Abstract
PURPOSE
We aimed to assess the severity of coronavirus disease 2019 (COVID-19) pneumonia on computed tomography (CT) using quantitative (QCT) and semiquantitative (SCT) assessments and compare with the clinical findings.
METHODS
Two observers independently examined the CT images of COVID-19 patients, and the SCT severity score was calculated. The SCT score was calculated as the sum of values ranging from 0 to 4, according to the volumetric rate of involvement for each lung lobe. In quantitative assessment, total lung volume (TLV) was automatically calculated from CT density values between −200 and −950 HU. Besides, healthy lung volume (HLV) was calculated from voxels between −800 and −950 HU. The QCT score was calculated with the following formula: (TLV – HLV / TLV) ×100. All patients were clinically divided into four groups: mild, common, severe, and critical. Interobserver agreement for SCT assessment was investigated using the Cohen's Kappa statistics (κ). Pearson's correlation coefficient was used for the relationship between continuous data. The diagnostic accuracy of SCT and QCT in the differentiation of clinically limited (mild, common) and extensive (severe, critical) disease was investigated using ROC analysis.
RESULTS
Seventy-six patients with a diagnosis of COVID-19 were included. There was good agreement between the two observers in the SCT evaluation of pulmonary disease severity (κ = 0.796; 95% CI, 0.751–0.841). A significant correlation was found between QCT and SCT scores (p < 0.001, r = 0.661). Both QCT and SCT scores showed a significant correlation with clinical severity score (p < 0.001, r = 0.620 and p = 0.004, r = 0.529, respectively). The ROC analysis revealed the AUC of QCT and SCT for differentiation of limited and extensive disease as 0.873 (95% CI, 0.774–0.972) and 0.816 (95% CI, 0.673–0.959), respectively.
CONCLUSION
The QCT assessment is an objective method in the evaluation of COVID-19 severity and is more successful than semiquantitative CT assessment to discriminate extensive from limited disease.
In December 2019, a new bat-origin coronavirus (SARS-CoV-2) capable of infecting humans has been identified, and the infection with SARS-CoV-2 has been named as coronavirus disease 2019 (COVID-19) (1). As of May 2, 2020, there were 3 175 207 confirmed cases with SARS-CoV-2 infection and 224 172 COVID-19 related deaths worldwide (2). COVID-19 is a rapidly spreading viral disease. Therefore, professional consensus, guidelines, and criteria have been published continuously to facilitate the diagnosis and management of patients (3–5). Although swab test and reverse transcription-polymerase chain reaction (RT-PCR) of the sample is the gold standard for diagnosis, chest computed tomography (CT) has an essential role in the follow-up, evaluation of disease severity, and complications of COVID-19 pneumonia (6–10). It has been shown that visual (semiquantitative) evaluation of disease severity on chest CT can reflect the clinical classification (mild, common, severe, or critical disease) and prognosis of patients with COVID-19 (8, 11). However, these semiquantitative CT (SCT) assessment methods are subjective, may take a few minutes, and may depend on the observer’s experience. Therefore, objective and rapid methods are needed. The use of quantitative CT (QCT) methods has been shown to be very successful in the detection, staging, and management of diffuse lung diseases (12, 13). However, the effectiveness and success of QCT in COVID-19 patients is unknown. Therefore, our aim is to compare the SCT and QCT methods in the assessment of COVID-19 pneumonia severity with reference to the clinical classification.
Methods
The local Clinical Research Ethics Committee approved this retrospective study with a protocol number of 60116787-020/28658 and the informed consent was waived.
Study population
We have investigated adult patients with RT-PCR confirmed COVID-19 who underwent chest CT from March 15, 2020, to April 8, 2020. Exclusion criteria of the study were the presence of lung mass (previously known or unknown), pulmonary edema, obvious pulmonary sequelae, known interstitial lung disease, history of lung surgery or radiotherapy, and the presence of major motion artifacts on CT. Besides, patients younger than 18 were excluded from the study. Two RT-PCR tests were performed within 24 hours for COVID-19 diagnosis. A third test was performed if the clinical and radiological findings were suspicious but the first two test results were negative.
Chest CT imaging
Chest CT images were obtained without contrast medium in the supine position and at full inspiration using a multidetector CT system (Brilliance 16, Philips Medical Systems). The CT scanner was dedicated only to patients suspected of having COVID-19. The CT room and CT scanner were sanitized using standard cleaning procedures and approved disinfectants after each procedure. A minimum of 20 minutes was provided between the two consecutive CT examinations. The parameters were 35 cm field of view, 512×512 matrix, 0.75 s rotation time, 16×0.75 mm slice collimation, 1.5 mm slice thickness, 50–90 mAs effective tube current-time product, and 100–120 kV tube voltage.
Visual (semiquantitative) CT analysis
First, all chest CT images were evaluated by a board-certified chest radiologist (F.U.) for suitability for the study according to the inclusion and exclusion criteria, and non-eligible patients were excluded. Then, all CT images were independently reviewed for the semiquantitative CT (SCT) analysis by the board-certified radiologist with seven years of experience in thoracic imaging and a senior radiology resident who completed thoracic imaging training. Observers were unaware of the patient’s laboratory and clinical findings. The semiquantitative CT (SCT) analyses were performed independently, and final decisions reached by consensus. In the presence of disagreement between the two observers for the SCT score, a final decision was made together with a third observer with 11 years of experience.
All chest CT images were evaluated at the lung window settings (window level: −500 HU, window width: 1400 HU), and the percentage of involvement in each lung lobe was calculated semiquantitatively, which was described by Chung et al. (8). Each of the five lung lobes was evaluated for the percentage of lobar involvement. In this evaluation, the percentage of each lobe involvement was calculated as follows: no involvement (0%) = 0 points, minimal involvement (1%–25%) = 1 point, mild involvement (26%–50%) = 2 points, moderate involvement (51%–75%) = 3 points, severe involvement (76%–100%) = 4 points. The SCT score was reached by summing the scores in five lobes (range, 0–20 points) (8, 11).
Evaluation of chest CT features
After semiquantitative evaluation, all chest CT images were re-evaluated by two radiologists in consensus for the following characteristics: (a) distribution: the presence of central (lesion >3 cm from the pleura), peripheral (lesion <3 cm from the pleura) or mixed; (b) attenuation of opacities: the presence of ground-glass opacities (GGOs), GGOs with consolidation, or consolidation; (c) the presence of cavitation, centrilobular nodules, air bronchogram, bronchial wall thickening, reversed halo sign, tree-in-bud pattern nodules, crazy paving pattern, vascular widening inside or around the opacity; (d) the number of involved lobes; (e) the presence of pleural effusion or thickening; (f) and the presence of mediastinal and/or hilar lymphadenopathy (a lymph node with a ≥10 mm diameter in short-axis). All subjective evaluations were made according to the terms guide for thoracic imaging (14).
Quantitative CT analysis
All CT images were anonymized prior to quantitative evaluation, and all patients were randomly numbered. Quantitative CT (QCT) analyses were performed using a free DICOM (digital imaging and communications in medicine) viewer (Horos software Version 3.3.3; Available at https://horosproject.org/) by a trained radiologist.
Fully automatic lung segmentation was applied to achieve an analysis of lung volume. Total lung volume (TLV) without emphysema was calculated from CT attenuation values between −200 and −950 Hounsfield unit (HU). Besides, mean lung attenuation (MLA), skewness, and kurtosis values were noted for TLV images. For each patient, healthy lung parenchyma volume (HLV) was calculated from voxels between −800 and −950 HU (Fig. 1). The quantitative CT (QCT) score was calculated with the following formula: (TLV – HLV / TLV) ×100. Although lung segmentations were performed automatically, minor user intervention was performed to exclude the main bronchi, esophagus, and trachea when needed.
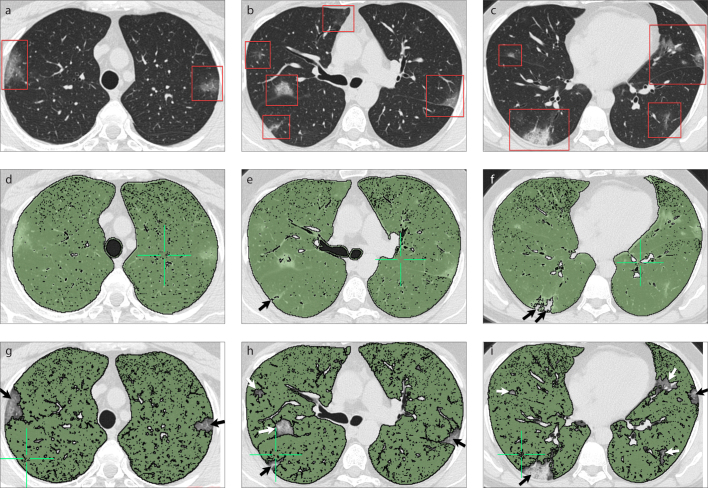
Figure 1. a–i.
A 36-year-old man with COVID-19 presented with fever and cough. Axial chest CT images (a–c) show bilateral, predominantly peripheral ground-glass opacities with a pronounced lower lobe distribution (red frames). Volumetric quantitative CT image (d) of the patient which analyzed voxels between −200 and −950 HU. Automatic segmentation of axial chest CT images (e–g) for the evaluation of healthy lung volume (pixel values between −800 and −950 HU) show bilateral, predominantly peripheral ground-glass opacities with a pronounced lower lobe distribution that were not included in automatic segmentation (arrows). Volumetric quantitative CT image (h, i) of the patient which analyzed voxels between −800 and −950 HU shows focal peripheral defects in the right lung (arrows).
Clinical classifications
All patients with COVID-19 were clinically divided into four groups at the time of initial presentation and CT imaging based on the clinical, radiological and laboratory findings which were defined by the Chinese National Health Commission as follows (4): Mild disease group, mild or minimal clinical symptoms without pneumonia on CT; Common disease group, fever, respiratory symptoms without respiratory distress, no need for supplemental oxygen and pneumonia in imaging; Severe disease group, fever or suspected respiratory infection and severe respiratory distress and/or increased respiratory rate ≥ 30 breaths/min and/or decreased oxygen saturation (SpO2) on room air with ≤ 93% and/or PaO2/FiO2 ≤ 300 mmHg; Critical disease group, respiratory failure requiring mechanical ventilation, septic shock and other organ failure requiring intensive care unit (ICU) admission.
Statistical analysis
To evaluate the data normality Shapiro-Wilk W test was used. Descriptive statistics of the data are presented with n (%) and, for non-normalized variables are shown as median (min–max range or interquartile percentiles [IQR]), and normal distributions are shown as mean ± standard deviation (SD). A Student’s t-test or Mann-Whitney U test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables were used. Relationships between the QCT score and SCT score were assessed using the Spearman’s correlation coefficient (r). An r value of 0–0.30 was considered weak, 0.31–0.50 moderate, 0.51–0.70 good, and 0.71–1.00 excellent correlation. Interobserver agreement was investigated using the Cohen’s Kappa statistics (κ). A κ value of 0–0.20 was considered poor, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 good, and 0.81–1.00 very good agreement (15). Mild and common disease groups were classified as limited disease, and severe and critical diseases were classified as extensive disease. The diagnostic performance of variables in the differentiation of limited and extensive disease was investigated using receiver operating characteristic (ROC) analysis, and the highest value of the Youden Index was obtained to determine an appropriate cutoff. The significance level is taken as α = 0.05. In the analysis MedCalc (version 16, MedCalc Software) and SPSS (v. 24.0, IBM) were used.
Results
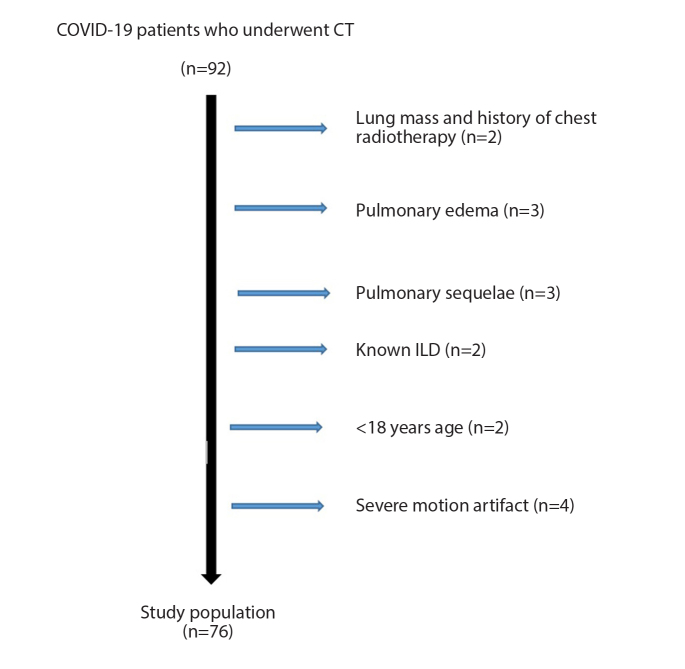
A total of 76 patients (45 male, 59.21%) were included in the study (Fig. 2). The median age of the patients was 48 years (range, 18–86 years). Total lung volume (TLV) and healthy lung volume (HLV) values were significantly higher in males than females (p < 0.001, for both). There was no significant difference between the two sexes in terms of age, disease severity, QCT, or SCT scores (Table 1). The median CT dose index (CTDIvol) and dose length product (DLP) values were 6.5 mGy (IQR, 5.2–6.5 mGy) and 136.5 mGy.cm (IQR, 119.1–169.3 mGy.cm), respectively.
Figure 2.
Patient selection and inclusion flow diagram of COVID-19 patients. ILD, interstitial lung disease.
Table 1.
Basic characteristics and measurement results according to sex
| Characteristics | Total population Median (range) |
Female Median (range) |
Male Median (range) |
p |
|---|---|---|---|---|
| Age (years) | 48 (18–86) | 48 (18–83) | 45 (20–86) | 0.606 |
| SCT Observer-1 | 4 (0–17) | 4 (0–17) | 4 (0–13) | 0.051 |
| SCT Observer-2 | 4 (0–15) | 4 (0–15) | 4 (0–14) | 0.059 |
| SCT Consensus | 4 (0–17) | 3 (0–14) | 5 (0–17) | 0.052 |
| TLV (cm3) | 4309 (1522–6952) | 3484 (1522–4866) | 5412 (2075–6952) | <0.001 |
| HLV (cm3) | 3330 (403–6554) | 2736 (403–4204) | 3924 (457–6554) | <0.001 |
| QCT (%) | 21.6 (5.7–78.9) | 21.4 (7.6–78.9) | 22.4 (5.7–78) | 0.565 |
| MLA (HU) | −777 (−840 to −523) | −767 (−759 to −596) | −784 (−840 to −523) | 0.619 |
| Skewness | 2 (−0.1 to 2.9) | 2 (0.4–2.8) | 2 (−0.1 to 2.9) | 0.823 |
| Kurtosis | 3.6 (−1.1 to 8.1) | 3.6 (−0.8 to 7.5) | 3.4 (−1.1 to 8.1) | 0.915 |
| Clinically severity score | 2 (1–4) | 2 (1–4) | 2 (1–4) | 0.195 |
SCT, semiquantitative computed tomography score; TLV, total lung volume; HLV, healthy lung volume; QCT, quantitative computed tomography score; MLA, mean lung attenuation; HU, Hounsfield unit.
Of 76 patients who underwent chest CT on admission, 67 (88.2%) had evidence of pneumonia on CT. Among 67 COVID-19 patients with pneumonia, 60 cases (89.6%) had GGOs, 22 (32.8%) had consolidation, and 39 (58.2%) had mixed GGOs and consolidation. Besides, 38 (56.7%) had a dominancy of peripheral, and 44 (65.7%) had a dominancy of mid-lower lung zone distribution. The left lower lobe was the most common involved lobe and was affected in 54 (71.1%) patients (Table 2). Seven (9.2%) of all patients had a single lesion, 34 (44.7%) had the crazy-paving pattern (GGO with interlobular septal thickening and intralobular lines), 7 (9.2%) had reversed halo sign, and 38 (50%) had vascular enlargement inside or around the opacity. Moreover, 7 (9.2%) patients had lymphadenopathy, and the mean age of patients with lymphadenopathy were significantly higher than those without (65.9±15.9 years and 47.8±17 years, p = 0.009). No patients had centrilobular nodules, tree-in-bud nodules or cavitation (Table 2).
Table 2.
CT findings in patients with COVID-19
| CT findings | n | Percentage of patients with positive CT findings (%) (n=67) | Percentage of total population (%) (n=76) | |
|---|---|---|---|---|
| Axial distribution of opacity | Central | 5 | 7.5 | |
| Peripheral | 38 | 56.7 | ||
| Mixed | 24 | 35.8 | ||
|
| ||||
| Craniocaudal distribution | Middle-upper | 12 | 17.9 | |
| Middle-lower | 44 | 65.7 | ||
| Mixed | 11 | 16.4 | ||
|
| ||||
| Number of opacity | Single | 7 | 10.4 | |
| Multiple | 60 | 89.6 | ||
|
| ||||
| Attenuation of opacity | Ground-glass | 60 | 89.6 | |
| Consolidation | 22 | 32.8 | ||
| Mixed | 39 | 58.2 | ||
|
| ||||
| Crazy paving pattern | Yes | 34 | 50.8 | 44.7 |
| No | 42 | 55.3 | ||
|
| ||||
| Reversed halo sign | Yes | 7 | 10.4 | 9.2 |
| No | 69 | 90.8 | ||
|
| ||||
| Air bronchogram | Yes | 26 | 38.8 | 34.2 |
| No | 40 | 52.6 | ||
|
| ||||
| Bronchial wall thickening | Yes | 37 | 55.2 | 48.7 |
| No | 39 | 51.3 | ||
|
| ||||
| Tree-in-bud pattern | Yes | 2 | 3.0 | 2.6 |
| No | 74 | 97.4 | ||
|
| ||||
| Vascular enlargement | Yes | 38 | 56.7 | 50.0 |
| No | 38 | 50.0 | ||
|
| ||||
| Cavity | Yes | 0 | 0 | 0.0 |
| No | 76 | 100 | ||
|
| ||||
| Pleural thickening/ effusion | Yes | 5 | 6.6 | |
| No | 69 | 90.8 | ||
|
| ||||
| Pleural thickening/ effusion | Unilateral | 1 | 1.3 | |
| Bilateral | 4 | 5.3 | ||
|
| ||||
| Mediastinal lymphadenopathy | Yes | 7 | 9.2 | |
| No | 69 | 90.8 | ||
|
| ||||
| Lobar involvement in limited disease group (n=53) | Right upper lobe | 32 | ||
| Right middle lobe | 28 | |||
| Right lower lobe | 32 | |||
| Left upper lobe | 32 | |||
| Left lower lobe | 36 | |||
|
| ||||
| Lobar involvement in extensive disease group (n=14) | Right upper lobe | 9 | ||
| Right middle lobe | 8 | |||
| Right lower lobe | 10 | |||
| Left upper lobe | 10 | |||
| Left lower lobe | 11 | |||
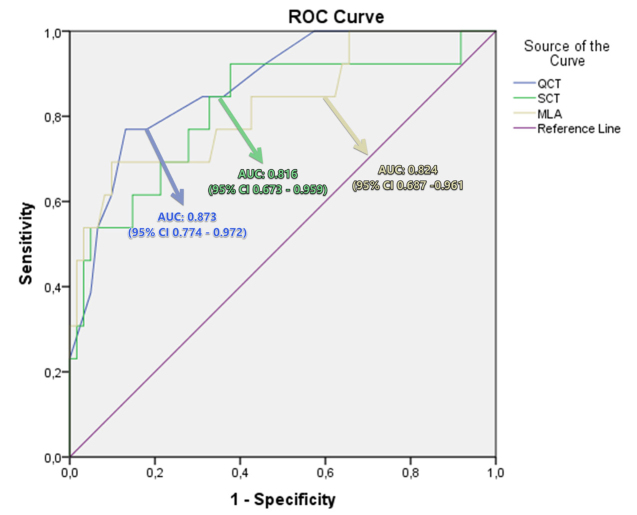
In the SCT evaluation, there was good agreement between the observers (p < 0.001, κ = 0.796, 95% CI, 0.751–0.841). The median semiquantitative CT (SCT) score was 4 (range, 0–15) (Table 1). The SCT score significantly correlated with clinical severity score (p = 0.004, r = 0.529) and quantitative values (Supplementary Table S1). The ROC analysis showed the area under the curve (AUC) of SCT for differentiation of limited and extensive disease as 0.816 (95% CI, 0.673–0.959) (p < 0.001) (Fig. 3). A SCT score cutoff of 6.5 had 76.9% sensitivity and 82% specificity for differentiating clinically extensive disease from limited disease.
Figure 3.
Receiver operating characteristic (ROC) curve analysis was obtained to differentiate extensive disease from limited disease in patients with COVID-19. Quantitative CT (QCT) score had an area under the curve (AUC) of 0.816 (95% CI 0.673–0.959), semiquantitative CT (SCT) score had an AUC of 0.873 (95% CI 0.774–0.972), and mean lung attenuation (MLA) value had an AUC of 0.824 (95% CI 0.687–0.961).
The median QCT score was 21.6% (range, 5.7%–78.9%) and QCT score showed significant correlation with clinical severity score (Supplementary Table S1, p < 0.001, r = 0.620). ROC analysis showed the AUC of QCT for differentiation of limited and extensive disease as 0.873 (95% CI, 0.774–0.972) (p < 0.001) (Fig. 3). A QCT score cutoff of 24.6% had 84.6% sensitivity and 77.2% specificity, and QCT scores 40.6% had 71.4% sensitivity and 100% specificity for differentiating clinically extensive disease from limited disease. SCT, QCT, and MLA values of patients with extensive disease were significantly higher than those with limited disease (Table 4).
Table 4.
Comparison of limited and extensive disease groups
| Parameters | Limited, median (range) | Extensive, median (range) | p |
|---|---|---|---|
| TLV (cm3) | 4335 (2185–6952) | 3695 (1522–5692) | 0.040 |
| HLV (cm3) | 3624 (1366–6554) | 2219 (403–4243) | 0.001 |
| SCT | 3 (0–11) | 10 (3–17) | <0.001 |
| QCT (%) | 20.4 (5.7–59.9) | 41.3 (10.9–78.9) | <0.001 |
| Skewness | 2 (0.6–2.9) | 1.2 (−0.1 to 2.1) | 0.001 |
| Kurtosis | 3.8 (−0.6 to 8.1) | 0.5 (−1.1 to 4.2) | <0.001 |
| MLA (HU) | −789 (−840 to −638) | −701 (−805 to −523) | 0.003 |
| Fever (Celcius, °C) | 36.8 (36.3–39.6) | 38 (36.5–39.5) | 0.026 |
| Onset to admission (days) | 4 (1–12) | 6 (3–10) | 0.071 |
TLV, total lung volume; HLV, healthy lung volume; SCT, semiquantitative computed tomography score; QCT, quantitative computed tomography score; MLA, mean lung attenuation; HU, Hounsfield unit.
While kurtosis (p < 0.001, r = −0.494) and skewness (p < 0.001, r = −0.477) values showed significant negative correlation with clinical severity score, MLA values showed significant positive correlation with clinical severity score (p < 0.001, r = 0.414) (Supplementary Table S1). ROC analysis showed the AUC of MLA for differentiation of limited and extensive disease as 0.824 (95% CI, 0.687–0.961) (p < 0.001). The MLA cutoff of −722.5 HU had 69.2% sensitivity and 90.2% specificity (Fig. 3). A total of five patients (6.6%) had a consolidation with a density of higher than −200 HU in the TLV evaluation. Moreover, the dense consolidation area in these patients was observed to be less than a quarter of the entire lesion volume.
There was at least one additional chronic disease in 32 of 76 patients (42.1%; 20 men). No significant difference was found between female and male patients in terms of additional chronic disease (p = 0.793) (Supplementary Table S2). The list of other chronic additional diseases is shown in Table 3. There was a significant correlation between the number of additional chronic diseases and SCT, QCT and clinically severity scores (p = 0.049; r = 0.229, p = 0.022; r = 0.266, and p = 0.009; r = 0.301, respectively). Limited disease was present in 57 patients (75%, n=32 males) and extensive disease in 19 patients (25%, n=13 males). No significant difference was found between male and female patients in terms of disease severity (p = 0.459). In patients with limited disease, fever was significantly lower than those with extensive disease (Table 4). The most common complaints in COVID-19 patients were cough in 49 patients (64.5%), high fever in 33 patients (43.4%), and fatigue in 30 patients (39.5%) (Table 3). The median time between symptoms onset to CT admission was 4 days (IQR, 2–7 days). When early admission was accepted as <7 days from symptom onset to CT admission, there was no significant difference between early and late admission groups in terms of GGO (p = 0.929), mixed opacity (p = 0.056), and consolidation on CT (p = 0.647).
Table 3.
Clinical characteristics of the patients
| Characteristics | All patients (n=76) | |
|---|---|---|
| Sex | Male | 45 (59.2) |
| Female | 31 (40.8) | |
|
| ||
| Age (years), mean±SD | Male | 49.2±17.4 |
| Female | 49.8±17.9 | |
|
| ||
| Chronic disease | Cardiovascular disease | 15 (19.7) |
| Hypertension | 15 (19.7) | |
| Diabetes | 13 (17.1) | |
| Chronic lung disease | 9 (11.8) | |
| Malignancy | 5 (6.6) | |
| Immunosuppression | 5 (6.6) | |
| Collagen vascular disease | 3 (4.0) | |
| Chronic renal failure | 1 (1.3) | |
|
| ||
| Smoking history | Never | 55 (72.4) |
| Former | 16 (21.0) | |
| Current | 5 (6.6) | |
|
| ||
| Pack-years of smoking, mean±SD | 22.4±20.6 | |
|
| ||
| Clinical symptoms | Cough | 49 (64.5) |
| High fever | 33 (43.4) | |
| Fatigue | 30 (39.5) | |
| Dyspnea | 25 (32.9) | |
| Myalgia | 18 (23.7) | |
| Diarrhea | 10 (13.2) | |
| Chest tightness | 10 (13.2) | |
| Nausea and vomiting | 10 (13.2) | |
| Headache | 9 (11.8) | |
| Sore throat | 9 (11.8) | |
| Sputum | 8 (10.5) | |
| Loss of taste/smell | 6 (7.9) | |
| Nasal congestion and runny nose | 3 (4.0) | |
| No obvious symptoms | 2 (2.6) | |
| Hemoptysis | 0 | |
| Dizziness | 0 | |
|
| ||
| Onset to admission (days) (n=74) | Median | 4 |
| P25 | 2 | |
| P75 | 7 | |
| Range | 1 to 12 | |
|
| ||
| Clinically severity score, median (range) | Male | 2 (1–4) |
| Female | 2 (1–4) | |
Data are presented as n (%) unless otherwise noted.
P25, 25th percentile; P75, 75th percentile.
Discussion
Herein, we investigated the effectiveness of QCT and SCT in assessment of COVID-19 patients: our results revealed a significant correlation between disease severity and QCT, SCT scores. Although QCT, SCT, and MLA were found to be successful in distinguishing between extensive and limited diseases, which may have a significant effect on patients’ prognosis and management, QCT showed the best discriminative performance. In the SCT assessment, there was good agreement between observers. The left lower lobe was the most common involved lobe and was affected in 54 (71.1%) patients and the number of additional chronic diseases was significantly correlated with SCT, QCT scores, and clinical disease severity score.
Chung et al. (8) examined 21 patients with COVID-19, and they showed that patients with high SCT scores had more extensive disease. However, in their study, the cutoff value was not specified, and the interobserver agreement for SCT assessment was not investigated. Li et al. (11) demonstrated an excellent interobserver agreement with ICC of 0.976 (95% CI, 0.962–0.985) for SCT assessment, and they reported that the SCT score was successful in diagnosis of severe-critical disease, with a cutoff value of 7.5 having 82.6% sensitivity and 100% specificity. Similarly, we found good agreement between observers with κ value of 0.796 (95% CI, 0.751–0.841), and SCT score was significantly correlated with disease severity. In our study, SCT score cutoff of 6.5 had 76.9% sensitivity and 82% specificity for differentiating extensive disease from limited disease. Besides, our results revealed that the SCT score significantly correlated with the QCT score, MLA, kurtosis, and skewness values.
In accordance with the literature (6–11), most of the lung opacities were predominantly distributed in the peripheral (56.7%), mid-lower lung zones (65.7%), and most patients had multiple opacities (89.6%) in our study. In a systematic meta-analysis involving 919 patients by Salehi et al. (16), the reported prevalence of consolidation was similar to our population (31% and 32.8%, respectively). Prevalence of consolidation was reported as 21.4% by Li et al. (11), while Caruso et al. (17) reported consolidation in 72% of the population. We think that the difference between the prevalence of consolidation between studies is due to the classification of the lesions on CT. While some studies in the literature divide the lesions in two according to CT attenuation values (consolidation or GGO), some examine the lesions in three groups (consolidation, GGO, or mixed) (11, 16, 17). The prevalence of GGO in our study was found to be quite similar to the meta-analysis by Salehi et al. (16) (89.6% vs. 89%, respectively). Recently, Caruso et al. (17) analyzed 58 patients with COVID-19 pneumonia, showing a higher frequency of GGO compared to our results (100% vs. 89.6%, respectively). Moreover, our findings differ from that of Zhu et al. (18), where GGO was found in only 15 of 32 patients. These differences between studies may be due to age difference of the populations or the difference in the time elapsed between disease onset and CT. While, Chung et al. (8) reported the prevalence of crazy paving pattern as 19%, Caruso et al. (17) reported it as 39% and Li et al. (11) as 44.6%. Similar to Li et al. (11), 34 patients (44.7%) in our study had lesions in the crazy-paving pattern. The reversed halo sign is a rare CT finding in COVID-19 patients (19), and the prevalence of reversed halo sign was found to be 9.2% in our population. Vascular enlargement, which is an interesting chest CT feature, was reported in 59% to 89% of COVID-19 patients in the literature (17, 19–21). Similarly, 56.7% of patients with pneumonia on CT had vascular enlargement in our population.
Quantitative CT assessment produces an objective, reproducible, and quantifiable evaluation of lung parenchymal changes associated with diffuse lung disease (12). It has been shown to be very successful in evaluating the disease severity and monitoring patients with idiopathic pulmonary fibrosis, collagen vascular disease-related interstitial lung disease, emphysema, and pulmonary sarcoidosis. Moreover, a QCT score of disease severity may serve as a useful tool or surrogate endpoint in evaluating the efficacy of therapy (12, 13). While voxel attenuation lower than −950 HU represents the volume of emphysema, attenuation values higher than −800 HU represent the interstitial lung disease and other diffuse lung diseases (13). However, as far as we know, the applicability of this method in COVID-19 patients is unknown. Our results revealed that QCT evaluation in patients with COVID-19 showed a stronger correlation than SCT evaluation for estimating the disease severity. We suggest that QCT is easily applicable in the evaluation of disease severity in patients with COVID-19 and helps to differentiate extensive disease from limited disease.
This study had several limitations. First, the study has a retrospective design, and the clinical data of the patients were collected retrospectively. Second, the number of patients between different disease severity groups was significantly different. Therefore, prospective studies with a larger population including different disease severity groups are needed to substantiate our results. Third, some of the COVID-19 patients who underwent chest CT were not included in the study as it can erroneously affect the quantitative analysis results (e.g., due to the presence of pulmonary edema or motion artifacts). Finally, some of the consolidation areas with high CT attenuation values were not included in the assessment of TLV, because CT attenuation values between −200 and −950 HU were used. However, this limitation was present in a small group of patients (n=5, 6.6 %).
In conclusion, quantitative CT assessment, with QCT score and MLA, is an objective method in the evaluation of COVID-19 severity and is more successful than semiquantitative CT assessment to discriminate extensive disease from limited disease.
Main points.
Quantitative CT assessment helps to objectively evaluate the disease extent in COVID-19 patients.
Quantitative CT assessment is more successful than semiquantitative CT assessment in the evaluation of disease severity in patients with COVID-19.
There was good agreement between observers in the semiquantitative evaluation of disease severity in patients with COVID-19.
Supplementary Information
Table S1.
Correlation between semiquantitative and quantitative results
| SCT | QCT | SKEW | KURT | MLA | HLV | TLV | CSS | ||
|---|---|---|---|---|---|---|---|---|---|
| SCT | r | 0.661 | −0.611 | −0.599 | 0.584 | −0.393 | −0.129 | 0.529 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 | 0.005 | 0.274 | 0.004 | ||
|
| |||||||||
| QCT | r | 0.661 | −0.657 | −0.658 | 0.614 | −0.638 | −0.365 | 0.620 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | ||
|
| |||||||||
| SKEW | r | −0.611 | −0.657 | 0.995 | −0.924 | 0.752 | 0.629 | −0.477 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
|
| |||||||||
| KURT | r | −0.599 | −0.658 | 0.995 | −0.914 | 0.752 | 0.626 | −0.494 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
|
| |||||||||
| MLA | r | 0.584 | 0.614 | −0.924 | −0.914 | −0.788 | −0.700 | 0.414 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
|
| |||||||||
| HLV | r | −0.393 | −0.638 | 0.752 | 0.752 | −0.788 | 0.928 | −0.246 | |
| p | 0.005 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.036 | ||
|
| |||||||||
| TLV | r | −0.129 | −0.365 | 0.629 | 0.626 | −0.700 | 0.928 | −0.122 | |
| p | 0.274 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.303 | ||
|
| |||||||||
| CSS | r | 0.529 | 0.620 | −0.477 | −0.494 | 0.414 | −0.246 | −0.122 | |
| p | 0.004 | <0.001 | <0.001 | <0.001 | <0.001 | 0.036 | 0.303 | ||
SCT, semiquantitative computed tomography score; QCT, quantitative computed tomography score; SKEW, skewness; KURT, kurtosis; MLA, mean lung attenuation; HLV, healthy lung volume; TLV, total lung volume; CSS, clinical severity score.
Table S2.
Categorical data distribution between male and female patients
| Total population n=76, n (%) | Male n=45, n (%) | Female n=31, n (%) | p | |
|---|---|---|---|---|
| Cardiovascular disease | 15 (19.74) | 11 (24.44) | 4 (12.9) | 0.214 |
| Hypertension | 15 (19.74) | 9 (20) | 6 (19.35) | 0.945 |
| Diabetes | 13 (17.11) | 8 (17.78) | 5 (16.13) | 0.851 |
| Chronic lung disease | 9 (11.84) | 7 (15.56) | 2 (6.45) | 0.227 |
| Malignancy | 5 (6.58) | 5 (11.11) | 0 | 0.075 |
| Immunosuppression | 5 (6.58) | 3 (6.67) | 2 (6.45) | 0.970 |
| Collagen vascular disease | 3 (3.95) | 1 (2.22) | 2 (6.45) | 0.352 |
| Chronic renal failure | 1 (1.32) | 1 (2.22) | 0 | 0.592 |
| Additional chronic disease | 32 (42.11) | 20 (44.44) | 12 (38.71) | 0.619 |
| Extensive disease | 19 (25) | 13 (28.89) | 6 (19.35) | 0.346 |
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus disease 2019 (COVID-19) situation report–87. World Health Organization; Geneva: 2020. [Accessed May 2, 2020]. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200501-covid-19-sitrep.pdf?sfvrsn=742f4a18_2. [Google Scholar]
- 3.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 Feb 18; doi: 10.1016/S2213-2600(20)30076-X. [Epub Ahead of Print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.China National Health Commission. [Accessed April 13, 2020];Diagnosis and treatment of pneumonitis caused by new coronavirus (trial version 7) Available at: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml. [Google Scholar]
- 5.World Health Organization. Country & Technical Guidance - Coronavirus disease (COVID-19) World Health Organization; Geneva: 2020. [Accessed April 13, 2020]. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance. [Google Scholar]
- 6.Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020. Feb 12, [Epub Ahead of Print] [DOI] [PMC free article] [PubMed]
- 7.Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, Ji W. Sensitivity of chest CT for COVID-19: Comparison to RT-PCR. Radiology. 2020. Feb 19, [Epub Ahead of Print] [DOI] [PMC free article] [PubMed]
- 8.Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang Y, Zhang H, Xu Y, Xie J, Pang P, Ji W. CT Manifestations of Two Cases of 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology. 2020;295:208–209. doi: 10.1148/radiol.2020200280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernheim A, Mei X, Huang M, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020. Feb 20, [Epub Ahead of Print] [DOI] [PMC free article] [PubMed]
- 11.Li K, Fang Y, Li W, et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020. Mar 25, [Epub Ahead of Print] [DOI] [PMC free article] [PubMed]
- 12.Ufuk F, Demirci M, Altinisik G. Quantitative computed tomography assessment for systemic sclerosis-related interstitial lung disease: comparison of different methods. Eur Radiol. 2020. Mar 19, [Epub Ahead of Print] [DOI] [PubMed]
- 13.Chen A, Karwoski RA, Gierada DS, Bartholmai BJ, Koo CW. Quantitative CT analysis of diffuse lung disease. Radiographics. 2020;40:28–43. doi: 10.1148/rg.2020190099. [DOI] [PubMed] [Google Scholar]
- 14.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 15.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22:276–282. doi: 10.11613/BM.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020. Mar 14, [Epub Ahead of Print] [DOI] [PubMed]
- 17.Caruso D, Zerunian M, Polici M, et al. Chest CT Features of COVID-19 in Rome, Italy. Radiology. 2020. Apr 3, [Epub Ahead of Print] [DOI] [PMC free article] [PubMed]
- 18.Zhu W, Xie K, Lu H, et al. Initial clinical features of suspected coronavirus disease 2019 in two emergency departments outside of Hubei, China. J Med Virol. 2020. Mar 13, [Epub Ahead of Print] [DOI] [PMC free article] [PubMed]
- 19.Li Y, Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am J Roentgenol. 2020. Mar 4, [Epub Ahead of Print] [DOI] [PubMed]
- 20.Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol. 2020. Mar 3, [Epub Ahead of Print] [DOI] [PubMed]
- 21.Bai HX, Hsieh B, Xiong Z, et al. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020. Mar 10, [Epub Ahead of Print] [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Correlation between semiquantitative and quantitative results
| SCT | QCT | SKEW | KURT | MLA | HLV | TLV | CSS | ||
|---|---|---|---|---|---|---|---|---|---|
| SCT | r | 0.661 | −0.611 | −0.599 | 0.584 | −0.393 | −0.129 | 0.529 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 | 0.005 | 0.274 | 0.004 | ||
|
| |||||||||
| QCT | r | 0.661 | −0.657 | −0.658 | 0.614 | −0.638 | −0.365 | 0.620 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | ||
|
| |||||||||
| SKEW | r | −0.611 | −0.657 | 0.995 | −0.924 | 0.752 | 0.629 | −0.477 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
|
| |||||||||
| KURT | r | −0.599 | −0.658 | 0.995 | −0.914 | 0.752 | 0.626 | −0.494 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
|
| |||||||||
| MLA | r | 0.584 | 0.614 | −0.924 | −0.914 | −0.788 | −0.700 | 0.414 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
|
| |||||||||
| HLV | r | −0.393 | −0.638 | 0.752 | 0.752 | −0.788 | 0.928 | −0.246 | |
| p | 0.005 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.036 | ||
|
| |||||||||
| TLV | r | −0.129 | −0.365 | 0.629 | 0.626 | −0.700 | 0.928 | −0.122 | |
| p | 0.274 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.303 | ||
|
| |||||||||
| CSS | r | 0.529 | 0.620 | −0.477 | −0.494 | 0.414 | −0.246 | −0.122 | |
| p | 0.004 | <0.001 | <0.001 | <0.001 | <0.001 | 0.036 | 0.303 | ||
SCT, semiquantitative computed tomography score; QCT, quantitative computed tomography score; SKEW, skewness; KURT, kurtosis; MLA, mean lung attenuation; HLV, healthy lung volume; TLV, total lung volume; CSS, clinical severity score.
Table S2.
Categorical data distribution between male and female patients
| Total population n=76, n (%) | Male n=45, n (%) | Female n=31, n (%) | p | |
|---|---|---|---|---|
| Cardiovascular disease | 15 (19.74) | 11 (24.44) | 4 (12.9) | 0.214 |
| Hypertension | 15 (19.74) | 9 (20) | 6 (19.35) | 0.945 |
| Diabetes | 13 (17.11) | 8 (17.78) | 5 (16.13) | 0.851 |
| Chronic lung disease | 9 (11.84) | 7 (15.56) | 2 (6.45) | 0.227 |
| Malignancy | 5 (6.58) | 5 (11.11) | 0 | 0.075 |
| Immunosuppression | 5 (6.58) | 3 (6.67) | 2 (6.45) | 0.970 |
| Collagen vascular disease | 3 (3.95) | 1 (2.22) | 2 (6.45) | 0.352 |
| Chronic renal failure | 1 (1.32) | 1 (2.22) | 0 | 0.592 |
| Additional chronic disease | 32 (42.11) | 20 (44.44) | 12 (38.71) | 0.619 |
| Extensive disease | 19 (25) | 13 (28.89) | 6 (19.35) | 0.346 |