uses enhanced visualization to achieve desired goals and outcomes, and evidence supports its use for smoking cessation [3–6]. Guided imagery is a multisensory cognitive process shown in previous research to increase motivation and facilitate goal achievement in sports and exercise [7–9]. This technique is used by about 2% of adults in the USA mainly for stress management [10] but there are a range of other known applications of this technique [11]. While racial and ethnic differences in the use of guided imagery may exist [12], we hypothesized that guided imagery might appeal to men and other groups given its popularity among elite athletes and other high profile individuals [13]. Research with racial/ethnic minorities and men found high acceptability of guided imagery for addressing stress in pregnant African American women [14,15], obesity in Latino adolescents [16], and insomnia in male veterans [17]. Guided imagery is being used in treatment for post-traumatic stress disorder in the primarily male-serving Veterans Administration [18]. In addition, guided imagery is novel compared to the standard behavioral approach used by quitlines [19,20] and may attract a more diverse group of smokers than is typical of quitline callers [21,22].
Using this technique, one imagines sights, sounds, tastes, smells, tactile sensations, and emotions associated with the desired outcome. Guided imagery for smoking cessation is focused on maintaining motivation, increasing self-efficacy, and coping with cravings, and has been shown to assist tobacco users in quitting [3,5,23]. Guided imagery as used in our study is based on a cognitive and motivational framework recently supported by psychometric analyses [24]. This perspective suggests that imagery serves cognitive and motivational functions to build self-efficacy for quitting and preventing relapse, expectations to cope with cravings, and self-image of being a nonsmoker. Cognitively focused imagery includes plans for obtaining information, images of specific strategies, and using resources to quit (e.g., quitlines). Motivationally focused imagery includes emotional and efficacy-enhancing messages associated with cigarette cravings and self-efficacy beliefs.
However, other than our prior work using mobile app-based delivery [3–6,25–27], guided imagery interventions have been delivered predominantly using an in-person approach. More scalable delivery methods are needed to make guided imagery a viable cessation approach. Little research has focused on how guided imagery can be delivered to smokers using remote or virtual methods. Therefore, the feasibility and acceptability of this implementation approach requires further study.
Telephone-based tobacco cessation quitlines are a standard of care for tobacco cessation [19,20,28,29]. The quitline infrastructure in the USA provides telephone cessation services to all 50 states plus Puerto Rico and Guam. Quitlines provide evidence-based care and according to the North American Quitline Consortium, had an average overall quit rate of 27.6% in 2017 [30]. However, quitlines reach only 1%–2% of smokers nationwide [30]. Adding an effective guided imagery intervention to quitline services might increase the reach of quitlines by appealing to tobacco users (including men and racial/ethnic minorities) who might not be interested in the quitline’s standard behavioral approach. Men are less likely to reach out for health help in changing health behaviors, and quitlines have limited reach among racial and ethnic minorities [21,22,31–34]. As stated above, we hypothesized that men might find an approach “used by athletes and their coaches” to be more appealing than a more traditional “counseling” one, and that certain racial/ethnic groups and other types of smokers might be enticed by an “alternative” approach to quitting versus standard “allopathic” behavioral treatment.
The present study (Be Smoke-Free) was designed to develop and test the feasibility and potential impact of a guided imagery-based tobacco cessation intervention delivered using a quitline model. The goals of the randomized feasibility trial were to: (i) develop intervention protocols, patient, and training materials; (ii) develop study protocols, procedures, and measures; (iii) test the feasibility of the protocols and procedures; and (iv) explore the potential impact of the guided imagery intervention versus an active behavioral control. The study protocol was published previously [35].
The study was conducted in two phases: (i) Development. During this formative phase, we developed and tested all intervention materials in conjunction with our Community Advisory Board, expert consultants, and focus testing with smokers. Details on the development process have been published elsewhere [36]. (ii) Randomized Feasibility Trial. During this phase, we conducted a randomized feasibility trial in collaboration with the Arizona Smokers’ Helpline (ASHLine), the statewide quitline. Participants were recruited through community-based methods (e.g., media, flyers, etc.) or the ASHLine. The ASHLine is operated by the University of Arizona College of Public Health. A co-investigator and one of the authors (UN) directed the ASHLine and served as the liaison between the research and the ASHLine staff. ASHLine staff participated in developing study protocols and procedures, screened participants for eligibility, collected data, and provided reports on quitline usage during the recruitment period. This manuscript describes the results of the randomized feasibility trial phase of the project.
METHODS
Study overview
The feasibility trial was conducted between 05/02/2018 and 6/11/2019 in Arizona. The study adheres to CONSORT guidelines for reporting clinical trials, was approved by the Institutional Review Board (Protocol # 1607731418A022), and study activities were monitored quarterly by an Independent Monitoring Committee (as required by the funding mechanism). Potential participants were eligible for the study if they were: daily smokers aged 18 or older; willing to receive telephone coaching; had a valid phone number and email address; and spoke English. Potential participants were excluded from the study if they: reported current schizophrenia; had used the ASHLine in the past 12 months; were currently receiving any form of tobacco cessation treatment; or refused to be randomized to one of the study conditions. Participants were recruited statewide through community-based methods (e.g., earned media, social media, presentations at local community organizations, and flyers) or the ASHLine. Recruitment materials described both study conditions (i.e., testing a new guided imagery tobacco cessation program versus a standard cognitive behavioral program). Recruitment material specifically described guided imagery in order to test whether this type of approach might be more appealing to men and/or racial/ethnic minority groups. In collaboration with ASHLine and their information technology staff, we added programming to their client intake system to screen for potential study participants and transmit eligible participants’ data into the project’s REDCap database. ASHLine intake staff were trained to assist in participant screening, and the ASHLine data manager oversaw data transfer and reporting. Our goal was to recruit 100 participants, a sample size determined to be sufficient for assessing feasibility outcomes (our primary outcomes) with reasonable precision: 50 participants per arm would yield a margin of error (half-width of 95% confidence interval [CI]) for binary outcomes [37]. We recruited participants through the ASHLine for 4 months and conducted community recruitment for 4 months. Participants were screened for eligibility and consented into the project by study staff via telephone. Potential participants received a detailed description of the study, including both study conditions (i.e., a summary of both study conditions and expectations for participation) prior to providing consent and completing the baseline survey. Those participants who completed the consent and baseline survey were automatically randomized to a guided imagery Intervention Condition (IC) or active behavioral Control Condition (CC) using a block randomization protocol programmed in Research Electronic Data Capture (REDCap; NCATS/NIH UL1TR000445) [35].
Intervention and Control Condition descriptions
We created the Be Smoke-Free IC and CC protocols based on existing quitline services and input from our Community Advisory Board, consultants, and audience of interest [36]. In each condition, we developed a six-session protocol to be delivered over 6 weeks. The sessions focused on: (i) reasons for and benefits of quitting; (ii) triggers for smoking and alternative strategies; (iii) coping with cravings and withdrawal; (iv) preparing for a quit day; and (v and vi) staying quit or recommitting to quit.
Four weeks of nicotine patches (21, 14, 7 mg) or nicotine lozenges (4, 2 mg) were offered to all participants. Nicotine replacement is recommended as a standard of care for smoking cessation and is offered by most tobacco quitlines [28]. It is available over the counter and by prescription (which is covered by most insurance plans). The recommended dosage is based on number of cigarettes smoked per day and other dependence measures (e.g., time to first cigarette after waking). Study coaches collected these data from the participants, screened for contraindications of use, and worked with participants to determine the proper product and dose during Session 3. Participants who were eligible to receive nicotine replacement were sent their product/dose by mail.
The IC focused on creating and using guided imagery audio files to visualize behavior change while CC utilized evidence-based behavioral techniques (see Table 1). Both study protocols were programmed into REDCap which the study “quit coaches” used to deliver the protocol and collect data on all intervention and control session activities.
Table 1 .
Session content by study condition
| Session | Intervention condition | Control condition |
|---|---|---|
| 1 Reasons for and benefits of quitting |
Identify reasons and benefits, choose top reason, develop two-part guided imagery script focusing on negative aspects of smoking and positive aspects of quitting. Set a practice schedule. Set a quit date 3–4 weeks away. Coach records audio file and sends to participant via text or email. Participant listens to audio file between sessions 1 and 2 | Identify reasons and benefits, choose top reason. Participant uses Be Smoke-Free post-it notes to write down negative aspects of smoking and positive aspects of quitting. Identify where to post notes and how often to review them for the next week. Set a quit date 3–4 weeks away. Participants review their post-it notes according to the schedule between session 1 and 2 |
| 2 Triggers for smoking and alternative strategies |
Review prior week’s progress. Make edits to guided imagery script, if needed. Identify situations that trigger smoking. Pick one trigger and discuss alternative coping strategies to use instead of smoking. Create a guided imagery script for the trigger and alternative strategies. Coach records/sends audio file(s) to participant to practice between sessions 2 and 3, and practice alternative strategies | Review prior week’s progress. Reinforce successes and troubleshoot challenges. Identify situations that trigger smoking. Complete an activity that lists all situations and pick one trigger. Discuss potential coping strategies to use instead of smoking. Assign an activity in the Be Smoke-Free Quitting Guide to complete between sessions 2 and 3, and practice alternative strategies |
| 3 Coping with cravings |
Review prior week’s progress and make edits to scripts, if needed. Discuss physical versus learned cravings. Identify alternative strategies for coping with learned cravings. Create script for coping strategies. Discuss NRT for dealing with physical cravings. Screen participant for use of NRT. Coach records/sends audio file(s) and sends NRT, if eligible. Participant listens to audio files between sessions 3 and 4, and practices coping skills | Review prior week’s progress. Reinforce successes and troubleshoot challenges. Discuss physical versus learned cravings. Identify alternative strategies for coping with learned cravings. Create script for coping strategies. Discuss NRT for dealing with physical cravings. Screen participant for use of NRT. Assign an activity in the Be Smoke-Free Quitting Guide to complete between sessions 3 and 4, and practice coping skills |
| 4 Withdrawal, NRT, and preparing for quit date |
Review prior week’s progress and make edits to scripts, if needed. Discuss withdrawal symptoms and how to manage them. Confirm receipt of NRT and review instructions for use, if applicable. Discuss how to prepare their environment for the quit date. Coach records/sends revised audio files, if applicable. Participant listens to audio files between sessions 4 and 5, practices skills, and uses NRT, if applicable | Review prior week’s progress. Reinforce successes and troubleshoot challenges. Discuss withdrawal symptoms and how to manage them. Confirm receipt of NRT and review instructions for use, if applicable. Discuss how to prepare their environment for the quit date. Assign an activity in the Be Smoke-Free Quitting Guide, practice skills between sessions 4 and 5, and use NRT, if applicable |
| 5 and 6 Preventing slips and/or recommitting to quit |
Review prior week’s progress and make edits to scripts, if needed. If the participant has quit, offer congratulations. If not, set a new quit date. Discuss challenging situations and alternative strategies. Define “slips” and discuss how to avoid them or deal with them in a constructive way. Send more NRT and updated audio files, if needed. Participant listens to audio files between sessions 5 and 6, practices skills, and uses NRT, if applicable | Review prior week’s progress. If the participant has quit, offer congratulations. If not, set a new quit date. Discuss challenging situations and alternative strategies. Define “slips” and discuss how to avoid them or deal with them in a constructive way. Send more NRT if needed. Participant completes an activity in the Smoke-Free Quitting Guide, practices skills, and uses NRT, if applicable |
| Staying smoke-free or recommit to quit | Review prior week’s progress and make edits to scripts, if needed. If the participant has quit, create a final script focusing on staying smoke-free. Focus on skills and reviewing audio files. If not quit, troubleshoot challenges and set a new quit date. Debrief the Be Smoke-Free program, and discuss additional resources, if needed. Coach records and sends final audio file | Review prior week’s progress. If the participant has quit, discuss staying smoke-free. Focus on skills, dealing with slips, anticipating challenges, and reviewing lessons and the Smoke-Free Quitting Guide. If not quit, troubleshoot challenges and set a new quit date. Debrief the Be Smoke-Free program, and discuss additional resources to use, if needed |
In the IC, one Masters-level (MPH) coach was trained to competency to work with participants to develop a guided imagery script in each session focusing on the weekly topic (described below). The coached worked with each participant individually via phone to develop a tailored guided imagery script in each session based on the topic covered (e.g., reasons for and benefits of quitting). The coach prompted the participant to identify situations that were most relevant to the topic, and elicited sights, sounds, smells, tastes, physical sensations, and emotions to create a vivid, evocative script. The coach and participant reviewed and revised the script during each session, and established a guided imagery practice schedule (e.g., once per day, seven days per week). After the session, the coach recorded the script as an electronic audio file (e.g., MP3) and sent the file to the participant via email or text. The participant was instructed to listen to the audio file according to the established schedule as well as to make behavioral changes identified during the sessions. At the beginning of sessions 2–6, the coach asked the participants to report on the number of times they listened to their guided imagery script during the previous week and recorded this data in the REDCap system. If the participant did not meet their practice goals, the coach and participant would engage in problem-solving to increase practice going forward. Self-reported practice was also collected during the 8-week follow-up assessment. During most sessions, participants were given the opportunity to revise a previously developed script in addition to creating a new one.
In the CC, the coach originally hired failed to achieve competency. Due to time constraints, we used the services of four part-time, experienced quit coaches (e.g., Masters-level, PhD-prepared, and/or Tobacco Treatment Specialists) throughout the duration of the study. The coaches worked with participants to identify behavioral skills relevant to each session’s topic, and to establish a schedule for practicing the skills. In each session, the coach and participant reviewed weekly progress, engaged in problem-solving activities to overcome barriers to quitting, and added new behavioral cessation strategies. The coaches assigned behavioral activities from the study-created project quit guide for participants to complete between sessions in addition to practicing behavioral skills.
Coach training, competency, and fidelity
We created training programs for both IC and CC coaches. Coaches for both conditions received 4 h of training in tobacco-specific information and general communication and coaching skills. Coaches then received 8 h of condition-specific training in the study protocol and either guided imagery or behavior change theory and related skills. The coaches engaged in role plays with J.G., P.G., U.N., and J.A. and received feedback. We developed standardized competency rating sheets for each session. Following the role plays, coaches were recorded while working with “standardized patients” and were rated by J.G., P.G., and U.N. until they achieved at least minimum competency. Finally, we enrolled nine “user test” participants (participants meeting all the study criteria) who were assigned to either IC or CC. All sessions were recorded and reviewed and rated by J.G., P.G., and U.N., and both coaches received weekly individual supervision by J.G. During the user test, the CC coach was unable to attain at least minimum competency for all sessions and was replaced by four, part-time coaches with extensive experience in delivering behavioral tobacco cessation treatment.
Intervention fidelity was monitored through standardized session protocols programmed into REDCap and session audio recordings. Each REDCap session protocol consisted of checklists, prompts, and text fields that all coaches were mandated to follow, review, and complete. Under the direction of J.A., study staff monitored the completion of each session protocol in REDCap and prompted coaches to improve documentation, when necessary. Session recordings were monitored weekly by J.G. and P.G., and feedback was provided to coaches during individual supervision.
Assessments
Self-reported tobacco use, guided imagery use, and attitudes towards quitting and guided imagery were assessed at baseline, 8-weeks, and 6-months post-enrollment. Demographics and other tobacco-related data (e.g., home smoking bans, number of smokers in the home, tobacco dependence, etc.) were collected at baseline. Tobacco-related data, guided imagery use and attitudes, and process data (e.g., consumer satisfaction) were collected at 8 weeks. Tobacco-related data, and guided imagery use and attitudes were also collected at 6 months. All of the measures used in this study were used successfully in our previous work [26,38]. The demographics collected at baseline included: gender, age, race/ethnicity, level of education, insurance, and prior use of tobacco cessation resources (including quitline and medication). We collected tobacco use status using a series of questions that have been standardized and employed in previous studies [39–41], and level of dependence using the Fagerström Tolerance Nicotine Dependence scale and the Shiffman Nicotine Dependence Syndrome Scale [42,43]. Self-efficacy for quitting was measured with the 15-item version of the Condiotte & Lichtenstein Confidence Questionnaire [44]. Imagery expectancies and credibility were measured using an adapted form of the Borokov and Nau Treatment Credibility Scale [45], used in our previous study to measure expectancies and perceived credibility of guided imagery for smoking cessation [26,38]. Consumer satisfaction was adapted from 8 items (using a five-point Likert scale) used in our previous research [26,27,38,41,46] that measured overall satisfaction with the program, perceived usefulness and relevance of the information, likeability, level of interest, ease of use, and whether they would recommend the program to others. For IC participants only, we assessed imagery mastery through ratings of imagery vividness and controllability collected weekly by the coach during each session [47].
For participants enrolled through the ASHLine, baseline data were collected by ASHLine staff and transferred electronically to the project REDCap system. For community recruits, data were collected in REDCap by study staff. Follow-up assessments were sent via text and/or email and administered online. Participants who did not complete assessments within 1 week were texted/emailed reminders. Participants who did not complete assessments within 2 weeks were called by study staff to complete the assessment via phone. Finally, participants who did not complete assessments within 4 weeks were given a “final option” to complete an abbreviated survey (7-day and 30-day tobacco use questions) via text. We attempted to obtain biochemical verification, via salivary cotinine (a metabolite of nicotine) assay, of smoking abstinence at the 6-month assessment from all participants reporting 7-day abstinence. The remote biochemical verification procedure was carried out by study staff. Eligible participants were mailed a salivary cotinine strip and collection kit with instructions. Study staff contacted the participants by text, email or phone to schedule a videoconference call to collect the saliva sample and interpret the results. During the videoconference calls, study staff verified the participants’ identity, instructed the participants in the collection protocol, witnessed the participant provide the sample, interpreted the results, instructed the participants to take a photo of the strip and text it to study staff, and recorded the results in REDCap (along with the photo).
Analyses
Summary statistics of baseline data were used to describe the study sample. Feasibility outcomes were summarized with means or proportions along with 95% CIs. Satisfaction with the program, coaching, and guided imagery at 8 weeks were summarized with medians and interquartile ranges, as these Likert scaled data were skewed. Comparisons between the IC and CC groups on tobacco outcomes at 6 months were estimated with chi-squared tests or t-tests and reported as odds ratios (OR) or differences. Time spent per day on visualization or meditation/relaxation was modeled with mixed models using time categorically, and a random effect for participant. Comparisons between the IC and CC groups in the difference in change from baseline at 8 weeks and 6 months were carried out using contrasts within these models. In IC, we used logistic regression to investigate a “dose–response” effect of adherence to the intervention on cessation, as measured by time spent on visualization or relaxation (as reported at 8 weeks). Demographics were used to assess the potential to increase reach to men and racial/ethnic minority groups by comparing these variables between participants recruited through the ASHLine versus those recruited via community-based methods. We compared baseline characteristics of those who dropped out or withdrew by 6 months and those who did not.
RESULTS
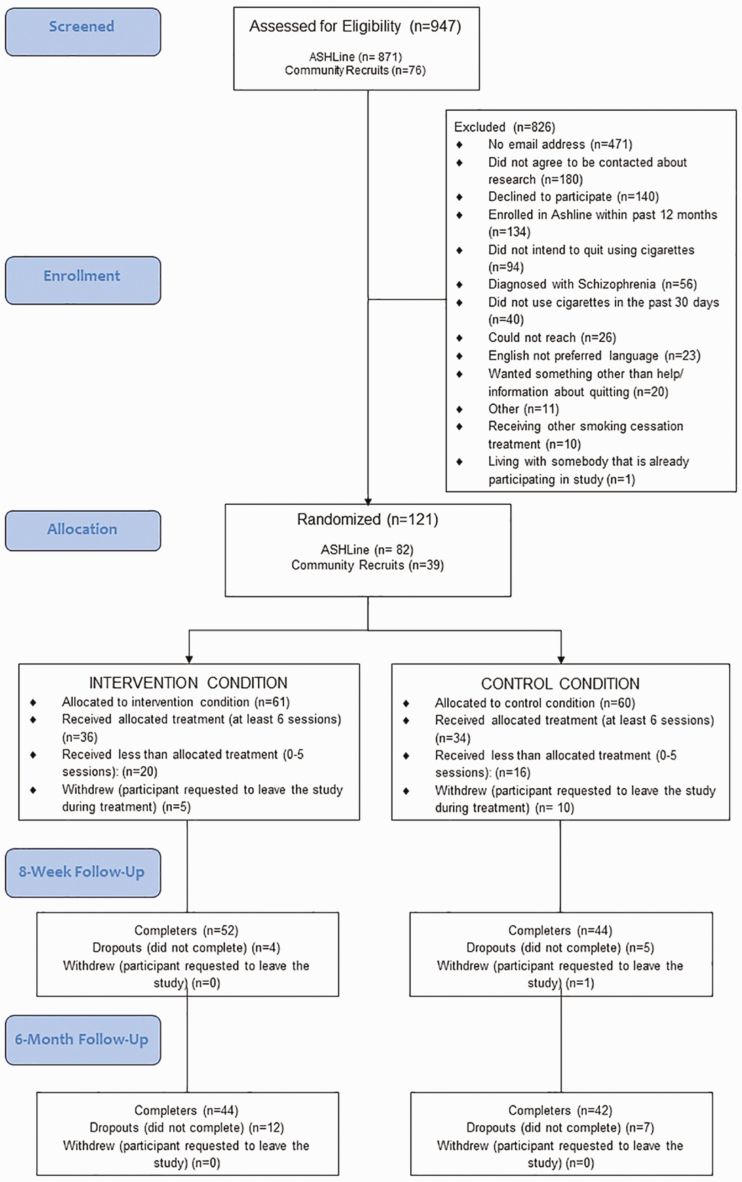
We recruited 121 participants (Fig. 1). The majority of participants (n = 82; 67.7%) were recruited through the ASHLine, and 39 (32.3%) through community-based methods. As displayed in Fig. 1 (CONSORT Diagram), after withdrawals, we obtained a sample of 105 (IC = 56; CC = 49).
Fig 1.
CONSORT diagram
Participant characteristics
As displayed in Table 2, participants were primarily female (65.7%), non-married (73.3%), non-Hispanic white (74.0%), with higher than a high school education (67.6%) and a mean age of 48.5 and 51.6 years in the IC and CC arm, respectively (combined sample mean = 50.5, SD = 14.4). All participants smoked cigarettes (mean cigarettes per day of 15.3 [SD = 7.5] and 16.3 [SD = 8.1]; combined mean = 15.8, SD = 7.8) and some also used e-cigarettes or vaped (23.2% and 16.3%, in IC and CC, respectively; combined 20.0%). Participants in CC were less likely to be employed (36.7% versus 53.6%) and less likely to be underinsured (have Medicaid or no insurance) (34.7% versus 46.4%). About a third in each study arm reported income below the federal poverty level. Most (71.4% and 77.6%; combined 74.3%) had full smoking bans in their homes, and were the only smokers in the household (75.0% and 64.6%; combined 70.2%). At baseline, participants in both arms reported high social support, cravings, concerns about relapsing, motivation to quit, and willingness to put effort into quitting. Confidence to quit, and beliefs that skills are effective to quit were slightly lower in IC.
Table 2.
Demographic and baseline characteristics
| Baseline characteristics | Guided imagery (n = 56) | Control (n = 49) |
|---|---|---|
| Values shown are n (%) unless otherwise noted | ||
| Age (y), mean (SD) | 48.5 (15.8) | 51.6 (14.5) |
| Male gender | 20 (35.7) | 16 (32.7) |
| Married | 13 (23.2) | 15 (30.6) |
| Non-Hispanic white | 44 (78.6) | 33 (68.8) |
| Hispanic ethnicity | 8 (14.3) | 9 (18.8) |
| Non-white, non-Hispanic | 4 (7.1) | 6 (12.5) |
| >High school education | 35 (62.5) | 36 (73.5) |
| Employment, full or part time | 30 (53.6) | 18 (36.7) |
| Uninsured or Medicaid | 26 (46.4) | 17 (34.7) |
| Below federal poverty level | 18 (32.1) | 18 (36.7) |
| Chronic health conditiona | 40 (71.4) | 32 (65.3) |
| Mental health condition | 34 (60.7) | 23 (46.9) |
| Nicotine dependence (Fagerström), mean (SD) | 4.0 (2.0) | 4.3 (1.6) |
| Cigarettes per day, mean (SD) | 15.3 (7.5) | 16.3 (8.1) |
| Tobacco/nicotine productsb | ||
| Cigarettes | 56 (100.0) | 49 (100.0) |
| E-cigarettes/vaping | 13 (23.2) | 8 (16.3) |
| Other nicotine products | 3 (5.4) | 0 (0) |
| Smoking allowed in home | ||
| Full ban | 40 (71.4) | 38 (77.6) |
| Partial ban | 7 (10.7) | 3 (6.1) |
| No ban | 8 (14.3) | 8 (16.3) |
| Other smokers in household | 14 (25.0) | 17 (35.4) |
| Perceived social support, meanc (SD) | 4.0 (1.2) | 3.7 (1.5) |
| Craving, meanc (SD) | 4.4 (1.2) | 4.7 (1.2) |
| Concern about relapsing, meanc (SD) | 3.9 (1.2) | 4.0 (1.2) |
| Motivation to quit, meanc (SD) | 4.6 (0.7) | 4.5 (0.8) |
| Confidence to quit, meanc (SD) | 3.8 (1.0) | 3.8 (1.1) |
| Belief that skills are effective, meanc (SD) | 2.8 (1.1) | 2.9 (1.1) |
| Effort willing to put into quitting, meanc (SD) | 4.8 (0.6) | 4.7 (0.6) |
aAsthma, COPD, hypertension, diabetes, and cancer.
bMay add to more than 100%; Other = cigars, pipes, smokeless, hookahs, and bidis.
cLikert response options ranged from 1 to 5, with 5 representing more positive scores, except craving, where response options ranged from 1 to 6, and higher values indicated stronger craving.
Feasibility
As displayed in Table 3, our initial goal was to recruit 100 participants in 10 months. However, we were able to randomize 121 participants in 8 months. Of those, 16 withdrew from the study prior to the 8-week assessment. Of the 105 remaining participants, retention at 8 weeks was 91.1% and 87.8% in the intervention and control arm, respectively; at 6 months, retention was 78.6% and 85.7%. Our retention rates exceeded our feasibility benchmarks of 75%. The mean number of sessions and length of treatment were 4.9 sessions and 37.9 days for the IC and 4.7 and 35.1 days for the CC. Our benchmark for program completion (six sessions) was at least 50%, which we exceeded with 64.3% (intervention) and 69.4% (control) attending all six sessions. We attempted to collect reasons from participants who did not complete all six sessions. The eight participants who had fewer than six IC coaching calls reported “personal issues” and “other” as the most common reasons. The 12 participants who had fewer than six CC coaching calls reported lack of time and feeling as if the sessions were not helpful or too stressful, in addition to personal issues. A majority of participants practiced skills (imagery or behavioral, respectively) five or more times per week (67.9% and 81.6%), which also exceeded our benchmark of at least 50%. Among participants in the IC, the most listened-to audio file was the final script recorded in session 5 or 6 (“Being Smoke-Free”). We were not able to meet our benchmark for biochemical verification (75%). We were able to complete biochemical verification with only 30.6% of participants. We were unable to contact 50.0% of participants. Of those who declined (19.4%), the most common reasons reported were started smoking again, lack of comfort in using technology, and distrust of providing a biological sample. In addition, the test strips we used (NicAlert) malfunctioned and produced mainly false positives; thus, we were unable to accurately verify abstinence using this approach.
Table 3 .
Feasibility outcomes
| Outcome | Value (%) | 95% CI | Feasibility criteria | Feasible? |
|---|---|---|---|---|
| Enrollment/screening Community ASHLine Total |
39/76 (51.3) 82/871 (9.4) 121/947 (12.8) |
39.6, 63.0 7.6, 11.6 10.7, 15.1 |
Enroll 10 participants per month for 10 months | Yes |
| Withdrawal rates Intervention Control |
5/61 (8.2) 11/60 (18.3) |
2.7, 18.1 9.5, 30.4 |
<20% | Yes |
| 8-week retention rate, n (%) Intervention Control |
52 (92.9) 44 (89.8) |
86.1, 99.6 81.3, 98.3 |
≥75% | Yes |
| 6-month retention rate, n (%) Intervention Control |
44 (78.6) 42 (85.7) |
67.8, 89.3 75.9, 95.5 |
≥75% | Yes |
| Length of treatment (days, mean) Intervention Control |
37.9 35.1 |
32.9, 43.0 29.4, 40.9 |
N/A | |
| Number of sessions (mean) Intervention Control |
4.9 4.7 |
4.4, 5.4 4.1, 5.4 |
N/A | |
| Attended 6 sessions, n (%) Intervention Control |
36 (64.3) 34 (69.4) |
50.4, 76.6 54.6, 81.7 |
≥50% | Yes |
| Participants who practiced ≥ 5 times/week, n (%) Intervention Control |
40 (67.9) 38 (81.6) |
55.2, 80.5 70.4, 92.9 |
≥50% | Yes |
| Biochemical validation (n = 36 eligible; n (%)) Agreed Completed Declined/could not contact |
18 (50.0) 11 (30.6) 19 (52.8) |
32.9, 67.1 16.3, 48.1 35.5, 69.6 |
≥75% | No |
Adherence
Adherence to the intervention was measured at 8 weeks by self-reported time spent per day on visualization and relaxation/meditation “in the last week.” As seen in Table 4, adherence was similar between the arms at all time points, with the majority of participants reporting little to no use of guided imagery at baseline. On average, participants reported weekly practice of 10–20 min at the 8-week assessment, and less than 10 min at the 6-month assessment. Self-reported use of relaxation/meditation was higher at baseline and 6 months (see Table 3).
Table 4 .
Adherence: time spent per day on visualization and relaxation/meditation in the last week
| Variable | Guided imagery | Control | Difference in change from baselinea (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| Imagery | Mean (SD) | Change from baseline | Mean (SD) | Change from baseline | ||
| Baseline | 1.7 (1.2) | – | 1.7 (1.3) | – | – | |
| 8 weeks | 3.0 (1.2) | 1.22 | 2.7 (1.3) | 0.94 | 0.29 (0.75, 1.71) | .42 |
| 6 months | 2.1 (1.2) | 0.36 | 2.1 (1.3) | 0.36 | 0.005 (−0.72, 0.73) | .99 |
| Relaxation/meditation | ||||||
| Baseline | 2.5 (1.5) | – | 2.2 (1.6) | – | – | |
| 8 weeks | 3.0 (1.3) | 0.52 | 2.5 (1.5) | 0.76 | −0.23 (−1.02, 0.55) | .05 |
| 6 months | 2.6 (1.2) | 0.13 | 2.8 (1.5) | 0.59 | −0.46 (−1.27, 0.35) | .27 |
Response options were 1 = never, 2 = less than 10 min, 3 = 10–20 min, 4 = 21–30 min, 5 = More than 30 min.
aGuided imagery–control.
Satisfaction
Participants in both arms were highly satisfied with the program and booklet, with median scores of 5 out of 5 for most of these measures (see Table 5). Coaching was also rated highly with median scores of 5 out of 5 for coach helpfulness, working as a team, and knowledge. Satisfaction with the guided imagery program was also high (5 out of 5 on all items), and participants rated guided imagery highly (4 out of 5) as an approach for quitting smoking.
Table 5.
Satisfaction at 8 weeks
| Guided imagery N = 51 | Control N = 43 | |
|---|---|---|
| Program satisfaction | Median (IQR)a | |
| Overall satisfaction with program | 5 (4, 5) | 5 (4, 5) |
| How likely are you to recommend the Be Smoke-Free program? | 5 (5, 5) | 5 (5, 5) |
| How helpful would you rate the booklet? | 5 (4, 5) | 4 (3.5, 5) |
| Coaching | ||
| How helpful would you rate the coaching sessions? | 5 (4, 5) | 5 (4, 5) |
| My coach and I were able to work together as a team to develop a quit smoking plan | 5 (4, 5) | 5 (4, 5) |
| My coach knew a lot about how to help me quit smoking | 5 (4, 5) | 5 (4, 5) |
| N (%) | ||
| Guided imagery (n = 51) | Median (IQR)a | N/A |
| How likely are you to continue using guided imagery? | 5 (3, 5) | |
| My coach knew a lot about guided imagery and explained it to me understandably | 5 (5, 5) | |
| My coach and I were able to work as a team to create my script and audio file | 5 (5, 5) | |
| How confident are you in guided imagery for quitting? | 4 (3, 5) | |
| How logical does guided imagery for quitting seem? | 4 (3, 5) |
aIQR, interquartile range; Likert response options ranged from 1 to 5, with 5 representing more positive scores.
Potential impact
Although not powered to test efficacy, we compared self-reported abstinence (7-day point prevalence and 30-day prolonged abstinence) at 6 months. As displayed in Table 6, self-reported 7-day abstinence at 6 months was 34.1% and 50.0% for the intervention and control arm (OR = 0.52, 95% CI: 0.22, 1.23), which was not statistically significant. The difference in 30-day cessation was similar; IC = 27.9% vs. CC = 38.1% (OR = 0.63, 95% CI: 0.25, 1.57). There was no evidence of a difference between the arms in 30-day other tobacco use, home bans, confidence, support, cravings, self-efficacy, concern about relapse (among quitters), or effort required. There was some evidence of a dose–response relationship on use of guided imagery on 7-day cessation, with an increase in cessation (estimated OR of 1.21 [95% CI:1.02, 1.45]) for each five times guided imagery was practiced. For the five-point Likert scaled measure of time spent per day on visualization, the OR was 1.28 (95% CI: 0.72, 2.28), indicating that more time listening to the guided imagery audio files was related to higher cessation. Among those not able to quit, participants in the IC smoked fewer cigarettes than those in the CC (OR = −2.1 95% CI: −6.4, 2.3).
Table 6 .
Preliminary efficacy outcomes at 6 months: cessation, home smoking bans, confidence, social support, cravings, self-efficacy, and effort
| Variable | Guided imagery N = 44 | Control N = 42 | OR (95% CI) |
|---|---|---|---|
| N (%) | N (%) | ||
| 7-day cessation (cigarettes) | 15 (34.1) | 21 (50.0) | 0.52 (0.22, 1.23) |
| 30-day cessation (cigarettes) | 12 (27.9) | 16 (38.1) | 0.63 (0.25, 1.57) |
| 30-day other tobacco use | 4 (9.5) | 7 (16.7) | 0.52 (0.14, 1.95) |
| Home bans (Full vs. partial or no bans) | 35 (62.5) | 36 (73.5) | 0.60 (0.26, 1.39) |
| Mean (SD)a | Mean (SD)a | Difference (95% CI) | |
| How confident are you that you will be able to stay off of using tobacco for at least 24 h? | 2.0 (1.2) | 2.6 (1.2) | −0.57 (−1.3, 0.13) |
| At this present moment, how confident are you that you will be able to stay smoke-free? | 4.1 (1.1) | 4.0 (1.2) | 0.02 (−0.78, 0.82) |
| How would you rate your support from others around you to quit tobacco and stay quit? | 3.2 (1.3) | 3.6 (1.2) | −0.47 (−1.0, 0.08) |
| How much have you craved tobacco today? | 2.1 (1.2) | 2.2 (1.7) | −0.14 (−0.83, 0.55) |
| Non-quitters | N = 26 | N = 21 | |
| Number of cigarettes smoked in last week | 9.1 (6.1) | 11.2 (9.0) | −2.1 (−6.4, 2.3) |
| If you stopped smoking cigarettes today, how concerned would you be that you might start smoking again? | 2.1 (1.0) | 2.0 (1.0) | 0.19 (−0.64, 0.68) |
| How confident are you in your ability to quit smoking at this time? | 3.7 (1.3) | 4.0 (1.2) | −0.31 (−1.2, 0.56) |
| How effective do you believe your skills are for quitting smoking at this time? | 4.4 (0.7) | 4.1 (1.2) | 0.30 (−0.30, 0.91) |
| How much effort are you willing to put into quitting smoking at this time? | 4.8 (0.4) | 4.9 (1.0) | −0.06 (−0.32, 0.21) |
| Quitters | N = 15 | N = 21 | |
| How concerned are you that you might start smoking again? | 2.0 (1.0) | 2.0 (1.0) | 0.02 (−0.64, 0.68) |
| How confident are you in your ability to stay smoke-free at this time? | 3.7 (1.3) | 4.0 (1.2) | −0.31 (1.30, −1.20) |
| How effective do you believe your skills are for staying smoke-free at this time? | 4.4 (0.7) | 4.1 (1.0) | 0.30 (−0.33, 0.94) |
| How much effort are you willing to put into staying smoke-free at this time? | 4.8 (0.4) | 4.9 (0.4) | −0.06 (−0.32, 0.20) |
aLikert response options ranged from 1 to 5, with 5 representing, for example, greater social support, concern, motivation, confidence, etc. Craving response options ranged from 1 to 6, and higher values indicated stronger craving.
Missing data/dropout
Completers (n = 86) were compared with dropouts (persons lost to 6-month follow-up, n = 19) on baseline characteristics, as shown in Table 7. Dropouts were less educated, below the federal poverty level, e-cigarette users and to allow smoking in the home than those retained. At baseline they performed more visualization than completers, but found it less logical.
Table 7.
Comparison of participants who dropped out versus those with those with 6-month follow-up
| Baseline characteristics | Completers (n = 86) n (%) or M (SD) | Dropouts (n = 19) n (%) or M (SD) |
|---|---|---|
| Male gender | 30 (34.9) | 6 (31.6) |
| Married | 24 (27.9) | 4 (21.1) |
| Non-Hispanic white | 66 (77.7) | 11 (57.9) |
| Hispanic ethnicity | 13 (15.3) | 4 (21.1) |
| Non-white, non-Hispanic | 6 (7.1) | 4 (21.1) |
| > High school education | 64 (74.4) | 7 (36.8) |
| Employment, full or part time | 38 (44.1) | 10 (52.6) |
| Uninsured or Medicaid | 33 (38.4) | 10 (52.6) |
| Below federal poverty level | 29 (33.7) | 13 (68.4) |
| Chronic health conditiona | 58 (67.4) | 14 (73.7) |
| Mental health condition | 46 (53.5) | 11 (57.9) |
| E-cigarettes/vaping | 13 (15.1) | 8 (42.1) |
| Perceived social supportb | 3.9 (1.4) | 3.8 (1.4) |
| Smoking not allowed in home | 67 (77.9) | 11 (57.9) |
| Other smokers in household | 25 (29.1) | 6 (33.3) |
| Age (y) | 51.6 (14.3) | 45.6 (15.6) |
| Nicotine dependence (Fagerström) | 4.0 (1.8) | 4.4 (1.9) |
| Cigarettes per day | 16.0 (8.3) | 15.0 (5.7) |
| Cravingb | 4.5 (1.3) | 4.7 (1.1) |
| Concern about relapsingb | 4.0 (1.2) | 3.7 (1.3) |
| Motivation to quitb | 4.5 (0.8) | 4.5 (0.6) |
| Confidence to quitb | 3.8 (1.0) | 3.6 (1.4) |
| Belief that skills are effectiveb | 2.8 (1.1) | 3.2 (1.0) |
| Effort willing to put into quittingb | 4.7 (0.6) | 4.7 (0.6) |
| Imagery visualization time b | 1.6 (1.1) | 2.4 (1.6) |
| Imagery confidenceb | 3.7 (0.9) | 3.4 (1.2) |
| Imagery logical b | 4.0 (0.9) | 3.6 (0.8) |
| Perceived supportb | 3.8 (1.4) | 3.8 (1.4) |
Bolded values show statistically significant difference.
aAsthma, COPD, hypertension, diabetes, and cancer.
bLikert response options ranged from 1 to 5, with 5 representing, for example, greater social support, concern, motivation, confidence, etc. Craving response options ranged from 1 to 6, and higher values indicated stronger craving. Imagery visualization time response options were 1 = never, 2 = less than 10 min, 3 = 10–20 min, 4 = 21–30 min, 5 = More than 30 min.
Discussion
Reach
Our primary form of recruitment was through the quitline, which has low reach among male smokers. When we used community-based strategies to recruit that highlighted guided imagery and its use among athletes, a larger percentage of the sample were male. In addition, our recruitment materials included a description of the “alternative” guided imagery approach and featured photos of diverse individuals. The participants recruited through community-based methods were slightly more diverse, indicating that there may be interest in guided imagery among those smokers who may not respond to traditional quitline media ads. In addition, we had differential attrition between conditions, with more participants withdrawing from the CC than IC. Anecdotal evidence from the coaches suggests that participants in the CC withdrew from the study once they learned that they had not been assigned to the guided imagery intervention because they did not want to participate in a standard behavioral cessation program. Unfortunately, the sample size in our study precluded analyzing for differences between the quitline versus community recruits (see Limitations). Therefore, a large-scale study is needed to determine the impact of guided imagery on reach of telephone cessation counseling.
Quit rates
Quitlines report quit rates per response rates to follow-up surveys (quit outcomes for those reached for a follow-up) as compared to intent-to-treat (ITT) rates in which those lost to follow-up are considered to be non-abstinent (a more conservative approach) [48]. Quitlines experience high (55%–65%) attrition in follow-up surveys. In 2017, the North American Quitline Consortium reported an average quit rate of 27.6% among those reached [49].
Little research has been conducted on quitlines using ITT analyses. However, in a recent study using an ITT approach, only 13.51% of clients calling the NY State quitline achieved 30-day point prevalence abstinence [50]. Our feasibility study that showed 30-day ITT abstinence rates of 27.9% in our IC group, suggesting that this approach if adopted by quitlines could significantly increase the quit outcomes compared to standard approaches.
Guided imagery for tobacco cessation
The present study extends the literature on guided imagery for smoking cessation. Few studies have systematically tested guided imagery in the last decade. Half of the studies used a more broad definition of “mindfulness” rather than guided imagery per se, and all but our own prior research relied on in-person delivery modalities [3–5,23,51]. Two trials relied on group formats which may introduce other social processes in addition to the exposure to guided imagery [5,51]. Wynd and colleagues demonstrated significant quit rates at 24 months for the guided imagery condition and 12% CC (25% and 12%, respectively). In another pilot randomized trial, quit rates of 36% and 30% at 6 and 12 weeks were observed compared to controls (18% and 12%). Both trials relied on standardized, and not tailored, guided imagery scripts which may preclude long-term use and practice with participants. Two trials used in-person individual therapy; one using a mindfulness approach and the other combined guided imagery with psychotherapy [3,23]. Our study used a public health approach which also allowed for personalization of the intervention. Using a quitline model, we were able to deliver guided imagery in a highly scalable manner. Having individual sessions allowed for tailoring of the guided imagery scripts to meet each participant’s needs. It is also noteworthy that the guided imagery audio files in the current study were delivered by text message and/or email, primarily to participants using mobile devices which allows for greater reach than conventional, in-person guided imagery interventions.
Feasibility
The results of this randomized feasibility study indicate that both the IC and CC protocols are feasible to deliver, and the continued study of guided imagery for tobacco cessation using a quitline model is warranted. We developed and finalized standardized protocols and materials for participants, coaches and study staff, obtaining iterative input from our Community Advisory Board, consultants with expertise in tobacco cessation for diverse populations, ASHLine coaches and enrollment staff, and focus groups/individual or group interviews with diverse smokers. We developed training materials for coaches in both conditions, as well as ratings and standardized metrics for assessing coach competency and monitoring fidelity of protocol fidelity. Using these metrics, we trained all but one of the coaches to competency and maintained protocol fidelity over time.
We developed successful methods for recruiting participants through a telephone quitline and at the community level. We surpassed our recruitment goals, and could have easily recruited more participants through the ASHLine. We “turned off” ASHLine recruiting periodically during the 4-month recruiting period because we did not have enough coaching staff to handle the participant volume. Through our community-based recruitment methods we attracted a slightly higher percentage of men and racial/ethnic minorities than through the ASHLine. However, community-based recruitment was more challenging and resulted in far fewer participants than through the ASHLine. We learned that targeted paid advertising and a comprehensive earned media campaign will be necessary to recruit participants in a larger-scale study.
Our study had high levels of retention. We had >90% retention at the 8-week follow-up and >80% at the 6-month follow-up assessment, which compares favorably to similar types of studies and far exceeds the 19.7%–56% retention experienced by quitlines [52]. Although relatively low, we experienced differential withdrawal, with twice as many participants requesting to leave the study in the CC versus the IC. The majority of these withdrawals took place prior to or during the first treatment session. The primary reason reported for withdrawal was “changed mind” which suggests that guided imagery might have attracted the smoker to enroll in the program. Anecdotally, several CC participants reported that they had wanted to be in the guided imagery program, and they also reported more displeasure with the sessions than did intervention participants.
Dropouts
The comparison of completers with dropouts showed that participants who dropped out were less educated and more likely to be below the federal poverty line. This may reflect the economic and time pressures faced by many persons of lower socio-economic status [36,53]. Dual users of e-cigarettes and combustible tobacco were more likely to drop out, possibly due to higher levels of nicotine dependence among this group [54,55]. Participants who allow smoking in the home were more likely to drop out. This may also reflect higher levels of nicotine dependence but may also indicate that members of the smokers’ social network also smoke. It has been shown that quitting is hampered by allowing smoking inside, living with a smoker, and lack of social support [56,57].
We also found high levels of adherence, with 67% of participants attending all six sessions (mean = 4.8 sessions) and almost 83% of participants using nicotine replacement therapy, with no differences between conditions. Satisfaction with the program was high, with participants rating most items with the highest score (5 out of 5). This suggests that the protocols we developed for both conditions are acceptable to smokers and may contribute to smoking abstinence.
Both conditions had quit rates that compare favorably to those of quitlines [30]. Although, the CC had higher quit rates than the guided imagery condition, the confidence intervals are wide and could potentially indicate differences in either direction. We also found that participants in the guided imagery condition who did not quit were able to reduce the number of cigarettes smoked per day more than those in the CC.
Limitations
Because the vast majority of participants were recruited through the ASHLine, we had a restricted pool from which to examine whether guided imagery would be more appealing to men and racial/ethnic minorities. However, we did recruit a slightly higher percentage of males and racial/ethnic minorities through our community recruitment activities than through the ASHLine.
There may have been a confounding effect of coach experience on cessation outcomes. In the guided imagery condition, we had one Masters-level coach who was trained and achieved competency in delivering the guided imagery protocol, but had no previous experience in behavior change or tobacco cessation counseling. In the CC, we employed four part-time experienced tobacco cessation coaches over the course of the project. These coaches had many years of training and experience in behavioral cessation counseling. We believe their expertise in helping tobacco users to quit may have affected the results, given the importance of experience and the therapeutic alliance.
Another study limitation may be lack of equivalence between the “homework” across the two study conditions. In the IC, participants were provided an audio file to listen to every day during the week while in the CC, participants were given assignments in the booklet or to practice behavioral strategies. There was no non-guided imagery audio file provided to participants in the control group, and there was more focus placed on listening to the audio file than on practicing behavioral strategies in the intervention group. Both conditions relied on self-reporting of practice between sessions.
We also experienced challenges in obtaining biochemical verification of abstinence. Participants were reluctant to provide biological samples, and the test we used produced a high percentage of false positives (a problem noted by many tobacco researchers during this same time period). Therefore, we were unable to accurately biochemically verify tobacco abstinence. This is an uncommon occurrence as the test is usually accurate [58,59], and we cannot speculate on why it malfunctioned during this period. More research is needed regarding the best method for obtaining accurate biochemical verification remotely (i.e., not using in-person collection methods).
Finally, we were not powered to detect efficacy. Thus, a fully powered, randomized trial is needed to establish efficacy of the guided imagery intervention.
Implications for research
Based on our findings and the limitations of our research, future research is warranted. The single-site feasibility trial was successful, demonstrating that our deliver model is both feasible to implement and acceptable to participants. A multi-site feasibility trial would establish feasibility across multiple quitlines serving diverse populations of tobacco users. The intervention appears to be promising, but this was a feasibility trial and not powered to detect efficacy. Therefore, a fully powered, randomized trial is needed before conclusions can be drawn regarding efficacy of the guided imagery intervention and the behavioral control. We discovered the need to match coaches on level of tobacco treatment experience across conditions to remove this potential confound. Finally, further research is needed to determine the best methods for remotely collecting biochemical verification of tobacco use status from all respondents at the follow-up assessment.
Conclusions
Our study indicates that a telephone-based guided imagery intervention for tobacco cessation is feasible. In addition, the exploratory analyses suggest both the IC and CC may be effective in producing abstinence rates that meet or exceed those of quitlines. Guided imagery may also expand the reach of telephone-based tobacco cessation services. We learned valuable lessons to apply to a future large-scale study. Further research to test this guided imagery tobacco cessation intervention is warranted.
Acknowledgments
We gratefully acknowledge the invaluable contributions of Yessenya Barraza, Kristina Souders, Crista Meinke, Catie Allen, The Arizona Smokers’ Helpline, the University of Arizona Cancer Center’s Behavioral Measures and Instrumentation Shared Resource, the University of Arizona Research Lab, Niraly Patel, Alejandra Zapien, and Anh Vu to the research study and/or preparation of this manuscript. The study was conducted at the University of Arizona Collaboratory for Metabolic Disease Prevention and Treatment. We dedicate this manuscript to Gayle Povis, RDN, project coordinator (1957–2019), our colleague and friend, without whom this project would not have been successfully completed. This study was funded by the National Center for Complementary and Integrative Health (Grant #R34AT008947).
Compliance with Ethical Standards
Conflicts of Interest: Judith S. Gordon, Melanie L. Bell, Julie S. Armin, Peter R. Giacobbi, and Uma S. Nair declare that they have no conflicts of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the University of Arizona Institutional Review Board (Protocol # 1607731418A022).
Informed Consent: Informed consent was obtained from all individual participants included in the study. This article does not contain any studies with animals performed by any of the authors.
References
- 1. Warren GW, Alberg AJ, Kraft AS, Cummings KM. The 2014 surgeon general’s report: “the health consequences of smoking–50 years of progress”: a paradigm shift in cancer care. Cancer. 2014;120(13):1914–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Babb S. Quitting smoking among adults—United States, 2000–2015. Morb mortal Wkly Rep, 2017;65. [DOI] [PubMed] [Google Scholar]
- 3. Brewer JA, Mallik S, Babuscio TA, et al. Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug Alcohol Depend. 2011;119(1–2):72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gordon JS, Armin J, D Hingle M, et al. Development and evaluation of the See Me Smoke-Free multi-behavioral mHealth app for women smokers. Transl Behav Med. 2017;7(2):172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wynd CA. Guided health imagery for smoking cessation and long-term abstinence. J Nurs Scholarsh. 2005;37(3):245–250. [DOI] [PubMed] [Google Scholar]
- 6. Zernig G. et al. A randomized trial of short psychotherapy versus sustained‐release bupropion for smoking cessation. Addiction. 2008;103(12):2024–2031. [DOI] [PubMed] [Google Scholar]
- 7. Morris T, Spittle M, Watt AP. Imagery in Sport, Champaign-Urbana, IL: Human Kinetics; 2005. [Google Scholar]
- 8. Martin KA, Hall CR. Using mental imagery to enhance intrinsic motivation. J Sport Exercise Psy, 1995;17(1):54–69. [Google Scholar]
- 9. Giacobbi PR Jr, et al. Mental imagery increases self-determined motivation to exercise with university enrolled women: a randomized controlled trial. Psychol Sport Exerc, 2014;15:374–381. [Google Scholar]
- 10. Clarke TC, et al. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl Health Stat Reports, 2015(79):1. [PMC free article] [PubMed] [Google Scholar]
- 11. Giacobbi PR Jr, Stewart J, Chaffee K, Jaeschke AM, Stabler M, Kelley GA. A scoping review of health outcomes examined in randomized controlled trials using guided imagery. Prog Prev Med (N Y). 2017;2(7):e0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Upchurch DM, Wexler Rainisch BK. Racial and ethnic profiles of complementary and alternative medicine use among young adults in the United States: findings from the national longitudinal study of adolescent health. J Evid-Based Compl Alt Med. 2012;17(3):172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Munroe KJ, et al. The four Ws of imagery use: where, when, why, and what. Sport Psychol, 2000;14(2):119–137. [Google Scholar]
- 14. Jallo N, Ruiz RJ, Elswick RK Jr, French E. Guided imagery for stress and symptom management in pregnant african american women. Evid-Based Compl Alt Med. 2014;2014:840923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jallo N, Salyer J, Ruiz RJ, French E. Perceptions of guided imagery for stress management in pregnant African American women. Arch Psychiatr Nurs. 2015;29(4):249–254. [DOI] [PubMed] [Google Scholar]
- 16. Weigensberg MJ, Lane CJ, Ávila Q, et al. Imagine HEALTH: results from a randomized pilot lifestyle intervention for obese Latino adolescents using Interactive Guided ImagerySM. BMC Compl Altern Med. 2014;14:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Epstein DR, Babcock-Parziale JL, Haynes PL, Herb CA. Insomnia treatment acceptability and preferences of male Iraq and Afghanistan combat veterans and their healthcare providers. J Rehabil Res Dev. 2012;49(6):867–878. [DOI] [PubMed] [Google Scholar]
- 18. Libby DJ, Pilver CE, Desai R. Complementary and alternative medicine in VA specialized PTSD treatment programs. Psychiatr Serv. 2012;63(11):1134–1136. [DOI] [PubMed] [Google Scholar]
- 19. Lichtenstein E, Zhu SH, Tedeschi GJ. Smoking cessation quitlines: an underrecognized intervention success story. Am Psychol. 2010;65(4):252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stead LF, et al. Telephone counselling for smoking cessation. Cochrane Database Syst Rev, 2013(8):CD002850. [DOI] [PubMed] [Google Scholar]
- 21. Sood A, Ebbert JO, Sood R, Stevens SR. Complementary treatments for tobacco cessation: a survey. Nicotine Tob Res. 2006;8(6):767–771. [DOI] [PubMed] [Google Scholar]
- 22. Carim-Todd L, Mitchell SH, Oken BS. Mind-body practices: an alternative, drug-free treatment for smoking cessation? A systematic review of the literature. Drug Alcohol Depend. 2013;132(3):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zernig G, Wallner R, Grohs U, Kriechbaum N, Kemmler G, Saria A. A randomized trial of short psychotherapy versus sustained-release bupropion for smoking cessation. Addiction. 2008;103(12):2024–2031. [DOI] [PubMed] [Google Scholar]
- 24. Giacobbi PR Jr, Tuccitto DE, Buman MP, Munroe-Chandler K. A measurement and conceptual investigation of exercise imagery establishing construct validity. Res Q Exerc Sport. 2010;81(4):485–493. [DOI] [PubMed] [Google Scholar]
- 25. Armin J, Johnson T, Hingle M, Giacobbi P Jr, Gordon JS. Development of a Multi-Behavioral mHealth App for women smokers. J Health Commun. 2017;22(2):153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Giacobbi P Jr, Hingle M, Johnson T, Cunningham JK, Armin J, Gordon JS. See me smoke-free: protocol for a research study to develop and test the feasibility of an mHealth App for women to address smoking, diet, and physical activity. JMIR Res Protoc. 2016;5(1):e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gordon JS, Armin JS, Cunningham JK, Muramoto ML, Christiansen SM, Jacobs TA. Lessons learned in the development and evaluation of RxCoach™, an mHealth app to increase tobacco cessation medication adherence. Patient Educ Couns. 2017;100(4):720–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fiore MC, et al. Treating Tobacco Use and Dependence: 2008. Update-Clinical Practice Guideline. Rockville, MD: P.H.S., United States Department of Health and Human Services; 2008. [Google Scholar]
- 29. Hopkins DP, Briss PA, Ricard CJ, et al. Reviews of evidence regarding interventions to reduce tobacco use and exposure to environmental tobacco smoke. Am J Prev Med. 2001;20(2 Suppl):16–66. [DOI] [PubMed] [Google Scholar]
- 30. Consortium NAQ. Results from the NAQC annual survey of quitlines, FY17.2018. Available at https://www.naquitline.org/page/2017survey. Accessibility verified September 9, 2019.
- 31. Trinidad DR, Pérez-Stable EJ, White MM, Emery SL, Messer K. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Am J Public Health. 2011;101(4):699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wallen J, et al. Engaging African Americans in smoking cessation programs. Am J Heal Educ, 2014;45(3):151–157. [Google Scholar]
- 33. Parent MC, Hammer JH, Bradstreet TC, Schwartz EN, Jobe T. Men’s mental health help-seeking behaviors: an intersectional analysis. Am J Mens Health. 2018;12(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marshall LL, et al. Race/ethnic variations in quitline use among US adult tobacco users in 45 states, 2011–2013. Nicotine Tob Res, 2016;19(12):1473–1481. [DOI] [PubMed] [Google Scholar]
- 35. Gordon JS, Giacobbi P Jr, Armin JS, Nair US, Bell ML, Povis G. Testing the feasibility of a guided imagery tobacco cessation intervention delivered by a telephone quitline: study protocol for a randomized controlled feasibility trial. Contemp Clin Trials Commun. 2019;16:100437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Armin JS, Giacobbi P, Nair U, Povis G, Gordon JS. Developing a guided imagery telephone-based tobacco cessation program for a randomized controlled trial. Tob. Use Insights. 2020. ( Under Review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gordon JS, et al. Development and evaluation of the See Me Smoke-Free multi-behavioral mHealth app for women smokers. Transl Behav Med, 2017;7(2):172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gordon JS, Andrews JA, Crews KM, Payne TJ, Severson HH. The 5A’s vs 3A’s plus proactive quitline referral in private practice dental offices: preliminary results. Tob Control. 2007;16(4):285–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Severson HH, Andrews JA, Lichtenstein E, Gordon JS, Barckley M, Akers L. A self-help cessation program for smokeless tobacco users: comparison of two interventions. Nicotine Tob Res. 2000;2(4):363–370. [DOI] [PubMed] [Google Scholar]
- 41. Severson HH, Gordon JS, Danaher BG, Akers L. ChewFree.com: evaluation of a Web-based cessation program for smokeless tobacco users. Nicotine Tob Res. 2008;10(2):381–391. [DOI] [PubMed] [Google Scholar]
- 42. Fagerstrom KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med. 1989;12(2):159–182. [DOI] [PubMed] [Google Scholar]
- 43. Shiffman S, Sayette MA. Validation of the nicotine dependence syndrome scale (NDSS): a criterion-group design contrasting chippers and regular smokers. Drug Alcohol Depend. 2005;79(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Condiotte MM, Lichtenstein E. Self-efficacy and relapse in smoking cessation programs. J Consult Clin Psychol. 1981;49(5):648–658. [DOI] [PubMed] [Google Scholar]
- 45. Borkovec TD, Nau SD. Credibility of analogue therapy rationales. J Behav Ther Exp Psychiatry, 1972;3(4):257–260. [Google Scholar]
- 46. Gordon JS, Mahabee-Gittens EM, Andrews JA, Christiansen SM, Byron DJ. A randomized clinical trial of a web-based tobacco cessation education program. Pediatrics. 2013;131(2):e455–e462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marks DF. New directions for mental imagery research. J Ment Imag, 1973;19:153–167. [Google Scholar]
- 48. NAQC. Calculating Quit Rates, 2015 Update, in NAQC Issue Papers. 2015. Available at https://www.naquitline.org/page/issuepapers?. Accessibility verified March 10, 2020. [Google Scholar]
- 49. NAQC. North American Quitline Consortium 2017 Survey. Available at https://www.naquitline.org/page/2017survey. Accessibility verified March 10, 2020.
- 50. Mann N, et al. The potential impact of the New York state Smokers’ quitline on population-level smoking rates in New York. Int J Environ Res Public Heal, 2019;16(22):4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tindle HA, et al. Guided imagery for smoking cessation in adults: a randomized pilot trial. Compl Health Pract Rev, 2006;11(3):166–175. [Google Scholar]
- 52. Consortium NAQ. Results from the FY 2016 NAQC Annual Survey of Quitlines.2017. Available at https://cdn.ymaws.com/www.naquitline.org/resource/resmgr/2015_survey/NAQC_FY2016_Annual_Survey.pdf. Accessibility verified September 16, 2019.
- 53. Hiscock R, Judge K, Bauld L. Social inequalities in quitting smoking: what factors mediate the relationship between socioeconomic position and smoking cessation? J Public Health (Oxf). 2011;33(1):39–47. [DOI] [PubMed] [Google Scholar]
- 54. Nardone N, et al. Nicotine intake, dependence, and characteristics of electronic cigarette and dual users. Tob Regul Sci, 2019;5(1):27–35. [Google Scholar]
- 55. Maglia M, et al. Dual use of electronic cigarettes and classic cigarettes: a systematic review. Addict Res Theory, 2018;26(4):330–338. [Google Scholar]
- 56. Yuan NP, Nair US, Crane TE, Krupski L, Collins BN, Bell ML. Impact of changes in home smoking bans on tobacco cessation among quitline callers. Health Educ Res. 2019;34(3):345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chandola T, Head J, Bartley M. Socio-demographic predictors of quitting smoking: how important are household factors? Addiction. 2004;99(6):770–777. [DOI] [PubMed] [Google Scholar]
- 58. Asha V, Dhanya M. Immunochromatographic assessment of salivary cotinine and its correlation with nicotine dependence in tobacco chewers. J Cancer Prev. 2015;20(2):159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cooke F, Bullen C, Whittaker R, McRobbie H, Chen MH, Walker N. Diagnostic accuracy of NicAlert cotinine test strips in saliva for verifying smoking status. Nicotine Tob Res. 2008;10(4):607–612. [DOI] [PubMed] [Google Scholar]