Abstract
PURPOSE
We aimed to evaluate the feasibility and safety of a modified technique for portal vein recanalization, percutaneous transluminal sharp recanalization (PTSR), when performing transjugular intrahepatic portosystemic shunt (TIPS) for the treatment of chronic portal vein occlusion (CPVO) and portal hypertension.
METHODS
Nine consecutive patients with CPVO and portal hypertension had undergone TIPS and PTSR procedure after failing in conventional percutaneous catheterization from March 2017 to July 2019. Technical success rates, effectiveness, and complications were evaluated. Follow-up of patients’ clinical outcomes and shunt patency were performed periodically. Primary and secondary shunt patency were analyzed by Kaplan-Meier method.
RESULTS
The occluded portal veins were successfully recanalized after failing in conventional percutaneous catheterization, and TIPS procedures were completed in all 9 patients. Two patients suffered from procedure-related complications. A portosystemic pressure gradient <12 mmHg, or a percent reduction of 25% to 50% of baseline, was achieved in all 9 patients after TIPS. During the median follow-up period of 28 months (range, 9–36 months), 1 patient experienced recurrent ascites and the other 8 patients remained asymptomatic. The cumulative rates of primary and secondary shunt patency were 66.67% and 100%, respectively, at 2 years.
CONCLUSION
As a supplementary method, PTSR is a feasible and safe method for portal vein recanalization when performing TIPS for patients with CPVO and portal hypertension.
Portal vein thrombosis is one of the important causes of extrahepatic portal vein obstruction and prehepatic portal hypertension (1). When the acute portal vein thrombosis becomes chronic, the occluded portal vein gradually atrophies, fibrosis develops and chronic portal vein occlusion (CPVO) ensues, eventually leading to the cavernous transformation of portal vein (2), which is a compensatory response to the portal vein occlusion whereby a collateral vein forms to help reduce portal pressure and maintain liver blood perfusion (3, 4). However, they are usually not completely effective in decompressing the portal system, and many patients have persistent portal hypertension and develop serious portal hypertensive complications, such as variceal bleeding and ascites (5).
Accumulating evidence has shown that transjugular intrahepatic portosystemic shunt (TIPS) (5–8) or modified TIPS combined with transhepatic or transsplenic approaches (9, 10) is technically feasible and effective to relieve portal hypertension in cirrhotic or non-cirrhotic patients with portal vein thrombosis or CPVO, with a technical success rate of 70% to 100%. Recanalization of the occluded portal vein is the key to the TIPS procedure for patients with portal hypertension and CPVO, while failed portal vein recanalization is the leading cause of TIPS failure (11). Conventional percutaneous catheterization techniques for portal vein recanalization mainly include percutaneous transhepatic and percutaneous transsplenic approaches. Recanalization can be achieved in most cases through either technique alone or in combination; however, they are not feasible in patients with portal vein atrophy and severe fibrosis.
Therefore, for the cases of failed portal vein recanalization by conventional percutaneous catheterization, we have developed a procedure of percutaneous transluminal sharp recanalization (PTSR) of the portal vein to complete TIPS. The purpose of this study is to introduce this technique when performing a TIPS procedure for patients with CPVO and portal hypertension, as well as to evaluate its feasibility and safety.
Methods
Patients
The imaging and clinical data of consecutive patients with CPVO and portal hypertension who had undergone TIPS procedure at our hospital from March 2017 to July 2019 were retrospectively analyzed. The inclusion criteria were: (a) CPVO diagnosis by Doppler ultrasound and enhanced computed tomography (CT); (b) recurrent variceal bleeding unresponsive to endoscopic therapy and medical treatment or refractory ascites. Patients with intrahepatic malignancies were excluded. This retrospective study was approved by the institutional review board of our hospital (decision number of ethics committee approval: 2017047 and [2018]02-275-01). Written patient informed consent was obtained before the procedure.
Interventions
All procedures were performed under fluoroscopy using local anesthesia. TIPS was performed by two of four interventional radiologists (Z.J., M.H., M.L. and C.W.), who had at least 10 years of experience with TIPS procedure. Indirect portography was performed via the superior mesenteric artery and the splenic artery to show the occlusion of the portal vein system and the branches of the intrahepatic portal vein. The right branch of the portal vein was punctured with a 22-gauge needle (Neff Percutaneous Access Set, Cook Medical Inc.) via the right midaxillary line. A 0.018-inch guidewire (V-18™ ControlWire™, Boston Scientific Corporation) was introduced through the needle into the branch of the intrahepatic portal vein. The needle was withdrawn and was exchanged for a 6 F sheath (Terumo Corporation).
All patients underwent the conventional percutaneous transhepatic catheterization for portal vein recanalization at first. With the use of a 5 F KMP catheter (Cook Medical Inc.), a V-18™ ControlWire™ was rotated to recanalize the occluded portal vein. If preoperative imaging showed patent splenic vein, percutaneous transsplenic puncture of the peripheral splenic vein (22-gauge needle) was performed for patients failing in the transhepatic approach. After access was achieved, a V-18™ ControlWire™ was advanced, and the needle was withdrawn and was exchanged for a 5 F sheath (Cook Medical Inc.). Percutaneous transsplenic catheterization, which was similar to the percutaneous transhepatic approach, was performed for portal vein recanalization.
Patients failing in portal vein recanalization by conventional percutaneous catheterization received PTSR (Figs. 1–3 and Videos 1, 2). A 20-gauge Chiba needle was bent 30°–40° at distal 1 cm and entered via guidewire into the transhepatic sheath and the 5 F KMP catheter. Under the roadmap of indirect portography and catheter positioning in the superior mesenteric artery or in the splenic vein guidance, the patent part of main portal vein or superior mesenteric vein or splenic vein was punctured through the residual trunk of the occluded portal vein. During the puncture procedure, the needle was slowly inserted and the puncture direction was adjusted according to the position of the guidance catheter, rotating the C-arm repeatedly. On the other hand, cone-beam CT might not be applicable during PTSR procedure owing to its low resolution. After confirmation of the needle tip in the trunk of superior mesenteric vein or splenic vein with the injection of contrast medium, a V-18™ ControlWire™ was introduced through the needle into the superior mesenteric vein or splenic vein. Then a 5 F KMP catheter was advanced over the guidewire and portal vein pressure was measured. A 6 mm diameter balloon catheter (RIVAL, Bard Peripheral Vascular, Inc.) was used for angioplasty and the balloon was positioned at the site of portal bifurcation.
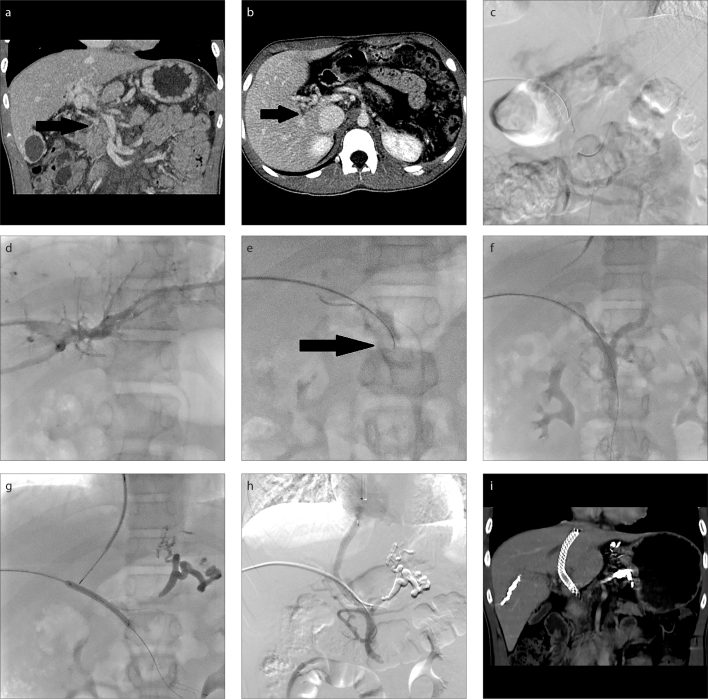
Figure 1. a–i.
A 14-year-old boy (Patient 2) with recurrent variceal bleeding, who underwent splenectomy 8 years ago due to cryptogenic splenomegaly and hypersplenism. CT images (a, b) demonstrate complete main portal vein occlusion with portal-portal collateral veins (black arrow) and patent superior mesenteric vein. Indirect portography (c) shows complete occlusion of the main portal vein. Image (d) shows peripheral branch of right portal vein accessed transhepatically and conventional percutaneous catheterization failed in recanalization. Image (e) shows the patent superior mesenteric vein punctured through the residual trunk of the occluded portal vein by a 20-gauge Chiba needle (black arrow) under indirect portography and catheter positioning guidance. Image (f) shows successful access to superior mesenteric vein as identified by direct portography. Image (g) shows portal vein punctured via transjugular approach after variceal embolization, with the contrast-filled balloon as a target. Image (h) shows venogram obtained after shunt creation with a covered stent implantation demonstrating shunt patency. CT image (i) demonstrates shunt patency after the procedure.
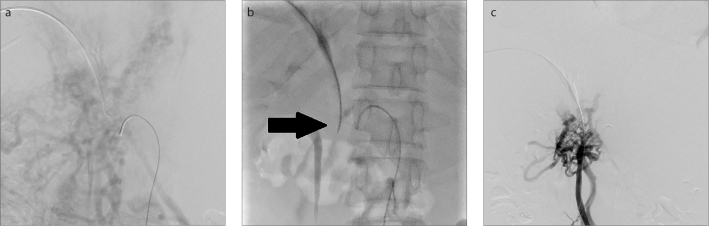
Figure 2. a–c.
A 44-year-old man (Patient 3) who suffered from ascites and protein S deficiency. Indirect portography (a) shows complete occlusion of the main portal vein. In image (b), under the indirect portography and catheter positioning guidance, the patent superior mesenteric vein was punctured through the residual trunk of the occluded portal vein by a 20-gauge Chiba needle (black arrow). Image (c) shows successful access to superior mesenteric vein as identified by direct portography.
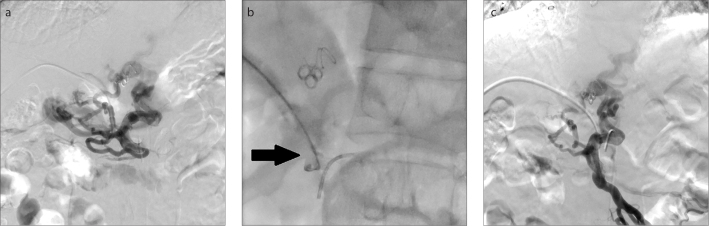
Figure 3. a–c.
A 52-year-old man (Patient 1) with recurrent variceal bleeding, who underwent liver transplantation and splenectomy 6 years ago due to cirrhosis. Direct portography via the transhepatic approach (a) shows complete occlusion of the main portal vein and conventional percutaneous catheterization failed in recanalization. In image (b), under the indirect portography and catheter positioning guidance, the patent superior mesenteric vein was punctured through the residual trunk of the occluded portal vein by a 20-gauge Chiba needle (black arrow). Image (c) shows successful access to superior mesenteric vein as identified by direct portography.
The RUPS-100 puncture set (Cook Medical Inc.) was used for establishing shunt. After successfully puncturing the right internal jugular vein, a 10 F sheath was introduced into the inferior vena cava, and the pre-TIPS portosystemic pressure gradient (PPG) was measured. After the middle or right hepatic vein was catheterized, the portal vein was punctured with the contrast-filled balloon as a target via the transjugular approach. A 0.035-inch guidewire (Terumo Corporation) was introduced along the catheter and entered into the patent part of the superior mesenteric vein or splenic vein via transjugular approach, followed by exchange with a 0.035-inch guidewire (Amplatz Super Stiff ™, Boston Scientific Corporation).
Variceal embolization was performed before shunt creation with a mixture of glue (NBCA; Histoacryl, B. Braun) and iodized oil (Lipiodol; Guerbet), or coils (Cook Medical Inc.).
After performing pre-dilation of the intrahepatic tract by a 6 mm diameter balloon catheter, an 8 mm diameter TIPS covered stent (VIATORR®, W.L. Gore and Associates) was deployed to complete the shunt. If necessary, additional 8 mm diameter covered stents (VIABAHN®, W.L. Gore and Associates) or 8 mm diameter bare metal stents (E•Luminexx™ Vascular Stent, Bard) were deployed to cover the occluded part of the portal vein. The stents were expanded with an 8 mm diameter balloon. The final portography was performed and the post-TIPS PPG was measured.
The transhepatic and transplenic tract was embolized with a mixture of glue and iodized oil.
Postoperative management
All patients underwent abdominal Doppler ultrasound and regular blood test immediately after the procedure to detect intra-abdominal hemorrhage. In order to avoid shunt dysfunction, low-molecular-weight heparin was given subcutaneously for the first 2 to 5 days after the procedure, and oral anticoagulants were prescribed for 6 to 12 months.
Follow-up
All patients were followed up at 1 week, 1, 3 and 6 months, and every 6 months until death or March 2020. Follow-up examinations included regular blood tests, coagulation function tests, Doppler ultrasound or CT. When shunt dysfunction was suspected (recurrence of variceal bleeding or ascites) or suggested by Doppler ultrasound and CT, TIPS venogram was obtained.
Statistical analysis
All qualitative variables were presented as frequency (percentage) and quantitative variables were expressed as mean ± standard deviation. The cumulative rate of shunt dysfunction was calculated by the Kaplan-Meier method. All analyses were performed using IBM SPSS V. 20 (IBM Corp.).
Results
Fourty consecutive patients with CPVO and portal hypertension underwent TIPS procedure from March 2017 to July 2019. A total of 9 patients (6 males and 3 females; median age, 43 years; IQR, 41.5–48.5 years) with CPVO and portal hypertension who had undergone PTSR and TIPS procedure after failing in conventional percutaneous catheterization were included in this study. Patients’ baseline clinical characteristics are summarized in Table 1. All patients received PTSR after failure of conventional percutaneous catheterization. Four patients (Patients 4, 6, 8, 9) failed in the combination of transhepatic and transsplenic approaches since preoperative images showed patent splenic vein, while the other 5 did not received a transsplenic approach due to the history of splenectomy (n=3) or totally occluded splenic vein (n=2).
Table 1.
Clinical characteristics of patients
| No. | Sex/Age (y) | Variceal bleeding | Ascites | Underlying diseases | Abdominal operations before TIPS | Thrombotic occlusion | ||
|---|---|---|---|---|---|---|---|---|
| MPV | SMV | SV | ||||||
| 1 | M/52 | + | − | − | Liver transplatation, splenectomy, cholecystectomy | Total | − | NA |
|
| ||||||||
| 2 | M/14 | + | − | − | Splenectomy | Total | − | NA |
|
| ||||||||
| 3 | M/44 | − | + | Protein S deficiency | − | Total | − | + |
|
| ||||||||
| 4 | F/42 | + | + | − | Liver transplatation, cholecystectomy | Total | + | − |
|
| ||||||||
| 5 | M/45 | + | − | Cirrhosis | Splenectomy | Total | − | NA |
|
| ||||||||
| 6 | M/43 | + | − | − | − | Total | + | − |
|
| ||||||||
| 7 | F/41 | − | + | − | − | Total | − | + |
|
| ||||||||
| 8 | F/57 | + | − | Primary myelofibrosis | − | Total | + | − |
|
| ||||||||
| 9 | M/43 | − | + | Cirrhosis | − | Total | + | − |
TIPS, transjugular intrahepatic portosystemic shunt; MPV, main portal vein; SMV, superior mesenteric vein; SV, splenic vein; M, male; F, female; NA, not applicable.
Patients’ clinical outcomes were summarized in Table 2. The median attempts of PTSR was 2 (range, 1–3). Two patients (2/9, Patients 5 and 7) suffered from procedure-related complications (13). Patient 5 had abdominal pain on the first postoperative day with an increased heart rate. Abdominal ultrasound showed peritoneal effusion, and blood examination showed a decrease in hemoglobin. Hepatic arteriography revealed bleeding from the branch of the right hepatic artery and superselective hepatic arterial embolization was performed. Patient 7 had increased heart rate during procedure, and the portography showed extravasated contrast medium. After the covered stent was implanted, the extravasation of contrast medium disappeared. Both patients recovered and were discharged after receiving medical treatments including transfusion of two unit of red blood cell and fluid administration and received anticoagulation therapy thereafter.
Table 2.
Clinical outcomes
| No. | Transsplenic catheterization | Attempts of PTSR | Pre-TIPS PPG (mmHg) | Post-TIPS PPG (mmHg) | Follow-up (months) | Procedure-related complication | Shunt dysfunction | Hepatic encephalopathy |
|---|---|---|---|---|---|---|---|---|
| 1 | − | 3 | 28 | 12 | 36 | − | − | − |
| 2 | − | 3 | 30 | 11 | 34 | − | + | − |
| 3 | − | 3 | 32 | 11 | 32 | − | + | − |
| 4 | + | 2 | 26 | 5 | 29 | − | − | − |
| 5 | − | 2 | 26 | 12 | 28 | + | − | − |
| 6 | + | 2 | 29 | 14 | 26 | − | − | − |
| 7 | − | 2 | 26 | 16 | 24 | + | + | − |
| 8 | + | 1 | 29 | 9 | 23 | − | − | − |
| 9 | + | 2 | 21 | 9 | 9 | − | − | − |
PTSR, percutaneous transluminal sharp recanalization; TIPS, transjugular intrahepatic portosystemic shunt; PPG, portosystemic pressure gradient.
In all 9 patients, the occluded portal veins were successfully recanalized to complete the TIPS procedure. After successfully establishing the shunt, a portosystemic pressure gradient <12 mmHg, or a percent reduction of 25% to 50% of baseline was achieved in all 9 patients. Immediately after the stent placement, portography showed patent shunt in all patients.
The median follow-up was 28 months (range, 9–36 months). Shunt dysfunction occurred in three patients (3/9), and all were identified within 12 months after the procedure. The cumulative rates of primary and secondary shunt patency were 66.7% and 100%, respectively, at 2 years. Patient 3 experienced recurrent mild ascites 11 months after TIPS and paracentesis was not required after shunt revision. Patients 2 and 7 were found to have shunt dysfunction during routine follow-up examination, and both of them were treated with shunt revision. Additionally, there was no death, overt hepatic encephalopathy, or episode of anticoagulation-related major hemorrhagic complication in any patient during the follow-up period.
Discussion
Portal hypertension due to extrahepatic portal vein obstruction and CPVO is recommended to be managed according to the guidelines elaborated for cirrhosis (1). In other words, TIPS should be considered for patients with recurrent variceal bleeding unresponsive to endoscopic therapy and medical treatment or refractory ascites (14, 15). When performing a TIPS procedure for patients with CPVO, recanalization of the occluded portal vein is the key to the success (12, 16). At present, conventional percutaneous catheterization via transhepatic or transsplenic approach is mainly used for portal vein recanalization, in which the guidewire and catheter enter the patent superior mesenteric vein or splenic vein through the occlusion lumen of the portal vein (5–10). However, for patients with fibrotic portal vein atrophy, conventional percutaneous catheterization cannot recanalize the portal vein and leads to TIPS procedure failure. In our study, 9 patients received PTSR after failing in conventional percutaneous catheterization and then TIPS was established successfully. Moreover, the incidences of procedure-related complications and shunt dysfunction in our study were similar to those seen in a previous study (17). Our results suggest that the PTSR is a feasible and safe supplementary method for portal vein recanalization in patients with CPVO.
Intra-abdominal hemorrhage is a serious complication of TIPS (13). Of the 9 patients in this study, 2 had intra-abdominal hemorrhage. One case was due to percutaneous transhepatic puncture-related injury to the hepatic artery. While the other case of intra-abdominal hemorrhage might be related to angioplasty for occluded portal vein after performing PTSR, since the punctured channel might be located outside the vessel lumen. Therefore, covered stent might be better than bare metal stent for covering the occluded portal vein after performing PTSR. According to our experiences, PTSR might have higher technical requirements for the interventional radiologists. By using a catheter in hepatic or superior mesenteric artery for positioning and adjusting the needle direction, the success rate of puncture can be improved and the risk of hemorrhage can be reduced.
Based on previous studies and our experiences, anticoagulation might be effective and safe for selected patients with or without cirrhosis after TIPS procedure (9, 18, 19). Therefore, all patients were prescribed postoperative anticoagulation therapy to reduce the risk of thrombosis in the shunt after excluding contraindication. However, there were still 3 cases of shunt dysfunction that occurred within 1 year after procedure. The reason might be related to the patient’s underlying diseases (20), as one of the patients was diagnosed with protein S deficiency. However, the reason might be insufficient decompression of PPG in another patient (from 26 mmHg to 16 mmHg).
Hepatic encephalopathy is a well-recognized complication of post-TIPS procedure due to changes in liver function and portosystemic shunt, with an incidence of 10%–50% (21). Post-TIPS hepatic encephalopathy affects morbidity and is an important reason for limiting the widespread application of TIPS. Nevertheless, none of the patients in our study developed overt hepatic encephalopathy during follow-up, which is in line with a previous study (9). The fact might be associated with the persistent hepatofugal flow due to CPVO, which caused minimal blood flow emptying into the liver before TIPS. On the other hand, most patients (7/9) in our study did not have cirrhosis, suggesting that patients in our study had better liver function than cirrhotic patients.
On the basis of previous studies and our experience, in cases of poor blood inflow into the shunt due to completely obliterated main portal vein with extensive superior mesenteric vein and splenic vein thrombosis, TIPS insertions might be clinically invalid (9, 12). Therefore, this technique has certain requirements for the patient’s condition: unobstructed superior mesenteric vein or splenic vein and short segment of occluded portal vein. According to our preliminary experience, occluded segment less than 5 cm should be defined as short occluded segment. The length of the occluded segment was measured before procedure according to pre-TIPS axial and coronal CT images. We never performed PTSR on patients with occluded segment more than 5 cm, since long segment might lead to PTSR failure and high risk of complication. These could guarantee a relatively high puncture success rate and low risk of postoperative shunt dysfunction.
We acknowledge that our study was limited by its retrospective nature and relative small sample size. However, as a preliminary study which provided evidence of the efficacy and safety of this modified technique, it is quite valuable. In the future, a large cohort study should be conducted to comprehensively evaluate the results of this study.
In conclusion, PTSR is a feasible and safe method for portal vein recanalization, which can be used as an effective supplement for conventional percutaneous catheterization to improve the success rate of TIPS in patients with portal hypertension and CPVO.
Main points.
Failing in portal vein recanalization may lead to transjugular intrahepatic portosystemic shunt (TIPS) failure in patients with chronic portal vein occlusion.
We have developed a modified percutaneous transluminal sharp recanalization (PTSR) technique for portal vein recanalization.
PTSR is feasible and safe for portal vein recanalization in TIPS for patients with chronic portal vein occlusion.
Supplementary Information
Video 1 shows the procedure of percutaneous transluminal sharp recanalization in Patient 3.
Video 2 shows the procedure of percutaneous transluminal sharp recanalization in Patient 1.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Vascular diseases of the liver. J Hepatol. 2016;64:179–202. doi: 10.1016/j.jhep.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Pagán JC, Hernández-Guerra M, Bosch J. Extrahepatic portal vein thrombosis. Semin Liver Dis. 2008;28:282–292. doi: 10.1055/s-0028-1085096. [DOI] [PubMed] [Google Scholar]
- 3.Wu M, Schuster M, Tadros M. Update on management of portal vein thrombosis and the role of novel anticoagulants. J Clin Transl Hepatol. 2019;7:154–164. doi: 10.14218/JCTH.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senzolo M, Tibbals J, Cholongitas E, Triantos CK, Burroughs AK, Patch D. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with and without cavernous transformation. Aliment Pharmacol Ther. 2006;23:767–775. doi: 10.1111/j.1365-2036.2006.02820.x. [DOI] [PubMed] [Google Scholar]
- 5.Van Ha TG, Hodge J, Funaki B, et al. Transjugular intrahepatic portosystemic shunt placement in patients with cirrhosis and concomitant portal vein thrombosis. Cardiovasc Intervent Radiol. 2006;29:785–790. doi: 10.1007/s00270-005-0090-4. [DOI] [PubMed] [Google Scholar]
- 6.Wils A, van der Linden E, van Hoek B, Pattynama PM. Transjugular intrahepatic portosystemic shunt in patients with chronic portal vein occlusion and cavernous transformation. J Clin Gastroenterol. 2009;43:982–984. doi: 10.1097/MCG.0b013e31819706a4. [DOI] [PubMed] [Google Scholar]
- 7.Fanelli F, Angeloni S, Salvatori FM, et al. Transjugular intrahepatic portosystemic shunt with expanded-polytetrafuoroethylene-covered stents in non-cirrhotic patients with portal cavernoma. Dig Liver Dis. 2011;43:78–84. doi: 10.1016/j.dld.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Han G, Qi X, He C, et al. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with symptomatic portal hypertension in liver cirrhosis. J Hepatol. 2011;54:78–88. doi: 10.1016/j.jhep.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 9.Qi X, Han G, Yin Z, et al. Transjugular intrahepatic portosystemic shunt for portal cavernoma with symptomatic portal hypertension in non-cirrhotic patients. Dig Dis Sci. 2012;57:1072–1082. doi: 10.1007/s10620-011-1975-5. [DOI] [PubMed] [Google Scholar]
- 10.Habib A, Desai K, Hickey R, et al. Portal vein recanalization–transjugular intrahepatic portosystemic shunt using the transsplenic approach to achieve transplant candidacy in patients with chronic portal vein thrombosis. J Vasc Interv Radiol. 2015;26:499–506. doi: 10.1016/j.jvir.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Ye P, Li Y, Ma S, Zhao J, Zeng Q. Percutaneous transhepatic balloon-assisted transjugular intrahepatic portosystemic shunt for chronic, totally occluded, portal vein thrombosis with symptomatic portal hypertension: procedure technique, safety, and clinical applications. Eur Radiol. 2015;25:3431–3437. doi: 10.1007/s00330-015-3777-1. [DOI] [PubMed] [Google Scholar]
- 12.Luo J, Li M, Zhang Y, et al. Percutaneous transhepatic intrahepatic portosystemic shunt for variceal bleeding with chronic portal vein occlusion after splenectomy. Eur Radiol. 2018;28:3661–3668. doi: 10.1007/s00330-018-5360-z. [DOI] [PubMed] [Google Scholar]
- 13.Boyer TD, Haskal ZJ. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology. 2010;51:386–400. doi: 10.1002/hep.20559. [DOI] [PubMed] [Google Scholar]
- 14.de Franchis R Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–335. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 16.Lombardo S, Espejo JJ, Pérez-Montilla ME, Zurera LJ, González-Galilea The keys to successful TIPS in patients with portal vein thrombosis and cavernous transformation. Radiologia. 2018;60:94–104. doi: 10.1016/j.rx.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Qi X, Han G. Transjugular intrahepatic portosystemic shunt in the treatment of portal vein thrombosis: a critical review of literature. Hepatol Int. 2012;6:576–590. doi: 10.1007/s12072-011-9324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delgado MG, Seijo S, Yepes I, et al. Efficacy and safety of anticoagulation on patients with cirrhosis and portal vein thrombosis. Clin Gastroenterol Hepatol. 2012;10:776–783. doi: 10.1016/j.cgh.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Jiang MS, Zhang HL, et al. Is post-TIPS anticoagulation therapy necessary in patients with cirrhosis and portal vein thrombosis? a randomized controlled trial. Radiology. 2016;279:943–951. doi: 10.1148/radiol.2015150369. [DOI] [PubMed] [Google Scholar]
- 20.Qi X, Chen H, Han G. Effect of antithrombin, protein C and protein S on portal vein thrombosis in liver cirrhosis: a meta-analysis. Am J Med Sci. 2013;346:38–44. doi: 10.1097/MAJ.0b013e31826485fc. [DOI] [PubMed] [Google Scholar]
- 21.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1 shows the procedure of percutaneous transluminal sharp recanalization in Patient 3.
Video 2 shows the procedure of percutaneous transluminal sharp recanalization in Patient 1.