Abstract
PURPOSE
We aimed to evaluate the safety and feasibility of the embolization of peripheral arteriovenous malformation (AVM) with non-adhesive liquid agents (NALA) injected by dual-lumen balloons (DLB).
METHODS
We conducted a multicenter retrospective study between January 2017 and June 2019, including patients with peripheral AVM embolized with NALA by DLB. Fourteen patients were included. The AVM classification, technical and clinical success were evaluated, as were nidus size, liquid agent used, volume and time of injection in DLB, complications, follow-up and need of surgical intervention.
RESULTS
The mean age of the patients was 37±22.5 years (range, 6–82 years). The mean nidus size was 5.2±2.4 cm (range, 3.0–12.0 cm). By Schobinger classification, 11 AVMs were classified in stage 3 and 3 AVMs were classified in stage 2. By Cho’s classification, 2 AVMs were in stage II, 4 AVMs were in stage I, 4 AVMs were in stage IIIa and 4 AVMs were in stage IIIb. Onyx was used in 11 patients (78.6%), while Squid, PHIL, and both Onyx and Squid were used in one patient each (7.1%). Seven patients (50%) required one session of embolization, 4 patients (28.6%) required two, 2 patients (14.3%) required three and 1 patient (7.1%) required four sessions. Complete nidus exclusion was achieved in 11 patients (78.6%), optimal clinical response in 12 patients (85.7%). Four patients (28.6%) exhibited minor complications, all controlled. No major complications were seen. Four patients underwent surgical intervention (28.6%).
CONCLUSION
The embolization of peripheral AVM with NALA in DLB appears to be safe and feasible, achieving high rates of technical and clinical success.
Mulliken and Glowacki classified vascular anomalies based on endothelial characteristics, dividing them in vascular tumors and vascular malformations in 1982 (1). The International Society for the Study of Vascular Anomalies (ISSVA) adopted this classification, providing agreement and standards in diagnosis and treatment. Arteriovenous malformations (AVMs) are high-flow vascular malformations characterized by abnormal shunt between pre-capillary arteries and post-capillary veins through a malformed vessel network called nidus (2–5). They are mostly congenital lesions (4) with variable clinical presentation, depending on the location and size. Typically, most peripheral AVMs present with local symptoms, such as skin color change, local pain and swelling, bruit, varicosities, ulceration, hemorrhagic and ischemic complications. In large AVMs several presentations may occur, such as compression of adjacent structures, high-output cardiac failure due to volume and pressure overload, and arterial steal phenomenon. This last presentation is secondary to blood flow deviation caused by the high-flow shunt, making the structures that usually would receive blood from the involved vessels of the AVM have less irrigation, leading to progressive dysfunction and atrophy of the target structure (2, 4, 6, 7).
The surgical management of peripheral AVMs is related with high morbidity and recurrence rates. Also, when AVMs recur, they are usually associated with a worsened condition, either by anatomy distortion due to previous surgical access, or by more severe clinical presentation (2, 7–9). Thus, endovascular approach has become the first-line treatment, with or without post-embolization surgical resection, aiming to exclude the nidus from the circulation, preventing collateral development and recurrence (7, 10–12). There are several reports in the literature with different solid, particulate, and liquid embolic agents for peripheral AVM treatment. Non-adhesive liquid agents (NALA) are well-established for the treatment of cerebral facial and vertebral AVMs (12–18) and have been used for peripheral AVMs with acceptable safety and improvement in clinical symptoms (4, 8, 19, 20). The challenge in NALA injection is the balance of volume injection in a rate that prevents excessive reflux of the material around the microcatheters. Usually, when reflux is present, a control injection is made after hardening of the proximal short plug to aid preferential anterograde flow of embolic agent into the malformation. Nevertheless, the proximal plug formation process can increase radiation exposure time and is associated with some risks, such as unwanted proximal vessel embolization, microcatheter entrapment and rupture of arterial feeders during microcatheter removal (17, 19, 21).
Controlled injection made with dual-lumen balloons (DLB) may aid NALA injections and prevent some disadvantages seen in widely used conventional embolization techniques (17, 19). We aim to present our initial experience in peripheral AVM embolization with NALA performed in DLB.
Methods
Patient selection
A multicenter retrospective review of clinical, demographic, and treatment characteristics of patients with peripheral AVM was performed in three Brazilian centers, between January 2017 and February 2019. We analyzed data from the hospitals’ systems database, which were prospectively maintained, and selected those patients with peripheral AVM treated with embolization with NALA injection by DLB, totaling 14 patients. Among those 14 lesions, 2 were uterine AVM, diagnosed right after delivery in a postpartum hemorrhage scenario and one was a lateral thoracic wall AVM diagnosed few months after local trauma. These 3 AVMs were considered acquired lesions and the other 11 AVMs were considered congenital lesions. An informed consent was obtained from each patient or their legal guardians before all procedures. The medical records and imaging studies were reviewed to evaluate the AVM: location, size, nidus morphology as described by Cho et al. (22) and Schobinger classification (23) (Tables 1 and 2), clinical symptoms, the procedure (total number of sessions and the number of sessions with DLB, volume of NALA injected, total time of injection in DLB and complications) and post-procedure follow-up (angiographic and clinical results and the need for surgery). All patients were diagnosed by computed tomography or magnetic resonance and submitted to digital subtraction angiography (DSA) before treatment. Angiographic results after treatment were defined as total or partial exclusion of the AVM nidus. Clinical results were defined as optimal, when there was regression of all symptoms referred by the patient with no permanent complications, and suboptimal, when there were partial or no regression of symptoms. In some patients, more than one session was necessary. The study was in accordance with the ethical standards of National and Institutional research committee (number 2.462.452).
Table 1.
Nidus morphology in peripheral arteriovenous malformations according to Cho et al. (22)
| Type | Description |
|---|---|
| I | No more than 3 separate arteries shunt to the initial part of a single venous component |
| II | Multiple arterioles shunt to the initial part of a single venous component, in which the arterial components show a plexiform appearance on angiography |
| IIIa | Fine multiple shunts are present between arterioles and venules and appear as a blush or fine striation on angiography |
| IIIb | Multiple shunts are present between arterioles and venules and appear as a complex vascular network on angiography |
Table 2.
Schobinger classification of arteriovenous malformations
| Stage | Features |
|---|---|
| 1 | Cutaneous blush (pink and/or blue)/warmth |
| 2 | Bruit/audible pulsation/expanding lesion |
| 3 | Pain/ulceration/bleeding/infection |
| 4 | Cardiac failure |
Inclusion criteria were: 1) presence of an AVM in peripheral territory (all those that do not have irrigation by arteries that feed the central nervous system, such as brain, cerebellum, brainstem and spinal cord); 2) first treatment performed by endovascular approach; 3) embolization using DLB, and when more than one session is required, the use of DLB in at least one session; 4) NALA as embolic agent used with DLB.
Exclusion criteria were: 1) the AVM involves any part of central nervous system vasculature; 2) surgical treatment preceding the endovascular treatment; 3) embolization not using DLB and/or NALA as embolic agent in any session; 4) follow-up time shorter than 6 months.
Embolization procedure
All procedures were performed under general anesthesia. A total of 8 interventional radiologists with at least 6 years of experience performed the interventions in the three centers. The access was made by right or left percutaneous femoral artery puncture, with a 6 F sheath and a 6 F guide catheter positioned in the parent artery. The DLB used to catheterize the feeding arteries of the AVM was a Scepter C 4×10 mm or 4×15 mm or a Scepter XC 4×11 mm (Microvention), which was prepared with a 50% solution of saline and Omnipaque 300 (GE Healthcare). In all embolization sessions, NALA was the only embolic agent used, as it is the first choice in our centers’ protocols. Mostly Onyx 18 (Medtronic) was used, with Squid 18 (Balt Extrusion) and PHIL (Microvention) as occasional alternatives. No other embolic agent, such as sclerosing, particulate or solid agents were used in any session of treatment. The choice of NALA that was used was dependent solely on operator preference. When Onyx or Squid were used, the vials were kept in a shaker for at least 20 min, to guarantee proper mixing of the tantalum powder. PHIL does not require any preparation and is pre-filled in syringes of 1 mL. The central lumen of DLB was slowly filled with 0.45 mL of dimethyl sufoxide (DMSO) prior to administration of NALA, then the balloon was inflated under road-map view until the balloon diameter matches artery size, to prevent overinflation and artery rupture. The administration of embolic agent started with NALA slowly substituting the DMSO inside the DLB within 60 seconds. The balloon was not deflated until the end of NALA injection, and then was withdrawn slowly under fluoroscopic control (Fig. 1).
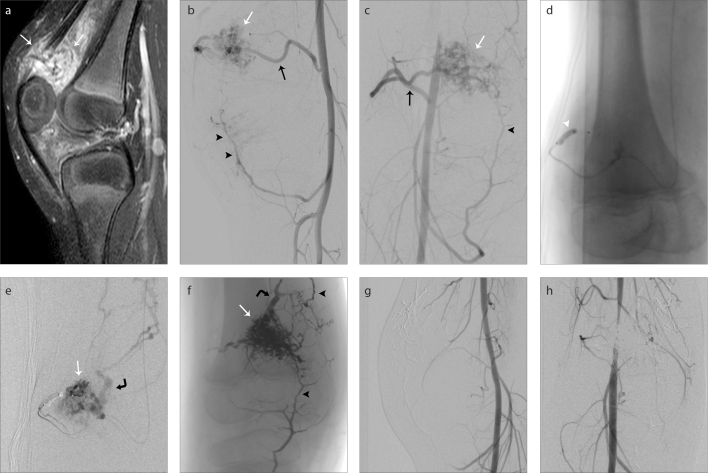
Figure 1. a–h.
A right knee intraarticular arteriovenous malformation (AVM) (a), with the nidus situated in suprapatellar recess (white arrows). Digital subtraction angiography (DSA) in lateral and anteroposterior views (b, c), respectively, show the main feeding artery (black arrow), the nidus (white arrow) and small collateral arteries reaching the nidus (black arrowheads). Image (d) shows dual-lumen balloon Scepter XC (white arrowhead) in the main feeding artery. DSA (e) performed from the dual-lumen balloon shows the AVM nidus (white arrow) and venous drainage (curved black arrow). Unsubtracted image (f) in anteroposterior view after the embolization, with the NALA cast filling the nidus (white arrow), the venous collector (curved black arrow) and the small collaterals arteries (black arrowheads). DSA performed in lateral (g) and anteroposterior (h) views after embolization show complete exclusion of the nidus from the circulation and no premature vein drainage.
No major complications, such as death, persistent organ disability or worsening of symptoms after treatment were seen. The continuous variables are presented as the mean ± standard deviation (SD), except for the follow-up time which is presented as median and range, and the categorical variables are presented as percentages.
Results
Fourteen patients (10 female and 4 male) were included in the study with a mean age of 37±22.5 years (range, 6–82 years) and a mean follow-up time of 12 months (range, 8–36 months). The AVM nidus were measured on DSA images, with a mean size of 5.2±2.4 cm (range, 3–12 cm). According to Cho et al. (22) classification, 7 patients were classified as type IIIa and 7 classified as type IIIb. When AVMs were classified according to Schobinger clinical grading system, 3 patients were in stage 2 (22.4%) and 11 in stage 3 (78.6%). A summary of demographic, clinical and AVM data is presented in Table 3.
Table 3.
Demographic, clinical and AVM data
| Patient no | Age (years)/Sex | Nidus size (cm) | Lesion location | Symptoms | Schobingera | Nidus morphologyb |
|---|---|---|---|---|---|---|
| 1 | 26/F | 12 | Uterine | Pain, metrorrhagia | 3 | IIIa |
| 2 | 68/F | 8.5 | Left gluteal | Swelling, pain, bleeding | 3 | IIIb |
| 3 | 42/F | 4.5 | Right foot | Swelling, pain, varicosity | 3 | IIIb |
| 4 | 23/M | 4.5 | Lateral thoracic wall | Swelling, pain | 3 | IIIb |
| 5 | 6/M | 3 | Right knee | Swelling, pain, knee movement limitation | 3 | IIIa |
| 6 | 36/F | 4 | Uterine | Pain, metrorrhagia | 3 | IIIa |
| 7 | 46/F | 3 | Inferior dental arch | Pain, bleeding, bone erosion, teeth loss | 3 | IIIa |
| 8 | 69/M | 4.5 | Right arm | Swelling, pain | 3 | IIIa |
| 9 | 25/F | 5.5 | Scalp – right parietal | Swelling, pain, bleeding, pulsatile mass, varicosity, bruit | 3 | IIIb |
| 10 | 11/F | 4 | Inferior dental arch | Pain, bleeding | 3 | IIIb |
| 11 | 23/F | 6 | Superior dental arch | Pain, bleeding | 3 | IIIa |
| 12 | 36/F | 5 | Submandibular | Swelling, pulsatile mass | 2 | IIIa |
| 13 | 82/M | 4.3 | Scalp – right parietal | Swelling, pulsatile mass, varicosity | 2 | IIIb |
| 14 | 23/F | 3.6 | Scalp – left frontal | Swelling, pulsatile mass, varicosity | 2 | IIIb |
A total of 25 procedures were performed in 14 patients. One embolization session was required in 7 patients (50%), two sessions in 4 patients (28.6%), three sessions in 2 patients (14.3%), and four sessions in 1 patient (7.1%). In 3 of the 4 patients submitted to two sessions of embolization, DLB was used in one session each and in one patient submitted to three sessions of embolization, DLB was used in one session. In all patients, the balloon was placed in the desired location, with no complication in the navigation or vascular lesions during balloon inflation.
Onyx was the NALA used in the majority of procedures (n=11, 85.7%). Squid was used in 2 patients (14.3%) and PHIL was used in 1 (7.1%). In one patient, both Onyx and Squid were used in different sessions. The mean total volume of NALA injected by the DLB was 8.5±8 mL (range, 1–21.5 mL), with a mean injection time of 41±38 min (range, 3–146 min). There were two patients in our series with a large nidus and dilatated vessels that required high volume of NALA: 31 and 21.5 mL of Onyx, with 107 and 146 minutes of injection, respectively. The mean volume of NALA and mean injection time of the other 12 patients were 5.6±2.8 mL (range, 1–9 mL) and 26±11 min (range, 3–44 min). The most illustrative procedure in terms of injection time was in a patient with a big uterine AVM submitted to only one session of embolization: we used a traditional detachable-tip microcatheter Apollo (Medtronic) in the right uterine artery and a DLB Scepter XC in the left uterine artery. In this specific case, 4.5 mL of Onyx was administered from each access with total time injection of 59 minutes from Apollo microcatheter and 24 minutes from Scepter XC (Fig. 2). There were no complications related to DLB retrieval after embolization and balloon deflation and there was no reflux of the NALA proximal to the balloon.
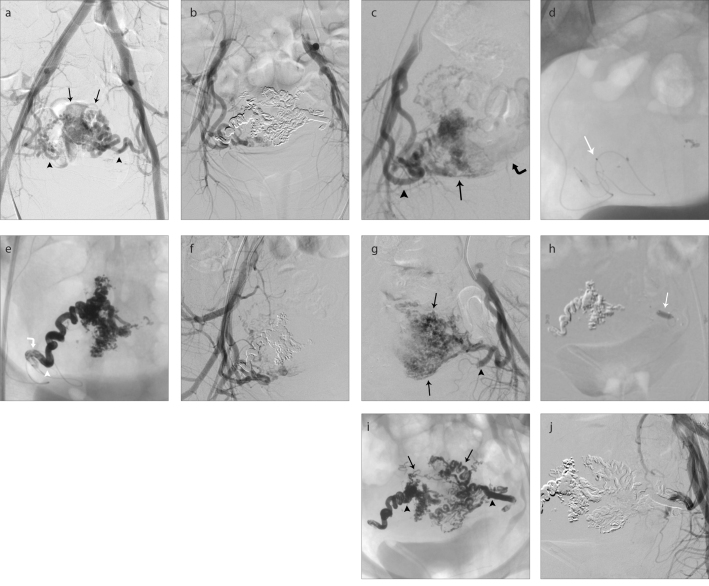
Figure 2. a–j.
A large uterine AVM causing massive post-partum bleeding. DSA (a) performed before embolization shows a large AVM nidus (black arrows) nourished by both uterine arteries (black arrowheads). DSA (b) performed after embolization shows complete exclusion of the nidus from the circulation. DSA (c) from right internal iliac artery shows tortuous and dilatated right uterine artery (black arrowhead) to the nidus (black arrow). There is a large venous collector (curved black arrow). Unsubtracted image (d) shows the detachable-tip microcatheter in the right uterine artery (white arrow). Unsubtracted image (e) after injection of 4.5 mL of NALA in the right uterine artery. The white arrowhead points to the location of the microcatheter tip to show the proximal plug formed around the microcatheter (curved white arrow). The injection of NALA by the microcatheter lasted 59 minutes. Image (f) shows DSA from right internal iliac artery with no more contribution of the right uterine artery to the AVM. DSA (g) from left internal iliac artery shows tortuous and dilatated left uterine artery (black arrowhead) to the nidus (black arrows). Image (h) shows DLB Scepter XC distally positioned in the left uterine artery (white arrow). Unsubtracted image (i) after injection of 4.5 mL of NALA in left uterine artery. There is the final NALA cast within the uterine arteries (black arrowheads) and AVM nidus (black arrows). The injection of NALA by the DLB lasted 24 minutes. DSA (j) from left internal iliac artery shows no more contribution of the left uterine artery to the AVM.
After endovascular treatment, 11 patients (78.6%) presented with complete exclusion of the AVM in DSA, with optimal clinical response. In 3 patients, partial occlusion of AVM was reached, with one patient having optimal clinical response (85.7% optimal clinical response in total) and the other two having suboptimal response (partial regression of local pain and swelling). Surgery was performed in one of the patients with suboptimal clinical response.
Four patients (28.6%) exhibited minor complications. One presented with a small focus of active bleeding in an inferior dental arch AVM during the embolization, which was immediately controlled with the balloon inflated in the external carotid and direct puncture with n-butyl-cyanoacrylate (n-BCA) administration. This patient had total AVM exclusion and optimal clinical response after treatment. The three patients with scalp AVM in our series showed up with local scalp necrosis that was not associated with pain, infection or bleeding, a few days after complete embolization of the AVM nidus. Those patients were also in follow-up with the plastic surgery team and were submitted to reconstructive surgery, all with optimal results. The procedural data and results are listed in Table 4.
Table 4.
Procedure data, results and follow-up
| Patient no | Total sessions/Sessions with DLB | EA | Volume (mL) | Time of injection (min) | Surgery | Complications | Angiographic results | Clinical results | FU (months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2/1 | Onyx | 4.5 | 24 | No | No | E | Optimal | 12 |
| 2 | 2/1 | Onyx | 6 | 32 | No | No | E | Optimal | 12 |
| 3 | 4/4 | Onyx/Squid | 7.5 | 29 | No | No | PE | Suboptimal | 24 |
| 4 | 1/1 | PHIL | 2.7 | 28 | No | No | E | Optimal | 12 |
| 5 | 1/1 | Onyx | 9 | 44 | No | No | E | Optimal | 12 |
| 6 | 1/1 | Onyx | 4.5 | 26 | No | No | E | Optimal | 8 |
| 7 | 3/1 | Onyx | 1 | 3 | No | No | E | Optimal | 18 |
| 8 | 1/1 | Onyx | 2 | 9 | No | No | E | Optimal | 12 |
| 9 | 3/3 | Onyx | 31 | 107 | Yes | No | E | Optimal | 18 |
| 10 | 2/2 | Onyx | 21.5 | 146 | No | Scalp necrosis | E | Optimal | 24 |
| 11 | 2/1 | Onyx | 9 | 43 | Yes | Bleeding | PE | Suboptimal | 36 |
| 12 | 1/1 | Onyx | 4.5 | 27 | No | No | PE | Optimal | 12 |
| 13 | 1/1 | Onyx | 9 | 32 | Yes | Scalp necrosis | E | Optimal | 12 |
| 14 | 1/1 | Squid | 7.5 | 24 | Yes | Scalp necrosis | E | Optimal | 12 |
EA, embolic agent; E, exclusion; PE, partial exclusion; PHIL, precipitating hydrophobic injectable liquid; FU, follow-up.
Discussion
AVMs are lesions with challenging diagnostic and treatment. The clinical presentation varies depending on location, size and morphology of the lesion, presenting from asymptomatic to extensive deformities and high-output cardiac failure or arterial steal phenomenon (2, 5–7). The Schobinger clinical staging not only measures the severity of presentation but also predicts the success of treatment (23, 24). The nidus morphology classification proposed by Cho et al. (22) predicts the difficulty of treatment and overall clinical response after treatment (9). Moreover, the indication for treatment has been extended to include low-grade Schobinger AVM, even for asymptomatic (grade 1) or oligosymptomatic (grade 2) cases, since it was described that these may progress over time into higher stages, making the resolution more difficult (6, 23, 24).
The endovascular treatment is the first-line approach in peripheral AVM, which is sometimes followed up with open surgery to reduce compression symptoms, assist in minor complications of embolization, such as skin necrosis, and also in aesthetic issues (7, 10–12, 18, 25–28). Surgical treatment alone is considered more harmful, associated to more complications, with a high morbidity and recurrence rates, often with a worsened condition (2, 7–9).
Several embolic agents are used in endovascular approach of AVM: particulate and sclerosing agents, adhesive agents, and NALA. The first group, such as ethanol, polidocanol and ethanolamine oleate are associated with lower therapeutic responses and higher recurrence rates compared with the liquid embolic agents (2, 5, 9, 29). N-BCA is used in AVM treatment with some disadvantages: the limited time of injection due to the speed of the agent polymerization, the need to catheterize multiple arterial feeders, and limited nidus filling. Moreover, the reflux of the adhesive agent around microcatheter tip may result in glued catheter and raise the risk of vessel injury during withdrawal maneuvers. The best curative results reported with intranidal embolization using n-BCA is 30% in pure plexiform brain AVM (12, 30).
NALA has been established as the first-line embolic agent for AVM in the central nervous system with higher rates of complete nidus embolization (12, 14, 17, 31–34) and its use has been extended for peripheral AVM with acceptable safety and effectiveness (4, 8, 19, 20). The low viscosity and prolonged solidification time allow longer injections and better penetration into the malformation, thus fewer branches need to be catheterized to reach nidus exclusion, promoting higher curative rates (12, 14, 17, 31–34). However, it is often necessary to form a plug along the microcatheter tip in order to establish a forward flow into the nidus (17, 32). The adequate plug formation not only requires increased total fluoroscopic time, but also, an excessive reflux can lead to embolization of unwanted territory and en-passage arteries, resulting in ischemia of normal tissue. Depending on the amount of NALA refluxed, it may cause the same effect of a glued microcatheter and the traction maneuvers in this scenario are related with increased risk of vascular injuries (12, 17, 19, 34, 35).
Numerous techniques were developed and described in an attempt of aiding the forward flow without excessive reflux: the “pressure cooker” technique and “modified pressure cooker” techniques (27, 36), balloon-assisted microcatheter navigation (28), double catheterization of the nidus (12), and the security catheter technique (37). The use of NALA in DLB has been described in central nervous system AVM (17, 38) and in peripheral AVM and arteriovenous fistulas (13, 19). In our experience, we were able to perform forward embolization into the AVM nidus without proximal reflux to the distal end of the balloon, which functions as a proximal plug (17, 38–40). In this way, it is possible not only to perform a more controlled and safe embolization of NALA in the vessels of interest, reducing the total fluoroscopy time, but also to reduce the risk of unwanted territory and en-passage arteries embolization (17, 38). Moreover, it is suggested that DLB leads to proximal blood flow arrest when inflated, reducing the probability of inadvertent embolization of draining veins (41), and enabling proximal artery control in cases of bleeding as exemplified by one of our minor complications.
The use of DLB with NALA, in our series, appeared to fulfill the advantages described in the literature. We obtained high rates of nidus exclusion (78.6%) and optimal clinical response (85.7%) with no major complications and an acceptable incidence of minor complications, most of them already expected and resolved. Despite lack of a direct comparison of embolization time between DLB and other devices, our results depicted lower mean injection time with DLB than we usually see when using traditional DMSO-compatible microcatheters. We exemplified this finding in one large uterine AVM submitted to only one session of embolization with double catheterization by an Apollo microcatheter in right uterine artery and a Scepter XC in left uterine artery and a significant difference in the embolization time, 59 minutes for the former and 24 minutes for the latter, even though the same volume of NALA was used in both. It is important to highlight the difficulty of directly comparing the embolization time with different materials: the AVMs are complex lesions, each with distinct anatomic characteristics, and differences in operator’s experience is another confounder.
The retrospective nature and the relatively small number of patients are the main limitations of our study. Further investigations with larger number of patients are necessary to determine the final role of DLB with NALA in peripheral AVM embolization.
In conclusion, our multicenter experience showed that the embolization of peripheral AVMs with DLB microcatheter devices and NALA is feasible and safe, with high rates of angiographic and clinical optimal results and low rates of complications. The technique may assist in the reduction of procedure time and total radiation dose. Further prospective investigations and studies with larger groups are necessary to provide better characterization of safety and reduction of total procedure time.
Main points.
DLB functions as a proximal plug and aids the forward flow of NALA into the AVM nidus, leading to a more controlled embolization, with high rates of optimal angiographic and clinical results and low rates of complications.
With less reflux of NALA, there is a decreased chance of reflux complications, such as catheter entrapment and embolization of unwanted territory and en-passage arteries.
The use of DLB seems to reduce total embolization time, resulting in less radiation exposure and shorter anesthetic time.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Mulliken J, Glowacki J. Hemangiomas and vascular malformations in infants and children - a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69:412–422. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Kitagawa A, Yamamoto T, Matsunaga N, Yamaji M, Ikeda S, Izumi Y. Polidocanol sclerotherapy combined with transarterial embolization using n-butyl cyanoacrylate for extracranial arteriovenous malformations. Cardiovasc Intervent Radiol. 2018;41:856–866. doi: 10.1007/s00270-017-1855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merrow AC, Gupta A, Patel MN, Adams DM. 2014 Revised Classification of Vascular Lesions from the International Society for the Study of Vascular Anomalies: Radiologic-Pathologic Update. Radiographics. 2016;36:1494–1516. doi: 10.1148/rg.2016150197. [DOI] [PubMed] [Google Scholar]
- 4.Kilani MS, Lepennec V, Petit P, et al. Embolization of peripheral high-flow arteriovenous malformations with Onyx. Diagn Interv Imaging. 2017;98:217–226. doi: 10.1016/j.diii.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Monsignore LM, Nakiri GS, Santos D, Abud G, Abud DG. Imaging findings and therapeutic alternatives for peripheral vascular malformations. Radiol Bras. 2010;43:185–194. doi: 10.1590/S0100-39842010000300011. [DOI] [Google Scholar]
- 6.Lam K, Pillai A, Reddick M. Peripheral arteriovenous malformations: Classification and endovascular treatment. Appl Radiol. 2017;46:15–21. [Google Scholar]
- 7.Lee B, Do YS, Yakes W, Kim DI, Mattassi R, Hyon WS. Management of arteriovenous malformations: A multidisciplinary approach. J Vasc Surg. 2004;39:590–600. doi: 10.1016/j.jvs.2003.10.048. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan KG, Vidyadharan R, Patel N, et al. Embolisation of high flow extracranial/peripheral arteriovenous malformations (AVMs) with ethylene vinyl alcohol copolymer (ONYX) in children — Birmingham Children’s Hospital experience. Eur J Plast Surg. 2014;37:129–134. doi: 10.1007/s00238-013-0900-x. [DOI] [Google Scholar]
- 9.Park KB, Do YS, Kim D, et al. Predictive factors for response of peripheral arteriovenous malformations to embolization therapy: analysis of clinical data and imaging findings. J Vasc Interv Radiol. 2012;23:1478–1486. doi: 10.1016/j.jvir.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Osuga K, Hori S, Kitayoshi H, et al. Embolization of high flow arteriovenous malformations: experience with use of superabsorbent polymer microspheres. J Vasc Interv Radiol. 2002;13:1125–1133. doi: 10.1016/S1051-0443(07)61954-X. [DOI] [PubMed] [Google Scholar]
- 11.Mulligan PR, Prajapati HJ, Martin LG, Patel TH. Vascular anomalies: classification, imaging characteristics and implications for interventional radiology treatment approaches. Br J Radiol. 2014;87:1–18. doi: 10.1259/bjr.20130392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abud DG, Riva R, Nakiri GS, Padovani F, Khawaldeh M, Mounayer C. Treatment of brain arteriovenous malformations by double arterial catheterization with simultaneous injection of onyx: retrospective series of 17 patients. Am J Neuroradiol. 2011;32:152–158. doi: 10.3174/ajnr.A2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paramasivam S, Niimi Y, Fifi J, Berenstein A. Onyx embolization using dual-lumen balloon catheter: Initial experience and technical note. J Neuroradiol. 2013;40:294–302. doi: 10.1016/j.neurad.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Pierot L, Cognard C, Herbreteau D, et al. Endovascular treatment of brain arteriovenous malformations using a liquid embolic agent: results of a prospective, multicentre study (BRAVO) Eur Radiol. 2013;23:2838–2845. doi: 10.1007/s00330-013-2870-6. [DOI] [PubMed] [Google Scholar]
- 15.Potts MB, Zumofen DW, Raz E, Nelson PK, Riina HA. Curing arteriovenous malformations using embolization. Neurosurg Focus. 2014;37:E19. doi: 10.3171/2014.6.FOCUS14228. [DOI] [PubMed] [Google Scholar]
- 16.Iosif C, de Lucena AF, Abreu-Mattos LG, et al. Curative endovascular treatment for low-grade Spetzler-Martin brain arteriovenous malformations: a single-center prospective study. J Neurointerv Surg. 2019;11:699–705. doi: 10.1136/neurintsurg-2018-014390. [DOI] [PubMed] [Google Scholar]
- 17.Jagadeesan BD, Grigoryan M, Hassan AE, Grande AW, Tummala RP. Endovascular balloon-assisted embolization of intracranial and cervical arteriovenous malformations using dual-lumen coaxial balloon microcatheters and onyx: initial experience. Neurosurgery. 2013;73:238–243. doi: 10.1227/NEU.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 18.Mounayer C, Hammami N, Piotin M, et al. Nidal embolization of brain arteriovenous malformations using onyx in 94 patients. Am J Neuroradiol. 2007;28:518–523. [PMC free article] [PubMed] [Google Scholar]
- 19.Jagadeesan BD, Mortazavi S, Hunter DW, et al. Endovascular balloon-assisted embolization of high-flow peripheral vascular lesions using dual-lumen coaxial balloon microcatheter and onyx: initial experience. J Vasc Interv Radiol. 2014;25:587–592. doi: 10.1016/j.jvir.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Numan F, Ömeroglu A, Kara B, Cantasdemir M, Adaletli I, Kantarci F. Embolization of peripheral vascular malformations with ethylene vinyl alcohol copolymer (Onyx) J Vasc Interv Radiol. 2004;15:939–946. doi: 10.1097/01.RVI.0000130862.23109.52. [DOI] [PubMed] [Google Scholar]
- 21.Castaneda F, Goodwin SC, Swischuk JL, et al. Treatment of pelvic arteriovenous malformations with ethylene vinyl alcohol copolymer (Onyx) J Vasc Interv Radiol. 2002;13:513–516. doi: 10.1016/S1051-0443(07)61532-2. [DOI] [PubMed] [Google Scholar]
- 22.Cho SK, Do YS, Shin SW, et al. Arteriovenous malformations of the body and extremities: analysis of therapeutic outcomes and approaches according to a modified angiographic classification. J Endovasc Ther. 2006;13:527–538. doi: 10.1583/05-1769.1. [DOI] [PubMed] [Google Scholar]
- 23.Kohout MP, Hansen M, Pribaz JJ, Mulliken JB. Arteriovenous malformations of the head and neck: natural history and management. Plast Reconstr Surg. 1998;102:643–654. doi: 10.1097/00006534-199809010-00006. [DOI] [PubMed] [Google Scholar]
- 24.Liu AS, Mulliken JB, Zurakowski D, Fishman SJ, Greene AK. Extracranial arteriovenous malformations: natural progression and recurrence after treatment. Plast Reconstr Surg. 2010;125:1185–1194. doi: 10.1097/PRS.0b013e3181d18070. [DOI] [PubMed] [Google Scholar]
- 25.Gross BA, Du R. Natural history of cerebral arteriovenous malformations: a meta-analysis. J Neurosurg. 2013;118:437–443. doi: 10.3171/2012.10.JNS121280. [DOI] [PubMed] [Google Scholar]
- 26.Saatci I, Geyik S, Yavuz K, Cekirge HS. Endovascular treatment of brain arteriovenous malformations with prolonged intranidal Onyx injection technique: long-term results in 350 consecutive patients with completed endovascular treatment course. J Neurosurg. 2011;115:78–88. doi: 10.3171/2011.2.JNS09830. [DOI] [PubMed] [Google Scholar]
- 27.Chapot R, Stracke P, Velasco A, et al. The pressure cooker technique for the treatment of brain AVMs. J Neuroradiol. 2014;41:87–91. doi: 10.1016/j.neurad.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Iosif C, Mendes GA, Saleme S, et al. Endovascular transvenous cure for ruptured brain arteriovenous malformations in complex cases with high Spetzler-Martin grades. J Neurosurg. 2015;122:1229–1238. doi: 10.3171/2014.9.JNS141714. [DOI] [PubMed] [Google Scholar]
- 29.Kaji N, Kurita M, Ozaki M, et al. Experience of sclerotherapy and embolosclerotherapy using ethanolamine oleate for vascular malformations of the head and neck. Scand J Plast Reconstr Surg Hand Surg. 2009;43:126–136. doi: 10.1080/02844310902840296. [DOI] [PubMed] [Google Scholar]
- 30.Valavanis A, Yasargil MG. The endovascular treatment of brain arteriovenous malformations. Adv Tech Stand Neurosurg. 1998;24:131–214. doi: 10.1007/978-3-7091-6504-1_4. [DOI] [PubMed] [Google Scholar]
- 31.van Rooji WJ, Sluzewski M, Beute GN. Brain AVM embolization with Onyx. Am J Neuroradiol. 2007;28:172–177. [PMC free article] [PubMed] [Google Scholar]
- 32.Weber W, Kis B, Siekmann R, Kuehne D. Endovascular treatment of intracranial arteriovenous malformations with onyx. Am J Neuroradiol. 2007;28:371–377. [PMC free article] [PubMed] [Google Scholar]
- 33.Warakaulle DR, Aviv RI, Niemann D, Molyneux AJ, Byrne JV, Teddy P. Embolisation of spinal dural arteriovenous fistulae with Onyx. Neuroradiology. 2003;45:110–112. doi: 10.1007/s00234-002-0936-2. [DOI] [PubMed] [Google Scholar]
- 34.Jahan R, Murayama Y, Gobin YP, Duckwiler GR, Vinters HV, Viñuela F. Embolization of arteriovenous malformations with onyx: clinicopathological experience in 23 patients. Neurosurgery. 2001;48:984–995. doi: 10.1227/00006123-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Arat A, Cil BE, Vargel I, et al. Embolization of high-flow craniofacial vascular malformations with onyx. Am J Neuroradiol. 2007;28:1409–1414. doi: 10.3174/ajnr.A0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abud DG, de Castro-Afonso LH, Nakiri GS, Monsignore LM, Colli BO. Modified pressure cooker technique: An easier way to control onyx reflux. J Neuroradiol. 2016;43:218–222. doi: 10.1016/j.neurad.2016.01.146. [DOI] [PubMed] [Google Scholar]
- 37.Abud DG, Abud TG, Nakiri GS. Management of brain AVM procedural hemorrhagic complication by the “security” catheter technique. J Neuroradiol. 2013;40:45–49. doi: 10.1016/j.neurad.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Spiotta AM, James RF, Lowe SR, et al. Balloon-augmented Onyx embolization of cerebral arteriovenous malformations using a dual-lumen balloon: a multicenter experience. J Neurointerv Surg. 2015;7:721–727. doi: 10.1136/neurintsurg-2014-011285. [DOI] [PubMed] [Google Scholar]
- 39.Spiotta AM, Miranpuri AS, Vargas J, et al. Balloon augmented Onyx embolization utilizing a dual lumen balloon catheter: utility in the treatment of a variety of head and neck lesions. J Neurointerv Surg. 2014;6:547–555. doi: 10.1136/neurintsurg-2013-010833. [DOI] [PubMed] [Google Scholar]
- 40.Spiotta AM, Hughes G, Masaryk TJ, Hui FK. Balloon-augmented Onyx embolization of a dural arteriovenous fistula arising from the neuromeningeal trunk of the ascending pharyngeal artery: technical report. J Neurointerv Surg. 2011;3:300–303. doi: 10.1136/jnis.2010.003095. [DOI] [PubMed] [Google Scholar]
- 41.Crowley RW, Ducruet AF, Mcdougall CG, Albuquerque FC. Endovascular advances for brain arteriovenous malformations. Neurosurgery. 2014;74:74–82. doi: 10.1227/NEU.0000000000000176. [DOI] [PubMed] [Google Scholar]