Abstract
In aging Caenorhabditis elegans, as in higher organisms, there is more than one cause of death. C. elegans exhibit early death with a swollen, infected pharynx (P death), and later death with pharyngeal atrophy (p death). Interventions that alter lifespan can differentially affect frequency and timing of each type of death, generating complex survival curve shapes. Here, we use mortality deconvolution analysis to investigate how reduction of insulin/IGF‐1 signaling (IIS), which increases lifespan (the Age phenotype), affects different forms of death. All daf‐2 insulin/IGF‐1 receptor mutants exhibit increased lifespan in the p subpopulation (p Age), while pleiotropic class 2 daf‐2 mutants show an additional marked reduction in P death frequency. The latter is promoted by pharyngeal expression of the IIS‐regulated DAF‐16 FOXO transcription factor, and at higher temperature by reduced pharyngeal pumping rate. Pharyngeal DAF‐16 also promotes p Age in class 2 daf‐2 mutants, revealing a previously unknown role for the pharynx in the regulation of aging. Necropsy analysis of daf‐2 interactions with the daf‐12 steroid receptor implies that previously described opposing effects of daf‐12 on daf‐2 longevity are attributable to internal hatching of larvae, rather than complex interactions between insulin/IGF‐1 and steroid signaling. These findings support the view that wild‐type IIS acts through multiple distinct mechanisms which promote different life‐limiting pathologies, each of which contribute to late‐life mortality. This study further demonstrates the utility of mortality deconvolution analysis to better understand the genetics of lifespan.
Keywords: aging, Caenorhabditiselegans, genetics, insulin/IGF‐1 signaling
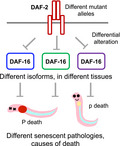
Different daf‐2 insulin/IGF‐1 receptor mutations differentially affect different forms of death in C. elegans. This involves tissue‐ and isoform‐specific action of the DAF‐16 FOXO transcription factor, notably in the pharynx where it protects not only against lethal pharyngeal pathology, but also aging more widely. Thus, wild‐type daf‐2 promotes death from old age through multiple effectors with distinct effects on the various senescent pathologies that cause it.
1. INTRODUCTION
Many genes have been identified manipulation of which can alter lifespan in the nematode Caenorhabditis elegans. By comparison, the proximate mechanisms that such genes influence to alter lifespan remain poorly understood. Lifespan is a function of senescent pathology that causes death (Blagosklonny, 2007; Gems, 2015). In C. elegans, a subset of aging wild‐type hermaphrodites die as the result of a lethal pharyngeal infection (Zhao et al., 2017). This is detectable by means of necropsy after death from old age, as corpses with an enlarged, swollen pharynx (P death ["big P"]). Worms that escape P death die later with an atrophied pharynx (p death ["small p"]) (Figure 1a). Genetic mutations or treatments that change lifespan act by differentially altering the frequency and/or timing of each form of death. By combining mortality and necropsy analysis, effects of interventions on P and p subpopulations can be assessed (mortality deconvolution) (Zhao et al., 2017) (Figure 1b).
Figure 1.
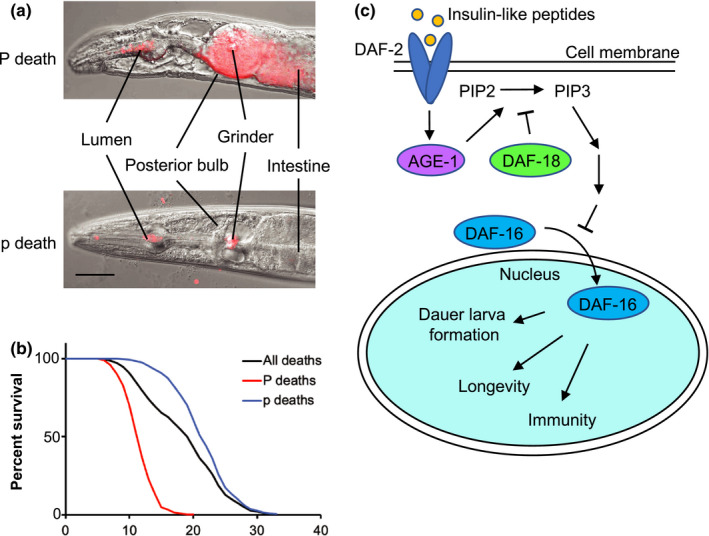
Schematic of mortality deconvolution and insulin/IGF‐1 signaling (IIS) pathway in Caenorhabditis elegans. (a) Representative Nomarski images of P and p corpses of worms fed RFP‐expressing E. coli. P corpses are characterized by the widespread RFP signal in the pharyngeal tissue, while p corpses contain either limited RFP foci or only signal in the grinder lumen. Scale bar 40 μm. (b) Schematic of mortality deconvolution for a typical wild‐type C. elegans lifespan assay. The black survival curve represents survival of all animals in the population, red and blue curves represent that of the P and p death subpopulation, respectively. (c) Simplified schematic of the IIS pathway in C. elegans. Under optimal conditions, insulin‐like peptides activate the DAF‐2 insulin/IGF‐1 receptor and its downstream kinase AGE‐1/PI3 K, which converts PIP2 to PIP3, an activity antagonized by DAF‐18/PTEN. Through a cascade of phosphorylation increased PIP3 level leads to phosphorylation and cytosolic sequestration of DAF‐16/FOXO. When IIS is inhibited, DAF‐16 enters the nucleus where it alters expression of genes involved in dauer formation, longevity, and innate immunity
The evolutionarily conserved IIS pathway affects a variety of biological processes, including development and aging (Figure 1c). In C. elegans, mutation of the daf‐2 insulin/IGF‐1 receptor or the age‐1 phosphatidylinositol 3‐kinase (PI3 K) catalytic subunit can cause constitutive formation of diapausal dauer larvae (the Daf‐c phenotype) (Murphy & Hu, 2013) and greatly extended lifespan (the Age phenotype) (Friedman & Johnson, 1988; Kenyon et al., 1993). Reduced IIS can also cause proportionally more modest increases in lifespan in higher organisms, including fruit flies, mice, and possibly humans (Kenyon, 2010). While the mechanisms mediating effects of IIS on lifespan remain uncertain, reduced IIS must act by altering the frequency and/or timing of P and/or p death. A further possibility is that effects of IIS may be mediated by mechanisms specific to either the P or p subpopulation.
Genetic analysis of daf‐2 is complicated by complex differences between daf‐2 alleles, which can be broadly grouped into two classes. Class 1 mutants have lesions in extracellular regions of the DAF‐2 receptor, and Age is partially suppressed by mutation of the daf‐12 nuclear hormone receptor. By contrast, class 2 mutants have lesions in either the DAF‐2 receptor ligand‐binding domain or the intracellular tyrosine kinase domain, are more pleiotropic, showing temperature‐sensitive reductions in feeding rate, movement rate and fertility, and the Age phenotype is often increased by mutation of daf‐12 (Antebi et al., 2000; Gems et al., 1998; Larsen et al., 1995; Patel et al., 2008).
Both the Daf‐c and Age phenotypes of IIS mutants require the DAF‐16/FOXO transcription factor (Kenyon et al., 1993; Murphy & Hu, 2013). In well‐nourished worms, DAF‐16 is phosphorylated and sequestered in the cytosol, but moves into the nucleus under stressed conditions and when IIS is reduced (Murphy & Hu, 2013), retarding aging (Figure 1c). The genetics of daf‐16 is complicated by the presence of multiple isoforms. Three sets of daf‐16 isoforms are transcribed from distinct promoters in distinct tissues, daf‐16a, daf‐16b, and daf‐16d/f/h (henceforth referred to as daf‐16f) (Bansal et al., 2014; Kwon et al., 2010; Murphy & Hu, 2013). Of these daf‐16a is the main mediator of daf‐2 Age, Daf‐c, and stress resistance, with daf‐16f also playing a lesser role; in tests with daf‐16 isoform‐specific mutations, daf‐16a(‐) but not daf‐16f(‐) partially suppressed daf‐2 Daf‐c and Age, but simultaneous abrogation of both isoforms strongly suppressed both phenotypes (Chen et al., 2015). Earlier evidence for a predominant role of daf‐16f (Bansal et al., 2014; Kwon et al., 2010) seems to have been an artifact of daf‐16f transgene overexpression and failure of the daf‐16a transgene to fully recapitulate daf‐16a isoform function (Chen et al., 2015). daf‐16b plays only a minor role in dauer development (Lee et al., 2001; Lin et al., 2001). Earlier studies focusing on daf‐16a demonstrated the importance of intestinal and neuronal DAF‐16 expression in daf‐2 longevity, but also suggested that the full extent of daf‐2 Age requires DAF‐16 function in other, unidentified tissues (Libina et al., 2003).
Here, we use mortality deconvolution analysis to reveal distinct effects of IIS on aging in P and p subpopulations, shedding new light on earlier lifespan genetic studies. These results reveal how distinct elements of IIS act differentially and in condition‐dependent ways upon distinct mechanisms that contribute to illness and death in elderly worms.
2. RESULTS
2.1. Reduced daf‐2 function reduces P death and causes p Age in an allele‐ and temperature‐dependent fashion
To investigate how IIS affects the two forms of death in C. elegans, we performed survival, necropsy, and mortality deconvolution analysis on a range of daf‐2 loss‐of‐function mutants: three class 1 (m41, m577, and e1368), two class 2 (e1370 and m120), and m596 which is at the boundary between class 1 and class 2 (Gems et al., 1998; Patel et al., 2008). Trials were conducted at 15˚C and 20˚C, which are permissive for development, and at 25˚C which induces dauer entry during development and dauer‐like behavior in class 2 daf‐2 mutant adults. All alleles were crossed into a fln‐2(+) background to avoid the confounding effects of the fln‐2(ot611) mutation, which reduces P frequency (Zhao et al., 2019). The resulting, deconvolved data includes survival data for the whole population, and for P and p subpopulations, plus the proportion of P deaths. However, because P sample sizes are often small, we discount P lifespan data in most cases, but present the data in Appendix S1. Raw survival data for all trials are available in Data S1.
At 20˚C, all six daf‐2 mutants showed an increase in overall lifespan whose magnitude varied both among and between the two classes (Figure 2a), similar to previously reported overall severity ranking of daf‐2 alleles (Gems et al., 1998). All mutants showed p Age and reduced P frequency, both of which were more marked in class 2 mutants (Figure 2a,b; Appendix S1: Table S1). A similar result was observed at 15˚C, where both e1368 and e1370 significantly reduced P frequency and increased p lifespan (Appendix S1: Figure S1; Table S2). The relative contribution of reduced P frequency and increased P and p lifespan to the overall Age phenotype was also calculated for each mutant. This showed that the greater lifespan of class 2 alleles was largely attributable to greater p Age (Figure 2c).
Figure 2.
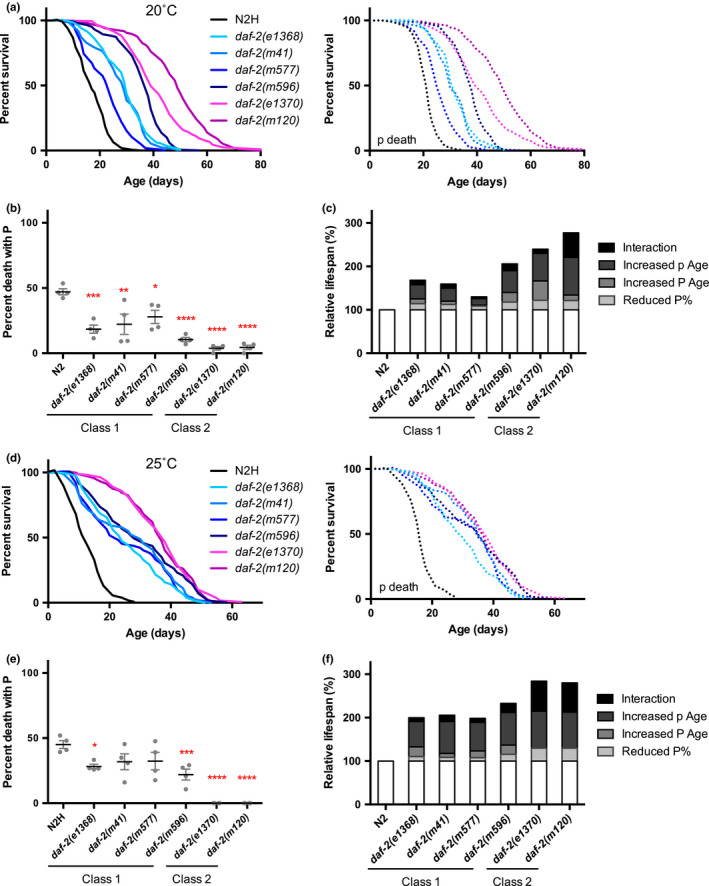
Effects of class 1 and 2 daf‐2 alleles on lifespan and P frequency at 20˚C and 25˚C. (a) Lifespan of the whole population or the p subpopulation and (b) frequency of P death of class 1 and class 2 daf‐2 mutants at 20˚C. N = 2 trials. (c) Contribution of increased p and P lifespan, and reduced P frequency, to daf‐2 mutants lifespan at 20˚C. The contribution of each factor is calculated one‐variable‐at‐a‐time, and interaction represents the residue effect due to the non‐linear nature of lifespan. (d) Lifespan and (e) frequency of P death of class 1 and class 2 daf‐2 mutants at 25˚C. N = 2 trials. (f) Contribution of increased p and P lifespan, and reduced P frequency, to daf‐2 mutants lifespan at 25˚C
At 25˚C, overall lifespan varied significantly between the two classes but, in contrast to 20˚C, was similar within each class of mutants (Figure 2d; Appendix S1: Table S3). Class 1 mutants again showed modest reduction in P frequency, but in class 2 mutants P death was entirely absent (Figure 2e). Moreover, the difference in p Age between the two classes is much smaller at 25˚C compared with 20˚C (Figure 2d,f; Appendix S1: Figure S2; Table S4). Thus, the greater longevity of daf‐2(e1370) and daf‐2(m120) is largely attributable to a reduction in the proportion of P deaths. Overall, these results show that the relative contribution of reduced P and of p Age to overall Age varies greatly between daf‐2 mutants depending on allele and ambient temperature.
Knockdown of daf‐2 function by RNA‐mediated interference (RNAi) also increases C. elegans lifespan (Arantes‐Oliveira et al., 2003). daf‐2 RNAi of wild‐type animals from either L4 or from the parental generation onward resulted in p Age but little reduction in P frequency (Appendix S1: Figure S3A, B). Similar results were obtained using the enhanced RNAi mutant rrf‐3(pk1426) (Simmer et al., 2002) (Appendix S1: Figure S3C, D). Differences between effects of daf‐2 mutant alleles and daf‐2 RNAi have been noted previously. For example, daf‐2 RNAi fails to induce dauer entry at 25˚C, and daf‐2 class 1 mutants and daf‐2 RNAi show additive effects on lifespan (Arantes‐Oliveira et al., 2003; Ewald et al., 2015). The lack of an effect of daf‐2 RNAi on P death could reflect non‐induction of the dauer program; however, reductions of pharyngeal infection and P death seen in daf‐2(e1370) at 15˚C (Figure 3b; Appendix S1: Figure S1) suggest that some suppression of P death can occur in the absence of the dauer program. The distinct effects of daf‐2 RNAi could reflect a difference in the way that IIS is disrupted within cells (see Discussion) and/or different levels of disruption in different tissues.
Figure 3.
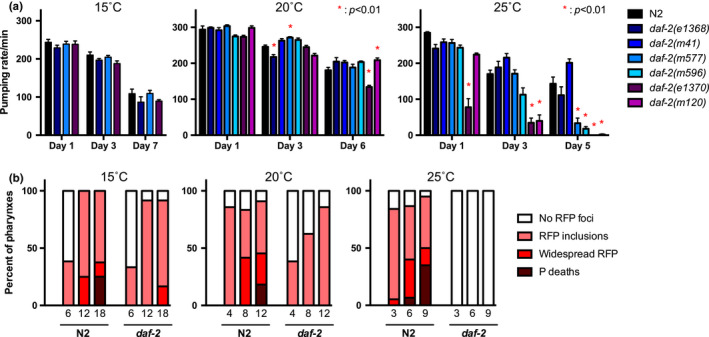
Temperature sensitivity of resistance to initial bacterial invasion and Eat in daf‐2(e1370). These results are consistent with the possibility that class 2 daf‐2 Eat contributes to resistance to P death. (a) Effects of mutation of daf‐2 on pharyngeal pumping rates in early adulthood at 15˚C, 20˚C, and 25˚C. A reduction in pumping rate was consistently observed in class 2 mutants at 25˚C only, consistent with earlier findings (Gems et al., 1998; Kenyon et al., 1993). n ≥ 10. Star indicates p < 0.01. (b) daf‐2(e1370) had little effect on initial bacterial invasion but suppressed widespread infection at 15˚C, delayed both bacterial invasion and further infection at 20˚C, and fully suppressed bacteria invasion at 25˚C. N = 2 trials
2.2. daf‐2 can reduce P death independently of effects on feeding rate
At 22.5˚C and 25˚C, class 2 daf‐2 mutants exhibit a near cessation of feeding (pharyngeal pumping) in class 2 mutants (Gems et al., 1998). Moreover, eat‐2 mutants exhibit reduced pharyngeal pumping rate and also reduced P frequency, apparently due to reduced mechanical damage to the pharyngeal cuticle (Zhao et al., 2017), an example of mechanical senescence. This suggests that the different levels of suppression of P death in daf‐2 mutants could reflect different degrees of suppression of pharyngeal pumping. To explore this, we assayed the effect of daf‐2 on pumping rate. At 25˚C, pumping rate in class 2 mutants was greatly reduced by day 3 of adulthood, but little reduction in pumping was seen at 15˚C or 20˚C, broadly consistent with previous findings (Gems et al., 1998). Thus, the reductions in P frequency seen in daf‐2 mutants at 15˚C and 20˚C are not attributable to reduced pharyngeal pumping rate.
During early adulthood, low‐level infection of pharyngeal tissue occurs, apparently due to activity‐dependent mechanical damage to the pharyngeal cuticle. This early infection leads in later life to the gross infection that causes P death and can be visualized using RFP‐expressing E. coli OP50 as red fluorescent inclusions or puncta within pharyngeal tissue (Zhao et al., 2017) (Figure 1a). We asked: does daf‐2(e1370) prevent early pharyngeal infection? As expected, at 25˚C initial bacterial invasion was fully suppressed, while at 20˚C daf‐2(e1370) caused a marked reduction in RFP inclusions in early adulthood (Figure 3b). Notably at 15˚C, early invasion was not suppressed but the progression to widespread infection is delayed and reduced (Figure 3b). Taken together these results suggest that daf‐2(e1370) acts in two way to reduce P death: by suppressing pumping, and also by a pumping rate‐independent mechanism, possibly improved innate immunity (see Discussion).
2.3. daf‐2 gain‐of‐function increases P death frequency
The effects of mutation of daf‐2 imply that IIS increases P death frequency and causes earlier p death. To test this further, we increased daf‐2 activity level using the gain‐of‐function allele daf‐2(gk390525gf), which increases DAF‐2 protein levels due to a defect in ubiquitylation‐mediated degradation and reduces lifespan (Tawo et al., 2017). As expected, this caused a modest but significant reduction in overall mean lifespan (−12%, p = 0.0028). Unexpectedly, this was largely due to a 40% increase in P frequency (p = 0.0486), while p lifespan was not significantly reduced (−4.8%, p = 0.103) (Figure 4a,b; Appendix S1: Figure S4A; Table S5). Consistent with this, when P death was suppressed using the antibiotic carbenicillin, p lifespan was not significantly reduced by daf‐2(gf) (−2.5%, p = 0.0906) (Appendix S1: Figure S4B). The increase in P frequency was not due to effects of daf‐2 gain‐of‐function on pharyngeal pumping rate, since no increase was observed in the mutant (Appendix S1: Figure S4C). The implications of these findings are discussed in the next section.
Figure 4.
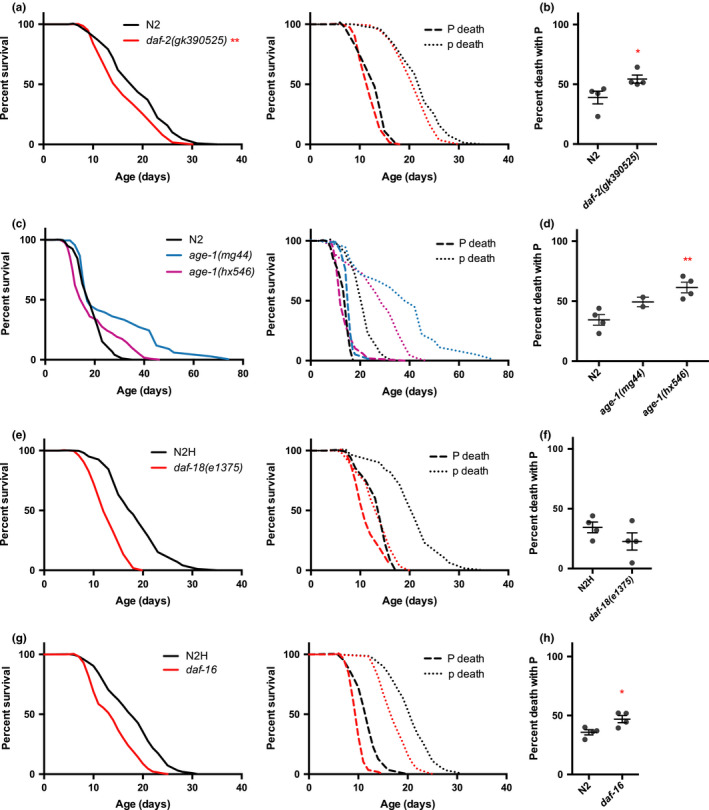
Effects of daf‐2 gain‐of‐function mutant, age‐1, and daf‐18 mutants on lifespan. (a) Lifespan and (b) P frequency of daf‐2(gk390525) gain‐of‐function mutant. daf‐2(gf) shortened lifespan largely by increasing P frequency. N = 2 trials. (c) Lifespan and (d) P frequency of two age‐1 mutant alleles. mg44 homozygotes were identified from among age‐1 −/+ mothers by singling worms and identifying those with all dauer progeny (see Methods). The increase in P frequency seen in the age‐1(hx546) strain was unexpected and could be an effect of hx546 or possibly a difference in the genetic background. N = 3 trials. Regarding the relatively small effect of hx546 on lifespan observed here: note that the effect of hx546 on lifespan is temperature sensitive, showing larger effects at 25˚C than 20˚C (Dorman et al., 1995; Friedman & Johnson, 1988). Moreover, the classic Friedman and Johnson (1988) study employed monoxenic liquid culture with a lower E. coli concentration than plate culture, which may have reduced P death. (e) Lifespan and (f) P frequency of daf‐18 mutant. N = 2 trials. (g) Lifespan and (h) P frequency of daf‐16 null allele. N = 2 trials
2.4. age‐1(rf) and daf‐18(rf) increase and decrease p lifespan, respectively
The AGE‐1 PI3 K catalytic subunit acts downstream of DAF‐2 (Figure 1c), and mutation of age‐1 increases lifespan (Friedman & Johnson, 1988; Morris et al., 1996). Mutation of age‐1, like that of daf‐2, caused p Age but did not reduce P frequency in either the age‐1(hx546) reduction‐of‐function mutant or the age‐1(mg44) null mutant (Figure 4c,d; Appendix S1: Table S6). The lack of a reduction in P death frequency is consistent with the neck‐and‐shoulder survival curve morphology seen in previous reports on age‐1 (Friedman & Johnson, 1988; Tissenbaum and Ruvkun 1998), that is, with an initial steep decline in survival (due to P deaths) followed by sustained survival in the longer‐lived p subpopulation. Mutation of glp‐1 also has this effect (Zhao et al., 2017).
Conversely, daf‐18(e1375), a reduction‐of‐function mutation affecting the C. elegans PTEN PIP3 phosphatase that antagonizes the PI3 K pathway (Ogg & Ruvkun, 1998), shortened p lifespan but did not increase P frequency (Figure 4e,f; Appendix S1: Table S7). These results, taken together with the effects of daf‐2(gf), could imply that p lifespan is a function of PIP3 levels, controlled by PI3 K and PTEN, while other outputs of the DAF‐2 receptor impact P frequency. Another possibility is that IIS increase by daf‐2(gk390525) is neomorphic in character.
2.5. Action of DAF‐16 in the pharynx reduces P death and promotes p Age
We have described how daf‐2(rf) increases lifespan by causing p Age and, in some contexts, by reducing P frequency. Both Daf‐c and Age phenotypes of daf‐2 require the DAF‐16 FOXO transcription factor. This raises the possibility that effects of IIS on P and p involve different functions of DAF‐16. We explored this first by testing effects of the null mutation daf‐16(mgDf50). In a daf‐2(+) background, daf‐16(0) reduced mean p lifespan (−16.8%, p < 0.0001) and modestly increased P frequency (Figure 4g,h; Appendix S1: Table S8). daf‐2 mutant longevity is to some extent attributable to resistance to E. coli infection (Garsin et al., 2003; Podshivalova et al., 2017), suggesting that the p life‐shortening effect of daf‐16(0) could reflect increased susceptibility to infection. But this is not the case, since daf‐16(0) shortened mean lifespan more when bacterial proliferation was blocked using antibiotics (−29.9%, p < 0.0001) (Appendix S1: Figure S5). We then tested whether the effects of daf‐2(rf) on both P and p deaths are daf‐16 dependent and found that they were, in both classes of daf‐2 mutant (Figure 5a,b; Appendix S1: Figure S6B, C; Table S9).
Figure 5.
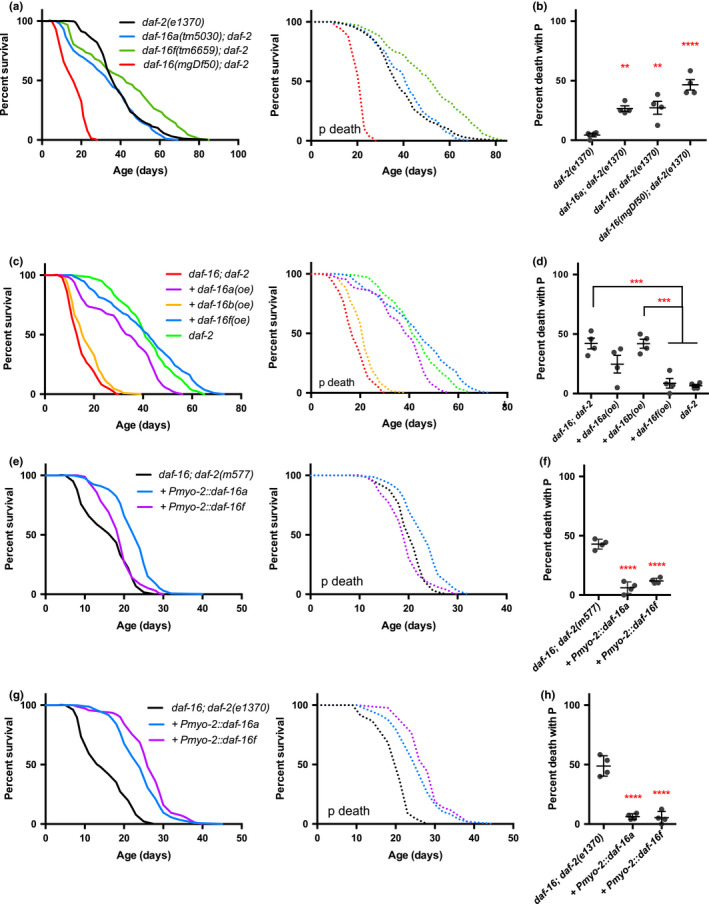
DAF‐16 expression in pharynx suppress P. (a) Lifespan and (b) P frequency of daf‐16 null or isoform‐specific mutant in daf‐2(e1370) mutant background. N = 2 trials. (c) Lifespan and (d) P frequency of isoform‐specific overexpression in daf‐16;daf‐2(e1370) double mutant background. N = 2 trials. (e) Lifespan and (f) P frequency of daf‐16;daf‐2(m577) double mutant overexpressing daf‐16 isoforms in the pharynx. N = 2 trials. (g) Lifespan and (H) P frequency of daf‐16;daf‐2(e1370) double mutant overexpressing daf‐16 isoforms in the pharynx. N = 2 trials
daf‐16 isoforms differ in their expression patterns, as do mammalian FOXOs, and also in their effects on C. elegans lifespan (Bansal et al., 2014; Chen et al., 2015; Kwon et al., 2010; Lin et al., 2001) (Appendix S1: Figure S6A). Pdaf‐16a drives expression in most tissues but not in the pharynx, Pdaf‐16b in the pharynx, spermathecae, and neurons, while Pdaf‐16f drives expression in almost all tissues including the pharynx (Kwon et al., 2010). At 20˚C in a daf‐16(0);daf‐2(e1370) background, the Pdaf‐16a::daf‐16a transgene array lpIs12 (Kwon et al., 2010) had little effect on P frequency but largely restored p Age, while the Pdaf‐16b::daf‐16b transgene array lpIs13 did not rescue either reduced P or p Age. By contrast, the Pdaf‐16f::daf‐16f transgene array lpIs14 both reduced P frequency and restored p Age (Figure 5c,d; Appendix S1: Table S10).
One possibility is that daf‐16f but not daf‐16a expression suppresses P due to the former's pharyngeal expression, rather than to differences in DAF‐16 isoform protein sequence. To test this, we constructed transgenic lines with expression of daf‐16a or daf‐16f limited to the pharynx using a myo‐2 promoter (Okkema & Fire, 1994). Pharyngeal overexpression of either DAF‐16A or DAF‐16F was found to reduce P frequency in wild type (Appendix S1: Figure S6E) and in daf‐16;daf‐2 mutants with both classes of daf‐2 mutation (Figure 5f,h), without reducing pharyngeal pumping rate in early adulthood (Appendix S1: Figure S7). Thus, daf‐16 isoform‐specific effects here are more attributable to spatial differences in expression than in gene‐regulatory specificity.
Restoration of daf‐2 p Age by Pmyo‐2::daf‐16 proved to be daf‐2 allele specific. Pmyo‐2::daf‐16a resulted in p Age in a wild‐type background, and both daf‐16;daf‐2 backgrounds. By contrast, Pmyo‐2::daf‐16f only restored p Age in the daf‐16;daf‐2(e1370) background (Figure 5e,g; Appendix S1: Figure S6D; Table S11; Table S12). This could reflect the fact that class 2 but not class 1 daf‐2 mutations cause DAF‐16F nuclear localization, whereas both classes cause DAF‐16A nuclear localization (Bansal et al., 2014).
Taken together, these results imply that daf‐16f expression in the pharynx protects against P in a tissue‐autonomous fashion. They also suggest that pharyngeal daf‐16 expression promotes p Age, since pharyngeal overexpression of daf‐16 extends lifespan to an extent comparable to that resulting from intestinal overexpression, as previously reported (Alic et al., 2014; Libina et al., 2003). However, there are two caveats with using multicopy transgene arrays to investigate gene function. First, their overexpression can lead to effects that do not reflect wild‐type gene action; this was suggested to be the case for the Pdaf‐16f transgene array lpIs14, which leads to a fourfold increase in mRNA compared with wild type (Bansal et al., 2014; Chen et al., 2015). Second, transgenes expressed from the cloned upstream promoter may not capture the full function of the isoform; this was shown for daf‐16a, which can be expressed from the daf‐16f promoter via alternative splicing (Chen et al., 2015).
To further investigate the role of daf‐16a and daf‐16f in IIS effects on P and p death while excluding these possible sources of artifact, we used the daf‐16a(tm5030) and daf‐16f(tm6659) isoform‐specific mutations which selectively abrogate expression of the isoform affected without changing mRNA levels of the other isoforms (Chen et al., 2015). While neither mutant increased P frequency in an otherwise wild‐type background (Appendix S1: Figure S6G), abrogation of either isoform restored wild‐type P frequency in daf‐2(1370) (Figure 5b; Appendix S1: Table S9). These results are consistent with pharyngeal expression of daf‐16 from the daf‐16f promoter protecting against P. Given that daf‐16a is expressed from the daf‐16f promoter, we postulate that at endogenous levels, Pdaf‐16f‐mediated expression in the pharynx of both daf‐16f and daf‐16a is required to reduce P. Here, the restoration of P by daf‐16f is particularly notable, since this mutation was previously found not to suppress daf‐2 Daf‐c, Age, or stress resistance (Chen et al., 2015). Thus, daf‐16f plays a more important role in preventing P than in other daf‐2 traits, including p Age.
daf‐16a(tm5030) but not daf‐16f(tm6659) partially suppressed class 1 daf‐2(m577) Age, consistent with previous observations (Chen et al., 2015), and the effect of daf‐16a(tm5030) was due to reduced p Age (Appendix S1: Figure S6B, C). Unexpectedly, daf‐16f(tm6659) modestly enhanced daf‐2(e1370) Age and markedly increased p Age (mean lifespan +28.7; p < 0.0001) (Figure 5a; Appendix S1: Table S9). A previous study using e1370 and the class 1 allele daf‐2(e1368) (which is similar to m577) saw no effect of daf‐16f(tm6659) on lifespan (Chen et al., 2015); the difference between our results could reflect the use in the prior study of the drug 5‐fluoro‐2′‐deoxyuridine (FUDR) to block progeny production. FUDR significantly extends daf‐2(e1370) lifespan at 20˚C (+19%, p < 0.0001) (Appendix S1: Figure S8) (Anderson et al., 2016). This could imply the presence of mildly life‐limiting pathologies caused by daf‐2(e1370) that are suppressed by both daf‐16f(tm6659) and FUDR.
2.6. Opposing effects on daf‐2 Age by daf‐12 are due to increased P and suppression of bagging
Combining two mutations affecting lifespan can produce complex effects that are difficult to interpret, as in the case of daf‐2 and the daf‐12 steroid hormone receptor (Larsen et al., 1995). One difference between the daf‐2 mutant classes is that daf‐12 partially suppresses Age in class 1 mutants, but can enhance it in class 2 mutants (Gems et al., 1998; Patel et al., 2008). How DAF‐12 signaling can have such opposite effects is a long‐standing puzzle. To understand the effects of daf‐12 on the two classes of daf‐2 mutants in terms of effects on P and p deaths, we performed mortality deconvolution on daf‐12(m20) in wild‐type and daf‐2 mutant backgrounds. Trials were performed at 25˚C and the class 1 allele daf‐2(m41) and class 2 allele daf‐2(e1370) were included, to be comparable to the original study in which effects on lifespan of allele class‐specific interactions between daf‐2 and daf‐12 were described (Larsen et al., 1995); we also included an additional class 1 allele, daf‐2(e1368). We recently reported that, when confounding effects of fln‐2 are excluded, daf‐12(m20) modestly reduces daf‐2(m41) lifespan by increasing P death frequency (Zhao et al., 2019). In the present study, necropsy analysis revealed that daf‐12(m20) increases P frequency in both wild type and class 1 but not class 2 daf‐2 mutant backgrounds (Figure 6a–c).
Figure 6.
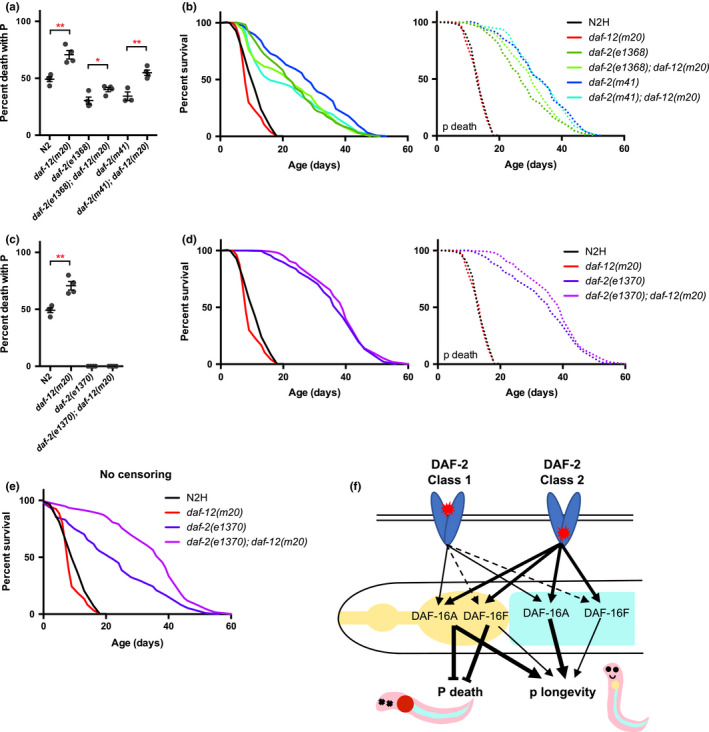
Effect of daf‐12(m20) mutation on daf‐2 Age at 25˚C, and model of the effects of daf‐2 mutations on C.elegans lifespan. (a) P frequency and (b) lifespan of wild type and two class 1 daf‐2 mutants without or with daf‐12(m20) at 25˚C, with internal hatching censored. daf‐12(m20) did not significantly affect p Age in class 1 daf‐2 mutant. N = 2 trials. (c) P frequency and (d, e) lifespan of wild type and class 2 daf‐2(e1370) mutant without or with daf‐12(m20) at 25˚C, with internal hatching censored (d) or not censored (e) after necropsy. daf‐12(m20) did not significantly affect lifespan in class 2 daf‐2 mutant. N = 2 trials. (f) Model for how class 1 and class 2 daf‐2 mutations affect C. elegans lifespan. Thickness of lines from DAF‐2 receptors represent severity of mutant effect. Red stars represent the lesion; in most cases, class 1 and class 2 mutations affect the extracellular and intracellular regions of the DAF‐2 receptor, respectively (Kimura et al., 1997; Patel et al., 2008). Class 1 and class 2 mutations exert differential effects on the two major isoforms of DAF‐16 in the pharynx and the intestine. Dashed lines indicate potential effect
An unexpected bonus of necropsy analysis using high magnification microscopy was better detection of internal hatching, specifically of dauer larvae which are difficult to observe within corpses since they are relatively immobile. Consistent with the previous observation of late progeny production by class 2 but not class 1 daf‐2 mutants (Gems et al., 1998), 18% of the corpses of daf‐2(e1370) mutants dying after day 10 were found to contain live dauer larvae (Appendix S1: Figure S9), but none of the daf‐2(e1368) or daf‐2(m41) mutants. By contrast, internally hatched dauer larvae were not seen in daf‐2(e1370);daf‐12 mutants, nor do they produce late progeny (Gems et al., 1998; Larsen et al., 1995; Vowels & Thomas, 1992).
Strikingly, when worms with internally hatched dauer larvae were censored, daf‐12(m20) no longer enhanced daf‐2(e1370) Age (Figure 6d,e; Appendix S1: Table S13). We conclude that daf‐12(m20) weakly suppresses daf‐2 Age by increasing P frequency, and that this effect is limited to class 1 daf‐2 mutants since P death is fully suppressed in class 2 mutants; and that daf‐12(m20) has little effect on daf‐2 p Age in either mutant class if corpses with internally hatched larvae are censored. This resolves the old puzzle of how daf‐12 can produce opposite effects on daf‐2 Age.
3. DISCUSSION
In this study, we have applied the new technique of mortality deconvolution to discover how effects of IIS on survival result from alteration of P and p death. The results provide new insights into earlier findings relating to the genetics of lifespan (Chen et al., 2015; D. Gems et al., 1998; Larsen et al., 1995; Nanji et al., 2005; Patel et al., 2008; Tawo et al., 2017). IIS pathway mutations strongly increase p lifespan, but in some cases also reduce P frequency in a daf‐2 allele‐ and temperature‐dependent manner. We present evidence that the reduction in P frequency results from expression from Pdaf‐16f in the pharynx of daf‐16f and probably also daf‐16a. Our findings identify the pharynx as a major tissue where DAF‐16 functions to regulate nematode lifespan. We also explain away a quarter‐century‐old enigma involving the effects on aging of allele‐specific interactions between daf‐2 and daf‐12. These new insights demonstrate the utility of mortality deconvolution as a technique for understanding the genetics of aging in C. elegans.
3.1. Distinct effects of IIS mutants on P and p
The large effects on lifespan of mutations in genes such as daf‐2 and age‐1 could imply action on core mechanisms of aging that affect all life‐limiting senescent pathology. Alternatively, IIS could act in different ways on different causes of death. Our findings presented here are consistent with the latter scenario, since IIS exerts differential effects on P and p death in a way that involves differential expression of daf‐16, both spatially and in terms of the daf‐16 isoforms involved (summarized in Figure 6f).
All daf‐2 and age‐1 mutants examined exhibited p Age, but marked reduction in P frequency was largely restricted to class 2 daf‐2 mutants. Like Eat and Unc (Gems et al., 1998), abrogation of P death is a temperature‐sensitive pleiotropic trait of class 2 daf‐2 mutants, such that P death is reduced at 15˚C and 20˚C, but absent at 25˚C (Figure 2). Given that mutation of eat‐2 reduces both pharyngeal pumping rate and P death (Zhao et al., 2017), it seems likely that cessation of pharyngeal pumping at 25˚C contributes to the temperature‐sensitive abrogation of P death in class 2 mutants. The fact that P frequency is also reduced at lower temperatures where pumping rate is normal (Figure 3a) implies the action of other protective mechanisms. Possibilities here are a more robust pharyngeal cuticle, consistent with elevated expression of extracellular matrix genes in daf‐2 mutants (Ewald et al., 2015), and enhancement of innate immunity, which is well documented in daf‐2 mutants (Evans et al., 2008; Garsin et al., 2003).
3.2. Distinct action of daf‐16 isoforms on P and p
Aging is a phenomenon of many diseases. It is therefore surprising that mutation of single genes such as daf‐2 is able so potently to delay the whole aging process. Our findings present an emerging picture in which daf‐2 acts via different daf‐16 isoforms in different tissues to suppress distinct pathologies, here the two forms of death, P and p. Overexpression of daf‐16f but not daf‐16a under their endogenous promoters reduced P frequency in daf‐16(0);daf‐2(e1370) worms (Figure 5d). Moreover, pharynx‐limited expression of either daf‐16a or daf‐16f reduced P frequency (Figure 5f,h). This implies that expression of DAF‐16 in the pharynx from Pdaf‐16f prevents P death. However, while DAF‐16F driven by its endogenous promoter is implicated in prevention of P death, it is likely that DAF‐16A also plays a role, since genetic abrogation of either daf‐16a or daf‐16f restored high P frequency in daf‐2(1370) mutants (Figure 5b). Consistent with this, daf‐16a has been shown to be also expressed from the daf‐2f promoter (Chen et al., 2015). Importantly, restoration of P in daf‐2(e1370) mutants by mutation of daf‐16f shows that the relative importance of daf‐16f compared with daf‐16A is greater for reducing P than for p Age, Daf‐c, and stress resistance (Chen et al., 2015) (Figure 5b, Figure 6f). This suggests that a major role of daf‐16f is protection against pharyngeal senescence.
The unexpected finding that pharynx‐limited daf‐16 rescue promotes daf‐2(e1370) p Age (Figure 5g) suggests that the worm pharynx plays a greater role in the regulation of senescence than previously realized. Previous studies established the importance for daf‐2 Age of DAF‐16 in the intestine, and to a smaller extent the neurons, though daf‐16 rescue at each site only partially restored Age in daf‐16;daf‐2 mutants (Libina et al., 2003). The role in aging of daf‐16 action in the pharynx, however, has not been investigated until now. Effects of pharyngeal DAF‐16 action on lifespan could operate in a tissue‐autonomous or nonautonomous fashion. The former would imply that pharyngeal pathology is life limiting. The latter scenario (which seems more plausible) would be consistent with established tissue‐nonautonomous effects of DAF‐16 on longevity and tumorigenesis, from the intestine and the hypodermis, respectively (Alic et al., 2014; Qi et al., 2012) (Figure 6f).
Phenotypic differences between daf‐2 alleles to some extent reflect the overall level of receptor impairment. However, daf‐2 mutants do not fall into a simple allelic series in terms of severity, and the existence of two classes of mutant suggests differential impairment of distinct elements of DAF‐2 receptor function (Gems et al., 1998). These elements appear to correspond to signaling through the PI3 kinase and Ras pathways (Nanji et al., 2005). Analysis of human insulin receptor mutants with lesions equivalent to those in daf‐2 alleles suggests that class 1 mutations may lead to reduced receptor protein levels, while class 2 alleles may cause a relatively greater decrease in Ras pathway signaling (Patel et al., 2008). Taken together with new findings presented here, this suggests that greater reduction in Ras signaling enhances pharyngeal resistance to infection in a manner dependent on pharyngeal action of DAF‐16. One possibility is that the increased DAF‐2 protein levels resulting from daf‐2(gk390525) (Tawo et al., 2017) cause a relatively greater increase in Ras signaling, thus accounting for the resulting increase in P death frequency.
3.3. daf‐2 daf‐12 interactions: explaining away an old conundrum
The daf‐16 dependence of daf‐2 Age (Kenyon et al., 1993) was an early illustration of the power of epistasis analysis applied to lifespan genetics. However, in many subsequent studies, combined effects of interventions affecting lifespan yielded results which, though intriguing, were hard to interpret (Gems et al., 2002). For example, daf‐12(m20) partially suppressed daf‐2(m41) Age, but enhanced daf‐2(e1370) Age, a somewhat paradoxical finding hinting at interesting and complex underlying molecular interactions between IIS and steroid signaling affecting aging (Gems et al., 1998; Larsen et al., 1995). However, application of mortality deconvolution analysis has yielded an explanation for daf‐2 daf‐12 interactions that is more mundane, if somewhat complex.
First, as described previously, mortality deconvolution analysis led to the discovery that the widely used N2 male stock (N2M) distributed by the Caenorhabditis Genetics Center, carries an X‐linked fln‐2(ot611) mutation that increases lifespan by reducing P frequency (Zhao et al., 2019). This mutation confounded previous comparisons of daf‐2;fln‐2+ vs daf‐2; +daf‐12 leading to a false impression that daf‐12(m20) can substantially suppress daf‐2 class 1 Age. However, in the absence of fln‐2, daf‐12 does still increase P frequency to a smaller extent, and by doing this weakly suppresses class 1 daf‐2 Age (Figure 6a,b) (Zhao et al., 2019). Suppression of class 1 daf‐2 Age by daf‐12 due solely to increased P frequency is consistent with neck‐and‐shoulder type survival curves seen previously (Larsen, 1993) (Figure 3b in that study).
Second, in the case of class 2 daf‐2 mutants, necropsy analysis revealed the presence of a proportion of adults with internally hatched larvae which had formed dauers. When these were censored (removed from survival curves), daf‐12(m20) did not enhance daf‐2 Age (Figure 6d,e). This suggests that the previously observed enhancement by daf‐12 of class 2 daf‐2 Age (Gems et al., 1998; Larsen et al., 1995; McCulloch & Gems, 2007) was due to prevention of death due to internal hatching in daf‐2(e1370) mutants. Death from internal hatching is caused by larvae devouring their mother from within; it is unclear how the presence of dauers, which are non‐feeding, increases maternal mortality. Possibilities are tissue damage from matriphagic activity by pre‐dauer stages, and secretions by mature dauers. It was previously reported that daf‐12(m20) strongly suppresses late progeny production and death by internal hatching in daf‐2(e1370) (Gems et al., 1998; Larsen et al., 1995). The mechanism by which daf‐12 prevents egg retention in daf‐2 mutants remains unknown.
Results presented here show that in the absence of internal hatching daf‐12(m20) has no impact on p Age in either class of daf‐2 mutant. For class 2 mutants, this is consistent with several previous reports in which daf‐12 did not enhance daf‐2(e1370) Age in contexts where internal hatching does not occur: in hermaphrodites treated with FUDR (Dumas et al., 2013), in axenic culture (Vanfleteren & Braeckman, 1999), and in males (McCulloch & Gems, 2007).
For class 1 mutants, published findings imply a more complex story. A lack of effect on p Age predicts that daf‐12 will not decrease maximum lifespan in class 1 daf‐2 mutants. That was observed for daf‐2(m41) in one study using conditions similar to those used here (25˚C, NGM plates) (Larsen, 1993). However, in another study using monoxenic liquid culture at 22.5˚C, daf‐2(m41) Age was fully suppressed by daf‐12(m20) (McCulloch & Gems, 2007), and in a third which used culture on NGM plates at 15˚C or 22.5˚C, maximum lifespan was reduced in most cases, though not that of daf‐2(m41) (Gems et al., 1998). This suggests that under different conditions to those used here, and in different class 1 alleles to those tested here, class 1 daf‐2 p Age can become partially or even fully daf‐12 dependent. Thus, some unresolved complexities remain.
A further consideration is that the canonical daf‐12(m20) allele used here is not nullimorphic. The daf‐12(rh61rh411) null allele shortens mean lifespan considerably more than m20, though it did not reduce maximum lifespan in several previous reports (Fisher & Lithgow, 2006; Gerisch et al., 2001). This lifespan shortening is partly due to a large increase in the frequency of P death (+59%, p = 0.0001), but also a significant reduction in p lifespan (−23%, p < 0.0001) (Appendix S1: Figure S10; Table S14).
These findings illustrate how the meaning of complex genetic phenomena, here, the opposite effects of daf‐12 on Age in the two classes of daf‐2 mutant, can sometimes be revealed by means of careful phenotypic analysis rather than molecular genetic investigation. More broadly, they illustrate the importance in understanding the genetic control of aging of discovering how the multiple pathologies that senescence is comprised of are determined.
4. EXPERIMENTAL PROCEDURES
4.1. Culture conditions and strains
Caenorhabditis elegans were maintained using standard protocols (Brenner, 1974). Unless otherwise stated, all strains were grown at 20˚C on nematode growth media (NGM) with plates seeded with E. coli OP50 to provide a food source. An N2 hermaphrodite stock recently obtained from the Caenorhabditis Genetics Center (CGC) was used as wild type (N2H). All strains were checked for the fln‐2 genotype. For those found to contain the fln‐2(ot611) background mutation derived from the CGC N2 male stock (Zhao et al., 2019), strains were outcrossed with N2H.
Caenorhabditis elegans strains used included the following. CB1375 daf‐18(e1375), DR20 daf‐12(m20), DR1296 daf‐2(e1370);daf‐12(m20), DR1547 daf‐2(m41);daf‐12(m20), FX5030 daf‐16(tm5030), FX6659 daf‐16(tm6659), GA91 daf‐16(mgDf50);daf‐2(m577), GA312 daf‐2(e1368);daf‐12(m20), GA1062 muEx211 [pNL213(ges‐1p::GFP::daf‐16)+rol‐6], GA1063 daf‐16(mu86) muEx211, GA1928 daf‐2(e1370), GA1945 daf‐2(m41), GA1946 wuEx304 [Pmyo‐2::daf‐16a+rol‐6(su1006)], GA1948 wuEx305 [Pmyo‐2::daf‐16f+rol‐6(su1006)], GA1952 daf‐16(mgDf50), GA1957 daf‐2(m120), GA1958 daf‐2(m596), GA1959 daf‐2(m577), GA1960 daf‐2(e1368), GA1990 daf‐2(gk390525), GR1168 age‐1(mg44)/mnC1 [dpy‐10(e128) unc‐52(e444)], HT1881 daf‐16(mgDf50);daf‐2(e1370) unc‐119(ed3);lpIs12 [daf‐16a::RFP+unc‐119(+)], HT1882 daf‐16(mgDf50);daf‐2(e1370) unc‐119(ed3);lpIs13 [daf‐16b::CFP+unc‐119(+)], HT1883 daf‐16(mgDf50);daf‐2(e1370) unc‐119(ed3);lpIs14 [daf‐16f::GFP+unc‐119(+)], HT1890 daf‐16(mgDf50);daf‐2(e1370), TJ1052 age‐1(hx546).
4.2. Construction of transgenic C. elegans strains
cDNA containing the coding regions of daf‐16a and daf‐16f were cloned into the L3790 vector (Addgene plasmid # 1596) containing the pharyngeal muscle‐expressed promoter Pmyo‐2. The resulting plasmids were injected using standard means at a concentration of 10 ng/μl into the gonad syncytium of wild‐type adult hermaphrodites, using a rol‐6(su1006) plasmid as a co‐injection marker to obtain roller lines with heritable extrachromosomal transgene arrays.
4.3. Lifespan and necropsy analysis (mortality deconvolution)
Unless otherwise specified, lifespan assays were carried out at 20˚C and without the use of 5‐fluoro‐2′‐deoxyuridine (FUDR). At the start of the assay, L4 animals were transferred to NGM plates seeded two days before with E. coli OP50, and transferred daily during the egg‐laying period or every 3–7 days thereafter. Worms were scored as dead when they fail to respond to gentle touch with a worm pick. The pharyngeal status of corpses (P or p) was scored using the highest magnification of a dissecting microscope or using Nomarski (differential interference contrast [DIC]) microscopy, as described (Zhao et al., 2017). Animals lost due to desiccation, matricidal hatching, or rupture were censored. All lifespan assays were conducted at least twice. Combined data from all trials were used to generate survival plots using the application GraphPad Prism (GraphPad Software, Inc.) unless otherwise specified, and statistical tests for significant difference in survival between populations were performed using the log‐rank test.
In order to estimate the proportion of total Age in daf‐2 mutants attributable to the three deconvolved determinants of overall lifespan (reduced P frequency, P Age, and p Age), we calculated the changes to wild‐type lifespan when each of the determinants changes to the level of the daf‐2 mutants.
For all lifespan experiments involving daf‐2 mutants, animals were raised at 15˚C and shifted to the test temperature at the L4 stage. For carbenicillin treatment, carbenicillin solution was added topically to plates with two‐day‐old E. coli lawns to a final concentration of 4 mM, and left to dry overnight.
Maternally rescued age‐1(mg44) homozygous segregants from GR1168 age‐1(mg44)/+ mothers were identified by singling L4 s and after 5 days testing for the presence of all dauer progeny (due to absence of maternal rescue) (Gottlieb & Ruvkun, 1994). mg44 homozygotes were picked onto the lifespan assay plate.
4.4. Microscopy
For measuring bacterial invasion of pharyngeal tissue, E. coli OP50‐RFP (Zhao et al., 2017) was grown overnight in LB broth supplemented with 25 μg/ml tetracycline, then pelleted and resuspended in OP50 media without tetracycline before seeding. Animals were transferred to NGM plates seeded with OP50‐RFP from L4 and transferred daily until imaging. Worms were then allowed to crawl on an unseeded plate for 5 minutes to remove surface fluorescent bacteria, before being mounted onto 2% agar pads with 0.1% levamisole. They were then viewed using a Zeiss Axioskop 2 plus microscope with a rhodamine filter to determine presence of RFP foci in pharyngeal tissue.
4.5. Pharyngeal pumping assays
Animals were examined on NGM plates without disturbance for 30 sec under a dissecting microscope, and the number of pharyngeal pumps scored using a manual click counter and a stopwatch.
4.6. Statistical analysis
Survival data were analyzed using GraphPad Prism. For whole populations (including both P and p deaths), animals lost due to causes other than aging were right censored. In the case of P and p subpopulations, deaths due to causes other than aging were excluded from the analysis rather than censored. The non‐parametric log‐rank test was used to detect statistically significant lifespan differences between cohorts. P frequencies between different cohorts were compared by one‐way ANOVA. Sample sizes and statistics for each lifespan are provided in the lifespan tables in the Supplementary Tables. Raw mortality data are provided in Ziehm tables (see Supplementary Data). Pumping rate between different cohorts were compared by two‐way ANOVA with multiple comparisons corrected with the Dunnett's test using GraphPad Prism.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
D. G. and Y. Z. conceived the study. F. A., E. R. G., I. M., H. W., B. Z., and Y. Z. performed the experiments. H. C. provided technical support. D. G. and Y. Z. analyzed and interpreted the data and wrote the manuscript.
Supporting information
Appendix S1
Data S1
ACKNOWLEDGEMENTS
We thank the Caenorhabditis Genetics Center, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440) and the National Bioresource Project C. elegans, for strains used in this study. We also thank T. Hoppe and P. Hu for strains. This work was supported by a Wellcome Trust Strategic Award (098565/Z/12/Z) and a Wellcome Trust Investigator Award (215574/Z/19/Z) to D. Gems.
DATA AVAILABILITY STATEMENT
All statistics relating to survival analysis are provided in the Supplementary tables. Raw mortality data are provided in Ziehm tables in Data S1.
REFERENCES
- Alic, N. , Tullet, J. M. , Niccoli, T. , Broughton, S. , Hoddinott, M. P. , Slack, C. , Gems, D. , & Partridge, L. (2014). Cell‐nonautonomous effects of dFOXO/DAF‐16 in aging. Cell Reports, 6, 608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, E. N. , Corkins, M. E. , Li, J.‐C. , Singh, K. , Parsons, S. , Tucey, T. M. , Sorkaç, A. , Huang, H. , Dimitriadi, M. , Sinclair, D. A. , & Hart, A. C. (2016). C. elegans lifespan extension by osmotic stress requires FUdR, base excision repair, FOXO, and sirtuins. Mechanisms of Ageing and Development, 154, 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antebi, A. , Yeh, W. H. , Tait, D. , Hedgecock, E. M. , & Riddle, D. L. (2000). daf‐12 encodes a nuclear receptor that regulates the dauer diapause and developmental age in C. elegans . Genes & Development, 14, 1512–1527. [PMC free article] [PubMed] [Google Scholar]
- Arantes‐Oliveira, N. , Berman, J. R. , & Kenyon, C. (2003). Healthy animals with extreme longevity. Science, 302, 611. [DOI] [PubMed] [Google Scholar]
- Bansal, A. , Kwon, E. , Conte, D. J. , Liu, H. , Gilchrist, M. , MacNeil, L. , & Tissenbaum, H. (2014). Transcriptional regulation of Caenorhabditis elegans FOXO/DAF‐16 modulates lifespan. Longevity & Healthspan, 3, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny, M. V. (2007). Paradoxes of aging. Cell Cycle, 6, 2997–3003. [DOI] [PubMed] [Google Scholar]
- Brenner, S. (1974). The genetics of Caenorhabditis elegans . Genetics, 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, A. T. , Guo, C. , Itani, O. A. , Budaitis, B. G. , Williams, T. W. , Hopkins, C. E. , Pande, M. , Grant, A. R. , Yoshina, S. , Mitani, S. , & Hu, P. J. (2015). Longevity genes revealed by integrative analysis of isoform‐specific daf‐16/FoxO mutants of Caenorhabditis elegans . Genetics, 201, 613–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman, J. B. , Albinder, B. , Shroyer, T. , & Kenyon, C. (1995). The age‐1 and daf‐2 genes function in a common pathway to control the lifespan of Caenorhabditis elegans . Genetics, 141, 1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas, K. J. , Guo, C. , Shih, H.‐J. , & Hu, P. J. (2013). Influence of steroid hormone signaling on life span control by Caenorhabditis elegans insulin‐like signaling. G3, 3, 841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, E. A. , Chen, W. C. , & Tan, M.‐W. (2008). The DAF‐2 insulin‐like signaling pathway independently regulates aging and immunity in C. elegans . Aging Cell, 7, 879–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald, C. Y. , Landis, J. N. , Porter Abate, J. , Murphy, C. T. , & Blackwell, T. K. (2015). Dauer‐independent insulin/IGF‐1‐signalling implicates collagen remodelling in longevity. Nature, 519, 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, A. L. , & Lithgow, G. J. (2006). The nuclear hormone receptor DAF‐12 has opposing effects on Caenorhabditis elegans lifespan and regulates genes repressed in multiple long‐lived worms. Aging Cell, 5, 127–138. [DOI] [PubMed] [Google Scholar]
- Friedman, D. B. , & Johnson, T. E. (1988). A mutation in the age‐1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics, 118, 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garsin, D. A. , Villanueva, J. M. , Begun, J. , Kim, D. H. , Sifri, C. D. , Calderwood, S. B. , Ruvkun, G. , & Ausubel, F. M. (2003). Long‐lived C. elegans daf‐2 mutants are resistant to bacterial pathogens. Science, 300, 1921. [DOI] [PubMed] [Google Scholar]
- Gems, D. (2015). The aging‐disease false dichotomy: understanding senescence as pathology. Frontiers in Genetics, 6, 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems, D. , Pletcher, S. , & Partridge, L. (2002). Interpreting interactions between treatments that slow ageing. Aging Cell, 1, 1–9. [DOI] [PubMed] [Google Scholar]
- Gems, D. , Sutton, A. J. , Sundermeyer, M. L. , Larsen, P. L. , Albert, P. S. , King, K. V. , & Riddle, D. L. (1998). Two pleiotropic classes of daf‐2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans . Genetics, 150, 129–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch, B. , Weitzel, C. , Kober‐Eisermann, C. , Rottiers, V. , & Antebi, A. (2001). A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, and life span. Developmental Cell, 1, 841–851. [DOI] [PubMed] [Google Scholar]
- Gottlieb, S. , & Ruvkun, G. (1994). daf‐2, daf‐16 and daf‐23: genetically interacting genes controlling dauer formation in Caenorhabditis elegans . Genetics, 137, 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon, C. (2010). The genetics of ageing. Nature, 464, 504–512. [DOI] [PubMed] [Google Scholar]
- Kenyon, C. , Chang, J. , Gensch, E. , Rudener, A. , & Tabtiang, R. (1993). A C. elegans mutant that lives twice as long as wild type. Nature, 366, 461–464. [DOI] [PubMed] [Google Scholar]
- Kimura, K. D. , Tissenbaum, H. A. , Liu, Y. , & Ruvkun, G. (1997). daf‐2, an insulin receptor‐like gene that regulates longevity and diapause in Caenorhabditis elegans . Science, 277, 942–946. [DOI] [PubMed] [Google Scholar]
- Kwon, E. S. , Narasimhan, S. D. , Yen, K. , & Tissenbaum, H. A. (2010). A new DAF‐16 isoform regulates longevity. Nature, 466, 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen, P. L. (1993). Aging and resistance to oxidative stress in Caenorhabditis elegans . Proceedings of the National Academy of Sciences of the United States of America, 90, 8905–8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen, P. L. , Albert, P. S. , & Riddle, D. L. (1995). Genes that regulate both development and longevity in Caenorhabditis elegans . Genetics, 139, 1567–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, R.Y.N. , Hench, J. , & Ruvkun, G. (2001). Regulation of C. elegans DAF‐16 and its human orthologue FKHRL1 by the daf‐2 insulin‐like signaling pathway. Current Biology, 11, 1950–1957. [DOI] [PubMed] [Google Scholar]
- Libina, N. , Berman, J.R. , & Kenyon, C. (2003). Tissue‐specific activities of C. elegans DAF‐16 in the regulation of lifespan. Cell, 115, 489–502. [DOI] [PubMed] [Google Scholar]
- Lin, K. , Hsin, H. , Libina, N. , & Kenyon, C. (2001). Regulation of the Caenorhabditis elegans longevity protein DAF‐16 by insulin/IGF‐1 and germline signaling. Nature Genetics, 28, 139–145. [DOI] [PubMed] [Google Scholar]
- McCulloch, D. , & Gems, D. (2007). Sex‐specific effects of the DAF‐12 steroid receptor on aging in Caenorhabditis elegans . Annals of the New York Academy of Sciences, 1119, 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, J. Z. , Tissenbaum, H. A. , & Ruvkun, G. (1996). A phosphatidylinositol‐3‐OH kinase family member regulating longevity and diapause in Caenorhabditis elegans . Nature, 382, 536–538. [DOI] [PubMed] [Google Scholar]
- Murphy, C. T. , & Hu, P. J. (2013). Insulin/insulin‐like growth factor signaling in C. elegans . In WormBook (pp. 1–43). [DOI] [PMC free article] [PubMed]
- Nanji, M. , Hopper, N. A. , & Gems, D. (2005). LET‐60 RAS modulates effects of insulin/IGF‐1 signaling on development and aging in Caenorhabditis elegans . Aging Cell, 4, 235–245. [DOI] [PubMed] [Google Scholar]
- Ogg, S. , & Ruvkun, G. (1998). The C. elegans PTEN homolog, DAF‐18, acts in the insulin receptor‐like metabolic signaling pathway. Molecular Cell, 2, 887–893. [DOI] [PubMed] [Google Scholar]
- Okkema, P. G. , & Fire, A. (1994). The Caenorhabditis elegans NK‐2 class homeoprotein CEH‐22 is involved in combinatorial activation of gene expression in pharyngeal muscle. Development, 120, 2175–2186. [DOI] [PubMed] [Google Scholar]
- Patel, D. S. , Garza‐Garcia, A. , Nanji, M. , McElwee, J. J. , Ackerman, D. , Driscoll, P. C. , & Gems, D. (2008). Clustering of genetically defined allele classes in the Caenorhabditis elegans DAF‐2 insulin/IGF‐1 receptor. Genetics, 178, 931–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podshivalova, K. , Kerr, R.A. , & Kenyon, C. (2017). How a mutation that slows aging can also disproportionately extend end‐of‐life decrepitude. Cell Reports, 19, 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, W. , Huang, X. , Neumann‐Haefelin, E. , Schulze, E. , & Baumeister, R. (2012). Cell‐nonautonomous signaling of FOXO/DAF‐16 to the stem cells of Caenorhabditis elegans . PLoS Genetics, 8(8), e1002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmer, F. , Tijsterman, M. , Parrish, S. , Koushika, S. P. , Nonet, M. L. , Fire, A. , Ahringer, J. , & Plasterk, R. H. A. (2002). Loss of the putative RNA‐directed RNA polymerase RRF‐3 makes C. elegans hypersensitive to RNAi. Current Biology, 12, 1317–1319. [DOI] [PubMed] [Google Scholar]
- Tawo, R. , Pokrzywa, W. , Kevei, É. , Akyuz, M. E. , Balaji, V. , Adrian, S. , Höhfeld, J. , & Hoppe, T. (2017). The ubiquitin ligase chip integrates proteostasis and aging by regulation of insulin receptor turnover. Cell, 169, 470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissenbaum, H. , & Ruvkun, G. (1998). An insulin‐like signaling pathway affects both longevity and reproduction in Caenorhabditis elegans . Genetics, 148, 703–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanfleteren, J. R. , & Braeckman, B. P. (1999). Mechanisms of life span determination in Caenorhabditis elegans . Neurobiology of Aging, 20, 487–502. [DOI] [PubMed] [Google Scholar]
- Vowels, J. J. , & Thomas, J. H. (1992). Genetic analysis of chemosensory control of dauer formation in Caenorhabditis elegans . Genetics, 130, 105–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Gilliat, A. F. , Ziehm, M. , Turmaine, M. , Wang, H. , Ezcurra, M. , & Gems, D. (2017). Two forms of death in aging Caenorhabditis elegans . Nature Communications, 8, 15458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Wang, H. , Poole, R. J. , & Gems, D. (2019). A fln‐2 mutation affects lethal pathology and lifespan in C. elegans . Nature Communications, 10, 5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data S1
Data Availability Statement
All statistics relating to survival analysis are provided in the Supplementary tables. Raw mortality data are provided in Ziehm tables in Data S1.


