Abstract
PURPOSE
We aimed to evaluate the role of adjunctive, solely nonthrombolytic endovascular therapy in treatment of acute lower-extremity ischemia by rotational percutaneous mechanical thrombectomy.
METHODS
A retrospective, single-center evaluation of 165 patients (167 limbs) that underwent rotational percutaneous mechanical thrombectomy between 2009 and 2016 was performed.
RESULTS
Rotational percutaneous mechanical thrombectomy was used as a single therapy in 9.0% (15 limbs), followed by percutaneous aspiration thrombectomy in 6.0% (10 limbs), percutaneous transluminal angioplasty in 19.8% (33 limbs) and stenting in 25.7% (43 limbs). Rotational percutaneous mechanical thrombectomy was followed by any combination of these three interventions in 39.5%. Clinical and technical success was documented in 92.2%, complications in 10.3% (n=17). No significant difference in clinical and technical success was observed using rotational percutaneous mechanical thrombectomy alone or with additional endovascular therapy. On a long-term basis, the re-ischemia-free survival was nearly twice as high as in previous studies that reported more cases treated by rotational percutaneous mechanical thrombectomy alone.
CONCLUSION
To assure a long-lasting primary patency after percutaneous mechanical thrombectomy, concomitant treatment of underlying lesions with adjunctive, nonthrombolytic endovascular methods should be considered.
Acute limb ischemia (ALI) is one of the most frequent causes for amputation and a potentially life-threatening condition, affecting approximately 1.5 persons per 10 000 per year (1). Endovascular management ranges from catheter-directed intraarterial thrombolysis (CDT), percutaneous aspiration thrombectomy (PAT) to percutaneous mechanical thrombectomy (PMT), all of which may be used independently or in combination. Surgical reperfusion therapy involves higher risks especially in elderly patients, with surgical mortality rates as high as 29% in high-risk populations (2–4). Several studies have shown that systemic administration of a thrombolytic agent alone to treat ALI carries high morbidity and mortality with poor clinical outcome (2, 5–7). Endovascular therapy by thrombolysis is known to be associated with hemorrhagic complications, and lower rates of technical success compared with open surgical repair (8, 9). The development of advanced aspiration techniques, such as PMT has helped to achieve a more complete and faster removal of the clot in a controlled selective angiography setting and possibly unmasks the underlying causative lesion (2, 10, 11). Supplemental stenting and percutaneous transluminal angioplasty (PTA) after rotational PMT or the need of additional fibrinolysis in cases of outflow occlusion have been reported (12, 13). Little evidence is found in the literature regarding the role of exclusively non-CDT based endovascular procedures in combination with rotational PMT.
In order to address the need for more data, the objective of this retrospective study was to evaluate the impact of additional, solely nonthrombolytic endovascular therapy in treatment of (sub-) acute lower-extremity ischemia by rotational percutaneous mechanical thrombectomy (rotational PMT).
Methods
Study design
Following institutional review board waiver for this retrospective single-center study, we included all patients with (sub-) acute infrainguinal limb ischemia who received rotational PMT between December 2009 and December 2016 at our tertiary care academic medical center (number of approval 20180905 01). Informed consent was obtained from all patients included in the study. Clinical data were extracted from the hospital’s clinical information system. Follow-up data were gathered by contacting the patients’ respective general practitioner. Follow-up information could be obtained in all patients, the median follow-up time was 27 months (range, 0–94 months). The registry data were collected in accordance with the Declaration of Helsinki.
Patients
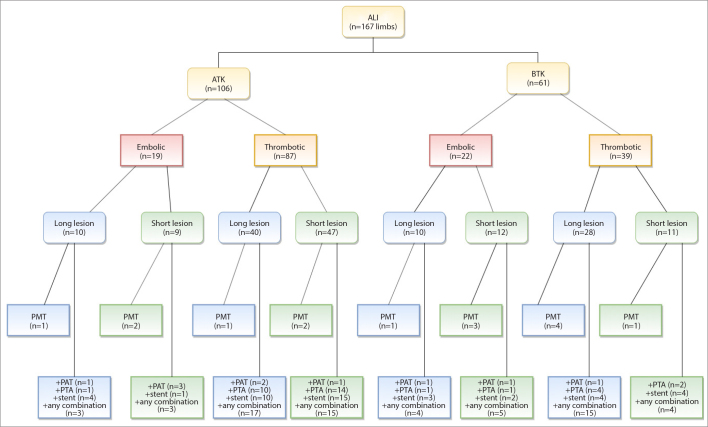
A total of 165 patients (89 male, 76 female) with a median age of 80 years (range, 36–99 years) were admitted by the Department of Vascular Surgery with peracute to chronic arterial occlusion of native or already treated femoropopliteal arteries. Treatment decisions were based on interdisciplinary consensus between vascular surgeons and interventional radiologists. The indication for endovascular therapy was ALI in all included cases. Patients were divided in five groups depending on the acuteness of the symptoms: Peracute (within 24 h), acute A (<7 days), acute B (8–14 days), subacute (15–90 days) and chronic (>90 days). Patients with a primary occlusion of the superficial femoral artery (SFA) and popliteal artery (including POP III segment) were considered for rotational PMT. If the occlusion reached distal to the POP III segment, additional PAT had to be performed. Demographic data and clinical presentation are displayed in Table 1. Fig. 1 shows a flowchart diagram of the usual workflow and treatment of lesions above and below the knee in our institution. Due to complex overall situations, there were some divergencies in the treatment of some patients in our study cohort.
Table 1.
General and vascular baseline characteristics of patients
| Demographics | |
|---|---|
| Age (years), median (range) | 80 (36–99) |
| Number of legs (patients) | 167 (165) |
|
| |
| Sex, n (%) | |
| Male | 89 (53.9) |
| Female | 76 (46.1) |
|
| |
| Treated side, n (%) | |
| Right | 81 (49.1) |
| Left | 82 (49.7) |
| Both | 2 (1.2) |
|
| |
| Risk factors, n (%) | |
| History of smoking | 87 (52.7) |
| Diabetes | 58 (35.2) |
| Hypertension | 159 (96.4) |
| Hypercholesterolemia | 159 (96.4) |
|
| |
| Comorbidities, n (%) | |
| Atrial fibrillation | 81 (49.1) |
| Transient cerebral ischemia or stroke | 35 (21.2) |
| Cerebral bleeding | 2 (1.2) |
| Ventricular tachycardia | 1 (0.6) |
| Renal insufficiency | 121 (73.3) |
| • Grade 1 | 12 (7.3) |
| • Grade 2 | 46 (27.9) |
| • Grade 3 | 51 (30.9) |
| • Grade 4 | 11 (6.7) |
| Rhabdomyolysis | 1 (0.6) |
|
| |
| Duration of symptoms, n (%) | |
| Peracute | 41 (24.6) |
| Acute A | 60 (35.9) |
| Acute B | 17 (10.2) |
| Subacute | 14 (8.4) |
| Chronic | 35 (21) |
|
| |
| Acute occlusion (Rutherford, TASC II), n (%) | |
| Category I | 0 (0) |
| Category IIa | 110 (65.9) |
| Category IIb | 46 (27.5) |
| Category III | 11 (6.6) |
|
| |
| Cause of arterial obstruction, n (%) | |
| Acute on chronic occlusive disease | 107 (64.1) |
| Embolism likely | 41 (24.6) |
| Other (popliteal aneurysm, Bypass) | 19 (11.4) |
|
| |
| Occlusion ending, n (%) | |
| SFA | 43 (25.8) |
| POP I/II | 63 (37.7) |
| POP III | 37 (22.2) |
| BTK | 24 (14.4) |
Percentages of sex, treated side, risk-factors and co-morbidities refer to the number of patients (n=165), the other variables in the table refer to the number of limbs (n=167).
SFA, superficial femoral artery; POP, popliteal artery; BTK, below the knee.
Figure 1.
Flowchart diagram of the workflow and treatment of lesions above and below the knee in our institution. ALI, acute limb ischemia; ATK, above the knee; BTK, below the knee; Long lesion, >10 cm; Short lesion, ≤10 cm; PMT, percutaneous mechanical thrombectomy; PTA, percutaneous transluminal angioplasty; PAT, percutaneous aspiration thrombectomy.
Catheter techniques
All endovascular interventions were performed under local anesthesia (Scandicain 2%, Aspen®) in our angiography suite with an ipsilateral (94.6%) or contralateral (5.4%) femoral approach (6 F to 8 F) using the Rotarex System (Straub). Details concerning the technical approach are described elsewhere (12, 14). After re-establishing blood flow, digital subtraction angiography (DSA) was performed to reevaluate the result (Siemens, Axiom Artis Zee). The following different scenarios were encountered: 1) PMT was performed without need of subsequent endovascular interventions; 2) PMT was performed with subsequent PTA or stenting (e.g., to address a residual stenosis or resistant / adherent thrombus); 3) PMT was performed with subsequent PAT (e.g., in case of distal embolization or occlusion distal to the POP III segment); 4) PMT was performed with subsequent combination of the scenarios 2 and 3.
Intravenous heparin was given to all patients in the emergency room and during the intervention (5000 IU Ratiopharm). After each successful approach, full-scale heparinization was established. Following the hospital stay, platelet inhibitors were prescribed for lifetime and dual platelet inhibitor therapy for 6 months was initiated in case of stenting.
Outcome criteria and definitions
Primary endpoints were technical and clinical success. Secondary endpoints were the rate of complications, limb salvage rate, mortality rate, the amputation-free survival and re-ischemia-free survival at 30 days, 12 and 36 months using rotational PMT alone or in combination with the abovementioned subsequent endovascular interventions.
Technical success was defined as the restoration of antegrade blood flow with complete or near complete (95% by volume) aspiration of the thrombus or embolus with at least one run-off vessel. For a better interpretation, technical results were graded according to the thrombolysis in myocardial infarction (TIMI) score, adapted from cardiac literature (15, 16). On a short-term basis, indication of success was reflected by an increase of at least ≥1 point relative to the baseline score: 0, no distal perfusion to the lesion; 1, faint flow beyond the occlusion without capillary bed filling; 2, delayed or sluggish distal perfusion with capillary bed filling; 3, normal perfusion.
Technical failure was defined as the incapability of restoration of antegrade blood flow by elimination of the thrombus or embolus and/or a missing run-off vessel. Also, a missing upward shift of the TIMI score of at least 1 point was interpreted as failure.
Clinical success was defined as a periinterventional relief (up to 30 days after intervention) of the acute ischemic symptoms and upward shift of at least one category on the Rutherford classification.
Complications were categorized as minor and major according to the reporting standards of the Society of Interventional Radiology (2). Amputations were graded according to the Society of Vascular surgery in minor (distal or through the tarsometatarsal joint) and major (proximal to the tarsometatarsal joint) amputations.
Statistical analysis
Statistical analysis was performed using SPSS (Statistical Package for Social Science, Version 25.0, IBM Corp.). Descriptive data are represented as follows: Normally distributed continuous variables are represented as mean ± standard deviation, non-normally distributed continuous variables are represented as median with ranges, categorical variables as frequencies and percentages. With regard to assessment of normality, the Anderson-Darling test was performed. The normality hypothesis was rejected with a p value less than 0.05. Times to events (amputation-free survival, re-ischemia free survival) were analyzed with Kaplan-Meier analysis (17). Group differences were assessed with the unpaired Student t-test, with Mann-Whitney U-test for nonparametric groups and the chi-square test for categorical variables. The Wilcoxon-test was used for ordinal data. A p value less than 0.05 was considered significant.
Results
The median length of the treated lesion was 11 cm (1–36 cm). In 25.8% of the limbs (n=43) the occlusion ended in the SFA, 37.7% (n=63) showed an occlusion ending in the POP I/II segments, 22.2% (n=37) had a lesion ending in POP III and 14.4% (n=24) showed an occlusion reaching the crural arteries (Table 1). Rotational PMT was used as a single therapy in 9.0% of the cases (15 limbs; Fig. 2), followed by stenting in 25.7% (Fig. 3), PTA in 19.8%, PAT in 6.0%. Rotational PMT was followed by any combination of these three interventions in 39.5%. The most common combination was rotational PMT + PTA + stenting (24.0%), followed by rotational PMT + PAT + PTA + stenting (6.5%), rotational PMT + PAT + PTA (4.8%) and rotational PMT + PAT + stenting (4.2%).
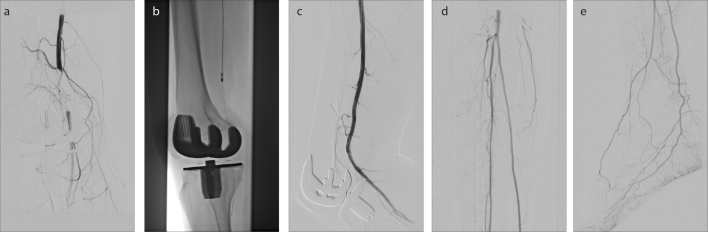
Figure 2. a–e.
A 60-year-old patient with known history of peripheral arterial occlusive disease who presented with symptoms of acute lower limb ischemia on the right side within <7 days (acute A). Panel (a) shows occlusion of the distal superficial femoral artery (SFA) reaching POP - II segment, measuring 5 cm, which was first treated by rotational PMT (b). Subsequent DSA revealed residual stenosis >30%. After additional stenting (c) a satisfactory result and 3 run-off vessels distal of the lesion could be achieved (d, e).
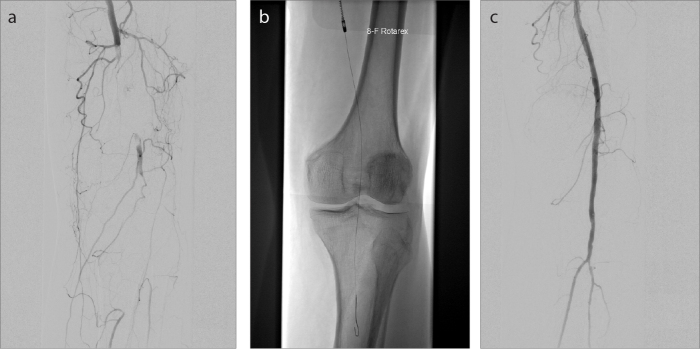
Figure 3. a–c.
An 87-year-old patient who presented with symptoms of acute lower limb ischemia on the left side within 8–14 days (acute B). DSA shows 10 cm measuring embolic occlusion of the SFA, reaching POP I segment (a). Complete removal of the clot by rotational PMT alone (b) with a subsequent 3 run-off vessel (c).
Primary endpoints of our study were technical success, clinical success, and adjunctive endovascular procedures. Before intervention, 47.3% of the limbs (n=79) had a TIMI-score 1, 44.9% (n=75) had a TIMI-score 2 and 7.8% of the limbs (n=13) showed no perfusion distal to the lesion (TIMI-score 0). We observed an increase of the TIMI-score of at least ≥1 point and restoration of antegrade flow with at least one run-off vessel in 154 of 167 limbs, resulting in a technical success rate of 92.2%. Clinical success was documented in 92.2% (154 of 167 limbs).
In those 13 patients/limbs (7.8%) in which the intervention was unsuccessful (median occlusion length 15 cm; range, 3–36 cm), one patient suffered from malignancy and presented with a paraneoplastic occlusion, three patients had a bypass- or in-stent occlusion, and four patients showed an occlusion length of ≥20 cm. In the remaining five patients we could not achieve an improvement of the TIMI-score of at least 1 point.
Before intervention, 6.6% of the patients (n=11) had no patent run-off vessel distal to the lesion, 55.7% (n=93) had one, 27.5% (n=46) had two and 10.2% (n=17) had three patent run-off vessels. After intervention two patients (1.2%) remained with no run-off, 40.1% (n=67) had one, 43.7% (n=73) had two, and 15.0% (n=25) had three patent run-off vessels distal of the lesion. Detailed information on technical and clinical outcome using rotational PMT alone or in combination with adjunct endovascular procedures is summarized in Tables 2 and 3. Details pertaining clinical and technical success depending on acuteness of symptoms are presented in Table 4.
Table 2.
TIMI score
| TIMI baseline Median (min–max) |
TIMI after intervention Median (min–max) |
p | |
|---|---|---|---|
| Rotational PMT alone | 1 (1–2) | 2 (1–3) | <0.001 |
| + PTA | 1 (1–2) | 3 (1–3) | <0.001 |
| + stent | 2 (0–2) | 3 (1–3) | <0.001 |
| + PAT | 1 (0–2) | 3 (2–3) | <0.001 |
| + any combination | 1 (0–2) | 3 (0–3) | <0.001 |
| Total | 1 (0–2) | 3 (0–3) | <0.001 |
| TIMI + 1 point, n (%) | 112 (67.1) | ||
| TIMI + 2 points, n (%) | 39 (23.4) | ||
| TIMI + 3 points, n (%) | 3 (1.8) |
Statistical significance was tested with the Wilcoxon-test.
TIMI, thrombolysis in myocardial infarction; PMT, percutaneous mechanical thrombectomy; PTA, percutaneous transluminal angioplasty; PAT, percutaneous aspiration thrombectomy.
Table 3.
Adjunct endovascular procedures
| Procedures | Legs treated | Technical success | Clinical success | Complications |
|---|---|---|---|---|
| Rotational PMT | 15 (9.0) | 12 (80.0) | 13 (86.7) | 2 (13.3) |
| + PTA | 33 (19.8) | 32 (97.0) | 32 (97.0) | 2 (6.0) |
| + stent | 43 (25.7) | 42 (97.7) | 43 (100) | 5 (11.6) |
| + PAT | 10 (6.0) | 10 (100) | 10 (100) | 1 (10.0) |
| Combination | 66 (39.5) | 56 (84.8) | 56 (84.8) | 7 (10.6) |
Data are presented as n (%)
Combination is defined as at least 2 adjunctive endovascular procedures after PMT.
PMT, percutaneous mechanical thrombectomy; PTA, percutaneous transluminal angioplasty; PAT, percutaneous aspiration thrombectomy.
Table 4.
Clinical and technical success depending on acuteness of symptoms
| Duration of symptoms | Clinical success (%) | Technical success (%) |
|---|---|---|
| Peracute | 39 (95.1) | 40 (97.6) |
| Acute A | 52 (86.7) | 55 (91.7) |
| Acute B | 17 (100) | 16 (94.1) |
| Subacute | 13 (92.9) | 13 (92.9) |
| Chronic | 33 (94.3) | 30 (85.7) |
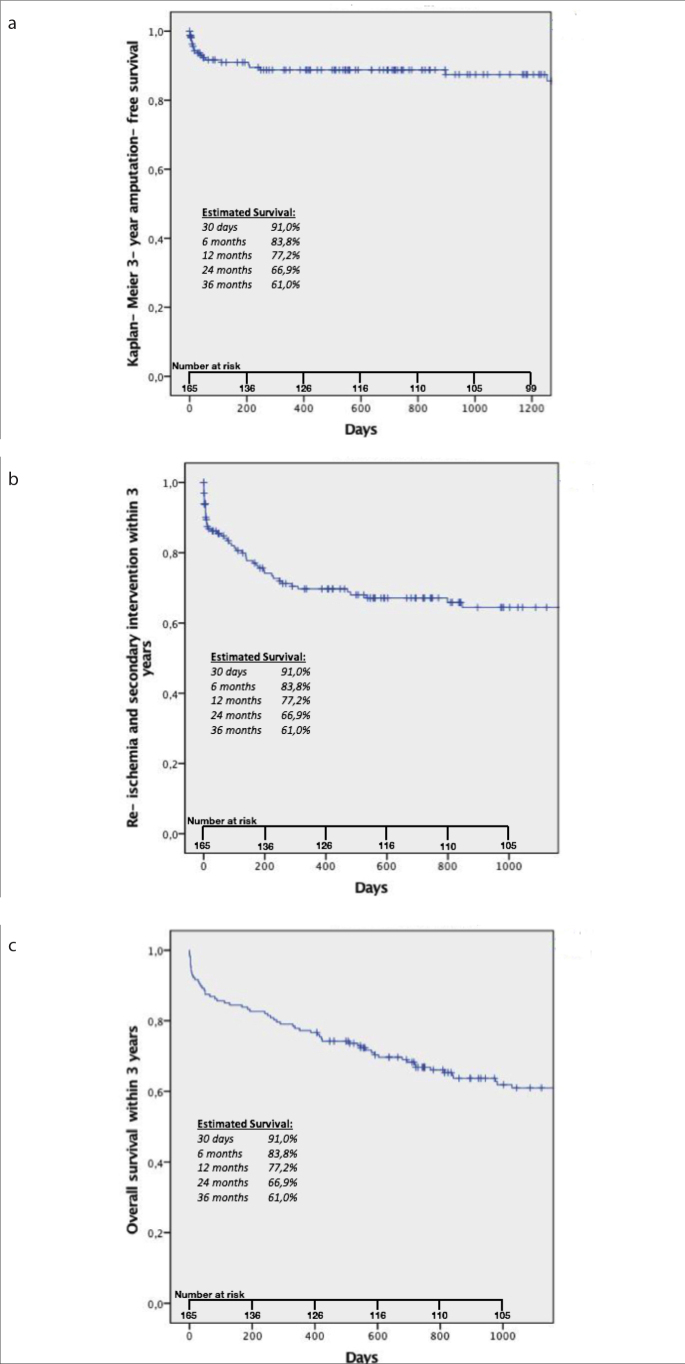
Secondary endpoints of our study were complication rate, limb salvage rate, mortality rate, amputation-free survival, and re-ischemia-free survival. The overall rate of complications for the study group was 10.3% (17 of 165 patients), with a minor complication rate of 4.2% (7 of 165 patients) and a major complication rate of 6.1% (10 of 165 patients). One patient with a minor complication class A developed a temporary spasm of a crural artery. Four patients categorized as class B had a distal embolization in which case the embolus could not be removed, but no further therapy was necessary. Other complications graded as class B occurred in a patient with a dissection of the crural arteries after rotational PMT alone and in another patient with self-limiting bleeding in one of the crural vessels. A major complication class D was observed in a patient with inguinal hematoma which needed surgical removal. Four patients developed a compartment syndrome, two of which were treated with fasciotomy (class D), while in the other two patients the extremity required major amputation (class E). Two patients suffered from spasm of the crural arteries; one patient needed a surgical bypass (class D) and another patient had to undergo major amputation (class E). Three other patients experienced a major complication graded as class F, as they died within 24 h, probably due to the intervention and/or the application of the contrast-medium or volume load: one patient had an acute on chronic renal failure after reperfusion, one patient suffered a cardiac arrest, and the third developed a lethal cardiac decompensation. During follow-up (median, 27 months after intervention; range, 0–94 months), a total of 20 major amputations were observed (75.0% of them had ≤1 run-off vessel following the intervention), resulting in a limb salvage rate of 88.0% (147 of 167 limbs). At the time of follow-up for this study, 76 of the initial 165 patients had deceased, yielding a mortality rate of 46.1%. Mortality rate after 30 days was 9.0% and after 12 months 22.8%. Amputation-free survival was 93.7% after 30 days, 88.8% after 12 months, and 87.4% after 36 months (mean±SE, 81.7±3 months; 95% CI, 75.9–87.5 months). Amputation-free survival within three years is presented in Fig. 4a. Comparing patients with Rutherford IIb and III categories to those with Rutherford IIa category, we found a higher amputation rate in the Rutherford IIb/III group with 18.0% vs. 9.0% in the latter group. This difference was significant (p = 0.02). Regarding the mortality rate, no significant difference could be found between the two groups: Rutherford IIb/III showed a mortality-rate of 56.0% compared with 42.0% in the Rutherford IIa group (p = 0.08). Patients suffering from renal insufficiency showed a mortality rate of 37.5%, compared with a mortality rate of 19.6% in patients without that comorbidity. This difference was significant (p < 0.001). Re-intervention was necessary in 31.0% for re-ischemia (patients who suffered amputation were not included in this group). Time between initial procedure to re-intervention was 160 days on average (median, 77 days; range, 0–1253 days). Indications were re-thrombosis in patients with known chronic occlusive disease (70.6%), cardiac embolism (23.5%), and others (bypass-occlusion, popliteal aneurysm 5.9%). Overall, 13.1% underwent surgery (bypass in 20 patients, surgical thrombectomy in 2 patients) and 17.4% underwent repeated intervention (PTA + stenting in 9 patients, re-rotational PMT in combination with any other endovascular procedure in 8 patients, PTA in 7 patients, PAT in 3 patients and intraarterial lysis in 2 patients). Re-ischemia-free survival after 30 days, 12 months, and 36 months was 86.2%, 69.7%, and 64.4%, respectively (mean±SE, 62.4±3.8 months; 95%CI, 54.9–69.8 months). Re-ischemia and secondary intervention within three years is presented in Fig. 4b, overall survival within three years is presented in Fig. 4c.
Figure 4. a–c.
Panel (a) shows 3-year amputation-free survival; panel (b) shows re-ischemia and secondary intervention within 3 years. Panel (c) shows 3-year overall survival.
Discussion
To date, endovascular thrombolysis has been the treatment of choice in patients with ALI (10, 11, 18). Several studies have discussed the strengths of a catheter-guided rotational thrombectomy, especially with respect to elderly patients with multiple cardiovascular risk factors (10, 12, 13, 19).
Beneficial primary revascularization rates have been reported previously ranging from 70% to 96% (12, 19, 20–24). However, the number of patients treated by rotational PMT alone was noticeably lower in our cohort with 9.0% compared with 29% in the cited literature (22). This fact is probably due to a more complex cohort of patients with more acute on chronic lesions, such as a higher number of patients with bent popliteal segment lesions. In the literature, additional PTA and/or stenting after PMT is reported in 22%–92% and 12%–49% (19, 20, 22, 25), vs. 58.0% and 70.0% in our study, respectively.
Heller et al. (13) previously published a comparable study analyzing the outcome after rotational PMT in ALI with a similar time-range, population-size and spectrum of comorbidities. However, our patients were 8.5 years older and we did not apply adjunctive CDT in any of our patients as compared with 22% CDT in their population.
Several studies discussed the disadvantages of fibrinolysis, especially in elderly patients with an associated high-risk profile (11, 26), e.g., a longer revascularization-time, the requirement of observation in an intensive care unit with highly trained personnel and associated higher costs, and vascular surgeons’ standby in case of bleeding complications. The risk of serious bleeding was reported in up to 12% in treatment of ALI (27).
Our patients were followed on a long-term basis (median, 27 months; range, 0–94 months). To our knowledge, there is only one other publication dealing with rotational PMT in ALI with a follow-up period of nearly three years (22). In our opinion, a long-term follow-up is crucial to exactly define the clinical outcome of all those critically ill patients.
We aimed to clearly determine our primary and secondary endpoints, especially the technical and clinical success, as those are lacking in most previous publications (10, 12, 13, 19, 20). In order to make the technical success more measurable, we used the TIMI-score (adapted from the cardiac literature). To our knowledge, there is only one other study using this score to grade technical success in a more sophisticated manner (16).
Furthermore, our definition of technical success implied at least one patent run-off vessel distal to the lesion, no exclusion was made in patients with initially no patent run-off vessel. Stanek et al. (22) showed that patients with poorer distal run-off had a significant higher re-thrombosis rate. Improvement of the run-off vessels was achieved after intervention upon rotational PMT; however, 2 patients remained with no patent run-off vessel after the intervention in our study.
When comparing the re-ischemia-free survival with the literature, our results are nearly twice as high as those reported so far on a long-term basis. Stanek et al. (22) reported re-ischemia-free survival in 74.6% after 30 days (vs. 86.2% in our study), 37.8% after 12 months (69.7% in our study) and 30.3% after 36 months (64.4% in our study). They used adjunctive treatment in 71% compared with 91.0% in our study. Re-ischemia rates were highest in the first year after intervention, whereas they seem to stabilize between 12 and 36 months. Amputation-free survival was within the average of the cited studies. After 12 months, amputation-free survival ranged from 65%–100% (12, 19, 20, 23–25, 28), vs. 88.8% in our study cohort, respectively.
However, most of cited studies excluded patients with occlusions reaching the POP-segments or the crural arteries, popliteal aneurysms, symptom duration >42 days and occlusions with no run-off vessel distal to the lesion (12, 20).
Long length or distal localization of the occlusion was no exclusion criterion in our study. Hence, 124 limbs (74.3%) showed an involvement of the popliteal arteries, seven limbs had a popliteal aneurysm and 24 limbs showed lesions reaching to the crural arteries which included segments of extreme bending. It would have been interesting to correlate length and localization of the occlusion with type of intervention, success, as well as “non-success”, and could be subject of future investigation.
Regarding the acuteness of symptoms, we observed slightly lower clinical success in the “acute A” group, and slightly lower technical success in the “chronic” group compared with the other subgroups of our study sample. To the best of our knowledge, there is no other study analyzing clinical and technical success depending on acuteness of symptoms in the endovascular treatment of patients with ALI so far.
Compared with the literature, our study population was relatively old with a median age of 80 years vs. 69 years in Heller et al. (13) and 72 years in Stanek et al. (22) and presented with a high number of comorbidities. For example, 73.3% of our patients suffered from renal insufficiency (vs. 13.6% in other studies) (13), which was linked to a higher mortality rate (p < 0.001). Furthermore, we had a high number of patients presenting with Rutherford IIb and III categories (TASC II) that showed a higher amputation-rate compared with patients with Rutherford-IIa category (18.0% vs. 9.0%, p = 0.02). This may possibly explain our higher mortality and amputation rate at 30 days compared with the cited study (9.0% and 6.0% vs. 0.7% and 2% respectively).
In a recent study by Langenskiöld et al. (29), demographic data of the recruited 195 patients with ALI were similar to our study population. Revascularization was performed in 88%; of those, 59% underwent surgical therapy, 38% catheter-directed thrombolysis and 3% a combination of both. Mortality rate within 30 days was 11.8% (vs. 9.0% in our study), amputation-free survival after 30 days was 81% (vs. 93.7%) and after one year 71% (vs. 89.0%). The cited study is a retrospective study analyzing weaknesses in the early management of patients with ALI. The high mortality rate within 30 days, and worse amputation-free survival after 30 days and after one year compared with our study show that even in a top hospital of a European country the urgency of treatment of ALI is widely underestimated: 72% of the patients received intravenous heparin in the emergency room, even though this is a level-I recommendation in the international guidelines; more than half of the patients underwent surgical revascularization, and the remaining patients received CDT, altogether leading to unfavorable outcome.
Complication rates have been reported between 7.5%–31.5% in previous studies (12, 19, 21, 30). Compared with the study of Heller et al. (13), the complication rate was less in our population, a fact that may be attributed to a less aggressive treatment strategy without any CDT. In addition to the mentioned observed complications in our study group, distal embolization which could be removed by subsequent PAT occurred in further 40 limbs. However, calcifications may hamper the aspiration of more distal emboli in crural vessels in a small number of patients. The use of PMT distal to the POP II segment is usually known to be off-label. However, and in contrary to most of the other studies, we did not exclude patients with POP III lesions or proximal crural lesions with a vessel diameter of 4 mm. Twenty-four limbs (14.4%) first presented with long lesions reaching the crural arteries, and the use of adjunctive endovascular procedures was necessary from the beginning. Unlike other studies, no vessel perforation caused by the rotational PMT catheter was observed. Presumably, this can be attributed to a more sophisticated design of the device provided by the manufacturer.
Compared with CDT, treatment with PMT in patients with ALI appears to reduce in-hospital time and revascularization time and is associated with fewer complications like bleeding, contributing to better cost-effectiveness. This minimally invasive procedure may be also advantageous in an aging society. However, those topics have not been addressed in our study and should therefore be the focus of future investigations. Furthermore, the vast majority of patients presenting with ALI (80%) has an underlying chronic vascular disease. As shown in our study, concomitant treatment of additional critical lesions can lead to a significant improvement of the long-term patency rate.
Two main limitations to this study need to be addressed. First, this trial is retrospective and lacks randomization; therefore, it may have been biased by patient selection. Setting up a prospective randomized study would be advantageous to define the exact value and clinical outcome of rotational PMT with or without adjunctive, non-CDT-based endovascular procedures in comparison with solely or additional CDT. However, from an ethical point of view, such a study would be difficult to design and perform, especially in patients with a higher risk of bleeding complications. Second, our study population included a heterogeneous number of underlying diseases (acute embolic, acute on chronic, and aneurysmatic) which may have influenced the clinical outcome and the overall number of major complications. Furthermore, we gathered the follow-up data by contacting the patients’ general practitioner. It would have been interesting to set up a follow-up protocol for each patient to better evaluate the long-term outcome.
In conclusion, endovascular treatment of (sub-) acute limb ischemia has excellent clinical and technical results with a low rate of technical failures and complications when rotational PMT is used along with other adjunctive, non-CDT-based endovascular procedures. Length and localization of the occlusion as well as the restoration of a run-off of at least one vessel might be relevant for the long-term outcome.
Main points.
Percutaneous mechanical thrombectomy (PMT) in acute and subacute lower-extremity ischemia requires adjunctive endovascular procedures in the vast majority of cases.
The adjunctive endovascular procedures to PMT were solely non-catheter-directed intraarterial thrombolysis based, and showed clinical and technical success rates in accordance with those found in clinical literature.
PMT is a safe procedure with low rates of minor or major complications.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45(Suppl):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 2.Nilesh H, Venkataramu N, Stanley K, et al. Quality improvement guidelines for percutaneous management of acute lower-extremity ischemia. J Vasc Interv Radiol. 2013;24:3–15. doi: 10.1016/j.jvir.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 3.Diffin DC, Kandarpa K. Assessment of peripheral intraarterial thrombolysis versus surgical revascularization in acute lower-limb ischemia: a review of limb-salvage and mortality statistics. J Vasc Interv Radiol. 1996;7:57–63. doi: 10.1016/S1051-0443(96)70734-0. [DOI] [PubMed] [Google Scholar]
- 4.Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–538. doi: 10.1016/S0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 5.Working Party on Thrombolysis in the Management of Limb Ischemia b Thrombolysis in the management of lower limb peripheral arterial occlusion-a consensus document. Working Party of Thrombolysis in the Management of Limb Ischemia. Am J Cardiol. 1998;81:207–218. [PubMed] [Google Scholar]
- 6.Patel N, Sacks D, Patel Rl, et al. SIR reporting standards for the treatment of acute limb ischemia with use of transluminal removal of arterial thrombus. J Vasc Interv Radiol. 2003;14(Suppl):S453–S465. doi: 10.1097/01.RVI.0000094619.61428.11. [DOI] [PubMed] [Google Scholar]
- 7.Berridge DC, Gregson RH, Hopkinson BR, et al. Randomized trial of intra-arterial recombinant tissue plasminogen activator, intravenous recombinant tissue plasminogen activator and intra-arterial streptokinase in peripheral arterial thrombolysis. Br J Surg. 1991;78:988–995. doi: 10.1002/bjs.1800780831. [DOI] [PubMed] [Google Scholar]
- 8.Davis FM, Albright J, Gallagher KA, et al. Early outcomes following endovascular, open surgical, and hybrid revascularization for lower extremity acute limb ischemia. Ann Vasc Surg. 2018;51:106–112. doi: 10.1016/j.avsg.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 9.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease. Int Angiol. 2007;26:81–157. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 10.Kronlage M, Printz I, Vogel B, et al. A comparative study on endovascular treatment of (sub)acute critical limb ischemia: mechanical thrombectomy vs thrombolysis. Drug Des Devel Ther. 2017;11:1233–1241. doi: 10.2147/DDDT.S131503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zehnder T, Birrer M, Do D, et al. Percutanous catheter thrombus aspiration for acute or subacute arterial occlusion of the legs: how much thrombolysis is needed? Eur J Vasc Endovasc Surg. 2000;20:41–46. doi: 10.1053/ejvs.2000.1117. [DOI] [PubMed] [Google Scholar]
- 12.Wissgott C, Kamusella P, Richter A, et al. Mechanical rotational thrombectomy for treatment thrombolysis in acute and subacute occlusion of femoropopliteal arteries: retrospective analysis of the results from 1999 to 2005. Rofo. 2008;180:325–331. doi: 10.1055/s-2008-1027144. [DOI] [PubMed] [Google Scholar]
- 13.Heller S, Lubanda JC, Varejka P, et al. Percutaneous mechanical thrombectomy using Rotarex® S device in acute limb ischemia in infrainguinal occlusions. BioMed Res Int. 2017;2017 doi: 10.1155/2017/2362769. 2362769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanek F, Ouhrabkova R, Prochazka D. Mechanical thrombectomy using the Rotarex catheter in the treatment of acute and subacute occlusions of peripheral arteries: immediate results, long-term follow-up. Int Angiol. 2013;32:52–60. [PubMed] [Google Scholar]
- 15.Khatri P, Neff J, Broderick JP, et al. Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke. 2005;36:2400–2403. doi: 10.1161/01.STR.0000185698.45720.58. [DOI] [PubMed] [Google Scholar]
- 16.Bauman F, Sharpe E, Peña C, et al. Technical results of vacuum-assisted thrombectomy for arterial clot removal in patients with acute limb ischemia. J Vasc Interv Radiol. 2016;27:330–335. doi: 10.1016/j.jvir.2015.11.061. [DOI] [PubMed] [Google Scholar]
- 17.StataCorp. Stata Statistical Software: Release 5. College Station TX: StataCorp LP; 1997. [Google Scholar]
- 18.Morrison HL. Catheter-directed thrombolysis for acute limb ischemia. Semin Intervent Radiol. 2006;23:258–269. doi: 10.1055/s-2006-948765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeller T, Frank U, Bürgerlein K, et al. Langzeitergebnisse nach Rekanalisation akuter und subakuter thrombotischer arterieller Verschlüsse der unteren Extremitäten mit einem Rotations-Thrombektomiekatheter. Fortschr Röntgenstr. 2002;174:1559–1565. doi: 10.1055/s-2002-35942. [DOI] [PubMed] [Google Scholar]
- 20.Duc SR, Schoch E, Pfyffer M, et al. Recanalization of acute and subacute femoropopliteal artery occlusions with the rotarex catheter: one year follow-up, single center experience. Cardiovasc Interven Radiol. 2005;28:603–610. doi: 10.1007/s00270-004-0339-3. [DOI] [PubMed] [Google Scholar]
- 21.Lichtenberg M. Percutaneous mechanical thrombectomy by means of rotational thrombectomy. current study situation. Medizinische Klinik. 2010;105:705–710. doi: 10.1007/s00063-010-1122-0. [DOI] [PubMed] [Google Scholar]
- 22.Stanek F, Ouhrabkova R, Prochazka D. Percutaneous mechanical thrombectomy in the treatment of acute and subacute occlusions of the peripheral arteries and bypasses. Vasa. 2016;45:49–56. doi: 10.1024/0301-1526/a000495. [DOI] [PubMed] [Google Scholar]
- 23.Ouriel K, Shortell CK, DeWeese JA, et al. A comparison of thrombolytic therapy with operative revascularization in the initial treatment of acute peripheral arterial ischemia. J Vasc Surg. 1994;19:1021–1030. doi: 10.1016/S0741-5214(94)70214-4. [DOI] [PubMed] [Google Scholar]
- 24.Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N Engl J Med. 1998;338:1105–1111. doi: 10.1056/NEJM199804163381603. [DOI] [PubMed] [Google Scholar]
- 25.Wissgott C, Kamusella P, Richter A, et al. Treatment of acute femoropopliteal bypass graft occlusion: comparison of mechanical rotational thrombectomy with ultrasound-enhanced lysis. Rofo. 2008;180:547–552. doi: 10.1055/s-2008-1027216. [DOI] [PubMed] [Google Scholar]
- 26.McNamara TO, Bomberger RA, Merchant RF. Intra-arterial urokinase as the initial therapy for acutely ischemic lower limbs. Circulation. 1991;83(2 Suppl):I106–119. [PubMed] [Google Scholar]
- 27.Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Ann Surg. 1994;220:251–268. doi: 10.1097/00000658-199409000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ansel GM, George BS, Botti CF, et al. Rheolytic thrombectomy in the management of limb ischemia: 30-day results from a multicenter registry. J Endovasc Ther. 2002;9:395–402. doi: 10.1177/152660280200900402. [DOI] [PubMed] [Google Scholar]
- 29.Langenskiöld M, Smidfelt K, Karlsson A, et al. Weak links in the early chain of care of acute lower limb ischaemia in terms of recognition and emergency management. Eur J Vasc Endovasc Surg. 2017;54:235–240. doi: 10.1016/j.ejvs.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Bérczi V, Deutschmann HA, Schedlbauer P, et al. Early experience and midterm follow-up results with a new, rotational thrombectomy catheter. Cardiovasc Intervent Radiol. 2002;25:275–281. doi: 10.1007/s00270-001-0095-6. [DOI] [PubMed] [Google Scholar]