Abstract
PURPOSE
We aimed to evaluate the feasibility, accuracy, and safety of Programmed Death–1/ Programmed Death–Ligand 1 (PD-1/ PD-L1) expression quantification in cytology cell-block samples obtained through transthoracic CT-guided fine-needle aspiration cytology (FNAC) from the interventional radiologist’s perspective.
METHODS
We performed a consecutive unselected series of 361 CT-guided biopsies of pulmonary nodules and masses which came to our observation from June 2017 to October 2018. For each case, exhaustive clinical, morphologic, molecular and tomographic data were available. All the material obtained was fixed in formalin to obtain a cell-block for the pathologist, who performed immunohistochemical analysis to detect PD-L1 expression levels on each sample.
RESULTS
Of all the analyzed samples, 93.6% (338/361) were defined to be diagnostic, including neoplastic (72%, 260/361) and non-neoplastic lesions (21.6%, 78/361); only 6.4% (23/361) of them resulted in nondiagnostic specimens. Non-small cell lung cancer (NSCLC) accounted for 73.8% of neoplastic lesions (192/260): most of them were adenocarcinoma (83%, 160/192), followed by squamous carcinoma (14%, 27/192) and poorly differentiated carcinoma (3%, 5/192). In 96% of NSCLC (184/192), the diagnosis was reached either in the absence of complications or with early minor complications. PD-L1 expression was evaluated in all 192 NSCLC cytology specimens: 180 immunostainings were found to be adequate for PD-L1 testing. In 76% of cases, PD-L1 expression level was lower than 50%.
CONCLUSION
The findings of our study indicate that PD-L1 quantification using a cell-block approach on CT-guided FNAC is a feasible and safe technique and should be taken into account alongside with core biopsy approach, especially in case of advanced disease and/or fragile and older patients.
Lung cancer continues to be the leading cause of cancer-related death worldwide (1). It can be divided into two major histological groups, namely small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), which feature different biological traits, prognosis and therapeutic approaches (2). Despite the improvement in the therapeutic options, NSCLC prognosis remains poor, with a 5-year survival rate around 15% in Europe and USA (3). Routine therapeutic approaches to NSCLC patients include surgery, radiotherapy, conventional chemotherapy, antikinase agents and more recently immunotherapy, and their combinations (4).
Immunotherapy has been shown to improve the prognoses of various cancer types (5, 6). Checkpoint molecules, namely Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) and Programmed Death–1/ Programmed Death–Ligand 1 (PD-1/PD-L1) are key factors by which tumors evade host immune response (5). PD-1 is a transmembrane cell receptor expressed on mature T cells and other components of the immune system. When PD-1 binds PD-L1, it results in a down-regulation of the proliferation of immune cells, which ultimately promotes the evasion from immune response in different types of cancer cells (7, 8). Thus, drugs which act by blocking the PD-1/PD-L1 interaction (PD-1 inhibitors, such as nivolumab and pembrolizumab, or PD-L1 inhibitors, such as atezolizumab, durvalumab, and avelumab) can restore anti-tumoral immune system activity (9). However the prescription of pembrolizumab requires a minimum of 50% of PD-L1-positive tumor cells for first-line administration, and 1% for second-line (10, 11). Recently, the FDA approved durvalumab as maintenance therapy in patients with unresectable stage III lesions, in the absence of progression after concurrent chemoradiotherapy (10).
Historically, core biopsy (CB) and fine-needle aspiration cytology (FNAC) represent the two methods of choice for diagnosing lung cancer. Cell-block preparations obtained from FNAC samples have been successfully used for ancillary tests in several studies (12) including the determination of predictive biomarkers. In the literature, FNAC is regarded as safer than CB (13).
For these reasons, over the last years, a return to FNAC with cell-block preparation has been advocated (14), not only for study protocols but even in everyday clinical practice.
The purpose of our study is to evaluate the feasibility, accuracy, and safety of PD-1/PD-L1 expression quantification in cell-block specimens obtained with transthoracic CT-guided FNAC in an ordinary clinical setting with a focus on the interventional radiology perspective.
Methods
Patients
We collected consecutive unselected series of 361 FNAC samples of pulmonary nodules and masses which came to our observation from June 2017 to October 2018. The study population comprised of 128 women and 233 men with a mean age of 71 years (range, 31–93 years). The research was performed according to the Declaration of Helsinki principles and approval of the Ethical committee was obtained (Melgene Study, protocol number 79456).
All patients signed the informed consent before the procedure. Patient and lesion characteristics are summarized in Table 1.
Table 1.
Clinical, morphological and safety data of the analyzed samples
| Unselected pool of patients | ||
|---|---|---|
| Total patients, n | 361 | |
| Age (years), mean (min–max) | 69.7 (31–93) | |
| Sex, n | 233 M, 128 F | |
| Diagnosis | Neoplastic | 260/361 (72%) |
| Non-neoplastic | 78/361 (21.6%) | |
| Nondiagnostic | 23/361 (6.4%) | |
|
| ||
| Tumoral lesions | ||
| Age (years), mean (min–max) | 70.8 (31–93) | |
| Sex, n | 176 M, 84 F | |
| Dimensions of the nodule (mm), min–max | 8–130 | |
| NSCLC | 192/260 (73.8%) | |
| Mix of SCLC | 68/260 (26.2%) | |
| Safety | ||
|
| ||
| Complications | No or early minor complications | 184/192 (96%) |
| Major complications | 8/192 (4%) | |
Data are presented as ratio of patients (percentage), unless otherwise noted.
NSCLC, non-small cell lung carcinoma; SCLC, small cell lung carcinoma.
CT-guided FNAC
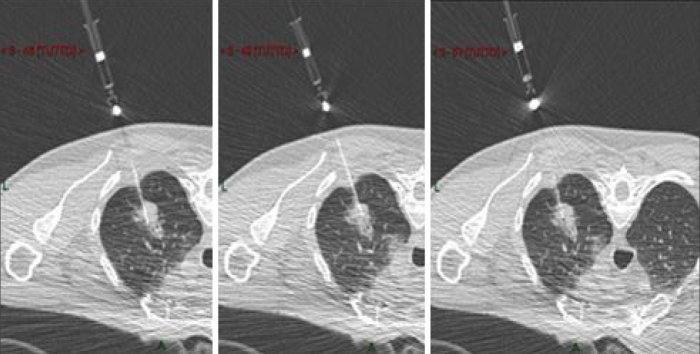
The decision to perform a CT-guided FNAC, over other diagnostic options, was taken by a multidisciplinary team composed by oncologists, pulmonologists, radiologists, radiotherapists, pathologists, and thoracic surgeons. CT-guided FNAC has been performed using a 16-row detector scanner (Somatom Sensation, Siemens) equipped with a fluoro-CT module (CareVision, Siemens). The gantry can be tilted up to ±18° to avoid ribs or vessels. FNAC has been performed using Chiba needles (HS Diva, HS) ranging from 24 G to 20 G (24 G and 22 G to reach nodules by crossing portions of the lung parenchyma, 20 G to reach nodules located more peripherally). The length of the needle was 10 or 15 cm. The aspiration system ensures the capillary draining of the material inside a small syringe of 2.5 mL connected to the needle. No automatic aspiration pistols were used because of the limited space inside the gantry. One advantage of FNAC is the possibility to reach different portions of the nodules, to guarantee aspiration of representative material by sampling different areas of each nodule, in particular in nonsolid lesions (Fig. 1). Most commonly, we sampled at least three different locations even if it is hypothetically possible to reach every portion of the nodule with the same needle insertion.
Figure 1.
CT-guided fine-needle aspiration cytology of mixed solid/nonsolid lesion in the upper lobe of the right lung on the axial plane in a patient on prone position. The needle reaches three different portions of the nodule to guarantee the aspiration of material by sampling different areas of the nodule.
Cytology
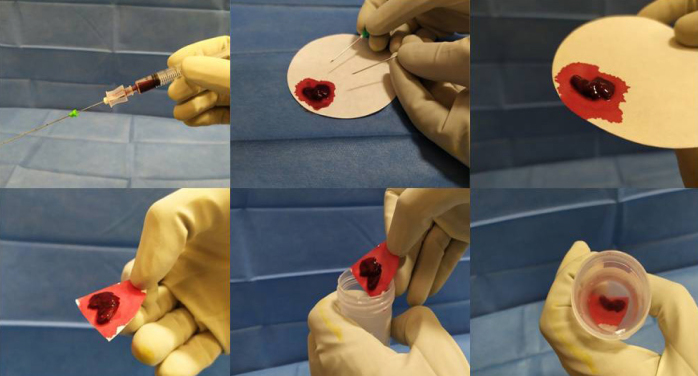
All the material obtained was fixed in formalin to obtain a cell-block. Briefly, the only difference with our previous approach based on smears is relative to “post-procedural” management of the biologic sample obtained at aspiration. It is dropped on tissue paper, let dry for few seconds, and then fixed by immersion of the paper on 10% buffered formalin (Fig. 2) instead of being smeared on slides (manual smears using a slide and immediate fixations). FNAC itself was conducted without any modification in hardware (e.g., needles, syringe) or procedure. The sample was sent to the Pathology Unit and processed according to routine procedures for small biopsy samples within 24 hours from sampling. Immunohistochemical (IHC) analysis to detect PD-L1 expression levels was performed on sequential unstained slides for all cases diagnosed as NSCLS with enough material according to guidelines, together with the analysis of ALK and ROS1 rearrangement when required by oncologists. IHC tests were performed with the 22C3 anti-PD-L1 monoclonal antibody (DAKO) on a DAKO Omnis autostainer, using a laboratory-developed test protocol, and appropriate negative and positive controls (15).
Figure 2.
Management of the biologic sample, which is slightly dried on tissue paper and then fixed in formalin to create a cell-block.
Based on the cytological analysis results, cases were divided into three groups: i) neoplastic lesions, ii) non-neoplastic lesions, and iii) nondiagnostic samples. Among neoplastic lesions, the adequacy for PD-L1 expression analysis and tumor proportion score (TPS), as provided in the cytopathology report, were recorded. According to the literature, TPS can be calculated on a sample with at least 100 viable cells; samples were therefore divided into adequate and not adequate. For adequate samples, the TPS itself was noted.
Statistical analysis
The statistical analysis was conducted using a dedicated software (Stata 16, StataCorp). A hypothesis test has been performed to validate study findings and mainly to evaluate if deviation of the observed frequencies was due to sampling or to the effect of the procedure through chi-squared test.
Results
Of the 361 consecutive pulmonary specimens included in the study, 93.6% provided diagnostic material, including 260 neoplastic (72%) and 78 non-neoplastic lesions (21.6%). Only 23 samples (6.4%) resulted as nondiagnostic.
From the radiologic point of view, 145 NSCLC nodules (76%) had solid appearance, and the remaining 47 nodules (24%) had mixed or nonsolid appearance; 159 lesions (83%) displayed a diameter ≤5 cm; according to the 8th edition of TNM there were 5 T1a (2.6%), 47 T1b (24.5%), 56 T1c (29.2%), 31 T2a (16.1%), 23 T2b (12%), 22 T3 (11.5%), and 8 T4 (4.2%).
NSCLC diagnosis accounted for 73.8% (192/260) of neoplastic lesions: most of them were adenocarcinomas (83%, 160/192), followed by squamous cell carcinoma (SCC, 14%, 27/192) and poorly differentiated carcinoma (negative for TTF1 and p40 immunostaining, 3%, 5/192). The remaining 26.2% (68/260) of neoplastic non-NSCLC lesions were a mix of SCLCs, neuroendocrine tumors, and other rare neoplastic lesions. PD-L1 was evaluated in all 192 NSCLC cytology specimens: 180 samples were found to be adequate for PD-L1 testing, while the remaining 12 cytological samples (6%) did not reach the cellularity threshold for evaluation. TPS was <1% in 64%, >1% and <50% in 12%, and >50% in 24% of NSCLC cases. The observed frequencies were coherent with the expected ones (16), thus allowing, through chi-squared test, the validation of the approach proposed.
According to the 8th edition of TNM, of the inadequate samples for PD-L1 evaluation, 4 were T2, 7 were T1, and 1 was T3. Two lesions had a nonsolid appearance, 4 had mixed appearances, and the last 6 presented a solid appearance. The FNAC of these samples have all been performed using Chiba needles 24G. Three of these lesions occurred peripherally within the lung parenchyma (<1 cm from the parietal pleura); 9 of them presented a distance from the parietal pleura between 1 and 3 cm. Results are summarized in Table 2. FNAC procedure was concluded in the absence of complications or with early minor complications (classified according to the latest CIRSE guidelines) (17) in the vast majority (96%) of NSCLC cases (184/192). Major complications occurred in 8 patients, including 4 hemoptysis and 3 hemothorax, both resolved after tranexamic acid infusion. In only one case the procedure was complicated by the occurrence of complete pneumothorax requiring chest tube insertion and drainage. The 3 patients who developed hemothorax and the patient who needed chest tube insertion required hospitalization (4/192, 2%), while the other complications (minor complications and hemoptysis) were managed as outpatients.
Table 2.
Morphological and molecular data of the analyzed samples
| NSCLC | ||
|---|---|---|
| Appearance | Solid/nonsolid | 145/47 |
|
| ||
| T stage | T1a (n=5), T1b (n=47), T1c (n=56), T2a (n=31), T2b (n=23), T3 (n=22), T4 (n=8) | |
|
| ||
| Histology | Adenocarcinoma, 160/192 | |
| Poorly differentiated carcinoma, 5/192 | ||
| Squamous carcinomas, 27/192 | ||
|
| ||
| PD-L1 expression | Adequate immunostainings, 180/192 | |
| TPS | <1% in 64%, | |
| >1% and <50% in 12% | ||
| >50% in 24% | ||
PD-L1, programmed death–ligand 1; TPS, tumor proportion score.
Statistical analysis confirmed that the differences between observed complication and the expected ones (18) were not statistically different (χ2=2.7757, p = 0.428). Details are reported in Table 3.
Table 3.
Major and minor complications in the cohort compared with the expected complication rates
| NSCLC | Major complications | Hospitalization | Minor complications | ||
|---|---|---|---|---|---|
| Pneumo-thorax with drainage | Hemothorax | Hemoptysis | Pneumo-thorax/ pleural hemorrhagic effusion | ||
| Observed (%) | 0.5 | 1.6 | 2 | 2 | 14.6 |
| Expected (%) | 1–2 | - | 0.5 | 2 | 12–45 |
NSCLC, non-small cell lung carcinoma.
Discussion
In our study, PD-L1 immunostaining documented the predominance of low/negative TPS (>1%–20%) in 72%, while high TPS (>20%) was documented in only 28% of cases. This distribution is following the literature data (19) resembling the expected distribution of PD-L1 expression in the tumor population.
According to CIRSE guidelines on percutaneous needle biopsy (PNB), our diagnostic technical success rate of PNB is 93.6%, far above the proposed threshold of 70%–75% (17). Only 6% of FNAC samples were found to be inadequate for PD-L1 expression quantification, while 94% of the immunostainings were adequate; even considering as “non-diagnostic” samples, those adequate to confirm the neoplastic nature and to assess the histotype of the lesion but unable to evaluate prognostic/response factors (e.g., PD-L1 expression quantification) the threshold is still exceeded by far (90% vs. 70%–75%). CB sample adequacy was reported to be approximately 96.4% (20). Even though the adequacy of CB samples is slightly higher than FNAC samples, both are far above the threshold of 70%–75% set by the guidelines for a diagnostic technical success rate of PNB. Within the limits of the cohort analyzed, the adequacy of PD-L1 expression quantification on FNAC samples seems to correlate with the T stage (seven inadequate samples were T1, four inadequate samples were T2 and only one inadequate sample was T3; no T4 were found inadequate). In addition, nodules that provide inadequate materials were generally located deeper in the lung parenchyma.
In the current era of precise medicine, lung cancer therapy design is increasingly based on the pathological and molecular tumor features (2). Cytological samples are now used for IHC and molecular testing of predictive biomarkers for actionable targets. Thus, the diagnostic workup of NSCLC cannot rely solely on traditional microscopic histotype definition. According to most recent guidelines, IHC assessment of histotype markers is important to classify poorly differentiated SCC or adenocarcinoma (21). IHC screening for ALK and ROS1 rearrangement should be performed on adenocarcinoma cytological samples (12); furthermore, clinical trials have validated IHC quantification of PD-1/PD-L1 expression (tumor proportion score, TPS), as a predictive biomarker for immune checkpoint inhibitor therapy (22). The development of the cell-block method, a complementary approach to conventional aspirate smear preparations, enabled 100% accuracy of tumor histotype definition on FNAC (23). The possibility to evaluate cell-block sections with IHC staining also allows for molecular subtyping and identification of predictive markers of response to immunotherapy. Many pathology studies already validate the PD-L1 testing with cytology cell block, providing accurate diagnoses and defining precise treatments in advanced NSCLC (12, 24). Notably, evaluation of PD-L1 staining in cytology has shown high concordance with histology specimens sampled from the same lesion (25).
Despite the recent advances in PD-L1 quantification on cytological samples, to the best of our knowledge, no reports of feasibility, accuracy, and safety of this technique from a “radiological” and “procedural” perspective has been published.
CT-guided percutaneous lung FNAC is a minimally invasive procedure to diagnose lung lesions, with low complication rates (21), especially concerning hemorrhagic complications. All four complications (1 case of hemoptysis and 3 cases of hemothorax) were solved after tranexamic acid infusion; only one patient developed a complete pneumothorax. Many studies showed a significantly higher complication rate for CB compared with FNAC. Tsai et al. (20) reviewed a cohort of 101 patients and reported procedure-related CB complications in 25.6%, pneumothorax being the most common (22.7%). In an extensive meta-analysis of studies reporting complications in CT-guided lung biopsy, Heerink et al. (13) demonstrated a higher complication rate for CB (38.8%) compared with FNAC (24%).
CT-guided FNAC provides the possibility to reach different areas of the same lesion, by moving the needle inside the nodule without crossing the pleura several times. This procedure may represent an advantage of FNAC over CB because such sampling is expected to more reliably represent spatial tumor heterogeneity of PD-L1 expression (24, 26).
The results of our study and a review of other clinical studies and meta-analyses suggest that FNAC is generally safer and better tolerated than CB but yields a slightly lower rate of samples adequate for IHC quantification of PD-L1 (13, 19, 21, 23). PD-L1 quantification is needed at the time of diagnosis, especially in advanced diseases and in fragile and older patients, to properly define the therapeutic strategy and identify those cases who will benefit from front-line immunotherapeutic regimens. However, based on its lower complication rates, FNAC may still be preferable to CB as a first-line diagnostic approach. The lower complication rates can allow prompt start of therapy reducing post-procedural hospitalization; the latter is especially important when facing an advanced disease even in fit patients.
The main limitations of our study are the relatively low number of FNAC specimens and the lack of a cross control of FNAC PD-L1 expression on surgical resection tumor samples. Nonetheless, cytological-surgical correlations available in the literature show a good correspondence of TPS in paired samples (25).
In conclusion, the findings of our study indicate that PD-L1 quantification by IHC by using a cell-block approach on CT-guided FNAC is a feasible and safe technique for PD-L1 quantification in routine clinical practice and should be taken into account alongside with CB approach, especially in advanced tumors and/or in fragile and older patients. From the radiologist’s perspective, no significant changes to the procedure are needed, making the cell-block technique easily implementable and acceptable by interventional radiologists.
Main points.
PD-L1 quantification using cell-block approach on fine-needle aspiration cytology (FNAC) is a feasible technique.
CT-guided FNAC is a safe approach especially in case of advanced disease.
The cell-block technique could be easily implemented by interventional radiologists.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II) J Vasc Surg. 2007;45(Suppl):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 2.Nilesh H, Venkataramu N, Stanley K, et al. Quality improvement guidelines for percutaneous management of acute lower-extremity ischemia. J Vasc Interv Radiol. 2013;24:3–15. doi: 10.1016/j.jvir.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 3.Diffin DC, Kandarpa K. Assessment of peripheral intraarterial thrombolysis versus surgical revascularization in acute lower-limb ischemia: a review of limb-salvage and mortality statistics. J Vasc Interv Radiol. 1996;7:57–63. doi: 10.1016/S1051-0443(96)70734-0. [DOI] [PubMed] [Google Scholar]
- 4.Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–538. doi: 10.1016/S0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 5.Working Party on Thrombolysis in the Management of Limb Ischemia b Thrombolysis in the management of lower limb peripheral arterial occlusion-a consensus document. Working Party of Thrombolysis in the Management of Limb Ischemia. Am J Cardiol. 1998;81:207–218. [PubMed] [Google Scholar]
- 6.Patel N, Sacks D, Patel Rl, et al. SIR reporting standards for the treatment of acute limb ischemia with use of transluminal removal of arterial thrombus. J Vasc Interv Radiol. 2003;14(Suppl):S453–S465. doi: 10.1097/01.RVI.0000094619.61428.11. [DOI] [PubMed] [Google Scholar]
- 7.Berridge DC, Gregson RH, Hopkinson BR, et al. Randomized trial of intra-arterial recombinant tissue plasminogen activator, intravenous recombinant tissue plasminogen activator and intra-arterial streptokinase in peripheral arterial thrombolysis. Br J Surg. 1991;78:988–995. doi: 10.1002/bjs.1800780831. [DOI] [PubMed] [Google Scholar]
- 8.Davis FM, Albright J, Gallagher KA, et al. Early outcomes following endovascular, open surgical, and hybrid revascularization for lower extremity acute limb ischemia. Ann Vasc Surg. 2018;51:106–112. doi: 10.1016/j.avsg.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 9.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease. Int Angiol. 2007;26:81–157. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 10.Kronlage M, Printz I, Vogel B, et al. A comparative study on endovascular treatment of (sub)acute critical limb ischemia: mechanical thrombectomy vs thrombolysis. Drug Des Devel Ther. 2017;11:1233–1241. doi: 10.2147/DDDT.S131503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zehnder T, Birrer M, Do D, et al. Percutanous catheter thrombus aspiration for acute or subacute arterial occlusion of the legs: how much thrombolysis is needed? Eur J Vasc Endovasc Surg. 2000;20:41–46. doi: 10.1053/ejvs.2000.1117. [DOI] [PubMed] [Google Scholar]
- 12.Wissgott C, Kamusella P, Richter A, et al. Mechanical rotational thrombectomy for treatment thrombolysis in acute and subacute occlusion of femoropopliteal arteries: retrospective analysis of the results from 1999 to 2005. Rofo. 2008;180:325–331. doi: 10.1055/s-2008-1027144. [DOI] [PubMed] [Google Scholar]
- 13.Heller S, Lubanda JC, Varejka P, et al. Percutaneous mechanical thrombectomy using Rotarex® S device in acute limb ischemia in infrainguinal occlusions. BioMed Res Int. 2017;2017 doi: 10.1155/2017/2362769. 2362769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanek F, Ouhrabkova R, Prochazka D. Mechanical thrombectomy using the Rotarex catheter in the treatment of acute and subacute occlusions of peripheral arteries: immediate results, long-term follow-up. Int Angiol. 2013;32:52–60. [PubMed] [Google Scholar]
- 15.Khatri P, Neff J, Broderick JP, et al. Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke. 2005;36:2400–2403. doi: 10.1161/01.STR.0000185698.45720.58. [DOI] [PubMed] [Google Scholar]
- 16.Bauman F, Sharpe E, Peña C, et al. Technical results of vacuum-assisted thrombectomy for arterial clot removal in patients with acute limb ischemia. J Vasc Interv Radiol. 2016;27:330–335. doi: 10.1016/j.jvir.2015.11.061. [DOI] [PubMed] [Google Scholar]
- 17.StataCorp. Stata Statistical Software: Release 5. College Station TX: StataCorp LP; 1997. [Google Scholar]
- 18.Morrison HL. Catheter-directed thrombolysis for acute limb ischemia. Semin Intervent Radiol. 2006;23:258–269. doi: 10.1055/s-2006-948765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeller T, Frank U, Bürgerlein K, et al. Langzeitergebnisse nach Rekanalisation akuter und subakuter thrombotischer arterieller Verschlüsse der unteren Extremitäten mit einem Rotations-Thrombektomiekatheter. Fortschr Röntgenstr. 2002;174:1559–1565. doi: 10.1055/s-2002-35942. [DOI] [PubMed] [Google Scholar]
- 20.Duc SR, Schoch E, Pfyffer M, et al. Recanalization of acute and subacute femoropopliteal artery occlusions with the rotarex catheter: one year follow-up, single center experience. Cardiovasc Interven Radiol. 2005;28:603–610. doi: 10.1007/s00270-004-0339-3. [DOI] [PubMed] [Google Scholar]
- 21.Lichtenberg M. Percutaneous mechanical thrombectomy by means of rotational thrombectomy. current study situation. Medizinische Klinik. 2010;105:705–710. doi: 10.1007/s00063-010-1122-0. [DOI] [PubMed] [Google Scholar]
- 22.Stanek F, Ouhrabkova R, Prochazka D. Percutaneous mechanical thrombectomy in the treatment of acute and subacute occlusions of the peripheral arteries and bypasses. Vasa. 2016;45:49–56. doi: 10.1024/0301-1526/a000495. [DOI] [PubMed] [Google Scholar]
- 23.Ouriel K, Shortell CK, DeWeese JA, et al. A comparison of thrombolytic therapy with operative revascularization in the initial treatment of acute peripheral arterial ischemia. J Vasc Surg. 1994;19:1021–1030. doi: 10.1016/S0741-5214(94)70214-4. [DOI] [PubMed] [Google Scholar]
- 24.Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N Engl J Med. 1998;338:1105–1111. doi: 10.1056/NEJM199804163381603. [DOI] [PubMed] [Google Scholar]
- 25.Wissgott C, Kamusella P, Richter A, et al. Treatment of acute femoropopliteal bypass graft occlusion: comparison of mechanical rotational thrombectomy with ultrasound-enhanced lysis. Rofo. 2008;180:547–552. doi: 10.1055/s-2008-1027216. [DOI] [PubMed] [Google Scholar]
- 26.McNamara TO, Bomberger RA, Merchant RF. Intra-arterial urokinase as the initial therapy for acutely ischemic lower limbs. Circulation. 1991;83(2 Suppl):I106–119. [PubMed] [Google Scholar]
- 27.Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Ann Surg. 1994;220:251–268. doi: 10.1097/00000658-199409000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ansel GM, George BS, Botti CF, et al. Rheolytic thrombectomy in the management of limb ischemia: 30-day results from a multicenter registry. J Endovasc Ther. 2002;9:395–402. doi: 10.1177/152660280200900402. [DOI] [PubMed] [Google Scholar]
- 29.Langenskiöld M, Smidfelt K, Karlsson A, et al. Weak links in the early chain of care of acute lower limb ischaemia in terms of recognition and emergency management. Eur J Vasc Endovasc Surg. 2017;54:235–240. doi: 10.1016/j.ejvs.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Bérczi V, Deutschmann HA, Schedlbauer P, et al. Early experience and midterm follow-up results with a new, rotational thrombectomy catheter. Cardiovasc Intervent Radiol. 2002;25:275–281. doi: 10.1007/s00270-001-0095-6. [DOI] [PubMed] [Google Scholar]