An integrated collaborative care intervention for obesity and depression led to improvements in lifestyle and cognitive behaviors that can inform intervention optimization.
Keywords: Obesity, depression, mediation, health behavior
Abstract
The RAINBOW trial demonstrated that an integrated collaborative care intervention was effective for improving weight and depression. This study examined mediation of the treatment effect by a priori specified lifestyle behaviors and cognitive functioning. Participants were randomized to a 12-month integrated intervention (n = 204) or usual care (n = 205). Body mass index (BMI) and 20-item Depression Symptom Check List (SCL-20) were co-primary outcomes (Y). To examine mediation, we assessed (a) the effect of the integrated intervention (X) on lifestyle behaviors (diet and physical activity) and cognitive functioning (problem-solving; M, X→M path a) and (b) the association of these behaviors with BMI and SCL-20 (M→Y path b). Mediation existed if paths a and b were significant or if path a and the product of coefficients test (paths a and b) were significant. Compared with usual care, the intervention led to significant improvements in leisure time physical activity (201.3 MET minutes/week [SD, 1,457.6]) and total calorie intake (337.4 kcal/day [818.3]) at 6 months but not 12 months (path a). These improvements were not significantly associated with improvements in BMI or SCL-20 (path b). However, avoidant problem-solving style score and increased fruit and vegetable intake significantly correlated with improvements in BMI at 6 and 12 months, respectively. Also, increased fruit and vegetable intake, higher dietary quality, and better problem-solving abilities significantly correlated with improvements in SCL-20 at 6 and 12 months. These findings did not support the hypothesized mediation, but suggest lifestyle behaviors and cognitive functioning to target in future intervention optimization.
Implications.
Practice: An integrated behavioral intervention for co-occurring obesity and depression improved diet and physical activity over 6 months; future efforts can focus on maintenance of behavior change.
Policy: An integrated behavioral intervention for co-occurring obesity and depression was not only effective for improving weight and depression but also for improving relevant health behaviors.
Research: There was no evidence of mediation of the positive effect of the integrated collaborative care intervention on either weight or depressive symptoms by theory- and empirically based hypothesized mediators.
INTRODUCTION
Adults with multiple chronic conditions account for almost three-quarters of total health care spending in the USA [1]. It is estimated that 25% [2, 3] to 40% [1] of Americans suffer from more than one chronic condition and the most prevalent contributors are those related to obesity (e.g., hypertension, dyslipidemia, and diabetes) and mental health (e.g., mood and anxiety disorders) [1]. Effective approaches for managing multiple chronic conditions are urgently needed to increase quality of care, improve population health, and address healthcare costs [4].
Obesity and depression are two highly prevalent chronic conditions that often co-occur. Obesity affects approximately 40% of US adults [5] and depression affects 21% of US adults at least once in their lifetime [6]. Adults with obesity are 32% more likely to be depressed than those who are normal weight [7]. Similarly, adults with depression are 30% more likely to be obese than adults without depression (43% versus 33%) [8]. Although generally treated as unrelated conditions [9], behaviors common to both conditions including physical inactivity, unhealthy diet, and maladaptive problem-solving abilities suggest possible shared mechanisms. Thus, an integrated treatment approach targeting shared mechanisms may be needed for effective treatment of both conditions. The recent RAINBOW (Research Aimed at Improving Both Mood and Weight) trial demonstrated that an integrated collaborative care intervention for comorbid obesity and depression resulted in significantly greater improvements in weight loss and depressive symptoms at 6 and 12 months compared with usual care [10]. However, the intervention effects were modest; the between-group mean difference at 12 months (primary endpoint) was −0.66 (95% CI: −1.13, −0.18; Cohen’s d = 0.28) for body mass index (BMI) and −0.19 (95% CI: −0.35, −0.03; Cohen’s d=0.23) for the 20-item Depression Symptom Check List (SCL-20). Moreover, intervention response (e.g., clinically significant improvement in primary outcome) varied. Among participants who engaged in the intervention, 30% achieved clinically significant weight loss (≥5%) and 32% achieved a clinically significant reduction in depressive symptoms (≥50%), whereas those who were not engaged 17% achieved ≥5% weight loss and 12% achieved ≥50% reduction in depressive symptoms at 12 months [11]. Thus, efforts to augment intervention effectiveness are needed to promote future translation of this intervention into primary care. A deeper understanding of the processes that influenced the intervention effect can inform strategies to augment intervention effectiveness.
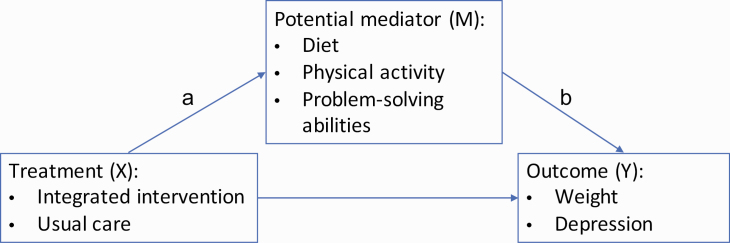
To understand these processes and to inform future optimization of the intervention, we examined mediation of the treatment effect on the primary outcomes according to an a priori specified conceptual framework (Fig. 1). The conceptual framework specified that engagement in the intervention would lead to improvements in lifestyle behaviors (physical activity and dietary intake) and cognitive functioning (problem-solving abilities) and that these improvements would lead to the observed improvements in the primary outcomes of weight and depressive symptoms. The lifestyle behaviors and cognitive functioning measures were selected based on the design of the integrated collaborative care intervention. The integrated collaborative care intervention targeted modest weight loss (5%–7%) and a 50% reduction in depression symptoms through improvements in diet, physical activity, and problem-solving abilities. Therefore, we hypothesized that these target measures would mediate the effect of the intervention on improving weight and depressive symptoms.
Fig. 1 .
Conceptual framework for mediation analysis.
METHODS
This study used data collected at baseline, 6 months, and 12 months from the RAINBOW trial. The trial protocol was previously published [12]. Briefly, 409 primary care patients (age ≥18 years) with obesity (BMI ≥30 or ≥27 if Asian) and depression (9-item Patient Health Questionnaire [PHQ9] ≥10) randomized to receive a 12-month integrated collaborative care intervention for obesity and depression (n = 204) or usual care (n = 205). The co-primary outcomes were changes in BMI and SCL-20 [13, 14] from baseline to 12 months, which were published [10].
Intervention
All participants continued to receive usual medical care from their primary care provider. Usual care also included information on mental health and weight management resources available at their clinic and an activity monitor. The integrated collaborative care intervention combined 2 evidence-based interventions: Group Lifestyle Balance (GLB) for obesity and Program to Encourage Active, Rewarding Lives (PEARLS) for depression. Adapted from the Diabetes Prevention Program, GLB is a behavioral lifestyle intervention aimed at modest weight loss (5%–7% of baseline weight) and at least 150 minutes of moderate-to-vigorous physical activity per week [15]. The PEARLS intervention utilizes problem-solving therapy as first-line, behavioral activation (including promotion of physical activity) and antidepressant medication as needed with a goal of reducing depressive symptoms by 50% or achieving PHQ-9<5 [16]. The integrated collaborative care intervention included a 6-month intensive phase comprised of 9 in-person individual sessions of 60 minutes each and 11 GLB videos of 20–30 minutes each viewed at home. The maintenance phase included monthly phone calls for another 6 months. Facilitated by a health coach dually trained on GLB and PEARLS, the intervention initiated with the PEARLS and added the GLB videos at the 5th session.
Data collection and measures
Trained research assistants blinded to intervention assignment conducted in-person assessments, including physical measurements, at baseline, 6, and 12 months. Participants filled out self-administered online questionnaires at the same time points.
The primary outcomes were BMI and SCL-20. Research assistants measured weight (and height at baseline only) at baseline and 6 and 12 month follow-up using a standardized protocol [17] and BMI was calculated by dividing weight by height in meters squared. Participants self-administered the SCL-20 to assess their depressive symptoms and a total score was calculated by averaging the 20 items; each item ranged from 0 (best) to 4 (worst). The SCL-20 has been validated and extensively used in primary care [14, 18].
Target measures of lifestyle behaviors and cognitive functioning were obtained by interview or self-report. Research assistants interviewed participants to conduct multiple-pass 24-hour recalls using the Nutrition Data System for Research (NDSR) [19] and 7-day Physical Activity Recalls per standardized protocol [20, 21]. As an index of overall diet quality, the DASH (Dietary Approach to Stop Hypertension) score was computed based on 9 nutrient targets (total fat, saturated fat, protein, cholesterol, fiber, magnesium, calcium, sodium, and potassium) [22] provided by the NDSR software. For each nutrient target, participants were assigned a point if they achieved the target and half a point if they achieved an intermediate target (i.e., half-way between the DASH target and the population mean), and the DASH score was the sum of points across all nine nutrients [23, 24]. Additional dietary target measures included daily servings of fruits and vegetables, daily total energy (kilocalories), and daily total fat consumption (g) given that the intervention emphasized increasing fruit and vegetable consumption and decreasing overall calories and fat.
Target measures of reported physical activity included Metabolic Equivalent Task (MET) minutes per week of leisure time physical activity based on the sum of the weighted physical activity minutes for moderate (weight: 4 METs), hard (weight: 6 METs), and very hard (weight: 10 METs) activities from the 7-day physical activity recall [25, 26]. Also, total energy expenditure in kilocalories per kilogram per day was derived from MET-minutes/day using the conversion 1 MET = 1 kcal/kg/h [25, 26].
Participants self-administered the 25-item Social Problem-solving Index-Revised Short Form (SPSI-R:S) to assess total problem-solving ability and 5 subscales including problem orientation (positive and negative) and problem-solving styles (rational, impulsive/careless, and avoidant), which has been previously validated [27, 28]. Each subscale was scored by summing the respective 5 items (each from 0 to 4), and the total problem-solving ability score ranged from 0 to 20 by averaging the subscale scores.
Statistical analysis
Longitudinal (e.g., change in target measure from baseline to 6 months and change in outcome from baseline to 12 months) and contemporaneous (e.g., changes in both target measure and outcome from baseline to 12 months) mediation were examined separately using the approach described by Kraemer et al. [29] and MacKinnon’s product of coefficients test [30]. According to Kramer and colleagues, mediation exists if there is a significant effect of the intervention (X) on the target measure (M, X→M path a) and the target measure is significantly associated with the outcome either as a main effect or an interaction effect with treatment group (M→Y path b; Fig. 1). Using MacKinnon’s product of coefficients test, mediation exists if there is a significant effect of the intervention on the mediator (path a) and the mediation indirect effect is significant. Path a was tested using repeated-measures linear mixed models to obtain between-group differences of the target measure and 95% confidence intervals (CIs) by tests of group-by-time interactions. The fixed effects of each model included baseline value of the target measure, randomization covariates (i.e., clinic, age, sex, race/ethnicity, education, any antidepressant medications if taken at the time of enrollment, and number of hospitalizations in the year pre-baseline), group (intervention or control), time point (6 or 12 months), and group-by-time interaction. The random effects accounted for repeated measures with an unstructured covariance matrix and clustering of patients within primary care physicians. Path b was tested using ordinary least square regression to test whether individual target measures (main effect) and/or the interaction of a target measure with group (intervention or control) predicted the corresponding outcome (BMI or SCL-20), adjusting for the baseline value of the outcome and randomization covariates. In addition, as a sensitivity analysis for path b for SCL-20, we included change in antidepressant medication prescription as a covariate to account for any effect of these medications. Change in prescription was defined as a new medication or a change in dose in the 12 months after randomization compared to the 12 months prior. MacKinnon’s product of coefficients test [30] for indirect effects was performed, allowing for group-mediator interaction as per Valeri and Vanderweele [31], 95% confidence limits calculated using bootstrapping.
We conducted two additional analyses of mediation. First, we tested for moderated mediation effects by moderators of age (i.e., ≥45 versus <45), sex, severity of comorbidities (e.g., baseline BMI and SCL-20: BMI ≥35 versus BMI<35 and SCL-20 ≥1.5 versus SCL-20 <1.5), and self-reported antidepressant medication use at baseline using the approach described by Hayes [32]. Second, we examined mediation according to adherence to the intervention because we hypothesized that if mediation by the target measures existed it would most likely occur among those who adhered to the intervention. Given that information on adherence in the control group did not exist, we created a propensity score of the probability of adhering to the intervention. The propensity score was created using all observed baseline profiles of sociodemographic, behavioral, clinical, and psychosocial characteristics. Participants in the intervention group were matched with control participants on an allowable absolute difference between exact propensity scores using 1:1 ratio [33]. Adherence was defined as attending intervention sessions consistently throughout the 12-month intervention and was based on a definition that we previously demonstrated to be related to clinically significant treatment outcomes [11]. Specifically, adherence was defined as having attended at least 1 intervention session in at least 3 out of 4 quarters of the 12-month intervention. Using this definition, those who were classified as adhering to the intervention attended an average of 14.5 sessions (SD 1.8) out of 15 sessions. We used the same mediation analyses as previously described for the subgroup of intervention adhered and propensity score matched control participants [34] and incorporated matched pairs information (e.g., matched pairs as random effects in path a model to generate the intervention effects on all the potential mediators.)
All analyses were conducted using SAS, version 9.4 (SAS Institute Inc., Cary, North Carolina). p < 0.05 (2-sided) was considered statistically significant. p-Values were not adjusted for multiple comparisons as this was an exploratory study, and additional dedicated studies are needed to confirm the results [35].
RESULTS
Participants were predominantly middle-aged (mean 51.0 [SD 12.1] years), female (70%), and college-educated (69% at least a college degree; Table 1). The average BMI was 36.7 (SD 6.4) and the average SCL-20 score was 1.5 (SD 0.5), indicating moderately severe obesity and depression.
Table 1.
Baseline characteristics by intervention group
| Characteristic | Overall (n = 409) | Intervention (n = 204) | Usual care (n = 205) |
|---|---|---|---|
| Age, years | 51.0 (12.1) | 50.9 (12.2) | 51.0 (11.9) |
| Female, No. (%) | 287 (70) | 144 (71) | 143 (70) |
| Race/ethnicity, No. (%) | |||
| Non-Hispanic White | 289 (71) | 147 (72) | 142 (69) |
| African American | 6 (1) | 3 (1) | 3 (1) |
| Asian/Pacific Islander | 40 (10) | 20 (10) | 20 (10) |
| Hispanic | 56 (14) | 26 (13) | 30 (15) |
| Other | 18 (4) | 8 (4) | 10 (5) |
| Education, No. (%) | |||
| High school/GED or less | 28 (7) | 10 (4) | 18 (9) |
| Some college | 98 (24) | 51 (25) | 47 (23) |
| College graduate | 150 (37) | 78 (39) | 72 (35) |
| Post college | 133 (32) | 65 (32) | 68 (33) |
| Annual family income, No. (%), n = 365 | |||
| <$75,000 | 93 (26) | 46 (26) | 47 (25) |
| $75,000–<$150,000 | 117 (32) | 54 (31) | 63 (33) |
| ≥$150,000 | 155 (42) | 76 (43) | 79 (42) |
| BMI | 36.7 (6.4) | 36.7 (6.9) | 36.6 (5.8) |
| SCL-20 score | 1.5 (0.5) | 1.5 (0.5) | 1.5 (0.6) |
Values are mean (SD) unless otherwise noted.
BMI, body mass index; GED, general educational development; SCL-20, Depression Symptom Checklist-20.
Path a (X→M): intervention effect on target measures
The intervention had a positive effect on physical activity and total calorie intake at 6 months (Table 2). Compared with control participants, intervention participants significantly increased their leisure time physical activity by 201.3 (SD 1,457.6) MET minutes more per week (p = 0.02) and decreased total calorie intake by 337.4 (SD 818.3) kilocalories more per day (p = 0.02) from baseline to 6 months. These between-group differences were no longer significant by 12 months (Table 2). Between-group differences also were not significant for changes in daily energy expenditure, DASH score, daily servings of fruits and vegetables, fat intake, or any of the problem-solving measures from baseline to 6 or 12 months.
Table 2.
Models testing association between intervention and potential mediators
| Outcome measures | Unadjusted estimates, mean (SD) | Intervention differences | ||
|---|---|---|---|---|
| Intervention | Usual Care | Coefficient | p Value | |
| n = 205 | n = 204 | (95% CI)a | ||
| Leisure time physical activity, MET minutes/weekb | ||||
| Baseline | 758.6 (955.3) | 668.1 (774.9) | ||
| Change from baseline to 6 months | 201.3 (1,457.6) | 9.7 (998.2) | 276.5 (39.0, 513.9) | 0.02 |
| Change from baseline to 12 months | −72.7 (1,283.7) | 9.6 (1,174.9) | 17.4 (−233.7, 268.9) | 0.89 |
| Total energy expenditure, kilocalories /kg/dayc | ||||
| Baseline | 33.4 (2.3) | 33.4 (2.2) | ||
| Change from baseline to 6 months | 0.4 (3.0) | 0.1 (2.5) | 0.3 (−0.3, 0.9) | 0.30 |
| Change from baseline to 12 months | −0.3 (2.8) | −0.1 (2.7) | −0.2 (−0.7, 0.4) | 0.54 |
| DASH scored | ||||
| Baseline | 2.3 (1.3) | 2.2 (1.3) | ||
| Change from baseline to 6 months | −0.1 (1.6) | 0.1 (1.7) | −0.1 (−0.4, 0.2) | 0.35 |
| Change from baseline to 12 months | 0.1 (1.7) | 0.2 (1.7) | −0.1 (−0.4, 0.2) | 0.42 |
| Fruit and vegetable, servings/day | ||||
| Baseline | 3.5 (3.2) | 3.7 (3.7) | ||
| Change from baseline to 6 months | −0.1 (3.9) | 0.1 (4.3) | −0.6 (−1.2, 0.1) | 0.09 |
| Change from baseline to 12 months | −0.0 (3.4) | 0.1 (4.1) | −0.2 (−0.8, 0.4) | 0.54 |
| Total calorie intake, Kilocalories/day | ||||
| Baseline | 1,812.4 (791.8) | 1,868.6 (792.3) | ||
| Change from baseline to 6 months | −337.4 (818.3) | −139 (832.3) | −160.5 (−293.7, −27.3) | 0.02 |
| Change from baseline to 12 months | −228.1 (892.6) | −262.6 (843.9) | −12.5 (−129.4, 154.4) | 0.86 |
| Total fat, g/day | ||||
| Baseline | 75.4 (44.3) | 79.7 (41.5) | ||
| Change from baseline to 6 months | −16.0 (48.2) | −6.1 (49.3) | −7.8 (−15.7, 0.2) | 0.06 |
| Change from baseline to 12 months | −13.0 (51.6) | −11.3 (47.4) | −3.3 (−11.6, 5.0) | 0.43 |
| Social Problem-solving Inventory-Revised Short Forme | ||||
| Baseline | 11.9 (2.6) | 11.8(2.6) | ||
| Change from baseline to 6 months | 0.8 (2.0) | 0.6 (1.7) | 0.2 (−0.2, 0.7) | 0.26 |
| Change from baseline to 12 months | 0.8 (2.2) | 0.9 (2.0) | −0.04 (−0.5, 0.4) | 0.86 |
| Positive Problem Orientationf | ||||
| Baseline | 10.1 (4.1) | 9.4 (4.5) | ||
| Change from baseline to 6 months | 1.3 (3.8) | 0.9 (4.3) | 0.7 (−0.1, 1.6) | 0.10 |
| Change from baseline to 12 months | 1.3 (4.0) | 1.7 (3.8) | 0.0 (−0.8, 0.8) | 0.99 |
| Negative Problem Orientationf | ||||
| Baseline | 8.6 (3.6) | 8.5 (3.7) | ||
| Change from baseline to 6 months | −0.9 (3.4) | −0.8 (3.3) | −0.2 (−0.8, 0.5) | 0.61 |
| Change from baseline to 12 months | −1.1 (3.2) | −0.9 (2.9) | −0.3 (−0.9, 0.2) | 0.25 |
| Rational Problem-solving styleg | ||||
| Baseline | 9.6 (4.5) | 9.4 (4.2) | ||
| Change from baseline to 6 months | 1.0 (3.9) | 0.7 (3.5) | 0.4 (−0.4, 1.1) | 0.42 |
| Change from baseline to 12 months | 0.7 (3.9) | 1.2 (4.0) | −0.4 (−1.3, 0.5) | 0.45 |
| Impulsivity/carelessness styleg | ||||
| Baseline | 4.4 (3.6) | 4.3 (3.4) | ||
| Change from baseline to 6 months | −0.1 (3.3) | −0.3 (3.0) | 0.3 (−0.3, 0.9) | 0.32 |
| Change from baseline to 12 months | −0.3 (3.3) | −0.1 (2.8) | 0.0 (−0.6, 0.7) | 0.90 |
| Avoidance styleg | ||||
| Baseline | 6.8 (4.6) | 6.9 (4.5) | ||
| Change from baseline to 6 months | −0.7 (3.4) | −0.6 (3.7) | −0.3 (−1.0, 0.4) | 0.37 |
| Change from baseline to 12 months | −0.6 (3.7) | −1.0 (4.0) | 0.2 (−0.6, 1.0) | 0.64 |
CI, confidence interval; DASH, Dietary Approaches to Stop Hypertension; MET, metabolic equivalent.
aAdjusted analysis for intervention versus usual care: adjusted coefficient (linear mixed model) and 95% CI. Mixed effect models accounting for primary care provider and repeated measure random effects were adjusted for baseline value of the outcome of interest, study site, sex, age, race/ethnicity, education, and therapeutic class of antidepressant medication taken, number of hospitalizations. Baseline data are mean (SD) scores, mean (95% CI) difference, and P value for the mean difference, derived from baseline t test differences without covariates.
bPhysical activity levels were measured by the interview-administered Stanford 7-day Physical Activity Recall. Leisure-time physical activity = non–work-related moderate activity min/wk × 4 METs + hard activity min/wk × 6 METs + very hard activity min/wk × 10 METs. One MET is defined as the energy expenditure for sitting quietly.
cThe Stanford 7-day Physical Activity Recall data also provided estimates of total daily energy expenditures. Total energy expenditure = sleep hours × 1 MET + light activity hours × 1.5 METs + moderate activity hours × 4 METs + hard activity hours × 6 METs + very hard activity hours × 10 METs.
dDASH scores were calculated based on combining nine nutrient targets (i.e., total fat, saturated fat, protein, cholesterol, fiber, magnesium, calcium, sodium and potassium). The intermediate target of each nutrient was half-way between the DASH target and population mean (based on the National Health and Nutrition Examination Surveys 2007–2008, latest data available at the inception of this study). For a nutrient, participants reaching the DASH target were assigned one point, those reaching the intermediate target were assigned a half-point, and those not meeting the intermediate target were given 0 points. The DASH score was the sum of points for all nine nutrients.
eAn increase in the composite score indicates an improvement in problem-solving skills.
fPositive and negative problem orientation were used to measure problem-solving orientation. An increased score for positive problem orientation is an improvement; while an increased score for negative problem orientation is a worsening.
gThree problem-solving styles: An increase in the rationale problem-solving style score is an improvement; while for the other two styles, an increase in the score is a worsening.
Path b (M→Y): effect of target measures on outcomes
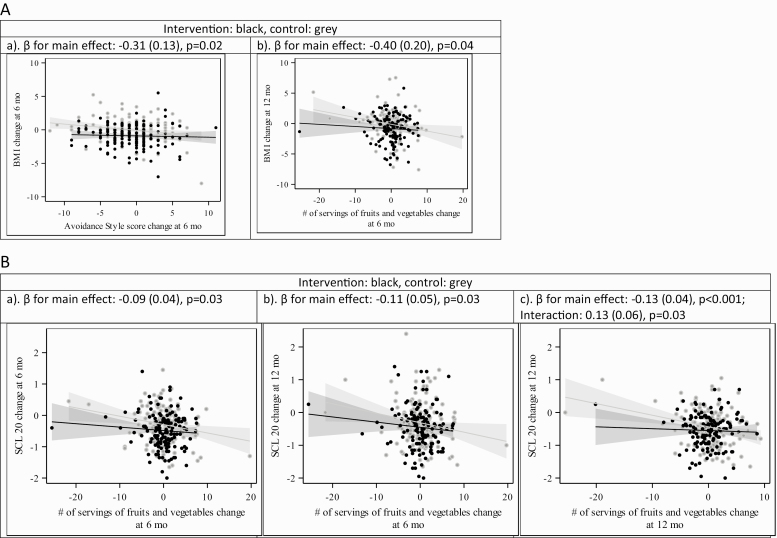
Daily servings of fruits and vegetables were associated with a change in BMI and SCL-20 score (Figs. 2 and 3, Appendix). For every increase of daily serving of fruits and vegetables from baseline to 6 months, BMI from baseline to 12 months decreased by 0.4 (SD 0.2) units (p = 0.04). For every increase in daily servings of fruits and vegetables at 6 months, SCL-20 decreased by 0.09 (SD 0.04) points at 6 months (p = 0.03) and 0.11 (SD 0.05) points at 12 months (p = 0.03). At 12 months, however, an increase in servings of fruits and vegetables was associated with a decrease in SCL-20 in the control group only (−0.13 [SD 0.04], p < 0.001) given the interaction term estimate of 0.13 (SD 0.06, p = 0.003). Likewise, at 12 months, an increase in the DASH score was associated with a decrease in SCL-20 in the control group only (−0.13 [SD 0.04], p = 0.001) given the interaction term estimate of 0.14 (SD 0.06, p = 0.02).
Fig. 2 .
(A) Behavior variables BMI. (B) Behavior variables SCL-20
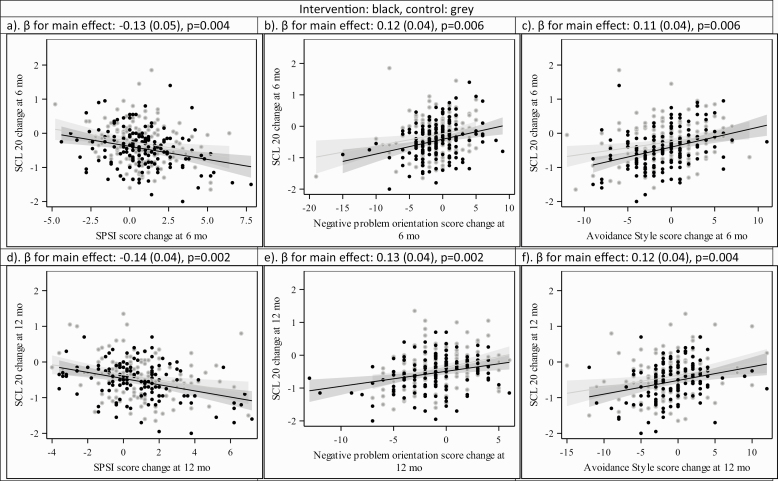
Changes in problem-solving overall, orientation, and style were not associated with changes in BMI with one exception (Fig. 2; Appendix). A unit increase in the avoidance style score was associated with a decrease of 0.31 (SD 0.13) BMI units (p = 0.02). Improvements in the overall problem-solving score as well as negative problem orientation and avoidance style from baseline to 6 and 12 months were significantly associated with improvements in SCL-20 over the same time periods (Fig. 3; Appendix). For every one-point improvement in the SPSI-R:S score, the SCL-20 decreased by 0.13 (SD 0.05) points from baseline to 6 months (p = 0.004) and 0.14 (SD 0.04) points from baseline to 12 months (p = 0.002). One-point improvements on the subscales of negative problem orientation and avoidance style from baseline to 6 and 12 months were significantly associated with contemporaneous decreases in SCL-20 scores from baseline to 6 and 12 months in the range of 0.11–0.13 points (p < 0.01). Similarly, a one-point improvement in the impulsivity/carelessness style subscale from baseline to 6 months was associated with a 0.11 (SD 0.05) point improvement in SCL-20 scores from baseline to 6 months (p = 0.02), yet there was no significant association by 12 months. Including change in antidepressant medication prescription as a covariate did not change the results for the associations of target measures and SCL-20.
Fig. 3 .
Problem-solving variables SCL-20
Additional mediation analyses
We examined the indirect effect for target measures that were significantly impacted by the intervention. The indirect effect of the change in leisure time physical activity and total calorie intake from baseline to 6 months on change in BMI and SCL-20 from baseline to 6 months and baseline to 12 months were not significant (data not shown). We also examined moderated mediation by the a priori moderators of age, sex, comorbidity severity, and antidepressant medication use at baseline and did not find evidence for moderation of the mediation effect of any of the target measures on the corresponding outcomes (data not shown). Finally, among those who adhered to the intervention and those in the control group who were matched using a propensity score to estimate those in the control group who would have likely adhered if they had been assigned to the intervention group, we did not find any significant mediation of the intervention effect of any of the target measures on the corresponding outcomes (data not shown).
DISCUSSION
In this secondary analysis of the RAINBOW trial, we examined a priori hypothesized mediators, including physical activity, diet, and problem-solving abilities of the benefit of the intervention on weight and depressive symptoms to support future translation. We did not find evidence of mediation of the effect of the intervention on either weight or depressive symptoms by these theory- and empirically based target measures. Nevertheless, the positive effect of the intervention on physical activity and total calorie intake from baseline to 6 months provides information that can inform optimization of the intervention. Similarly, associations of fruit and vegetable intake with weight and depressive symptoms as well as problem-solving abilities with depressive symptoms can contribute to intervention optimization. This is important for future translation into primary care because although the intervention resulted in significant improvements in weight and depressive symptoms at 12 months, the improvements were modest and variable [10].
The reasons for our findings that the beneficial intervention effect was not mediated by the a priori identified lifestyle behaviors and cognitive functioning may be related to measurement and selection of the mediators. In regard to measurement, it is possible that the mediators were not measured with adequate precision and validity and/or that they were not measured at adequate time intervals. The 7-day physical activity recall and single multiple pass 24-hour dietary recall may not have been able to accurately capture the changes that participants made over the 12-month follow-up period to lose weight. Similarly, it is possible that the SPSI-R:S was not sensitive to the improvements in problem-solving skills that the participants achieved as a result of participating in the intervention. It is also possible that the measures of behaviors related to diet, physical activity, and problem-solving abilities at only a few discrete time points over 12 months and by self-report were not able to capture the timing of changes associated with the intervention and with outcomes. Future trials can include additional, ecologically and temporally more precise and objective measurements of diet, physical activity, and problem-solving to improve the ability to understand their mediating effects on intervention outcomes. In regard to selection of mediators, the intervention may have been mediated by factors other than those that we measured. For example, the effectiveness of the PEARLS intervention could be due to behavioral activation and/or changes in antidepressant medications. However, behavioral activation and antidepressant use (e.g., prescriptions filled, medications taken) were not measured. Data on antidepressant medication use was limited to self-reported use at baseline and prescriptions from Electronic Health Record during the follow-up period. Future studies should include these measures and examine their mediating effect on outcomes.
Despite the fact that we did not find evidence of mediation, the findings can inform efforts to optimize the intervention and translate the intervention into practice. The intervention had a positive effect on diet (total calorie intake) and physical activity (leisure time physical activity) relative to the control group at 6 months but not 12 months. This appeared to be due, at least in part, to a lack of maintenance of these behaviors in the second 6 months of the intervention. Other studies of behavioral obesity interventions have also documented challenges with behavior maintenance in the second 6 months [36–38]. Given that the strategies to support behavior adoption and behavior maintenance are distinct, the intervention could increase focus on the skills that support maintenance [39, 40]. For example, overcoming relapses is an important skill for maintaining successful behavior change. Additionally, given that the intervention was designed with a decrease in direct participant contact in the second 6 months, as in the majority of behavioral lifestyle interventions, the intervention could incorporate feasible strategies to support continued contact for successful maintenance of behavior changes. Technology-mediated strategies via mobile applications and text messages may be feasible for continued contact. Increasing focus on behavioral maintenance and identifying feasible approaches for continued participant contact will be critical for supporting translation in primary care.
Other strategies for intervention optimization based on our findings relate to fruit and vegetable consumption and problem-solving abilities. Increases in daily servings of fruits and vegetables at 6 months were associated with improvements in weight at 12 months and depressive symptoms at 6 and 12 months in both treatment groups. Thus, to optimize effectiveness, the intervention could emphasize the importance of fruit and vegetable consumption for improving both health outcomes. While interventions targeting weight loss typically focus on nutrition, this is less common for interventions targeting depression. In addition, the contemporaneous associations of changes in problem-solving abilities with changes in SCL-20 scores over 6 and 12 months underscore the importance of using problem-solving therapy for treating depressive symptoms. Other studies have also shown that patients with lower problems solving abilities as well as maladaptive problem orientations and problem-solving styles tend to have higher levels of depressive symptoms [41–43]. To augment the effectiveness of problem-solving therapy, the intervention could incorporate alternative or additional emotion regulation strategies. Future research can examine the effectiveness of these strategies to augment intervention, which will be important for translation into primary care.
Several notable weaknesses may have limited our findings. As noted, measurement error of the hypothesized mediators may have limited our ability to detect significant mediation if it was indeed present. Measurement of diet and physical activity relies on individual’s ability to recall, which may have differed by group—it is possible that participants randomized to the intervention may recall their diet and physical activity more accurately compared to those randomized to usual care given that the intervention recommended self-tracking of these behaviors. Thus, for example, if those randomized to usual care tended to overestimate their consumption of healthy foods and amount of physical activity and underestimate their consumption of unhealthy foods, this could result in an overestimate of improvements in the control group, an underestimate of the intervention effect on the hypothesized mediators, and an underestimate of the mediator effect on weight. Additionally, mediation may vary according to specific subgroups such as age, sex, obesity and/or depression severity, and antidepressant medication use. Although we examined moderated mediation, it remains possible that we lacked sufficient power to detect significant mediation in these smaller subgroups. Finally, findings may not be generalizable to more diverse populations given that participants were primarily white and college educated.
Despite these limitations, this study has several strengths that enabled a thorough analysis of potential mediators of the integrated collaborative care intervention for comorbid obesity and depressive symptoms to inform intervention optimization and support future translation into primary care. We used validated measures of diet, physical activity, and problem-solving abilities as well as blinded assessment of outcomes. Although we did not detect significant mediation of the intervention effect by our a priori hypothesized mediators, the findings provide evidence that can be used to optimize the intervention prior to translating into primary care practice. Future research can examine the effectiveness of the strategies identified to optimize the integrated collaborative care intervention. Additionally, future research to understand behavioral mediators of an integrated intervention for obesity and depression can improve measurement of the mediators and increase sample size to allow for increased power for moderated mediation analysis.
Acknowledgments
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R01HL119453 and UH3HL132368. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank the participants and their families who made this study possible.
APPENDIX
Potential Mediator Effects On Primary Outcomes (Behavior Measures Change of Bmi From Baseline, and Physical Activity And Problem-Solving Ability Assessment Change of Scl-20 From Baseline)
| Potential mediator | Timepoint | Outcome timepoint | Change for BMI estimate (standard error) | Change for SCL-20 estimate (standard error) | ||
|---|---|---|---|---|---|---|
| Main effect | Interaction term | Main effect | Interaction term | |||
| Physical activity MET | 6 | 6 | 0.09 (0.17) | −0.25 (0.21) | 0.03 (0.05) | −0.07 (0.06) |
| 6 | 12 | 0.24 (0.24) | −0.56 (0.32) | −0.05 (0.06) | 0.05 (0.08) | |
| 12 | 12 | 0.16 (0.22) | −0.57 (0.29) | −0.05 (0.05) | 0.01 (0.06) | |
| Energy expenditure | 6 | 6 | −0.008 (0.15) | −0.11 (0.20) | 0.01 (0.05) | −0.03 (0.06) |
| 6 | 12 | 0.08 (0.21) | −0.21 (0.30) | −0.02 (0.06) | −0.10 (0.08) | |
| 12 | 12 | 0.10 (0.21) | −0.40 (0.30) | −0.03 (0.04) | −0.004 (0.06) | |
| DASH score | 6 | 6 | 0.07 (0.14) | 0.03 (0.20) | −0.03 (0.04) | 0.05 (0.06) |
| 6 | 12 | −0.26 (0.20) | 0.32 (0.29) | −0.09 (0.05) | 0.06 (0.07) | |
| 12 | 12 | −0.27 (0.21) | 0.22 (0.30) | −0.13 (0.04)*** | 0.14 (0.06)* | |
| Servings of fruit and vegetable | 6 | 6 | −0.10 (0.14) | −0.06 (0.20) | −0.09 (0.04)* | 0.07 (0.06) |
| 6 | 12 | −0.40 (0.20)* | 0.26 (0.29) | −0.11 (0.05)* | 0.09 (0.08) | |
| 12 | 12 | −0.30 (0.20) | 0.28 (0.31) | −0.13 (0.04)*** | 0.13 (0.06)* | |
| Total calorie intake | 6 | 6 | 0.05 (0.14) | −0.17 (0.20) | 0.05 (0.04) | −0.10 (0.06) |
| 6 | 12 | 0.11 (0.21) | −0.22 (0.29) | 0.01 (0.05) | −0.05 (0.07) | |
| 12 | 12 | 0.11 (0.22) | 0.10 (0.30) | 0.01 (0.04) | 0.005 (0.06) | |
| Total fat intake | 6 | 6 | 0.008 (0.14) | −0.12 (0.20) | 0.02 (0.04) | −0.07 (0.06) |
| 6 | 12 | 0.08 (0.21) | −0.13 (0.30) | −0.02 (0.05) | −0.02 (0.08) | |
| 12 | 12 | 0.04 (0.22) | 0.00 (0.31) | 0.02 (0.04) | −0.03 (0.06) | |
| SPSI-R:S score | 6 | 6 | 0.08 (0.15) | −0.04 (0.20) | −0.13 (0.05)** | −0.006 (0.06) |
| 6 | 12 | −0.21 (0.22) | 0.10 (0.30) | 0.01 (0.06) | −0.10 (0.08) | |
| 12 | 12 | −0.15 (0.23) | 0.15 (0.31) | −0.14 (0.04)** | 0.001 (0.06) | |
| Positive problem orientation | 6 | 6 | −0.14 (0.13) | 0.01 (0.19) | −0.03 (0.04) | −0.03 (0.06) |
| 6 | 12 | −0.24 (0.19) | 0.03 (0.28) | 0.02 (0.05) | −0.06 (0.07) | |
| 12 | 12 | −0.18 (0.21) | −0.02 (0.30) | −0.07 (0.04) | −0.01 (0.06) | |
| Negative problem orientation | 6 | 6 | −0.16 (0.14) | 0.05 (0.19) | 0.12 (0.04)* | 0.03 (0.06) |
| 6 | 12 | −0.19 (0.20) | 0.20 (0.28) | 0.07 (0.05) | 0.05 (0.08) | |
| 12 | 12 | −0.003 (0.22) | 0.04 (0.30) | 0.13 (0.04)** | −0.04 (0.06) | |
| Rational problem-solving style | 6 | 6 | −0.16 (0.14) | 0.22 (0.20) | 0.05 (0.05) | −0.04 (0.06) |
| 6 | 12 | −0.34 (0.21) | 0.29 (0.29) | 0.07 (0.06) | −0.03 (0.08) | |
| 12 | 12 | −0.32 (0.21) | 0.40 (0.30) | −0.06 (0.04) | 0.004 (0.06) | |
| Impulsivity/carelessness style | 6 | 6 | −0.12 (0.14) | 0.11 (0.20) | 0.11 (0.05)* | −0.06 (0.06) |
| 6 | 12 | 0.24 (0.22) | −0.14 (0.30) | −0.04 (0.06) | 0.05 (0.08) | |
| 12 | 12 | −0.28 (0.23) | 0.32 (0.30) | 0.04 (0.05) | 0.05 (0.06) | |
| Avoidance style | 6 | 6 | −0.31 (0.13) | 0.25 (0.20) | 0.11 (0.04)* | 0.08 (0.06) |
| 6 | 12 | −0.21 (0.20) | 0.18 (0.30) | 0.03 (0.05) | 0.14 (0.08) | |
| 12 | 12 | 0.06 (0.20) | −0.29 (0.30) | 0.12 (0.04)** | −0.03 (0.06) |
DASH, Dietary Approaches to Stop Hypertension; MET, metabolic equivalent; SCL-20, 20-item Depression Symptom Check List; SPSI-R:S, Social Problem-Solving Index-Revised Short Form.
*p ≤ 0.05, **p ≤ 0.005, ***p ≤ 0.001.
Compliance with Ethical Standards
Authors’ Contributions: J.M., L.G.R., N.L., L.X., P.W.L. conceptualized and designed the study. L.X. carried out the statistical analysis with expert input from P.W.L.; J.M., L.GR., N.L., and L.X. interpreted the data; L.G.R. drafted the manuscript; J.M., L.X., N.L., P.W.L., E.M.V., M.B.S., J.M.S., M.A.L., L.M.W., T.S., and A.N.G.P. critically revised the manuscript for important intellectual content; and J.M., L.G.R., E.M.V., and M.B.S. obtained funding. J.M. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest: The authors have no conflicts of interest to report. LMW has received fees as a consultant from BlackThorn Therapeutics for work unrelated to this study.
Ethical Approval: All procedures performed were in accordance with the ethical standards of Sutter Health IRB and with the 1964 Helsinki declaration and its later amendments.
Informed Consent: Informed consent was obtained from all individual participants included in the study. This article does not contain any studies with animals performed by any of the authors.
References
- 1. Buttorff C, Ruder T, Bauman M. Multiple chronic conditions in the United States. Santa Monica, CA: RAND; 2017. [Google Scholar]
- 2. Ward BW, Schiller JS. Prevalence of multiple chronic conditions among US adults: estimates from the National Health Interview Survey, 2010. Prev Chronic Dis. 2013;10:E65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: a 2012 update. Prev Chronic Dis. 2014;11:E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood). 2008;27(3):759–769. [DOI] [PubMed] [Google Scholar]
- 5. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics NCHS data brief; no. 288 ; DHHS publication; no. (PHS) 2018–1209; 2017. [Google Scholar]
- 6. Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 Major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75(4):336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pereira-Miranda E, Costa PRF, Queiroz VAO, Pereira-Santos M, Santana MLP. Overweight and obesity associated with higher depression prevalence in adults: a systematic review and meta-analysis. J Am Coll Nutr. 2017;36(3):223–233. [DOI] [PubMed] [Google Scholar]
- 8. Pratt LA, Brody DJ. Depression and obesity in the US adult household population, 2005–2010. Women. 2014;20:39. [PubMed] [Google Scholar]
- 9. Faith MS, Matz PE, Jorge MA. Obesity-depression associations in the population. J Psychosom Res. 2002;53(4):935–942. [DOI] [PubMed] [Google Scholar]
- 10. Ma J, Rosas LG, Lv N, et al. Effect of integrated behavioral weight loss treatment and problem-solving therapy on body mass index and depressive symptoms among patients with obesity and depression: the RAINBOW Randomized Clinical Trial. JAMA. 2019;321(9):869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lv N, Xiao L, Majd M, et al. Variability in engagement and progress in efficacious integrated collaborative care for primary care patients with obesity and depression: within-treatment analysis in the RAINBOW trial. PLOS One. 2019. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma J, Yank V, Lv N, et al. Research aimed at improving both mood and weight (RAINBOW) in primary care: a type 1 hybrid design randomized controlled trial. Contemp Clin Trials. 2015;43:260–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goldberg DP, Rickels K, Downing R, Hesbacher P. A comparison of two psychiatric screening tests. Br J Psychiatry. 1976;129:61–67. [DOI] [PubMed] [Google Scholar]
- 14. Glass RM, Allan AT, Uhlenhuth EH, Kimball CP, Borinstein DI. Psychiatric screening in a medical clinic. An evaluation of a self-report inventory. Arch Gen Psychiatry. 1978;35(10):1189–1195. [DOI] [PubMed] [Google Scholar]
- 15. Ma J, Yank V, Xiao L, et al. Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med. 2013;173(2):113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ciechanowski P, Chaytor N, Miller J, et al. PEARLS depression treatment for individuals with epilepsy: a randomized controlled trial. Epilepsy Behav. 2010;19(3):225–231. [DOI] [PubMed] [Google Scholar]
- 17. Ober C, Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun. 2006;7(2):95–100. [DOI] [PubMed] [Google Scholar]
- 18. Derogatis L. The Hopkins symptom checklist: a measure of primary symptom dimensions. In Pichot P ed. Psychological Measurement: Modern problems in Pharmacopsychiatry. Basle: Karger; 1973. [DOI] [PubMed] [Google Scholar]
- 19. Blair SN, Haskell WL, Ho P, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122(5):794–804. [DOI] [PubMed] [Google Scholar]
- 20. Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. 2003;77(5):1171–1178. [DOI] [PubMed] [Google Scholar]
- 21. Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: an observational validation study. J Am Diet Assoc. 2004;104(4):595–603. [DOI] [PubMed] [Google Scholar]
- 22. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713–720. [DOI] [PubMed] [Google Scholar]
- 23. Sacks FM, Obarzanek E, Windhauser MM, et al. Rationale and design of the Dietary Approaches to Stop Hypertension trial (DASH). A multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann Epidemiol. 1995;5(2):108–118. [DOI] [PubMed] [Google Scholar]
- 24. Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3–10. [DOI] [PubMed] [Google Scholar]
- 25. Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121(1):91–106. [DOI] [PubMed] [Google Scholar]
- 26. Wilson PW, Paffenbarger RS Jr, Morris JN, Havlik RJ. Assessment methods for physical activity and physical fitness in population studies: report of a NHLBI workshop. Am Heart J. 1986;111(6):1177–1192. [DOI] [PubMed] [Google Scholar]
- 27. D’Zurilla TJ, Nezu AM, Maydeu-Olivares A. The Social Problem-Solving Inventory-Revised (SPSI-R): Technical Manual. North Tonawanda, NY: Multi-Health Systems, Inc; 2002. [Google Scholar]
- 28. Dreer LE, Berry J, Rivera P, et al. Efficient assessment of social problem-solving abilities in medical and rehabilitation settings: a Rasch analysis of the Social Problem-Solving Inventory-Revised. J Clin Psychol. 2009;65(7):653–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59(10):877–883. [DOI] [PubMed] [Google Scholar]
- 30. MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7(1):83–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hayes AF. Partial, conditional, and moderated mediation: Quantification, inference, and interpretation. Communication Monographs. 2018;85(1):4–40. [Google Scholar]
- 33. Fraeman KH, Evidera B. A General SAS® Macro to Implement Optimal N: 1 Propensity Score Matching Within a Maximum Radius. Pharma SUG 2015-Paper HA05. 2015. [Google Scholar]
- 34. Jo B, Stuart EA, Mackinnon DP, Vinokur AD. The use of propensity scores in mediation analysis. Multivariate Behav Res. 2011;46(3):425–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Althouse AD. Adjust for multiple comparisons? it’s not that simple. Ann Thorac Surg. 2016;101(5):1644–1645. [DOI] [PubMed] [Google Scholar]
- 36. Kramer MK, Vanderwood KK, Arena VC, et al. Evaluation of a diabetes prevention program lifestyle intervention in older adults: a randomized controlled study in three senior/community centers of varying socioeconomic status. Diabetes Educ. 2018;44(2):118–129. [DOI] [PubMed] [Google Scholar]
- 37. Goode RW, Ye L, Sereika SM, et al. Socio-demographic, anthropometric, and psychosocial predictors of attrition across behavioral weight-loss trials. Eat Behav. 2016;20:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Middleton KR, Anton SD, Perri MG. Long-term adherence to health behavior change. Am J Lifestyle Med. 2013;7(6):395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sciamanna CN, Kiernan M, Rolls BJ, et al. Practices associated with weight loss versus weight-loss maintenance results of a national survey. Am J Prev Med. 2011;41(2):159–166. [DOI] [PubMed] [Google Scholar]
- 40. Rothman AJ. Toward a theory-based analysis of behavioral maintenance. Health Psychol. 2000;19(1S):64–69. [DOI] [PubMed] [Google Scholar]
- 41. Dixon WA, Heppner PP, Burnett JW, Anderson WP, Wood PK. Distinguishing among antecedents, concomitants, and consequences of problem-solving appraisal and depressive symptoms. J Counsel Psychol. 1993;40(3):357. [Google Scholar]
- 42. Haaga DA, Fine JA, Terrill DR, Stewart BL, Beck AT. Social problem-solving deficits, dependency, and depressive symptoms. Cogn Ther Res. 1995;19(2):147–158. [Google Scholar]
- 43. Kant GL, D’Zurilla TJ, Maydeu-Olivares A. Social problem solving as a mediator of stress-related depression and anxiety in middle-aged and elderly community residents. Cogn Ther Res. 1997;21(1):73–96. [Google Scholar]