Abstract
Successful translation of genetic information into patient-centered care and improved outcomes depends, at least in part, on patients’ genetic knowledge. Although genetic knowledge is believed to be an important facilitator of familial communication of genetic risk information, empirical evidence of this association is lacking. We examined whether genetic knowledge was related to frequency of current familial communication about colorectal cancer and polyp (CRCP) risk, and future intention to share CRCP-related genomic test results with family members in a clinical sample of patients. We recruited 189 patients eligible for clinical CRCP sequencing to the eMERGE III FamilyTalk randomized controlled trial and surveyed them about genetic knowledge and familial communication at baseline. Participants were primarily Caucasian, 47% male, average age of 68 years, mostly well educated, and with high-income levels. Genetic knowledge was positively associated with future-intended familial communication of genetic information (odds ratio = 1.11, 95% confidence interval: 1.02–1.23), but not associated with current communication of CRC risk (β = 0.01, p = .58). Greater current communication of CRC risk was associated with better family functioning (β = 0.04, p = 8.2e-5). Participants’ genetic knowledge in this study was minimally associated with their intended familial communication of genetic information. Although participants have good intentions of communication, family-level factors may hinder actual follow through of these intentions. Continued focus on improving proband’s genetic knowledge coupled with interventions to overcome family-level barriers to communication may be needed to improve familial communication rates.
Keywords: Genetic knowledge, Familial communication, Cancer, Communication intention
IMPLICATIONS.
Practice: Patients’ technical genetic knowledge only has a modest impact on improving their familial commutation of genetic information in clinical oncology, and as such, specific prompts for familial communication (e.g., specifying at-risk relatives or communication aids) that are not focused on increasing knowledge may be needed to overcome family-level barriers of communication.
Policy: Policymakers who want to increase familial communication of genetic risk information should consider this evidence in intervention design and implementation.
Research: Possible future research to be conducted includes observational studies that consider the age of relatives and closeness of relationship between proband and relative in determining frequency of communication as well as, studies with a prespecified hypothesis to confirm the present findings.
INTRODUCTION
Successful translation of genetic information into patient-centered care and improved outcomes depends, at least in part, on patients’ genetic knowledge [1]. Differing levels of basic and applied genetic knowledge may have an impact on individuals’ decision making at various phases of the clinical genetic testing continuum [1]. This belief is reflected in the education-centered goals of both older and more recent definitions of genetic counseling practice which aims to increase the counselee’s genetic knowledge [2] and enable them “to appreciate the way heredity contributes to the disorder and the risk of recurrence in specified relatives” [3]. This presumption also justifies the widespread practice of assessing participants’ genetic and genomic knowledge in genomic research studies [4,5]. Studies measure genetic or genomic knowledge for various purposes—to describe baseline knowledge, knowledge gains after intervention, and relationship between knowledge and use of genetic services [1]. However, little empirical work has been done on how or if patients’ genetics-related knowledge affects downstream outcomes in genomic medicine.
Familial communication of an individual’s genomic risk information is one important outcome of genomic medicine which, based on qualitative studies, is also partly dependent on patients’ knowledge of genetics [6]. Communication of genetic risk is influenced by family-level factors (e.g., level of openness in family communication) as well as individual-level factors such as genetic knowledge and gender [7]. The importance of genetic knowledge in achieving greater family communication is supported by prior research including a proband’s need for more information before telling relatives [6], misunderstanding about inheritance [8,9], and belief that relatives will not benefit from the information [6,10]. Knowledge of genetic information was also found to have a positive effect on family communication through a qualitative exploration of individuals carrying pathogenic genetic variants for a cardiac condition [11]. These pieces of evidence suggest that greater genetic knowledge may facilitate familial communication of genetic risk by enabling patients to overcome the knowledge barrier that impedes communication. To test these qualitative observations, quantitative studies are needed to directly examine the association between genetic knowledge and familial communication.
Although communication of DNA-based risk estimates alone is generally not sufficient to drive health behavior change [12,13], some amount of genetic knowledge and appreciation of genomics is necessary to effectively adopt genomic medicine services and engage in familial communication behaviors. The relative importance of knowledge compared to other factors that influence familial communication of genetic risk information remains unknown. On the one hand, the studies mentioned above indicate that greater knowledge may increase familial communication, but evidence to the contrary also exists. Higher genomic literacy (which includes genetic knowledge) was not associated with individual’s confidence in their ability to communicate about genomics topics (i.e., their self-efficacy) [14]. Furthermore, the process of familial communication is not always verbal and may occur through summary letters provided by genetic counselors [15]. Such means of communication limit the necessity for patients to relay facts or recommendations about genetics and thus diminish the importance of self-efficacy. Only limited attempts have been made to associate patients’ genetic proficiency with genomics-related outcomes. In one related study, lower genomic literacy was not associated with familial communication of family health history, but was associated with greater frequency of communication with providers [14]. However, the exact influence of patients’ genetic knowledge on familial communication of genetic risk information remains an open question.
We therefore conducted a study to examine the association between individuals’ knowledge about genetics and familial communication intentions and behaviors. Based on the prior literature and our conceptualization of genetic knowledge and familial communication, we hypothesized that individuals with higher genetic knowledge would report increased intention to communicate with family about genetic risk information.
MATERIALS AND METHODS
Recruitment
The electronic MEdical Records and GEnomics (eMERGE) Network is a National Human Genome Research Institute funded consortium of research institutions across the United States, which has been described in detail elsewhere [16]. Briefly, this consortium of research institutions recruits patients and leverages biorepositories linked to electronic health records for genomic discovery and implementation studies [17,18]. One of its main aims is to assess the health impact; cost effectiveness; and ethical, legal, and social implications of reporting genetic variants on a broader population scale for patients, clinicians, and healthcare institutions. We report baseline survey results from a randomized control trial (FamilyTalk [19]) conducted at the Seattle, WA site, namely, University of Washington (UW) and Kaiser Permanente Washington as part of eMERGE III. Eligibility criteria for all probands included being an active patient at either institution, agreeing to receive either sequencing results or a panel test for Lynch syndrome, and previously receiving a diagnosis of either colorectal cancer or polyp formation from the institution. In Seattle, we approached patients by email to inquire about their interest in being part of a family communication study. We sent a baseline survey to all interested probands via email for electronic completion. We conducted consent procedures and collected written consent from all participants who expressed interest in the study. Participants who provided baseline data and consented to be in the randomized trial were included in this sample. This study was approved by the University of Washington Institutional Review Board.
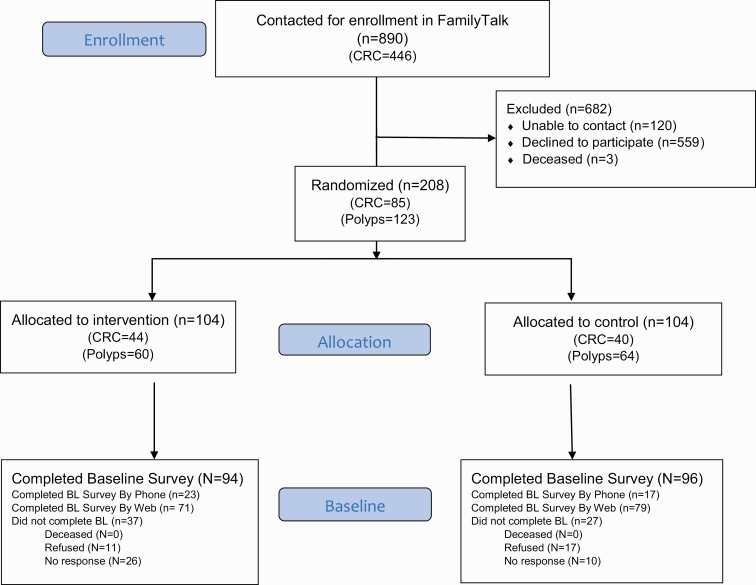
The goal of FamilyTalk is to assess the effectiveness and social and economic impact of an innovative online tool to increase family communication about colorectal cancer and polyp (CRCP) risk and screening. The clinical study population were identified from existing eMERGE participants or UW Medical Genetics patients with laboratory testing at UW for CRCP. Consenting participants completed baseline surveys and underwent sequencing for clinically relevant CRCP-associated genes, either as part of the eMERGE Seq panel [20] or a UW Laboratory Medicine clinical test. The participants were randomized to one of the two study arms: (i) receive access to a family-based website (FamilyTalk) to encourage communication about their genomic sequencing results among their family members or (ii) receive standard of care as control probands. This study reports results from the survey administered at baseline, before randomization and intervention, from patients who consented to participate. Figure 1 contains the Consolidated Standards of Reporting Trials (CONSORT) document detailing study recruitment.
Fig 1.
CONSORT diagram of participant flow through the phases of the FamilyTalk randomized trial.
Measures
At baseline, each participant completed a survey, either via a website (REDCap) or via telephone, which included questions about intention to share their genomic result(s) with family members, knowledge and understanding of genetics, family functioning related to CRC risk communication, and background and demographic data. At the time of data collection, study participants had not undergone genetic counseling as a part of the study.
Independent variables
Participant demographics and clinical variables
Participants’ sociodemographic characteristics including education, age, race, marital status, and income were measured for the study. We also collected clinical variables such as self-reported history of genetic testing and personal/family history of a genetic disorder.
Knowledge about genome sequencing
Knowledge of genetic sequencing and impact on disease diagnosis and treatment was measured using an 11-item validated genetic knowledge measure [21]. Items included “Genome sequencing may find variants in a person’s genes that they can pass on to their children” and “Genome sequencing is a routine test that most people can have through their physician’s office” (see Supplementary Appendix). This measure has two subscales, items 1–5 indicate knowledge of sequencing limitations (e.g., “scientists know how all variants of genes will affect a person’s chances of developing diseases”) and items 6–11 indicate knowledge of sequencing benefits (e.g., “genome sequencing may find variants in a person’s genes that will increase their chance of developing a disease in their lifetime”). Participants responded to these items on a 5-point Likert scale ranging from strongly agree to strongly disagree. Four negatively worded items were reverse scored, so that “agree” reflected a correct response and “strongly agree” reflected a more confident correct response in the correct direction for all items. To create knowledge scale scores, responses of “strongly agree” were assigned a value of 2 and “agree” a value of 1. The maximum possible point from these 11 items was therefore 22. Genetic knowledge score was treated as continuous in the analyses.
Communication and flow of cancer information within families:
The Cancer Family Impact Scale (CFIS) is a validated instrument for use in studies investigating relationships among family factors and CRC prevention behaviors. It consists of five latent constructs—Negative effects of cancer on the family, positive effects of cancer on the family, how families communicate about cancer, how information about cancer is conveyed in families, and how individuals react to family norms about cancer. All five subscales of the CFIS were used to measure family functioning related to CRC—that is, interpersonal communication among family members and flow of cancer information within the family [22]. Participants responded to the 18 items of CFIS on a 5-point Likert scale ranging from strongly agree (4) to strongly disagree (0). Negatively worded items were reverse scored, so that “agree” reflected a correct response favoring better familial communication for all items. Higher summed scores indicated a greater communication and flow of about cancer information within family and therefore better family functioning.
Outcome variables
We examined two different familial communication behaviors: (i) current frequency of communication about CRC-related risk and (ii) intent to share CRC-related genomic test results in future.
Frequency of current communication about CRC risk
Frequency of communication about CRC risk was measured with a previously developed scale [23]. We asked participants about how frequently in the past year they had communicated with each of the following family members about colon cancer risk: mother, father, sister, brother, children, grandchildren. Communication was rated on a 4-point Likert scale, from 1 (not at all) to 4 (a lot). An option for “I do not currently have this relative” was provided. As previously described by Bowen and colleagues [23], for all living relatives, an overall frequency of communication score was computed by summing responses within person and calculating an average.
Intention to share CRC-related genetic test result
Participants’ future intention to share genetic test results from the clinical trial with biological family members was investigated using one question: “When I receive my genetic research results, I plan to share them with: My children, My mother, My father, My siblings.” Responses were rated on as follows: 2 (yes), 1 (unsure or have not decided), 0 (no), 3 (not applicable). We adapted the method from Bowen and colleagues [23] to sum responses across all applicable relatives within a person and calculating an average. Intention to share was dichotomized into two categories: lower intention (average score < 2) versus higher intention (average score = 2).
Data analysis
We conducted analyses using R Version 3.4.4. We examined descriptive statistics for all variables. We conducted bivariate analyses between genomic knowledge score and each familial communication behavior using chi-square tests for categorical outcomes. We then created multivariable logistic regression models to examine the association between two outcome variables (frequency of current familial communication of CRC risk and future intention to share CRC-related genomic test results) and independent variable (genetic knowledge). Both multivariable models included gender, age (modeled continuously), education (categorized into college or less and some graduate school or higher), income, CRC diagnosis, genetic knowledge (modeled continuously), prior genetic testing (yes or no), and CFIS (modeled continuously). Statistical significance was assessed as p ≤ .05. Due to the exploratory nature of this analysis, it was not adjusted for multiple comparisons [24–26].
RESULTS
Study sample
In total, 189 participants were included in the final study sample (one observation was dropped due to greater than 90% missingness). As shown in Table 1, study participants were primarily Caucasian and older, with an average age of 67.4 years, roughly evenly split between sexes (47% male and 53% female), and 38% had been diagnosed with colon cancer. In terms of socioeconomic status, participants were mostly highly educated with 67% having a bachelor’s degree or higher, and had high-income levels (47% earned >$90k per year). Some participants (11.4%) reported having a personal or family history of a genetic disorder and 18.9% reported having undergone genetic testing before joining the study. Participants who answered the survey via website (n = 150) were younger (p = .02) and more educated (p ≤ .01), but otherwise comparable to participants to answer the survey over phone (n = 39). To account for response bias, we controlled for age and educational status in our analyses.
Table 1.
Demographic and clinical characteristics of participants (N = 189)
| Total | |||
|---|---|---|---|
| Variable | Categories | N | % |
| N | 189 | 100.0 | |
| Age | Mean (years) | 67.4 | |
| Range | [32–103] | ||
| Sex | |||
| Female | 103 | 54.5 | |
| Male | 86 | 45.5 | |
| Race (n = 184) | |||
| White or Caucasian | 151 | 79.1 | |
| Black or African American | 4 | 2.1 | |
| Asian | 17 | 8.9 | |
| Mixed/Other | 19 | 9.9 | |
| Education (n = 185) | |||
| ≤ Some high school (grades 9–12) | 4 | 2.2 | |
| High school graduate or GED | 9 | 4.9 | |
| Post high school training other than college | 9 | 4.9 | |
| Some college | 39 | 21.1 | |
| Bachelor’s degree or equivalent | 59 | 31.9 | |
| Master’s degree | 45 | 24.3 | |
| Doctoral or other professional degree | 20 | 10.8 | |
| Marital status (n = 185) | |||
| Now married | 134 | 72.4 | |
| Widowed | 22 | 11.9 | |
| Divorced/separated/never married | 29 | 15.3 | |
| Income (n = 174) | |||
| <45k | 27 | 15.5 | |
| 45–90k | 65 | 37.4 | |
| >90k | 82 | 47.1 | |
| Living first-degree relatives (n = 189) | |||
| Mother | 52 | 27.5 | |
| Father | 35 | 18.5 | |
| Sister | 111 | 58.7 | |
| Brother | 118 | 62.4 | |
| Children | 162 | 85.7 | |
| Prior genetic testing (n = 185) | |||
| Had genetic testing before | 35 | 18.9 | |
| Never had genetic testing | 145 | 78.4 | |
| Don’t know/decline to answer | 5 | 2.7 | |
| Personal/family history of genetic disorder (n = 185) | |||
| Yes | 21 | 11.4 | |
| No | 161 | 87.0 | |
| Don’t know/decline to answer | 3 | 1.6 | |
| Clinical characteristic (n = 178) | |||
| CRC diagnosis | 69 | 38.7 | |
| Colon polyp | 103 | 61.2 |
CRC colorectal cancer.
Genetic sequencing knowledge
On average participants scored 48.2% (10.6 of 22 total possible points) in knowledge of genetic sequencing questionnaire overall and scored higher in the benefits of sequencing subscale compared to the limitations subscale (p = 4.4e-6). There was considerable variation between proportions of individuals who answered each question correctly (Supplementary Table S1). For example, item 7 “Genome sequencing may give a person information about their chances of developing several different diseases” was most often correctly answered (89.7% of participants), whereas item 5 “Genome sequencing is a routine test that most people can have through their physician’s office” was the least often answered correctly by participants (53.3%). Welch two sample t-test showed a statistically significant difference between participants’ knowledge of risk variants (item 8 “variants in a person’s genes that will increase their chance of developing a disease in their lifetime”) and protective variants (item 9 “variants in a person’s genes that will decrease their chance of developing a disease in their lifetime”; p = .001). In bivariate analysis, we found that participants with higher genetic knowledge were more educated (p = 1.2e-6), and younger in age (p = .04). However, previous experience with genetic testing or personal history of genetic disorder was not associated with genetic sequencing knowledge (p = .25).
Familial communication
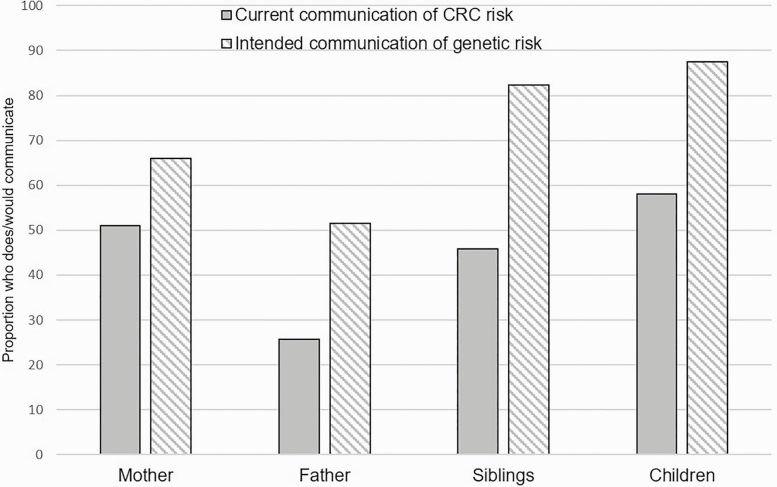
Average frequency of current communication of CRC risk was 1.27 on a scale of 0–4, and average frequency of intended communication of genetic risk was 1.79 on a scale of 0–2, with higher numbers indicating greater sharing. Across all applicable first-degree relatives (FDRs), that is, parents, siblings, and children, these communication frequencies correspond to an average current communication of CRC risk of 0.55 FDRs/proband (range: 0–1), and average intention for future communication of 0.89 FDRs/proband (range: 0–1). As shown in Fig. 2, participants reported higher intended future familial communication of sequencing results from the study compared with their current communication of CRC across all FDRs.
Fig 2.
Family communication responses to survey questions about current communication frequency of CRCP risk and intention of future communication about genetic test results (n = 189). *Statistically significant difference.
Knowledge and familial communication
In adjusted analysis, genetic knowledge was positively associated with the intent of sharing genetic information with family members in the future (odds ratio [OR] = 1.11, 95% confidence interval [CI]: 1.02–1.23); however, genetic knowledge was not associated with current communication of CRC risk within the family (β = 0.01, p = .58; Table 2). In the bivariate analysis, current communication frequency was higher among participants who were older (β = 0.02, p = .02), who had a diagnosis of colorectal cancer (β = 0.81, p = 1.8e-16), and who reported better family functioning (β = 0.07, p = 2.9e-10). However, reported frequency of current communication was lower among those with lower educational attainment (β = −0.40, p = .02) and those with lower income (β = −0.13, p = .01). In the adjusted model, better family functioning remained as a significant predictor of communication frequency (β = 0.40, p = 8.2e-5). In a separate adjusted model, intention of future communication of genetic test results was higher among females (OR = 2.4, 95% CI: 1.05–6.0) and those with better genetic knowledge (OR = 1.11, 95% CI: 1.02–1.23). Prior genetic testing was neither associated with current familial communication of CRC risk nor intended future communication of genetic information.
Table 2.
Bivariate and multivariate regression models showing factors related to current and future intention of familial communication (N = 189)
| Current communication of CRC risk | Intent to communicate genetic risk | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Categories | Bivariate model | Multivariate model | Bivariate model | Multivariate model | ||||
| β | p-value | β | p-value | χ 2 | p-value | OR [95% CI] | p-value | ||
| Gender | Femalea | 0.13 | .41 | 0.16 | .34 | 2.80 | .08 | 2.4 [1.05–6.0] | .04 |
| Age | 0.02 | .02 | 0.01 | .46 | 46.7 | .40 | 1.01 [0.97–1.06] | 34 | |
| Education | College or lessb | −0.40 | .02 | −0.16 | .34 | 2.2e-31 | 1.00 | 1.1 [0.45–2.78] | .82 |
| Income | −0.13 | .01 | −0.05 | .27 | 10.7 | .21 | 0.93 [0.72–1.21] | 0.61 | |
| Diagnosis | CRCc | 0.81 | 1.8e-16 | 0.37 | .08 | 2.80 | .09 | 1.72 [0.58–5.4] | .33 |
| Total genetic knowledge | −0.01 | .87 | 0.01 | .58 | 15.4 | .74 | 1.11 [1.02–1.23] | .02 | |
| Knowledge of sequencing limitations | −0.03 | .22 | 14.8 | .13 | |||||
| Knowledge of sequencing benefits | 0.02 | .49 | 12.0 | .40 | |||||
| Prior genetic testing | −0.03 | .88 | 0.07 | .69 | 1.37 | .50 | 0.97 [0.36–2.51] | .96 | |
| Family functioning (CFIS) | 0.07 | 2.9e-10 | 0.04 | 8.2e-5 | 24.4 | .69 | 1.04 [0.97–1.1] | .20 |
Significant results are in bold. CFIS Cancer Family Impact Scale; CI confidence interval; CRC colorectal cancer; OR odds ratio.
aCompared with male.
bCompared with some graduate school or higher.
cCompared with polyp.
DISCUSSION
This study examined relationships between genetic knowledge and familial communication of CRCP-related risk. Among 189 eMERGE III FamilyTalk participants, technical knowledge about genetics was minimally associated with intended familial communication about genetic risk information, but not current communication of CRC-related risk within the family, which was instead associated with better family functioning. This is the first quantitative study to examine the association between knowledge and familial communication in cancer, an outcome that is of increasing importance due to the prevalence of genetic testing in clinical oncology and the field’s reliance on familial communication to initiate cascade genetic testing. In contrast to results from qualitative studies on familial communication, genetic knowledge was not related to better communication. Family communication is a complex multifactorial phenomena that is largely influenced by the nature of family relationships between proband and relatives [27–29], which is consistent with our finding of higher current familial communication of CRC risk reported in families with better family functioning. In addition to family-level factors, individual-level factors such as knowledge of genetic sequencing allow probands to understand the concept of shared familial risk of heritable diseases and appreciate the importance of engaging in familial communication of genetic test results. Knowledge of pathogenic genetic variants and their associated lifetime cancer risks likely increase probands’ concern for their relative’s health, and the resulting feeling of obligation to inform relatives about genetic test results may supersede some commonly reported barriers to familial communication, such as worry about negatively impacting family relationships [27,30]. Although genetic knowledge alone may not be sufficient for familial communication, it may influence the urgency with which probands communicate results with relatives as it may allow better appreciation of shared genetic risk and understanding of the potential for disease prevention.
Probands in this study reported moderate current and intended communication with FDRs, which is comparable to communication rates in Lynch syndrome families from other studies in the absence of interventions to increase communication [31]. The wide gap between participants’ intention to communicate CRC-related sequencing results and their current CRC-related communication behaviors in our study exemplifies the well-established phenomena of intention-behavior gap, that is, incomplete correlation between health intentions and health behavior [32]. Although intention offers superior prediction of behavior compared to other cognitions (attitudes, self-efficacy, risk perceptions) [33], and forming an intention is vital to initiate new behaviors, factors that influence intention realization are varied and should be explored in the context of familial communication of genetic information in future studies. Female probands in this study reported higher intention of future communication of genetic test results, which is consistent with prior research on familial communication of genetic information [6,34]. Women are more likely to be “kinkeepers” in the family (i.e., individuals who keep in touch with other family members) [35] and thus more likely to communicate genetic test results [36–38] to their family members compared with men. This study adds to the literature on familial communication by reporting higher communication among females in the context of CRC, a nongendered disease; much of the existing familial communication studies focus on gendered diseases such as hereditary breast and ovarian cancer [27,39,40] although similar patterns have also been reported among hereditary CRC mutation carriers [41].
In agreement with prior research on public understanding of genetics and genomics [42], overall, participants in our study demonstrated good understanding of the role played by genetics in disease causation (85% or more correct on items 4, 6, 7, and 8). Better understanding of risk conferring variants compared to protective variants likely reflects the motivation of clinical genetic testing, which focuses on identifying susceptibility variants for a disease and not, protective genetic variants. In addition, our results support prior research and show that higher educational attainment [43,44] and younger age [43,45,46] were associated with higher genetic knowledge. This genetic knowledge gap, especially lower knowledge of limitation of genetic sequencing, may affect personal and perceived clinical utility of clinical genomics, and consequently impact familial communication and will be an issue of increasing importance as genomic medicine is more widely adopted and used in diverse clinical settings. Technical genetic knowledge is seldom sought out by counselees during genetic counseling [47]. Although counselors focus on dissemination of technical genetic knowledge, patients generally want to know less technical (e.g., what are genes? What is a genetic test) and more personally applicable information (e.g., How does it affect my family?) [47]. This mismatch of information need and delivery indicates that patients may not fully appreciate the importance of technical genetic knowledge for personal clinical decision making. However, genetic knowledge is important for downstream outcomes in genomic medicine such as familial communication and may also affect other patient-centered outcomes such as uptake of testing and adherence to screening or surgical recommendations.
The findings from this study should be considered in light of its limitations. This sample of FamilyTalk patients were enriched for well-educated, older, cancer-undiagnosed participants who as participants of a clinical genetics randomized controlled trial are likely more interested in genetic sequencing than the general population and as a result genetic knowledge may be overestimated in this study. Although this demographic is somewhat representative of patients who undergo cancer genetic testing, the findings likely do not generalize to all patients undergoing clinical sequencing. Future research should investigate the impact of genetic knowledge on intention to communicate in a more demographically diverse population, where the distribution of participants’ education is more representative of the general population. Knowledge of shared familial risk was not a validated subscale of the knowledge instrument used in this study. The instrument contained a single item on familial sharing of genomic variants that specifically focused on sharing with children (item 6) and may not accurately capture patients’ knowledge about the importance of sharing variants with other FDRs. Combining different genders of siblings and children in our analyses has likely prevented us from observing differences in familial communication between genders within relative types. In addition, the cross-sectional analysis of the baseline data cannot establish temporality between the knowledge and familial communication explored in this study and there is potential for residual confounding by other psychosocial variables not included in the analyses. Most participants in this study reported a very high intention of sharing, suggesting a celling effect that may have affected the study outcomes.
Practice implications
The results presented here show that genetic knowledge is important for intended familial communication of genetic information. However, despite patients’ best intentions, rates of intention realization, that is, actual familial communication, may be affected by quality of intentions (intentions based on personal beliefs about the outcomes of communicating vs. intentions based on social pressure to communicate) as well as family-level factors. Increasing genetic knowledge through discussion of general and cancer genetics during genetic consultation visits conveys the importance of familial communication to patients. Thus, genetic consultations should continue to focus on personally applicable genetic information that can improve the quality (what is communicated) and quantity (who it is communicated to) of familial communication in clinical cancer genetics. In addition, specific prompts for familial communication (e.g., specifying at-risk relatives or communication aids) could also be built into regular counseling sessions in order to overcome family-level barriers of communication. Primary care providers, who likely have more knowledge of familial issues, could also play an active role in cascade testing discussions.
Future research
Possible future research to be conducted based on these findings includes observational studies that consider the age of relatives and closeness of relationship between proband and relative in determining frequency of communication. In addition, studies with a prespecified hypothesis to confirm the present findings should be conducted. Further research is also needed on relationships between different domains of genetic knowledge (e.g., technical genetic knowledge vs. knowledge of shared familial risk) and frequency and content of familial communication. A strength of our study is utilization of intention to communicate as one of the study outcomes measured at baseline (i.e., before return of study results) as it minimizes interaction with potential confounders such as affect, which is known to be important in decision making and information processing. It will be important to examine the concordance of expressed intention to communicate and actual familial communication once genomic results have been returned to these, and similar, research participants.
In conclusion, participants in this study had modest genetic knowledge and those with lower educational attainment had lower knowledge about the limitations of genetic sequencing. The results presented here show that patients’ technical knowledge about genetic sequencing may affect future intention of communicating genetic test results to family members, but not current communication of CRC risk. Despite the best intentions of communicating genetic test results to family members, actual communication may be hindered by family-level factors. Additional investigation of the relative importance of general genetic knowledge compared with other family-level factors in improving familial communication rates and subsequently maximizing cascade genetic testing is warranted.
SUPPLEMENTARY MATERIAL
Supplementary data are available at Translational Behavioral Medicine online.
Table S1: Frequency of correct responses to genomic knowledge questions among study participants (N=189).
Table S2: Familial communication responses to survey questions about current and future intention of familial communication of CRCP related risk.
Funding:
This study was funded by National Human Genome Research Institute (3U01HG008657-02S1).
COMPLIANCE WITH ETHICAL STANDARDS
Conflicts of Interest: Sukh Makhnoon, Deborah J. Bowen, Brian H. Shirts, Stephanie M. Fullerton, Hendrika W. Meischke, Eric Larson, James Ralston, Kathleen Leppig, David R. Crosslin, David Veenstra, and Gail P. Jarvik declare that they have no conflicts of interest.
Authors’ Contributions: All authors were involved in the preparation of this manuscript and read and approved the final version.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1. Kaphingst KA, Peterson E, Zhao J, et al. Cancer communication research in the era of genomics and precision medicine: A scoping review. Genet Med. 2019;21(8):1691–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Resta R, Biesecker BB, Bennett RL, et al. , A new definition of genetic counseling: National Society of Genetic Counselors’ Task Force report. J Genet Couns. 2006;15(2):77–83. [DOI] [PubMed] [Google Scholar]
- 3. Fraser FC. Genetic counseling. Am J Hum Genet. 1974;26(5):636–659. [PMC free article] [PubMed] [Google Scholar]
- 4. Richman AR, Tzeng JP, Carey LA, Retèl VP, Brewer NT. Knowledge of genomic testing among early-stage breast cancer patients. Psychooncology. 2011;20(1):28–35. [DOI] [PubMed] [Google Scholar]
- 5. Lipkus IM, Vadaparampil ST, Jacobsen PB, Miree CA. Knowledge about genomic recurrence risk testing among breast cancer survivors. J Cancer Educ. 2011;26(4):664–669. [DOI] [PubMed] [Google Scholar]
- 6. Wiseman M, Dancyger C, Michie S. Communicating genetic risk information within families: A review. Fam Cancer. 2010;9(4):691–703. [DOI] [PubMed] [Google Scholar]
- 7. Gaff CL, Clarke AJ, Atkinson P, et al. Process and outcome in communication of genetic information within families: A systematic review. Eur J Hum Genet. 2007;15(10):999–1011. [DOI] [PubMed] [Google Scholar]
- 8. Adelswärd, V. and Sachs L., The messenger’s dilemmas—Giving and getting information in genealogical mapping for hereditary cancer. Health Risk Soci. 2003;5(2):125–138. [Google Scholar]
- 9. Finlay E, Stopfer JE, Burlingame E, et al. Factors determining dissemination of results and uptake of genetic testing in families with known BRCA1/2 mutations. Genet Test. 2008;12(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mellon S, Berry-Bobovski L, Gold R, Levin N, Tainsky MA. Communication and decision-making about seeking inherited cancer risk information: Findings from female survivor-relative focus groups. Psychooncology. 2006;15(3):193–208. [DOI] [PubMed] [Google Scholar]
- 11. White CB, Moyer CA, Stern DT, Katz SJ. A content analysis of e-mail communication between patients and their providers: Patients get the message. J Am Med Inform Assoc. 2004;11(4):260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baranowski T, Cullen KW, Nicklas T, Thompson D, Baranowski J. Are current health behavioral change models helpful in guiding prevention of weight gain efforts? Obes Res. 2003;11Suppl:23S–43S. [DOI] [PubMed] [Google Scholar]
- 13. Hollands GJ, French DP, Griffin SJ, et al. The impact of communicating genetic risks of disease on risk-reducing health behaviour: Systematic review with meta-analysis. BMJ. 2016;352:i1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaphingst KA, Blanchard M, Milam L, Pokharel M, Elrick A, Goodman MS. Relationships between health literacy and genomics-related knowledge, self-efficacy, perceived importance, and communication in a medically underserved population. J Health Commun. 2016;21Suppl 1:58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. VandenBoom E, Trepanier AM, Carmany EP. Assessment of current genetic counselor practices in post-visit written communications to patients. J Genet Couns. 2018;27(3):681–688. [DOI] [PubMed] [Google Scholar]
- 16. Chisholm RL. At the interface between medical informatics and personalized medicine: The emerge network experience. Healthc Inform Res. 2013;19(2):67–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCarty CA, Chisholm RL, Chute CG, et al. ; eMERGE Team . The eMERGE Network: A consortium of biorepositories linked to electronic medical records data for conducting genomic studies. BMC Med Genomics. 2011;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gottesman O, Kuivaniemi H, Tromp G, et al. ; eMERGE Network . The electronic medical records and genomics (eMERGE) network: Past, present, and future. Genet Med. 2013;15(10):761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bowen DJ, Shinn EH, Gregrowski S, et al. Patient-reported outcomes in the translational breast cancer research consortium. Cancer. 2020;126(5):922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. eMERGE Network. Available at https://www.genome.gov/Funded-Programs-Projects/Electronic-Medical-Records-and-Genomics-Network-eMERGE. Accessibility verified August 28, 2019.
- 21. Kaphingst KA, Facio FM, Cheng MR, et al. Effects of informed consent for individual genome sequencing on relevant knowledge. Clin Genet. 2012;82(5):408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sinicrope PS, Vernon SW, Diamond PM, et al. Development and preliminary validation of the cancer family impact scale for colorectal cancer. Genet Test. 2008;12(1):161–169. [DOI] [PubMed] [Google Scholar]
- 23. Bowen DJ, Hay JL, Harris-Wai JN, Meischke H, Burke W. All in the family? Communication of cancer survivors with their families. Fam Cancer. 2017;16(4):597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 25. Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316(7139):1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol. 2002;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Foster C, Watson M, Moynihan C, Arden-Jones A, Eeles R. Juggling roles and expectations: Dilemmas faced by women talking to relatives about cancer and genetic testing. Psychol Health. 2004;19(4):439–455. [Google Scholar]
- 28. Chivers Seymour K, Addington-Hall J, Lucassen AM, Foster CL. What facilitates or impedes family communication following genetic testing for cancer risk? A systematic review and meta-synthesis of primary qualitative research. J Genet Couns. 2010;19(4):330–342. [DOI] [PubMed] [Google Scholar]
- 29. Smith KR, Zick CD, Mayer RN, Botkin JR. Voluntary disclosure of BRCA1 mutation test results. Genet Test. 2002;6(2):89–92. [DOI] [PubMed] [Google Scholar]
- 30. Sobel SK, Cowan DB. Impact of genetic testing for Huntington disease on the family system. Am J Med Genet. 2000;90(1):49–59. [DOI] [PubMed] [Google Scholar]
- 31. Menko FH, Ter Stege JA, van der Kolk LE, et al. The uptake of presymptomatic genetic testing in hereditary breast-ovarian cancer and Lynch syndrome: A systematic review of the literature and implications for clinical practice. Fam Cancer. 2019;18(1):127–135. [DOI] [PubMed] [Google Scholar]
- 32. Sheeran P, Webb TL. The intention–behavior gap. Social Person Psychol Compass. 2016;10(9):503–518. [Google Scholar]
- 33. Sheeran P, Klein WM, Rothman AJ. Health behavior change: Moving from observation to intervention. Annu Rev Psychol. 2017;68:573–600. [DOI] [PubMed] [Google Scholar]
- 34. Peterson SK, Watts BG, Koehly LM, et al. How families communicate about HNPCC genetic testing: Findings from a qualitative study. Am J Med Genet C Semin Med Genet. 2003;119C(1):78–86. [DOI] [PubMed] [Google Scholar]
- 35. Wilson BJ, Forrest K, van Teijlingen ER, et al. Family communication about genetic risk: The little that is known. Community Genet. 2004;7(1):15–24. [DOI] [PubMed] [Google Scholar]
- 36. d’Agincourt-Canning L. Experiences of genetic risk: Disclosure and the gendering of responsibility. Bioethics. 2001;15(3):231–247. [DOI] [PubMed] [Google Scholar]
- 37. Forrest K, Simpson SA, Wilson BJ, et al. To tell or not to tell: Barriers and facilitators in family communication about genetic risk. Clin Genet. 2003;64(4):317–326. [DOI] [PubMed] [Google Scholar]
- 38. Hughes C, Lerman C, Schwartz M, et al. All in the family: Evaluation of the process and content of sisters’ communication about BRCA1 and BRCA2 genetic test results. Am J Med Genet. 2002;107(2):143–150. [DOI] [PubMed] [Google Scholar]
- 39. Kenen R, Arden-Jones A, Eeles R. We are talking, but are they listening? Communication patterns in families with a history of breast/ovarian cancer (HBOC). Psychooncology. 2004;13(5):335–345. [DOI] [PubMed] [Google Scholar]
- 40. Koehly LM, Peters JA, Kenen R, et al. Characteristics of health information gatherers, disseminators, and blockers within families at risk of hereditary cancer: Implications for family health communication interventions. Am J Public Health. 2009;99(12):2203–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koehly LM, Peterson SK, Watts BG, Kempf KK, Vernon SW, Gritz ER. A social network analysis of communication about hereditary nonpolyposis colorectal cancer genetic testing and family functioning. Cancer Epidemiol Biomarkers Prev. 2003;12(4):304–313. [PubMed] [Google Scholar]
- 42. Lea DH, Kaphingst KA, Bowen D, Lipkus I, Hadley DW. Communicating genetic and genomic information: Health literacy and numeracy considerations. Public Health Genomics. 2011;14(4–5):279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Calsbeek H, Morren M, Bensing J, Rijken M. Knowledge and attitudes towards genetic testing: A two year follow-up study in patients with asthma, diabetes mellitus and cardiovascular disease. J Genet Couns. 2007;16(4):493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Haga SB, Barry WT, Mills R, et al. Public knowledge of and attitudes toward genetics and genetic testing. Genet Test Mol Biomarkers. 2013;17(4):327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ashida S, Goodman M, Pandya C, et al. Age differences in genetic knowledge, health literacy and causal beliefs for health conditions. Public Health Genomics. 2011;14(4–5):307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Henneman L, Timmermans DR, van der Wal G. Public experiences, knowledge and expectations about medical genetics and the use of genetic information. Community Genet. 2004;7(1):33–43. [DOI] [PubMed] [Google Scholar]
- 47. Joseph G, Pasick RJ, Schillinger D, Luce J, Guerra C, Cheng JKY. Information mismatch: Cancer risk counseling with diverse underserved patients. J Genet Couns. 2017;26(5):1090–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.