Abstract
PURPOSE
We aimed to evaluate the effect on the radiation dose to the patient by reducing the tube current during the placement of the ablation needles (reduced dose group) compared with the patient doses delivered when scanning at the standard fully diagnostic level (full dose group) in computed tomography (CT)-guided percutaneous cryoablation.
METHODS
We conducted a retrospective study of 103 patients undergoing cryoablation in a tertiary cancer center. Overall, 62 patients were scanned with standard exposure parameters (full dose group) set on a 64-slice multidetector CT scanner, while 41 patients were scanned on a reduced dose protocol. Dose levels were retrieved from the hospital picture and archiving communication system including the volumetric CT dose index (CTDIvol), total dose length product (DLP), length of cryoablation procedure, number of cryoablation needles and patient size. Wilcoxon Mann-Whitney (rank-sum) tests were used to compare the median DLP, CTDIvol and skin dose between the two groups.
RESULTS
Median total DLP for the full dose group was 6025 mGy·cm (1909–13353 mGy·cm) compared with 3391 mGy·cm (1683–6820 mGy·cm) for the reduced dose group. The reduced dose group had a 44% reduction in total DLP and 42% reduction in total CTDIvol (p < 0.001). The estimated skin doses were 384 mGy for the full dose group and 224 mGy for the reduced dose group (42% reduction) (p < 0.001). At 12-month follow-up, the technical success for the full dose (n=62) was 97% with 2 patients requiring a further cryoablation treatment for residual tumor. The technical success for the reduced dose group (n=41) was 100%.
CONCLUSION
CT dose reduction technique during image-guided cryoablation treatment of renal tumors can achieve significant radiation dose reduction whilst maintaining sufficient image quality.
Renal cell carcinoma is the most common kidney cancer and has a rising incidence (1–4), with obesity and smoking being major risk factors (5–8).
Image-guided ablation offers a more minimally invasive option compared with surgery and the current evidence base shows that it is a safe and effective treatment for T1a tumors, with a low rate of complications (9–11). The major advantage of cryoablation over other modalities is the ability to accurately visualize the iceball and therefore zone of ablation on intraprocedural imaging, either with computed tomography (CT) or magnetic resonance imaging (MRI) (12, 13). However, renal cryoablation involves the placement of more ablation probes and can have almost three times the radiation exposure compared with CT-guided radiofrequency ablation procedures (14).
In addition to this substantial radiation dose per cryoablation, estimated to be between 32 and 39.7 mSv, the follow-up CT imaging will also add to the total radiation burden (15, 16). Whilst this level of radiation dose and associated stochastic risk may be a lesser concern in the older patients, greater consideration needs to be given to younger patients (<50 years old) and in patients requiring lifelong follow-up imaging, in particular those with hereditary diseases such as Von Hippel-Lindau syndrome (15). To our knowledge, the potential for reducing radiation dose for cryoablation patients.
The principle aim of this study was to evaluate the effect on the radiation dose to the patient by reducing the tube current during the placement of the ablation needles (reduced dose group) compared with the patient doses delivered when scanning at the standard fully diagnostic level (full dose group) in CT-guided percutaneous cryoablation.
Methods
The study involved retrospective analysis of a prospectively collected renal cryoablation database.
Formal ethics approval or patient consent was not required as this was a retrospective data review and was not classified as research under the United Kingdom National Health Service Health Research Authority and a waiver is granted at our institution.
Consecutive percutaneous image-guided renal cryoablation procedures between June 2008 and June 2014 in a single institution specialist cancer centre were reviewed. Cryoablation procedures which treated more than one tumor were excluded (n=6). A total of 103 cryoablation procedures were included in the study. Overall, 62 procedures were performed with the standard ablation procedure scanning protocol (full dose group) and 41 cryoablation procedures were performed with a dose reduction scanning protocol (reduced dose group). Baseline clinical and tumor characteristics were recorded.
Procedure
All cryoablations were performed using an argon gas-based system (CryoHit, Galil Medical) under CT guidance. All patients were scanned on a 64-slice MDCT scanner (Somatom Sensation 64, Siemens). All procedures were performed by one of three consultant interventional radiologists (over 15 years of combined experience).
The number of cryoprobes was decided based on the size of the tumor and the number needed to achieve an effective ablation margin to cover the entire tumor volume including at least a 5 mm margin. All patients received a general anesthetic for the procedure.
Prior to insertion of the cryoprobes, three helical acquisitions were performed. The first was a noncontrast enhanced scan, followed by arterial and portal venous phase acquisitions, which were performed 30 and 65 s after injection of 100 mL of Niopam 300 intravenous contrast agent (Bracco). The scanned volume for each of these CT scans included the entire abdomen volume to provide an up to date image of the renal tumor for treatment planning. Scanning parameters for these acquisitions are shown in Table 1.
Table 1.
The acquisition and reconstruction parameters used for the three helical CT scan immediately prior to image-guided cryoablation needle insertion
| Non-contrast phase | Arterial and portal venous phases | |
|---|---|---|
| Tube voltage (kV) | 120 | 120 |
| Quality reference mAs used with CARE dose 4D | 180 | 180 |
| Beam collimation (mm) | 24×1.2 | 64×0.6 |
| Helical pitch | 1.2 | 1.2 |
| Image slice thickness (mm) | 3 | 3 |
| Reconstruction kernel | B30f medium smooth | B30f medium smooth |
kV, kilovoltage; mAs, milliampere-second; CARE, combined applications to reduce exposure (Siemens Healthineers).
Once the contrast acquisitions had been undertaken the cryoprobes were placed and treatment monitored under CT guidance. This involved a wide volume acquisition; 40 mm collimation with 64 thin slices to be reformatted later. A helical acquisition covering the entire tumor was undertaken following each needle placement to allow for coronal and sagittal reformats.
Dose reduction protocol
The defined dose reduction scanning protocol was constructed in January 2013 following a departmental cryoablation patient dose audit leading to a period of step-wise dose reduction of mAs throughout the probe targeting phase of the scans acquired during the cryoablation procedure with continuous feedback on the acquired image quality by the interventional radiology team. For all scans the tube kilovoltage (kVp) was fixed at 120 kV.
In the full dose group each of these scans was performed using the exposure parameters given in Table 1, with CARE Dose 4D activated (automated dose modulation), whilst in the reduced dose group the mAs for each scan was manually reduced in a progressive manner by initially halving the effective mAs (mAs/pitch) that had been selected for the contrast enhanced scans by the Care Dose 4D system and then subsequently reducing this mAs value by 20 mAs per acquisition until either the interventional radiologist deemed the images to be too low quality or 50 mAs had been reached. It was determined by consensus from the interventional radiology team that 50 mAs was the minimum setting at which clinically adequate image quality could be achieved, irrespective of patient size.
Radiation dose measurements
All patient radiation dose levels were evaluated by two CT radiographers (combined 20 years of experience) and one medical physicist (10 years of experience) using the dose records stored in the hospital picture and archiving communication system (IMPAX, AGFA Healthcare). The CT dose index volume (CTDIvol) and dose length product (DLP) were recorded per procedure. The individual CTDIvol values from each of the acquisitions were summed up to calculate the total CTDIvol. The length of procedure (minutes), number of cryoablation needles and all ancillary procedures such as pneumodissection or hydrodissection to displace surrounding vital structures were recorded. In addition, patient size was measured at the level of the tumor by recording the maximum anteroposterior diameter and the maximum lateral diameter of the patient. These two measurements were combined in order to calculate the patient effective diameter, a surrogate marker for patient size.
Measurements performed locally showed that the dose measured at the surface of a 32 cm diameter CTDI phantom at 120 kV, as a broad estimate of skin dose, was equal to 1.1 times the CTDIvol under the same exposure conditions. This value of 1.1 was used to make a broad estimate of patient skin doses during cryoablation procedures in order to determine whether any deterministic effects were likely.
Clinical outcomes
Complications were classified as per the Clavien-Dindo classification system in relation to cryoablation with major complications representing those requiring surgical, endoscopic, or radiologic intervention (e.g., Clavien-Dindo Grade 3 or more) (17, 18).
The technical effectiveness was reviewed at 12 months post-ablation with technical success defined as no evidence of recurrent disease on imaging at 12 months post-ablation.
Statistical analysis
Descriptive statistics are presented with n (%). Non-normally distributed variables are shown as median (minimum-maximum). The Pearson chi-square test was used to analyze categorical variables between both groups and the Wilcoxon Mann-Whitney (rank-sum) test was used to compare the median values of the continuous variables along with the radiation dose metrics (DLP, CTDIvol and skin dose) between the two groups. All data was tabulated in Microsoft Excel (Office 365, 2017) and statistical analysis was performed using SPSS (Version 16, 2016; SPSS Inc.).
Results
Baseline distribution of characteristics for the full dose and reduced dose groups are shown in Table 2.
Table 2.
Baseline characteristics for the full dose and reduced dose groups
| Full dose (n=62) | Reduced dose (n=41) | p | |
|---|---|---|---|
| Sex, n (%) | 0.860 | ||
| Female | 25 (40) | 16 (39) | |
| Male | 37 (60) | 25 (61) | |
|
| |||
| Age (years), median (range) | 67 (21–86) | 76 (44–86) | 0.007 |
|
| |||
| Patient effective diameter* (cm), median (range) | 44 (23–59) | 40 (27–52) | 0.079 |
|
| |||
| Tumor size* (cm), median (range) | 3 (1.0–5.4) | 3 (1.5–5.0) | 0.409 |
|
| |||
| Number of cryoprobes, median (range) | 6 (1–11) | 6 (3–11) | 0.609 |
|
| |||
| Ancillary procedure performed, n (%) | 0.953 | ||
| Yes | 20 (32) | 13 (32) | |
| No | 42 (68) | 28 (68) | |
|
| |||
| Number of scans, median (range) | 27 (14–53) | 26 (13–41) | 0.518 |
|
| |||
| Procedure time (min), median (range) | 34 (17–64) | 34 (20–52) | 0.685 |
The data is presented as n (%), unless otherwise noted. The p values from the Pearson chi-square test (categorical variables) and the Wilcoxon Mann-Whitney (rank-sum) test (continuous) comparisons between the two groups are provided. Significance was taken if p < 0.05.
Patient effective diameter and tumor size were calculated according to Nguyen et al. (23).
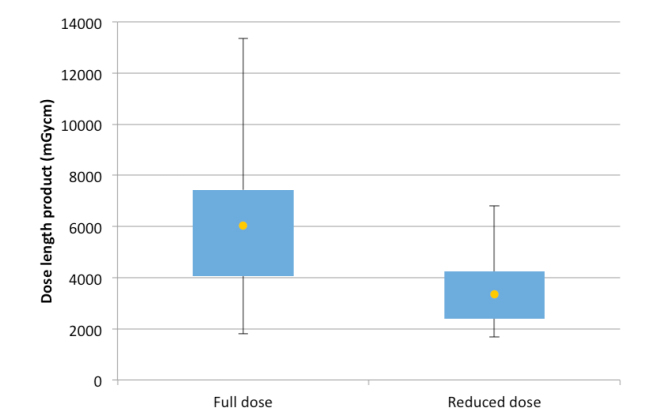
The distribution of the total DLP values between the full dose and reduced dose groups are shown in Fig. 1. This demonstrates that for the reduced dose group there is both an overall reduction in median dose and less variation in the range of doses. The difference of 43.72% between the median total DLP values across both groups was statistically significant (p < 0.001). The other dose metric values (median CTDIvol, CTDIvol per acquisition and estimated skin dose) in the reduced dose group compared with the full dose group (Table 3) were all lower and the differences were statistically significant (p < 0.001). In addition, the maximum DLP in the study was substantially lower in the dose reduction group (p < 0.001).
Figure 1.
A box and whisker plot of the dose distributions in the full dose and reduced dose groups. Yellow marker represents the median result.
Table 3.
Summary of the dose metrics for the full dose and reduced dose patient cohorts
| Full dose group | Reduced dose group | % reduction | |
|---|---|---|---|
| Total DLP (mGy·cm) | 6025 (1909–13353) | 3391 (1683–6820) | 43.72 |
| Total CTDIvol (mGy) | 349 (143–968) | 203 (121–331) | 41.83 |
| CTDIvol per acquisition (mGy) | 12.8 (5.6–36.9) | 8.1 (4.0–15.0) | 36.72 |
| Estimated skin dose (mGy) | 384 (157–1065) | 224 (133–364) | 41.67 |
The numerical data are presented as median (range).
DLP, dose length product; CTDIvol, volume CT dose index.
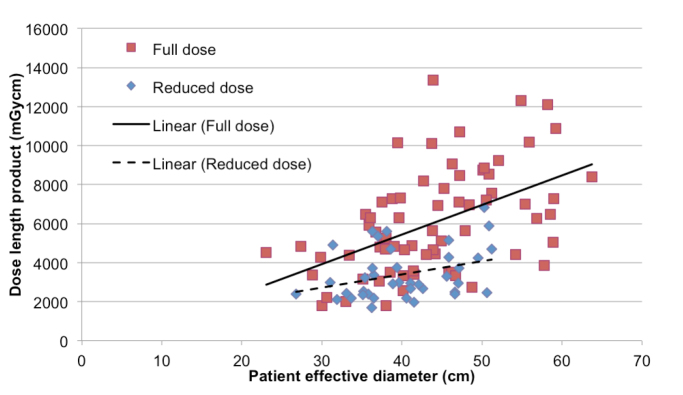
The relationship between median total DLP and patient effective diameter for both groups are shown on a scatter graph (Fig. 2). For both groups, the total DLP increased with increasing patient effective diameter, with a greater incremental increase observed in the total DLP for the full dose group with respect to patient size. No statistical difference in patient size was observed between the full dose and reduced dose groups (p = 0.079).
Figure 2.
Scatter graph demonstrating the variation in total DLP against patient effective diameter for both the full dose and reduced dose groups.
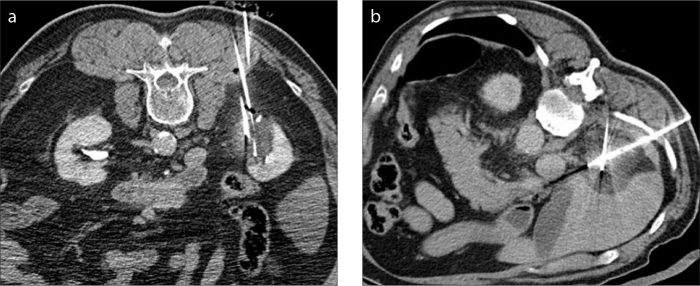
The interventional radiologists were able to successfully manually adjust the mAs values on a patient by patient basis, as discussed in the methods section, accounting for the patient’s overall body habitus whilst still achieving adequate image quality (Fig. 3).
Figure 3. a, b.
Images of a reduced dose CT image (a) from a cryoablation procedure compared with a full dose image (b).
There were no major complications associated with the 103 cryoablation procedures. At 12-month follow-up, the technical success for the full dose (n=62) was 97% with 2 patients requiring a further cryoablation for residual tumor. The technical success for the reduced dose group (n=41) was 100%.
Discussion
This study found that an average dose reduction of 44% could be achieved following a cryoablation dose reduction scanning protocol whilst maintaining adequate image quality and procedural technical success, reinforcing the existing literature that cryoablation for renal cell carcinoma, although beneficial to the patient, comes with a high radiation dose penalty which must be addressed (15, 16, 19).
Leng et al. (14) and Arnold et al. (15) previously found radiation doses could be reduced by up to 50% whilst maintaining acceptable image quality for interventional procedures. Leng et al. (14) consistently obtained doses of up to 7814 mGy·cm, comparable to the full dose group in this study. The total CTDIvol values reported by Leng et al. (14) were a mean of 515 mGy in a smaller sample size which were higher than both our full and reduced dose groups (349 and 202 mGy, respectively) (14). We acknowledge the differences in study design that make it difficult to further compare our study with the Leng et al. (14) study, who used a simulation model to calculate dose savings. Other potential differences include scan length, desired image quality which can be subjective, and the total number of scans taken during each procedure which would ultimately impact on patient dose. Levesque et al. (19) had also demonstrated a significant dose reduction of 54% following introduction of a reduced dose scanning protocol (29). Compared to this study, there were notable differences in the Levesque et al. (19) study contributing to the greater dose reduction including a lower peak kilovoltage of 100 kVp and the greater use of CT fluoroscopy during cryoprobe placement in the reduced dose group (85% of cases) compared with only 28% of the full dose group (19). However, using CT fluoroscopy comes with a radiation penalty to the operator, and Stewart et al. (20) found the estimated annual radiation dose to the operator without lead shielding was 3.9 mGy (20).
Any dose reduction possible in high dose procedures such as CT-guided cryoablation, which requires multiple monitoring scans is important as there is a risk that patients could receive skin doses that approach the threshold of 2Gy at which transient skin effects may occur (14, 16). This study found median estimated “skin doses” of 384 mGy for the full dose group and 224 mGy for the reduced dose group, both well below the 2 Gy threshold for deterministic effects. The maximum recorded skin dose was 1065 mGy, which is only 53% of the minimum dose required for transient erythema.
Dose reduction strategies during post-ablation imaging follow-up remain equally important (21); Arnold et al. (15) found that patients received effective doses of greater than 50 mSv when assuming a 3-, 6- and 12-month follow-up CT scanning regime.
Although we have successfully adjusted mAs values according to patient size, a reduction in kVp, especially for smaller patients, should be considered in a future study as a further method of dose reduction. Furthermore, reducing the scan length to the minimum needed to cover the area of interest would also lower the total dose. This aspect was not directly addressed although it is standard practice for the radiographer to review the scanned volume based on the CT scout images.
Whilst a diagnostic scan needs to yield images of an acceptable quality, the placement of cryoablation needles does not need the same level of image quality hence the dose can be reduced (22). Ultrasound should also be considered as a useful adjunct given the lack of ionizing radiation and benefit of real-time imaging to help with needle placement. A recent study found the use of ultrasound instead of CT for ablation probe placement reduced the mean total DLP to as low as 805 mGy; however, the renal tumors treated were small with a mean number of cryoprobes used only 2.6 compared with 6 in our study, which is a further factor accounting for the lower total DLP. The usual limitations of ultrasound also apply in this instance such as obscuration of the tumor by bowel gas artifact, difficult to visualize tumors due to body habitus and obscuration of the cryoablation iceball due to acoustic shadowing (19).
The limitations of this study, apart from its retrospective design, were that radiation dose was estimated using DLP which does not accurately quantify dose to the individual patient. However, DLP has been used in previous studies on dose reduction (19). Given the study included historic procedures from over 5 years ago with older CT scanners, this accounts for the higher DLP values than would be expected in current practice where more dose reduction techniques are available. The patient cohorts were not matched; however, there was no statistical significance in the baseline clinical characteristics, including the number of scans required per cryoablation procedure, providing additional evidence supporting the use of a reduced dose procedure. Given the reduced dose group was treated after the standard dose group, greater operator experience is a potential confounding factor and may have led to increased accuracy of probe positioning and limiting the anatomical coverage of each CT scan during the targeting phase, although the number of scans did not differ between the groups. Assessment of imaging quality by the interventional radiologist can be subjective; therefore, selecting 50 mAs as the minimum setting at which clinically adequate images quality could be achieved was a consensus decision by the entire interventional radiology team.
In conclusion, a CT dose reduction technique during image-guided cryoablation treatment of renal tumors can achieve significant radiation dose reduction when compared with the full dose cohort whilst maintaining sufficient image quality.
Main points.
CT-guided cryoablation for renal tumors can involve high radiation doses.
This study found that an average dose reduction of 43.72% could be achieved following a CT dose reduction technique during cryoablation treatment of renal tumors whilst maintaining sufficient image quality and procedural technical success.
At 12-month follow-up, the technical success for the full dose (n=62) was 97% with 2 patients requiring a further cryoablation treatment for residual tumor. The technical success for the reduced dose group (n=41) was 100%.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Statistics OfN. Cancer statistics registrations: Registrations of cancer diagnosed in 2008. England: Office for National Statistics, National Statistics London; 2010. [Google Scholar]
- 3.Zagoria RJ. Imaging of small renal masses: A medical success story. AJR Am J Roentgenol. 2000;175:945–955. doi: 10.2214/ajr.175.4.1750945. [DOI] [PubMed] [Google Scholar]
- 4.Smittenaar CR, Petersen KA, Stewart K, Moitt N. Cancer incidence and mortality projections in the UK until 2035. Br J Cancer. 2016;115:1147–1155. doi: 10.1038/bjc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998;51:203–205. doi: 10.1016/S0090-4295(97)00506-2. [DOI] [PubMed] [Google Scholar]
- 6.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: A need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen MM, Gill IS, Ellison LM. The evolving presentation of renal carcinoma in the united states: Trends from the surveillance, epidemiology, and end results program. J Urol. 2006;176:2397–2400. doi: 10.1016/j.juro.2006.07.144. [DOI] [PubMed] [Google Scholar]
- 8.Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001;166:1611–1623. doi: 10.1016/S0022-5347(05)65640-6. [DOI] [PubMed] [Google Scholar]
- 9.Atwell TD, Carter RE, Schmit GD, et al. Complications following 573 percutaneous renal radiofrequency and cryoablation procedures. J Vasc Interv Radiol. 2012;23:48–54. doi: 10.1016/j.jvir.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Deng W, Chen L, Wang Y, et al. Cryoablation versus partial nephrectomy for clinical stage t1 renal masses: A systematic review and meta-analysis. J Cancer. 2019;10:1226–1236. doi: 10.7150/jca.28881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmit GD, Atwell TD, Callstrom MR, et al. Percutaneous cryoablation of renal masses >or=3 cm: Efficacy and safety in treatment of 108 patients. J Endourol. 2010;24:1255–1262. doi: 10.1089/end.2009.0328. [DOI] [PubMed] [Google Scholar]
- 12.Breen DJ, Bryant TJ, Abbas A, et al. Percutaneous cryoablation of renal tumours: Outcomes from 171 tumours in 147 patients. BJU Int. 2013;112:758–765. doi: 10.1111/bju.12122. [DOI] [PubMed] [Google Scholar]
- 13.Oguro S, Tuncali K, Elhawary H, Morrison PR, Hata N, Silverman SG. Image registration of pre-procedural MRI and intra-procedural CT images to aid CT-guided percutaneous cryoablation of renal tumors. Int J Comput Assist Radiol Surg. 2010;6:111–117. doi: 10.1007/s11548-010-0485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leng S, Christner JA, Carlson SK, et al. Radiation dose levels for interventional CT procedures. AJR Am J Roentgenol. 2011;197:W97–W103. doi: 10.2214/AJR.10.5057. [DOI] [PubMed] [Google Scholar]
- 15.Arnold DC, II, Schroeder G, Smith JC, et al. Comparing radiation exposure between ablative therapies for small renal masses. J Endourol. 2013;27:1435–1439. doi: 10.1089/end.2013.0209. [DOI] [PubMed] [Google Scholar]
- 16.Leng S, Atwell TD, Yu L, et al. Radiation dose reduction for CT-guided renal tumor cryoablation. AJR Am J Roentgenol. 2011;196:W586–W591. doi: 10.2214/AJR.10.5144. [DOI] [PubMed] [Google Scholar]
- 17.Schmit GD, Schenck LA, Thompson RH, et al. Predicting renal cryoablation complications: New risk score based on tumor size and location and patient history. Radiology. 2014;272:903–910. doi: 10.1148/radiol.14132548. [DOI] [PubMed] [Google Scholar]
- 18.Zargar H, Atwell TD, Cadeddu JA, et al. Cryoablation for small renal masses: Selection criteria, complications, and functional and oncologic results. Eur Urol. 2016;69:116–128. doi: 10.1016/j.eururo.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Levesque VM, Shyn PB, Tuncali K, et al. Radiation dose during CT-guided percutaneous cryoablation of renal tumors: Effect of a dose reduction protocol. Eur J Radiol. 2015;84:2218–2221. doi: 10.1016/j.ejrad.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Stewart JK, Looney CB, Anderson-Evans CD, et al. Percutaneous cryoablation of renal masses under CT fluoroscopy: Radiation doses to the patient and interventionalist. Abdom Imaging. 2015;40:2606–2612. doi: 10.1007/s00261-015-0456-2. [DOI] [PubMed] [Google Scholar]
- 21.Eisenberg JD, Gervais DA, Singh S, et al. Radiation exposure from ctguided ablation of renal masses: Effects on life expectancy. AJR Am J Roentgenol. 2015;204:335–342. doi: 10.2214/AJR.14.13010. [DOI] [PubMed] [Google Scholar]
- 22.Yu L, Liu X, Leng S, et al. Radiation dose reduction in computed tomography: Techniques and future perspective. Imaging Med. 2009;1:65–84. doi: 10.2217/iim.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen PL, Chen RC, Clark JA, et al. Patient-reported quality of life after salvage brachytherapy for radio-recurrent prostate cancer: A prospective phase II study. Brachytherapy. 2009;8:345–352. doi: 10.1016/j.brachy.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]