Abstract
PURPOSE
Computed tomography (CT) premises are one of the strategic points in the spread of hospital-acquired infections. Ultraviolet germicidal irradiation (UVGI) is an effective method that could potentially be used to purify the ambient air in them. However, it cannot be directly used in the presence of humans and, therefore, it is not operationally suitable in such units with continuous human circulation. Newer devices have been developed to purify air with more efficient and shielded UV-C sources. This study aims to assess the microbial air contamination in CT scanning rooms and investigates the efficacy and technical considerations of shielded UV-C arrays.
METHODS
Two shielded UVGI systems, each equipped with 15 Watt UV-C LED arrays, were tested in a very busy CT unit. Initially, a pilot study was performed to determine ambient microorganisms under routine conditions before UVGI installation, followed by three basic scenarios of UVGI use under normal and abnormal conditions: A, UVGI, with both air-conditioning (AC) and ventilation on; B, UVGI, with AC on and ventilation off; C, UVGI, with both AC and ventilation off. Ambient air was sampled in various time points before and after the initialization of UV irradiation and analyzed for colony formation.
RESULTS
The mean total colony count in the pilot study was 1360±450 CFU/m3. Pre-UVGI colony count was 3510 CFU/m3 for Scenario A, ~10000 CFU/m3 for Scenario B and 990 CFU/m3 for Scenario C. Thirty minutes after UVGI, total colony counts in all three scenarios dropped to 30 to 70 CFU/m3. Under normal operating conditions and UVGI, the mean colony count was found as 21.4±13.5 CFU/m3 and the average efficacy of the UVGI was found as 99.39%.
CONCLUSION
This study identified substantial microbial air contamination in CT scanning rooms during normal and abnormal operating conditions. UV-C LED arrays effectively eliminate these microbiological contaminants. This effect is also observed under abnormal operating conditions where no other means of ventilation or air conditioning exists.
Computed tomography (CT) premises theoretically comprise one of the strategic points in the spread of hospital-acquired infections (1, 2). There is an increased use of these systems during COVID-19 pandemic as they play a key role in assisting diagnosis and have even been used as a primary diagnostic tool in some countries on the basis of case definitions (2–4). Therefore, these facilities have gained recognition as being of major importance during the pandemic regarding disease transmission (5, 6). Several attempts, mostly empirical, were undertaken to ensure microbiological decontamination in these units. Not only surfaces, but air in the scanning rooms may theoretically contain a significant amount of pathogenic microorganisms (1, 5). Various methods exist for atmospheric decontamination, one of which is the process of purification by ultraviolet (UV) radiation.
The antimicrobial effects of UV radiation have been known for a long time (7). Radiation is classified according to wavelength, as A, B, and C; the shorter the wavelength, the more severe its biological effects. Ultraviolet C (UV-C) radiation, consisting of wavelengths shorter than 280 nm, is completely absorbed by the atmosphere and is unable to reach the earth’s surface. Nevertheless, UV-C radiation is artificially produced by low-pressure mercury vapor discharge lamps or light-emitting diode (LED) lamps, and is used particularly to decontaminate the water sources from bacteria, viruses, fungi, and certain protozoa through photochemical reactions. These systems are referred to as UV germicidal irradiation (UVGI), in which naked-type mercury lamps are used in health institutions for temporary irradiation of environments such as laboratories, operating rooms, and biological safety cabinets. However, the harmful effects of UV-C radiation in humans, especially on the skin and eyes, is well known (8). For this reason, uncovered (naked) systems are not directly used in the presence of patients and personnel and are not operationally suitable in environments with continuous patient circulation.
In order to eliminate the abovementioned disadvantages of conventional UVGI systems, newer devices have been developed to purify ambient air with much more efficient UV-C LED sources and shielded systems. The potential use of these systems in the crowded environments of health institutions have gained importance throughout the pandemic period. However, the quantification of microorganisms in the air in CT scanning rooms and the effectiveness of UVGI was not scientifically evaluated to this date. This is an initial study that investigates the use and the efficacy of shielded UVGI systems in CT scanning rooms with continuous and intense use.
Methods
Setting
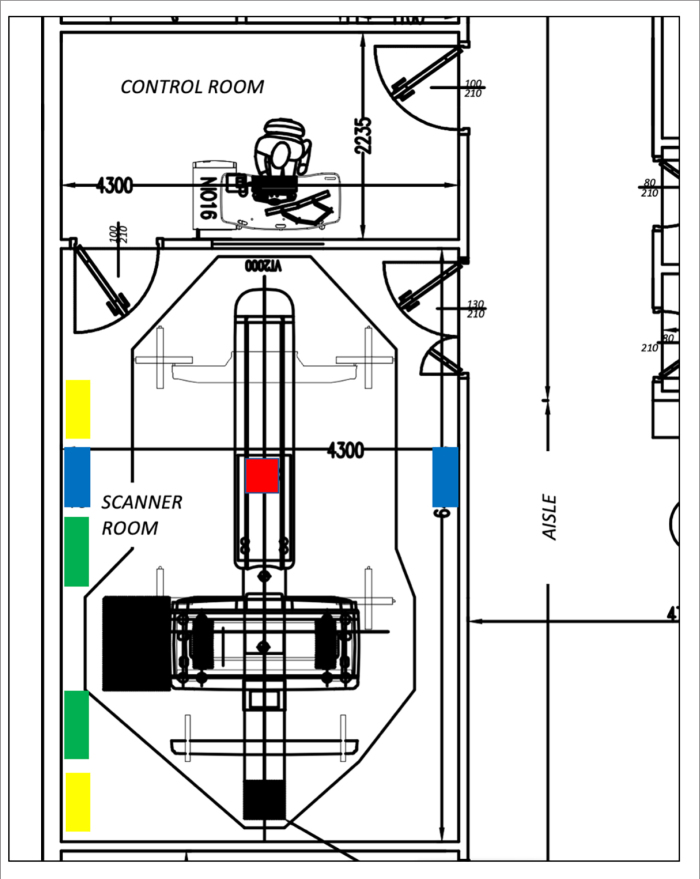
This study was conducted in a CT scanning room located in the emergency radiology center of a tertiary referral hospital: the institution is officially designated as a pandemic hospital. An average of 320 daily thoracic examinations have been performed in that particular unit during the climax of the pandemic. The CT device was a 128-slice scanner (Optima 660 SE, GE Healthcare). Usable dimensions of the designated room were 654×417×310 cm (L×W×H) and its gross volume was 84.54 m3 (Fig. 1). The room had a false ceiling with acoustic ceiling tiles. There were two doors to the room. The door between the scanning and the control room was 210×100 cm in size and had a hydraulic closing mechanism to keep it always closed except during personnel passage. The main door for the patient entrance was 210×130 cm in size and was kept open for ventilation between each patient. The room contained two split-type air conditioner (AC) units. These units were located 250 cm above the floor, each with 24000 BTU/hour capacity and set to 20ºC internal room temperature. Compressors and outdoor air intakes for these systems were at least 180 cm above ground (9). However, they run in recirculation mode to dissipate the heat at maximal efficiency. With the use of those systems, the temperature was usually kept below 23°C and relative humidity level was kept below 60% (9, 10). ACs did not have filtration, as they were independent and served only the scanner room. The room also had two duct-type fans to remove ionized air. One of these fans was located 30 cm above the floor and absorbed 880 m3 air per hour, and the other was located 30 cm below the ceiling and emitted 880 m3 air per hour, hence providing more than 12 air change per hour for the useful volume of the room. Exhaust outlet from the room was situated above the roof level and arranged to minimize the recirculation of exhausted air back into the building (9).
Figure 1.
Architectural design of the CT scanning room and placement of the devices: UVGI (blue); AC (green); ventilation (yellow); active air sampler (red).
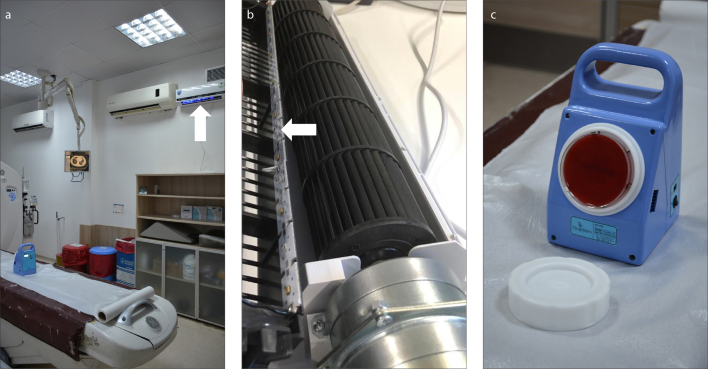
Along the long walls of the room, two shielded UVGI fan systems (UVC FAN-15, AeroRad) were positioned 250 cm above the floor, aligned to the approximate center of the CT table at both sides (Fig. 2a). Each of these devices used a linear array of 3.5×3.5×1.36 mm LED elements, producing UV-C radiation of 270–280 nm in wavelength and 15 Watt in power. They also provided air circulation at a rate of 286 m3/hour per device. The LED arrays, used as a source of UV-C in these devices were positioned before the output duct of the device, and they were therefore shielded from outside environment (Fig. 2b). Within the framework of the specified system and the setup schematized in Fig. 1, the entire room volume was theoretically processed in 8.86 minutes.
Figure 2. a–c.
Study environment and devices. Panel (a) shows the CT scanning room and UVGI system (arrow); panel (b) shows the placement of the UV-C LED elements behind the fan (arrow); panel (c) shows the air sampling device and blood agar.
Pilot study and operating scenarios
All studies were conducted in the same room during four separate workdays with almost equivalent workloads. Initially, a pilot study was conducted to determine the actual presence of microorganisms in the air of the CT scanning room that may merit decontamination, as well as their variability in numbers and types. In this pilot study, UVGI was not installed yet, and AC and ventilation were on as usual. The pilot study lasted for 6 hours, from noon to 6 PM. During that run, 57 patients were examined and the number of scans was 94. During the pilot study, 18% of the scans were thoracic examinations for COVID-19.
During the operating scenarios, three basic tests representing normal and abnormal operating conditions were investigated: (i) Scenario A (normal): UVGI, AC, and ventilation on; (ii) Scenario B (abnormal): UVGI and AC on, ventilation off; (iii) Scenario C (abnormal): UVGI on, AC and ventilation off. The room continued to be in routine use as each of the scenarios was run. Each scenario, except Scenario C, lasted for 6 hours, from noon to 6 PM. Number of patients imaged during these test periods were 45, 52, and 16, and number of scans were 73, 88, and 23, respectively.
During the operating scenarios, approximately 28% of scans were thoracic examinations for COVID-19. During the pilot study and the operating scenarios, all patients, irrespective of their COVID-19 status suspected or confirmed, were provided with disposable medical masks upon their arrival for CT imaging and were admitted to CT scanning room after covering their mouth and nose (11). Gantry and the patient couch of the CT scanner was cleaned and disinfected after encounter with such patients by focusing on high-touch surfaces and by using a fast-acting disinfecting solution that contained 5% 2-Propanol (CAS registry number: 67-63-0) and 2% didecyldimethylammonium chloride (CAS registry number: 7173-51-5) as active substrates. The floor of the scanning room was cleaned and disinfected three times a day with 1/10 diluted sodium hypochlorite solution with 6%–14% active chlorine (6, 4, 11, 12). Neither surface nor floor decontamination took place during the actual microbiological sampling. A terminal cleaning and disinfection of the scanning room occurred at the end of each day.
Air sampling
Air measurements were performed with a device known as an active air sampler (Hytest Air, Diatek). The sampler was brand-new and had factory-certified initial calibration before its use. This equipment is used to sample a previously determined quantity of air of between 10 and 1000 liters to quantitate the microbiological status of air in the environment. There is no internationally recognized standard for air sampling in the radiology units (13). Therefore, choice of media, sampling volume, frequency, sampling position, and incubation time were based on a recent paper of Stauning et al. (14) on operating rooms and recommendations from infection control staff at our institution. A sampling volume of 1000 L, as suggested for nonradiological settings was not possible since a pilot study with this volume returned all samples with too many colonies to give reliable counts (15). We have therefore used a sampling volume of 100 L as recommended by Staunning et al. (14). Under each of the conditions described above, the sampler was placed in the center of the room on the patient couch and the air were sampled on blood agar plates with 5% defibrinated sheep blood immediately before (0 minutes) and 30, 60, 120, 180, 240, 300, and 360 minutes after initialization of UVGI (Fig. 2c). Samples for “0” minutes were obtained in order to get a “reference value” of ambient bacteria conditions for each scenario. For that reason, the scenario conditions-other than the UVGI, were in place for 2 hours before the initial reference sampling at 0 minutes was performed. The UVGI, on the other hand, was completely operational at all times except during each testing period of 6 hours and was made operational immediately after obtaining initial reference sampling at the beginning of the experiment (i.e., 0 min).
Data analysis
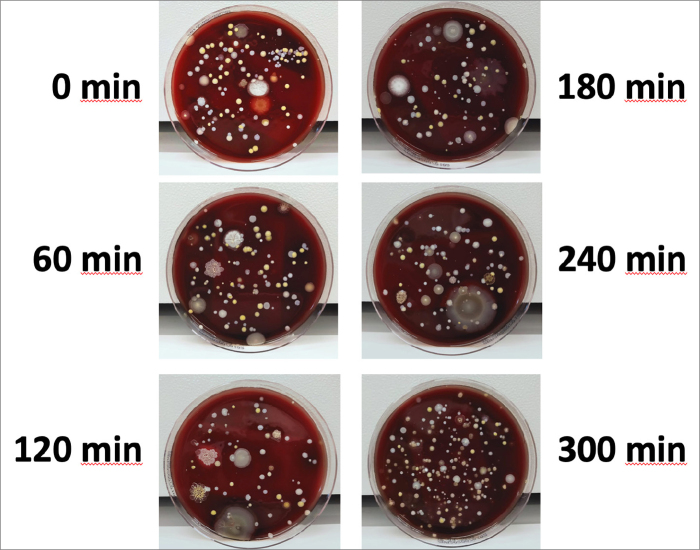
For the analysis of bacteria an incubation period of 48 hours is required. Therefore, plates were incubated for 48 hours at 35ºC and were photographed at the end of that period (Fig. 3). They were read by a medical microbiologist blinded to the experimental settings. Laboratory analysis was done to determine the total amounts of bacteria and fungi and to identify 3 major species of bacteria (i.e., diphtheroids, bacilli sp., Staphylococcus spp.) that was indicated in relevant literature (1). These types were also found to be prevalent in our pilot study and they were known to have particular resistance to low level of disinfection (12). The limit of detection (LOD) of this sampling and analysis method was 1 colony forming unit (CFU) per 100 liters. Therefore, colony counts were indicated as CFU per 100 liters (CFU/100 L) of air. However, data regarding total colony count were transformed to CFU per metric cube (CFU/m3) by multiplying them with 10 to allow comparison with relevant literature. The incubation of fungi takes up to 7 days for differential analysis. Therefore, samples were further incubated at room temperature up to 7 days and re-photographed at the end of that period for better visual demonstration of fungi.
Figure 3.
Appearance of the medium in the pilot study exposed to air sampling with one hour intervals.
Data were presented using descriptive methods. Minimum, maximum, mean ± standard deviation for total colony counts were indicated for each scenario. The efficacy (E) for each scenario was calculated as described below and was based on the average number of total colony counts at 30 to 360th minutes during each scenario and the number of total colony counts at 0 minute for each scenario which served as the control count.
Research ethics standards compliance
The study was approved by Institutional Review Boards (Approval no: 17073117_050.06_E.114 and 2020-06-15T16 _41_08). This study was not a clinical investigation on human subjects or a laboratory experiment animal subjects, sample collection did not prolong the radiological examination or alter the scanning protocols and UVGI was not a part of regulatory or customary air purification measures for the premise where the study was conducted. Informed consent was obtained for radiological investigation precluding these factors.
Results
The mean total colony count in the pilot study was 1360±450 CFU/m3 (Fig. 3, Table 1). Pre-UVGI total colony count (i.e., reference value) was 3510 CFU/m3 for Scenario A, 10000 CFU/m3 for Scenario B and 990 CFU/m3 for Scenario C. Thirty minutes after UVGI, colony counts for Scenarios A, B and C were dropped to 30, 70, and 60 CFU/m3, respectively. At the end of six hours, colony counts of Scenario A and B were 40 and 20 CFU/m3, respectively. In Scenario C, in which AC was disabled, the room temperature reached 26.8ºC at 120 minutes. Since maximum permissible room temperature for CT system was 27ºC, the study was discontinued at that point in time (Fig. 4, Table 2). According to Table 2, total colony counts for Scenario A were between 0 and 40 CFU/m3 (21.4±13.5 CFU/m3). Total colony counts for Scenario B were between 10 and 70 CFU/m3 (37.1±26.3 CFU/m3). Total colony counts for Scenario C were between 30 and 60 CFU/m3 (46.7±15.3 CFU/m3). The number of colonies detected during each test period continued to be at very low levels (at most +1 colonies per 100 L of air compared to colony count at 30 min for same volume) despite the intense use of the room and subsequent flow of patients (45 patients for Scenario A, 52 patients for Scenario B and 16 patients for Scenario C) and personnel in and out of the room (Fig. 3).
Table 1.
Colony count and its distribution at 48 hours in the pilot study
| Experimental condition | Time point (min) | UVGI | Ventilation | AC | Temperature (°C) | Humidity (%) | Total colonies (CFU/100 L) | Diphtheroid (CFU/100 L) | Bacilli sp. (CFU/100 L) | Staphylococcus species (CFU/100 L) | Fungi (CFU/100 L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pilot study | 0 | None | On | On | 23.7 | 49 | 198 | 19 | 2 | 176 | 1 |
|
| |||||||||||
| 60 | None | On | On | 23.6 | 49 | 113 | 9 | 10 | 93 | 1 | |
|
| |||||||||||
| 120 | None | On | On | 23.8 | 49 | 94 | 25 | 6 | 63 | 0 | |
| 180 | None | On | On | 24.1 | 50 | 109 | 22 | 6 | 80 | 1 | |
|
| |||||||||||
| 240 | None | On | On | 24.1 | 47 | 114 | 31 | 12 | 71 | 0 | |
|
| |||||||||||
| 300 | None | On | On | 24.0 | 48 | 187 | 51 | 20 | 116 | 0 | |
|
| |||||||||||
| Mean | None | On | On | 23.8 | 48.7 | 136 | 26 | 9 | 100 | 1 | |
UVGI, ultraviolet germicidal irradiation; AC, air conditioning; CFU, colony forming unit.
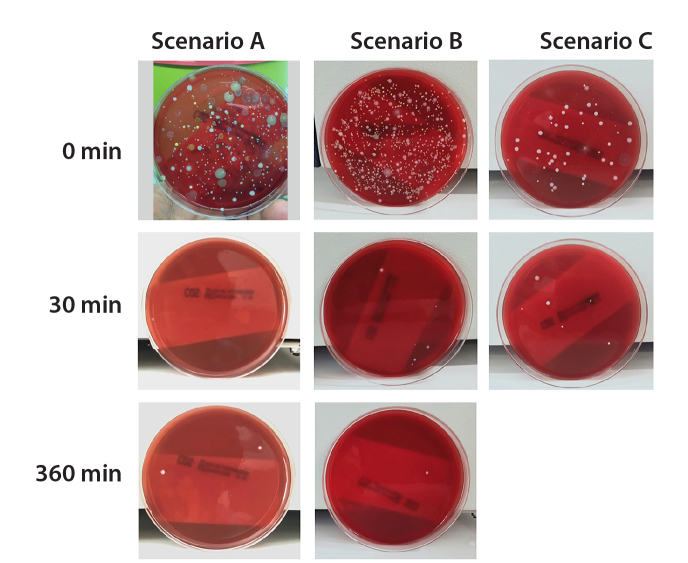
Figure 4.
Appearance of the medium exposed to air sampling before UV-C exposure and 30 and 360 minutes after UV-C exposure. UV-C significantly decreases the number of microorganisms in the environment under all three conditions (Scenario C was brought to an end at the end of two hours, in which ambient air temperature had reached the maximum permissible temperature specified for the CT device).
Table 2.
Colony count and its distribution at 48 hours in three different experimental conditions
| Experimental condition | Time point (min) | UVGI | Ventilation | AC | Temperature (°C) | Humidity (%) | Total colonies (CFU/100 L) | Diphtheroid (CFU/100 L) | Bacilli sp. (CFU/100 L) | Staphylococcus species (CFU/100 L) |
|---|---|---|---|---|---|---|---|---|---|---|
| Scenario A (normal condition) | 0 | Off (1) | On | On | 20.3 | 48 | 351 | 310 | 15 | 25 |
|
| ||||||||||
| 30 | On | On | On | 20.7 | 49 | 3 | 0 | 0 | 2 | |
|
| ||||||||||
| 60 | On | On | On | 21.4 | 49 | 2 | 0 | 0 | 1 | |
| 120 | On | On | On | 21.9 | 47 | 1 | 0 | 0 | 1 | |
|
| ||||||||||
| 180 | On | On | On | 21.8 | 47 | 0 | 0 | 0 | 0 | |
|
| ||||||||||
| 240 | On | On | On | 21.8 | 48 | 3 | 0 | 1 | 0 | |
|
| ||||||||||
| 300 | On | On | On | 21.2 | 48 | 2 | 0 | 0 | 1 | |
|
| ||||||||||
| 360 | On | On | On | 20.8 | 49 | 4 | 0 | 3 | 0 | |
|
| ||||||||||
| Scenario B (abnormal condition) | 0 | Off (1) | Off (2) | On | 19.3 | 56 | ~1000 | 40 | 5 | ~900 |
|
| ||||||||||
| 30 | On | Off | On | 19.2 | 53 | 7 | 0 | 0 | 7 | |
|
| ||||||||||
| 60 | On | Off | On | 19.1 | 53 | 1 | 0 | 0 | 1 | |
|
| ||||||||||
| 120 | On | Off | On | 19.3 | 52 | 7 | 0 | 0 | 7 | |
|
| ||||||||||
| 180 | On | Off | On | 19.1 | 50 | 3 | 0 | 0 | 3 | |
|
| ||||||||||
| 240 | On | Off | On | 19.6 | 49 | 5 | 0 | 0 | 5 | |
|
| ||||||||||
| 300 | On | Off | On | 19.8 | 49 | 1 | 0 | 0 | 1 | |
|
| ||||||||||
| 360 | On | Off | On | 19.9 | 50 | 2 | 0 | 0 | 2 | |
|
| ||||||||||
| Scenario C (abnormal condition) | 0 | Off (1) | Off (2) | Off (3) | 22.5 | 43 | 99 | 58 | 1 | 39 |
|
| ||||||||||
| 30 | On | Off | Off | 23.4 | 46 | 6 | 2 | 0 | 4 | |
|
| ||||||||||
| 60 | On | Off | Off | 25.1 | 46 | 5 | 0 | 0 | 5 | |
|
| ||||||||||
| 120 | On | Off | Off | 26.8 | 44 | 3 | 0 | 1 | 2 | |
UVGI, ultraviolet germicidal irradiation; AC, air conditioning; CFU, colony forming unit.
UVGI was activated only during the test periods;
Ventilation was deactivated 2 hours before the test;
AC was deactivated immediately before the test.
The most abundant (3100 CFU/m3) microorganism observed at the beginning of Scenario A was the diphtheroid bacillus. In Scenario B, with the ventilation system turned off, Staphylococcus sp. were more common (9000 CFU/m3). In Scenario C, in which the ventilation and AC turned off, there was no significant predominance of any specific microorganism (Fig. 4, Table 2).
According to these findings, total efficacy of the UVGI fan system was highest in Scenario B (99.63%), and lowest in Scenario C (95.28%). In normal operating conditions (i.e., Scenario A) the efficacy was 99.39%, almost equal to the efficacy in Scenario B.
Discussion
This study demonstrates that the air in the CT room contains a considerable amount of microorganisms in routine working conditions. Conventional ventilation systems can theoretically reduce particles suspended in the air by removing contaminated air from the room and providing fresh air in return (16). In this regard, they may act as a simple measure against infection by diluting indoor air and evacuating infectious agents from around a source (17). In fact, in Scenario B during which ventilation was deactivated, the initial number of ambient microorganisms in the environment was significantly above the numbers in normal working conditions (10000 CFU/m3 vs. 3510 CFU/m3, respectively). However, even in well-ventilated rooms, number of microorganism in the air is still very high, as shown in the pilot study (mean, 1360 CFU/m3), and at the start of Scenario A (3510 CFU/m3). Moreover, the primary role of the ventilation systems is not to control the infection but to provide fresh air to patients and workers and, at least for the country where this study was conducted, to prevent the accumulation of possibly negligible amount of ionized air in the environment. These systems are deactivated by the technologist in very hot days to keep the scanner room temperature within specified limits, as ventilation fans emit fresh but warm air directly from outside. To summarize, ventilation plays only a collateral role in infection control, but it still complies with the recent advice of World Health Organization (WHO) that recommends CT scanning rooms to be adequately ventilated, and if aerosol-generating procedures are to be performed at least 12 air changes per hour to be provided (6, 18).
In Scenario C, in which ventilation and AC were simultaneously deactivated, the initial number of ambient microorganisms in the air was much lower than in Scenario A during which these systems were fully operational (990 CFU/m3 vs. 3510 CFU/m3). This observation indicates that AC may actually be an important factor leading to microbiological contamination. Microorganisms are known to proliferate in environments where air, dust, and water are present, and AC systems can be ideal environments for microbial growth if not properly maintained (18). Partly for that reason and partly because of their perturbation effects on ambient air layers, these systems are not permitted by many guidelines during the pandemic, if not working at very low flow levels (19). However, such an operating condition (i.e., operating without AC) is not practical, as it is impossible to keep the scanner room’s temperature within specified limits during intense use and in very hot days without cooling the ambient air.
Not only total numbers, but also predominant species were noted to vary between different operating conditions. In that context, Staphylococcus sp. became more prevalent and significantly increased in number after deactivating the ventilation and normalized after deactivating AC units. It is known that accumulation of dust and moisture within heating-ventilation-air-conditioning (HVAC) systems increases the risk for environmental fungi and bacteria, and clusters of infections caused by Aspergillus spp., P. aeruginosa, S. aureus, and Acinetobacter spp. have been linked to poorly maintained and/or malfunctioning air conditioning systems (20, 21) (Fig. 5). In that context, it may again be hypothesized that a high capacity ventilation dilutes and partially removes such microorganisms but cannot effectively eliminate them.
Figure 5.
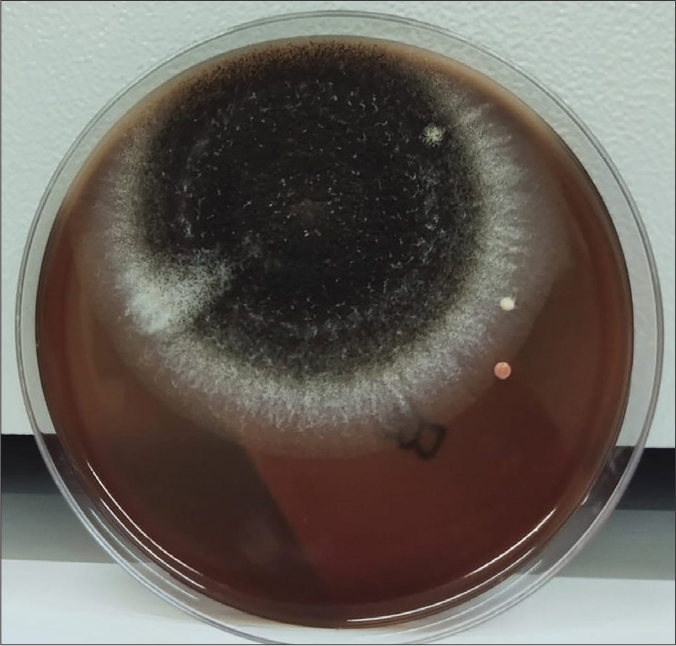
Mold colonies on day 7. Aspergillus colony was observed at 60 min in Scenario B during which AC was fully operational. This is a major fungal microorganism with airborne transmission in healthcare facilities (20).
Four different methods are currently used to reduce the amount of microorganisms in the air, hence the risk of airborne transmission, including pressurization, dilution, filtration, and purification. As stated in the introduction, UVGI is an important method used under the latter method. Studies on the use of UV-C lamps in health facilities have been conducted in the past (22). It has been determined that UVGI was especially effective against infectious agents in the air, preventing the replication and disrupting vital cellular functions of bacteria and viruses by damaging nucleic acids (22, 23). These studies were based on the longterm irradiation with mercury UV-C lamps in human-free environments. The current study differs from other studies in four main aspects: (i) it was conducted in a CT scanning room, (ii) in routine use, (iii) under three different scenarios representing normal and abnormal working conditions, and (iv) using shielded type LED UV-C source (22, 24).
This study demonstrates that UV-C exposure, the biological mechanism of which is summarized above, has decontaminating effect on airborne microorganisms under normal and many abnormal conditions. This effect reaches up to 99.39% under normal conditions where the CT scanning room is functioning in the specified manner (i.e., ventilation and AC on), with equipment routinely operating and with the specified number of personnel present. Although the routine flow of patients and personnel continued in the CT scanning room, the ongoing UV-C exposure preserved the level of microbiological decontamination not to exceed 1 CFU/100 L in colony count. Due to human circulation in the indoor environment and the presence of two doors, indoor and outdoor air were constantly mixed and it was theoretically impossible to reduce the number of microorganisms in the air to absolute zero in normal conditions. However, the mean total colony count of 21.4 CFU/m3 during normal operating conditions was still within the limits for industrial clean rooms and may be classified microbiologically as U.S. Customary Class of 10000 according to former Federal Standard 209E (25). It is also within the recommended limits of current ISO Class 8 (26) and Grade C of European Union Guide to Good Manufacturing Practices (27). Within the context of healthcare facilities, there is no internationally recognized standard for air contamination levels in radiology units. However, there are certain but varying recommendations for other healthcare units. As an example, acceptable air contamination in operating rooms varies from 180 CFU/m3 as suggested by the Healthcare Infection Society to 100 CFU/m3 which is widely used by the Scandinavian health authorities (15, 28, 29). In the present study, CFU levels that were reached after UVGI were lower than these levels that are designated for good surgical practices. This is even true for abnormal conditions. In this study, UVGI effectively lowered total colony counts in Scenario B and C, and decreased Staphylocococcus sp. colonies almost 1000 folds after ventilation was deactivated (i.e., from 9000 CFU/m3 to 10 CFU/m3, in Scenario C). Ventilation by itself is not as effective as UVGI, as in Scenario B, the reference colony number for Staphylocococcus sp. was 250 CFU/m3. UVGI, therefore significantly decontaminates the air with regard to several microorganisms even in the absence of proper ventilation. This observation is also valid for the absence of proper AC with ventilation and may act as a contingency measure in case of disruption of such conditions. Reactivation of ventilation and AC systems are known to cause a transient burst of microorganism and necessitate all air filters to be changed (20). UVGI, may also counteract such burst as it was shown to effectively eliminate several microorganisms within 30 minutes in all scenarios. On the other hand, as stated above, the use of AC systems with air recirculation are not recommended during the pandemic (19). Nevertheless, in CT scanning rooms that houses scanners with high scan rates, they need to be present to help cool the machine. UVGI efficiently eliminates total number of microorganisms in such situations and reaches to over 95% efficacy as shown in Scenario C.
Direct exposure to UV-C radiation is harmful to human health (8, 30). The most common precaution against these effects is the placement of UVGI systems near the ceiling and screen their lower sections (i.e., upper room irradiation) (16, 17). Another method is to hide the UV-C source inside the ventilation device or ducts (i.e., duct irradiation) (20, 24, 31). The UVGI system used in our study was designed according to the second method, which does not require personnel or patients to be removed from the room during irradiation. Therefore, active use of the room can be continued and a great operational advantage is achieved. Another advantage of the second method is that, when combined with LED technology, it grants the opportunity to apply radiation at much higher levels in actively used rooms, thereby irradiating suspended particles more intensely and inactivating them faster (16). LED systems present much higher radiant power and allow emission of higher doses in shorter periods of time. An important point to be considered in shielded systems is that the air flow through the device must not exceed 6 air changes per hour (16). This is because faster air flow shortens the amount of time the particles are exposed to UV-C radiation. Therefore, in the UVGI systems used in our study, the rate of air changes per hour was adjusted to be approximately 3.4 air changes per hour for each device. The specified level should be calculated and adjusted independently for each space. Since the purification effect depends on air mixing via convection between the room’s irradiated upper zone and the lower patient-care zones, this should be taken into account during calculations (32, 33).
Apart from contamination of bacteria and fungi, air in CT scanning rooms may theoretically contain viral microorganisms. However, very few of them are consistently airborne in transmission (i.e., are routinely suspended in an infective state in air and capable of spreading great distances) and hospital-infections of airborne viral diseases are very limited (20). Therefore, infection-control measures used to prevent spread of these viral diseases in healthcare facilities primarily involve patient isolation, vaccination of susceptible persons, and antiviral therapy as appropriate rather than measures to control air flow or quality (34). As such, in COVID-19 international guidelines recommended droplet and contact precautions to be followed as droplets are known not to stay in the air for such distances, and droplet transmission followed by contaminated surfaces are believed to be the main modes of spread for SARS-CoV2 in radiology suites (11, 35, 36). However, the disease is known to have high concealment and rapid transmission (37). Aerosol propagation, therefore, is another theory yet to be proved (5). Such mode is thought to be important for premises where aerosol-generating procedures such as cardiopulmonary resuscitation or non-invasive ventilation are performed (6). These droplet nuclei can be suspended in the air for longer periods and be transported over longer distances (38). Also, it is recommended to turn off all ACs to prevent air contamination as tested in Scenario C (5). However, in CT scanning rooms AC systems are needed to keep the ambient temperature below certain limits to ensure scanner’s functionality, and this may theoretically cause dissemination of the infectious agent. There is a limited number of studies on the sensitivity of SARS-CoV-2 towards the germicidal effect of UV radiation. Studies on the effects of UV-C on members of the coronavirus family, including members of SARS-CoV, have demonstrated high efficacy of this radiation on the specified microorganisms (39, 40). In experiments, the necessary UV dosage for SARS-CoV reduction was investigated and it was found that UV-C light completely inactivated the SARS virus at a distance of 3 cm for 15 minutes at 4016 μW/cm2 (39). The fact that the genome of SARS-CoV-2 is 80% homologous with SARS-CoV suggests that SARS-CoV-2 could be deactivated by UV-C of similar magnitude and duration (41).
In conclusion, during the COVID-19 pandemic, many national and international recommendations for disinfection of radiology departments have been published and constantly updated, most of which are based on empirical approach (6). In light of the findings in this study, shielded UV-C LED fan systems stand out as an effective method in the decontamination of air in CT units for several microorganisms, where contact with certain or potential cases is inevitable. This effect is observed both for normal and abnormal operating conditions where no other means of ventilation or air conditioning exist or permitted. However, it should be kept in mind that decreasing germ numbers may not necessarily decrease the number of airborn contagious diseases in patients and workers. This technology, therefore, should further be evaluated regarding the end results and the protection of workers and patients from airborn contagious infections should be demonstrated on large scale comparative studies. Also, more specific studies on protection from COVID-19 infection are needed.
Main points.
Air in the CT room contains a significant amount of microorganisms, regardless of ventilation, under routine working conditions.
Microorganisms are known to proliferate in environments where air, dust, and water are present, and air-conditioning may be an important factor leading to microbiological contamination.
Ultraviolet C radiation exerts a significant decontaminating effect on airborne microorganisms under normal and many abnormal conditions with an efficacy of more than 99.39% in routine use, reducing mean colony counts to clean room standards. But it should not be used as a stand-alone technology.
LED ultraviolet C sources present much higher radiant power and with a shielded design they can be used while people are present in the room.
In CT scanning rooms air-conditioning is needed to keep the ambient temperature below certain limits to ensure scanner’s functionality, which may cause dissemination of SARS-CoV-2 during the COVID-19 pandemic. Theoretically, SARS-CoV-2 may be deactivated by ultraviolet C irradiation.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Ilyas F, Burbridge B, Babyn P. Health care–associated infections and the radiology department. J Med Imaging Radiat Sci. 2019;50:596–606. doi: 10.1016/j.jmir.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao Y, Xiang C, Wang S, Peng C, Zou Q, Hu J. Radiology department strategies to protect radiologic technologists against COVID19: Experience from Wuhan. Eur J Radiol. 2020 doi: 10.1016/j.ejrad.2020.108996.. doi: 10.1016/j.ejrad.2020.108996.. Published online 20 April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao C, Liu X, Zhang H, Li Y, Liu J. COVID-19 Computed tomography findings: a systematic review and meta-analysis. J Am Coll Radiol. 2020;17:701–709. doi: 10.1016/j.jacr.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H, Liu S, Xu H, et al. Guidelines for imaging diagnosis of COVID-19. 2nd ed. People’s Republic of China: Professional Committee of Radiology for Infectious Disease; 2020. [Google Scholar]
- 5.Huang Z, Zhao S, Li Z, et al. The battle against coronavirus disease 2019 (COVID-19): emergency management and infection control in a radiology department. J Am Coll Radiol. 2020;17:710–716. doi: 10.1016/j.jacr.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Use of chest imaging in COVID-19: A rapid advice guide. Geneva: World Health Organization; 2020. Cited July 2020. Available from: https://www.who.int/publications/i/item/use-of-chest-imaging-in-covid-19. [Google Scholar]
- 7.Perkins JE, Bahlke AM, Silverman HF. Effect of ultra-violet irradiation of classrooms on spread of measles in large rural central schools. Am J Public Health. 1947;37:529–537. doi: 10.2105/AJPH.37.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohania D, Chandel S, Kumar P, et al. Ultraviolet radiations: skin defense-damage mechanism. Ultraviolet Light in Human Health, Diseases and Environment. Ahmad S, editor. Adv Exp Med Biol. 2017;996:71–87. doi: 10.1007/978-3-319-56017-5_7. [DOI] [PubMed] [Google Scholar]
- 9.American Institute of Architects. Guidelines for design and construction of hospital and healthcare facilities. Washington, DC: American Institute of Architects Press; 2001. [Google Scholar]
- 10.Orme I. Patient impact. In: Hansen W, editor. A guide to managing indoor air quality in healthcare organizations. Oakbrook Terrace: Joint Commission on Accreditation of Healthcare Organizations Publications; 1997. pp. 43–52. [Google Scholar]
- 11.Kooraki S, Hosseiny M, Myers L, Gholamrezanezhad A. Coronavirus (COVID-19) outbreak: What the department of radiology should know. J Am Coll Radiol. 2020;17:447–451. doi: 10.1016/j.jacr.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ontario Agency for Health Protection and Promotion (Public Health Ontario) Provincial Infectious Diseases Advisory Committee. Best practices for cleaning, disinfection and sterilization of medical equipment/devices. 3rd ed. Toronto: Queen’s Printer for Ontario; 2013. [Google Scholar]
- 13.Grinshpun SA, Buttner MP, Willeke K. Sampling for airborne microorganisms. In: Yates MV, Buttner MP, editors. Manual of environmental microbiology. 4th ed. Washington, DC: ASM Press; 2016. pp. 414–430. [Google Scholar]
- 14.Stauning MT, Bediako-Bowan A, Andersen LP, et al. Traffic flow and microbial air contamination in operating rooms at a major teaching hospital in Ghana. J Hosp Infect. 2018;99:263–270. doi: 10.1016/j.jhin.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman PN, Williams J, Stacey A, et al. Microbiological commissioning and monitoring of operating theatre suites. J Hosp Infect. 2002;52:1–28. doi: 10.1053/jhin.2002.1237. [DOI] [PubMed] [Google Scholar]
- 16.Schoen LJ, Hodgson MJ, et al. ASHRAE position document on airborne infectious diseases. Atlanta: American Society of Heating, Refrigerating and Air-Conditioning Engineers; 2014. [Google Scholar]
- 17.CDC. Guidelines for preventing the transmission of mycobacterium tuberculosis in health-care settings. Atlanta: Centers for Disease Control and Prevention; 2005. pp. 1–140. [Google Scholar]
- 18.Streifel AJ. Design and maintenance of hospital ventilation systems and prevention of airborne nosocomial infections. In: Mayhall CG, editor. Hospital epidemiology and infection control. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 1211–1221. [Google Scholar]
- 19.Scientific Advisory Comitee. COVID-19 pandemy management guideline. Ankara: Turkish Ministry of Health; 2020. Precautions regarding climatization/airconditioning systems; pp. 303–307. [Google Scholar]
- 20.CDC Guidelines for environmental infection control in health-care facilities. Atlanta: Centers for Disease Control and Prevention; 2003. [Google Scholar]
- 21.Shaffer JG, McDade JJ. Airborne Staphylococcus aureus: a possible source in air control equipment. Arch Environ Health. 1963;5:547–551. doi: 10.1080/00039896.1962.10663329. [DOI] [PubMed] [Google Scholar]
- 22.Memarzadeh F, Olmsted RN, Bartley JM. Applications of ultraviolet germicidal irradiation disinfection in health care facilities: effective adjunct, but not standalone technology. Am J Infect Control. 2010;38:S13–24. doi: 10.1016/j.ajic.2010.04.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fletcher LA, Noakes CJ, Beggs CB, Sleigh A. The importance of bioaerosols in hospital infections and the potential for control using germicidal ultraviolet irradiation. In: Monedero MAS, Garcia-Ferrandez AR, editors. Proceedings of the First Seminar on Applied Aerobiology; 2004 May 20; Murcia: Centro de Edafologia y Biologia Aplicada del Segura; 2002. [Google Scholar]
- 24.VanOsdell D, Foarde K. Defining the effectiveness of UV lamps installed in the circulation air ductwork. Air Conditioning and Refrigeration Technology Institute US Department of Energy Office of Scientific and Technical Information; Cited July 2020. Available from: https://www.osti.gov/servlets/purl/810964. [Google Scholar]
- 25.Federal Standard. FS 209E, Airborne particulate cleanliness classes in clean rooms and clean zones. United States: U.S General Service Administration; 1992. [Google Scholar]
- 26.ISO. Cleanrooms and controlled environments-part 1: classification of air cleanliness by particle concentration. Geneva: International Organization for Standardization; 2015. International Standard ISO 14644-1:2015. [Google Scholar]
- 27.EU. Guidelines to good manufacturing practice medicinal products for human and veterinary use. Brussels: European Commission Enterprise and Industry Directorate General; 2008. [Google Scholar]
- 28.Swedish Standards Institute. Mikrobiologisk renhet i operationsrum - förebyggande av luftburen smitta - vaegledning och grundlaeggande krav. Stockholm: SSI; 2012. [Google Scholar]
- 29.Danish Ministry of Health - Statens Serum Institut. Nationale infektionshygiejniske retningslinjer (National infection control guidelines) 11th edition. Copenhagen: 2015. Cited July 2020. Available from: http://www.ssi.dk/w/media/Indhold/DK-dansk/Smitteberedskab/Infektionshygiejne/NIR/NIROperativ.ashx. [Google Scholar]
- 30.Reed NG. The history of ultraviolet germicidal irradiation for air disinfection. Public Health Reports. 2010;125:15–27. doi: 10.1177/003335491012500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menzies D, Pasztor J, Rand T, Bourbeaau J. Germicidal ultraviolet irradiation in air conditioning systems: effect on office worker health and wellbeing - a pilot study. Occup Environ Med. 1999;56:397–402. doi: 10.1136/oem.56.6.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riley RL, Permutt S. Room air disinfection by ultraviolet irradiation of upper air: air mixing and germicidal effectiveness. Arch Environ Health. 1971;22:208–219. doi: 10.1080/00039896.1971.10665834. [DOI] [PubMed] [Google Scholar]
- 33.Nicas M, Miller SL. A multi-zone model evaluation of the efficacy of upper-room air ultraviolet germicidal irradiation. Appl Occup Environ Hyg. 1999;14:317–328. doi: 10.1080/104732299302909. [DOI] [PubMed] [Google Scholar]
- 34.Garner JS Hospital Infection Control Practices Advisory Committee. Guideline for isolation precautions in hospitals. Infect Control Hosp Epidemiol. 1996;17:53–80. doi: 10.1086/647190. [DOI] [PubMed] [Google Scholar]
- 35.Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages Interim guidance. Geneva: World Health Organization; 2020. Cited May 2020. Available from: https://www.who.int/publications-detail/rational-use-of-personal-protective-equipment-for-coronavirus-disease-(covid-19)-and-considerations-during-severe-shortages. [Google Scholar]
- 36.Siegel JD, Rhinehart E, Jackson M, Chiarello L. Health Care Infection Control Practices Advisory Committee, 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35:S65–164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan JF, Yuan S, Kok KH, To KKW, Chu H, Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osterholm MT, Hedberg CW, Moore KA. Epidemiology of infectious diseases. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 5th ed. Philadelphia: Churchill Livingstone; 2000. pp. 156–167. [Google Scholar]
- 39.Darnell MER, Subbarao K, Feinstone SM, Taylor DR. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J Virol Meth. 2004;121:85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker C, Gwangpyo K. Effect of ultraviolet germicidal irradiation on viral aerosols. Environ Sci Technol. 2007;41:5460–5465. doi: 10.1021/es070056u. [DOI] [PubMed] [Google Scholar]
- 41.Fisher D, Heymann D. Q&A: The novel coronavirus outbreak causing COVID-19. BMC Med. 2020;18:57. doi: 10.1186/s12916-020-01533-w. [DOI] [PMC free article] [PubMed] [Google Scholar]