Abstract
PURPOSE
The prominent vessel sign (PVS) on susceptibility-weighted imaging (SWI) can be dichotomized into prominent cortical veins (PCV) and prominent medullary veins (PMV). This study was designed to compare the predictive value of PCV and PMV in the evaluation of the severity of acute ischemic stroke (AIS) in patients within the reperfusion window.
METHODS
Forty-seven consecutive patients with AIS within the middle cerebral artery territory were recruited. Magnetic resonance imaging was performed within 8 hours of symptom onset and at 7 days after stroke onset. Infarct volume was measured, and the early clinical outcome at 7 days was assessed using the modified Rankin Scale. PVS was dichotomized into cases with both PCV and PMV and cases with only PCV according to location.
RESULTS
Patients with both PCV and PMV (n=32) had higher admission National Institutes of Health Stroke Scale scores (p = 0.020), larger infarct volumes at baseline (p = 0.026) and 7 days (p = 0.007), and larger infarct growth at 7 days (p = 0.050) than those with PCV only. Multivariate regression analysis showed that both the time of onset at baseline (p = 0.013) and infarct growth at 7 days (p = 0.014) could independently predict poor early clinical outcome.
CONCLUSION
PMV may predict poor early clinical outcome in AIS patients, and reperfusion therapy may, therefore, be required more urgently in patients with PMV.
Susceptibility-weighted imaging (SWI) is increasingly used in the assessment of ischemic stroke (1) because of the differences in magnetic susceptibility among various components such as blood and blood products (2). The prominent vessel sign (PVS) on SWI sequences has been correlated with the amount of deoxyhemoglobin in the cerebral venous compartments secondary to an increased oxygen extraction fraction in the ischemic tissue (3). It has been reported that patients with PVS have larger infarct volumes and worse clinical outcomes when compared with those without PVS (4). Although the PVS has been reported in patients with ischemic stroke beyond the reperfusion window (5), magnetic resonance changes in patients with acute ischemic stroke (AIS) beyond the time window were not as important as those within the time window. Meanwhile, several methods have been used to assess the PVS and their association with the clinical outcome. However, a more sensitive predictor of the prognosis of patients with AIS is still unknown. The most common method involves dichotomizing PVS into prominent cortical veins (PCV) and prominent medullary veins (PMV) according to their location. In most previous studies, the clinical significance of PCV and PMV have been analyzed, but rarely did the studies provide conclusive results on whether they had the same clinical value. Hence, in our study, we analyzed PVS in AIS patients within the time window and compared the PCV and PMV in the evaluation of the severity of stroke at baseline and the prognosis at follow-up.
Methods
Patients
The clinical and imaging databases of consecutive patients presenting with AIS in our hospital, which were recruited prospectively between January 2012 and December 2015, were retrospectively reviewed. The inclusion criteria were as follows: time between stroke onset and magnetic resonance imaging (MRI) scan <8 hours; PVS in the middle cerebral artery (MCA) territory; age 18–80 years; no history of stroke or premorbid symptoms; and premorbid modified Rankin Scale (mRS) score ≤2. The exclusion criteria were as follows: intracranial hemorrhage or brain tumors; serum glucose level <2.7 mmol/L or >22.2 mmol/L; and inadequate image quality. Ethics approval for this project was obtained from the institutional review board (Protocol Number: ky2011-009-02). Written consent was obtained from patients or their next of kin.
Clinical assessment and follow-up protocol
Patients underwent a multimodal MRI examination at baseline and a conventional MRI scan at 7 days after stroke onset. Treatment strategies included reperfusion therapy (endovascular treatment or intravenous recombinant tissue plasminogen activator [rt-PA] thrombolysis) and routine treatment according to their MRI findings and clinical presentations. All patients who had thrombolysis or endovascular treatment underwent follow-up multimodal MRI within 24 hours of the intervention. According to predefined criteria (6), vascular risk factors such as history of atrial fibrillation, transient ischemic attack, hypertension, diabetes and hyperlipidemia were obtained when the patients just arrived at the hospital. National Institutes of Health Stroke Scale (NIHSS) scoring was evaluated at baseline before treatment, 7 days after stroke onset, and mRS scores were assessed at 7 days. Early good and poor functional outcomes were defined by the mRS scores of 0–2 and 3–6, respectively.
Imaging
MRI was performed using a 3.0 Tesla scanner (Trio-Tim, Siemens). The multimodal MRI protocol included time-of-flight MRI angiography (TOF MRA), axial diffusion-weighted imaging (DWI), fluid attenuation inversion recovery (FLAIR), SWI, and perfusion-weighted imaging (PWI). DWI was acquired with a single-shot echo-planar imaging with b values 0 s/mm2 and 1000 s/mm2, repetition time/echo time (TR/TE) 3000/75 ms, field-of-view (FOV) 23×23 cm2, matrix 128×128, and slice thickness 5 mm. FLAIR parameters were TR/TE/inversion time (TI) 8000/94/2500 ms, FOV 20×17.6 cm2, matrix 256×179, flip angle 150°, and slice thickness 5 mm. SWI parameters were TR/TE 27/20 ms, FOV 23×17.25 cm2, matrix 256×243, flip angle 15°, and slice thickness 2.5 mm. TOF MRA parameters were TR/TE 28/3.04 ms, FOV 20×18 cm2, matrix 256×179, slice thickness 0.7 mm, slices per slab=40, and flip angle 13°. The follow-up MRI protocol included T1-weighted image, T2-weighted image, DWI, FLAIR, gradient echo (GRE), and TOF MRA.
Image analysis
Images were reviewed independently by two experienced neuroradiologists who were blinded to the patient’s clinical information. Disagreement between neuroradiologists was resolved by a consensus.
The minimum intensity projection (mIP) SWI of 12.8 mm thickness was used for the evaluation of PVS. PVS on SWI was defined as a regional prominence of hypointense vessels with either more numerous or larger veins and greater signal loss in the target area compared with the contralateral hemisphere, which included prominent cortical and medullary veins. The PVS Alberta stroke program early CT score (ASPECTS) in the MCA territory were divided into eight areas including one deep white-matter area and 7 cortical areas (Insular, M–M6) (7). PCV were assessed in the seven cortical areas; meanwhile, PMV were assessed in the white matter. The PVS score ranged from 0 (no PVS) to 8 (PVS in all ASPECTS areas). PVS was dichotomized into extensive PVS (scores 6–8) and non-extensive PVS (scores 1–5). PVS was also dichotomized into two groups: patients with both PCV and PMV and patients with PCV only. SWI-DWI mismatch was determined by the presence of PVS on SWI in the involved MCA territory extending beyond the region of DWI hyperintensity. The susceptibility vessel sign (SVS) was defined as the presence of hypointensity within the culprit intracranial artery lumen in which the thrombus diameter exceeded the contralateral normal vessel diameter (8). The SVS was classified as SVS positive and SVS negative. Lengths of SVS were measured on SWI mIP images.
Both baseline infarct volume (volume 1) and follow-up infarct volume (volume 2) were measured on DWI series. Infarct growth was defined as the difference between volume 2 and volume 1. These analyses were independently performed by a third neuroradiologist using the 3D Slicer version 4.8 (http://www.slicer.org). For each patient, the semi-automatically measured infarct volumes were assessed for a subsequent quantitative comparison. Arterial status was determined on TOF MRA according to the modified thrombolysis in myocardial infarction (TIMI) scale (9): TIMI 0, complete occlusion; TIMI 1, severe stenosis; TIMI 2, mild or moderate stenosis; and TIMI 3, normal. We categorized vascular status into 2 subgroups: TIMI 0–1 and TIMI 2–3. Hemorrhagic transformation (HT) was evaluated on GRE series in 7 days. Patients with HT were divided into 4 groups (10): hemorrhagic infarct type 1 (HI1), hemorrhagic infarct type 2 (HI2), parenchymal hematoma type 1 (PH1), and parenchymal hematoma type 2 (PH2), (Table 1). Our data were dichotomized into 2 groups for analysis: PH1+PH2 and no-HT+HI1+HI2.
Table 1.
The classification of hemorrhagic transformation
| Type | Description |
|---|---|
| HI1 | Small hypointensity petechiae |
| HI2 | More confluent hypointensity throughout the infarct zone without mass effect |
| PH1 | Homogeneous hypointensity occupying <30% of the infarct zone, some mass effect |
| PH2 | Homogeneous hypointensity occupying >30% of the infarct zone, significant mass effect. Or, any homogeneous hypointensity located beyond the borders of the infarct zone |
HI1, hemorrhagic infarct type 1; HI2, hemorrhagic infarct type 2; PH1, parenchymal hematoma type 1; PH2, parenchymal hematoma type 2.
Statistical analysis
All statistical analyses were performed in SPSS version 23.0 (IBM Corp.). The Kolmogorov-Smirnov normality test was used for continuous data. Descriptive statistics for non-normal variables are shown as median (25th–75th percentiles). Descriptive statistics for normal variables are shown as mean ± standard deviation. The ordinal and categorical variables are expressed as median (25th–75th percentiles) and number (percentage), respectively. Student t test was used for continuous variables that fit normal distribution, Mann-Whitney U test was used for continuous variables that did not fit the normal distribution. Ordinal variables were compared using Mann-Whitney U test. Categorical variables were tested using the chi-square test or Fisher’s exact test, where appropriate. Binary logistic regression models were used to determine independent predictors of poor clinical outcome. p < 0.05 was considered statistically significant.
Results
A total of 47 patients with PVS on SWI (Fig. 1) were included. Clinical and imaging characteristics of the study population are presented in Table 2.
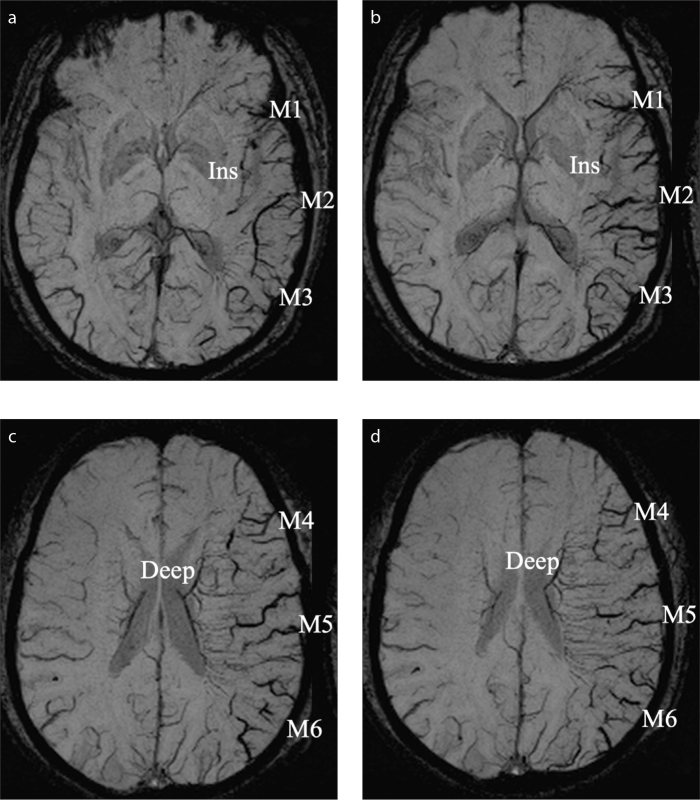
Figure 1. a–d.
Illustrative examples of grading prominent vessel sign (PVS) on susceptibility-weighted imaging (SWI). Axial SWI minimum intensity projection images (a–d) demonstrate the extensive grade of PVS which are visible on eight areas of the left middle cerebral artery (MCA) territory. Ins, insular cortex; M1, anterior MCA cortex; M2, MCA cortex lateral to the insular cortex; M3, posterior MCA cortex; M4, M5 and M6, the anterior, lateral and posterior MCA territories which are superior to M1, M2 and M3, respectively; Deep, deep white matter, also known as prominent medullary veins.
Table 2.
Clinical and imaging characteristics of the study population (n=47)
| Baseline | Age (years) | 57.3±11.8 |
|
| ||
| Male sex | 78.7% (37/47) | |
|
| ||
| Risk factors | ||
| Atrial fibrillation | 14.9% (7/47) | |
| TIA before stroke | 10.6% (5/47) | |
| Hypertension | 46.8% (22/47) | |
| Diabetes | 6.4% (3/47) | |
| Hyperlipidemia | 12.8% (6/47) | |
|
| ||
| Onset-to-MRI time (min) | 256.5±97.8 | |
|
| ||
| NIHSS | 6 (3, 11) | |
|
| ||
| PVS | ||
| Extensive | 63.8% (30/47) | |
| Non-extensive | 36.2% (17/47) | |
| Cases with PCV only | 31.9% (15/47) | |
| Cases with both PCV and PMV | 68.1% (32/47) | |
| PVS-DWI mismatch | 97.9% (46/47) | |
|
| ||
| SVS positive | 59.6% (28/47) | |
|
| ||
| SVS length (mm) | 15.0±6.5 | |
|
| ||
| ICA/MCA | ||
| Intracranial ICA TIMI 0 | 12.8% (6/47) | |
| M1 segment TIMI 0 | 57.5% (27/47) | |
| M1 segment TIMI 1 | 10.6% (5/47) | |
| M1 segment TIMI 2 | 10.6% (5/47) | |
| M1 segment TIMI 3 | 4.3% (2/47) | |
| M2 segment TIMI 0 | 4.3% (2/47) | |
|
| ||
| Volume 1 (mL) | 6.2 (1.9, 24.3) | |
|
| ||
| Treatment | ||
| Intravenous thrombolysis | 34.0% (16/47) | |
| Endovascular treatment | 17.0% (8/47) | |
| Routine treatment | 48.9% (23/47) | |
|
| ||
| 7-day follow-up | NIHSS | 4 (1, 8)a |
|
| ||
| 7-day good outcome | 50.0% (23/46) | |
|
| ||
| Volume 2 (mL) | 31.3 (8.8, 67.6)b | |
|
| ||
| Infarct growth (mL) | 15.0 (1.7, 36.6)b | |
|
| ||
| ICA/MCA TIMI 2–3 | 66.0% (31/47) | |
|
| ||
| HT (PH1+PH2) | 21.7% (10/46) | |
Data are presented as mean ± standard deviation, median (interquartile range), or percentage (number/total).
TIA, transient ischemic attack; MRI, magnetic resonance imaging; NIHSS, National Institutes of Health Stroke Scale; PVS, prominent vessel sign; PCV, prominent cortical veins; PMV, prominent medullary veins; DWI, diffusion-weighted imaging; SVS, susceptibility vessel sign; ICA, internal carotid artery; MCA, middle cerebral artery; TIMI, thrombolysis in myocardial infarction; HT, hemorrhagic transformation; PH1, parenchymal hematoma type 1; PH2, parenchymal hematoma type 2.
Analyses were performed in 45 patients;
Analyses were performed in 46 patients.
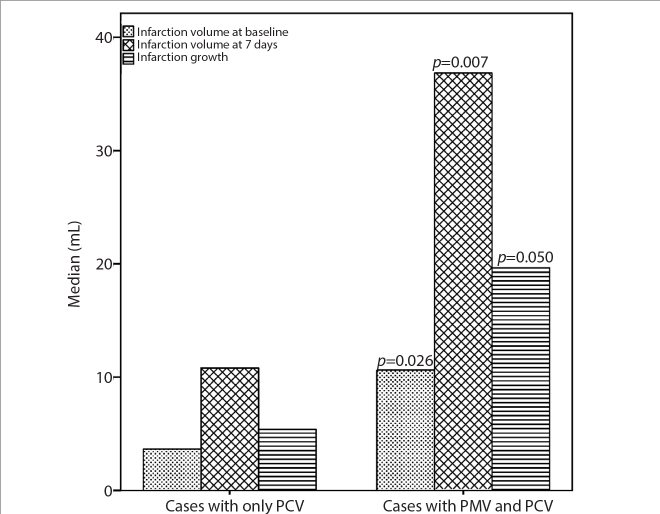
Patients with both PCV and PMV had higher NIHSS at baseline (p = 0.020), larger infarct volumes at baseline (p = 0.026) and at 7 days (p = 0.007), and larger infarct growth (p = 0.050) than did those with PCV only (Table 3; Figs. 2 and 3). Although patients with extensive PVS were younger than those without extensive PVS, the former had higher NIHSS scores (p = 0.013) and larger infarction volumes at baseline (p = 0.019) (Table 4). SWI-DWI mismatch was found in 97.9% (46/47) of patients (Fig. 2).
Table 3.
Comparison of demographic characteristics and clinical presentation between patients with only PCV and patients with PCV and PMV
| Characteristics | Cases with PCV only (n=15) | Cases with PCV and PMV (n=32) | p | |
|---|---|---|---|---|
| Baseline | Age (years) | 60.5±11.4 | 55.8±11.8 | 0.207 |
| Male sex | 73.3% (11/15) | 81.3% (26/32) | 0.704 | |
| Onset-to-MRI time (min) | 249.2±96.9 | 256.9±99.5 | 0.730 | |
| NIHSS | 4 (2, 7) | 8 (4,12) | 0.020 | |
| Volume 1 (mL) | 2.7 (0.9, 6.2) | 10.6 (1.9, 33.9) | 0.026 | |
| ICA/MCA TIMI 0–1 | 86.7% (13/15) | 84.4% (27/32) | 1.000 | |
| SVS positive | 60.0% (9/15) | 59.4% (19/32) | 0.968 | |
| SVS length (mm) | 14.0±3.9 | 15.5±7.5 | 0.498 | |
| Basal ganglia and deep white matter infarction | 53.3% (8/15) | 50.0% (16/32) | 0.831 | |
| Reperfusion therapy | 33.3% (5/15) | 59.4% (19/32) | 0.096 | |
| 7-day follow-up | NIHSS (n=45) | 2 (1, 4) | 4 (2, 9) | 0.081 |
| 7-day good outcome (n=46) | 66.7% (10/15) | 41.9% (13/31) | 0.116 | |
| Volume 2 (mL) (n=46) | 10.8 (5.6, 33.6) | 36.8 (16.4, 89.2) | 0.007 | |
| Infarct growth (mL) (n=46) | 5.4 (0.2, 25.5) | 19.6 (5.6, 56.9) | 0.050 | |
| HT (PH1+PH2) | 7.1% (1/14) | 28.1% (9/32) | 0.143 | |
| ICA/MCA TIMI 2–3 | 60.0% (9/15) | 68.8% (22/32) | 0.555 |
Data are presented as mean±SD, median (interquartile range), or percentage (number/total).
PCV, prominent cortical veins; PMV, prominent medullary veins; MRI, magnetic resonance imaging; NIHSS, National Institutes of Health Stroke Scale; ICA, internal carotid artery; MCA, middle cerebral artery; TIMI, thrombolysis in myocardial infarction; SVS, susceptibility vessel sign; HT, hemorrhagic transformation; PH1, parenchymal hematoma type 1; PH2, parenchymal hematoma type 2.
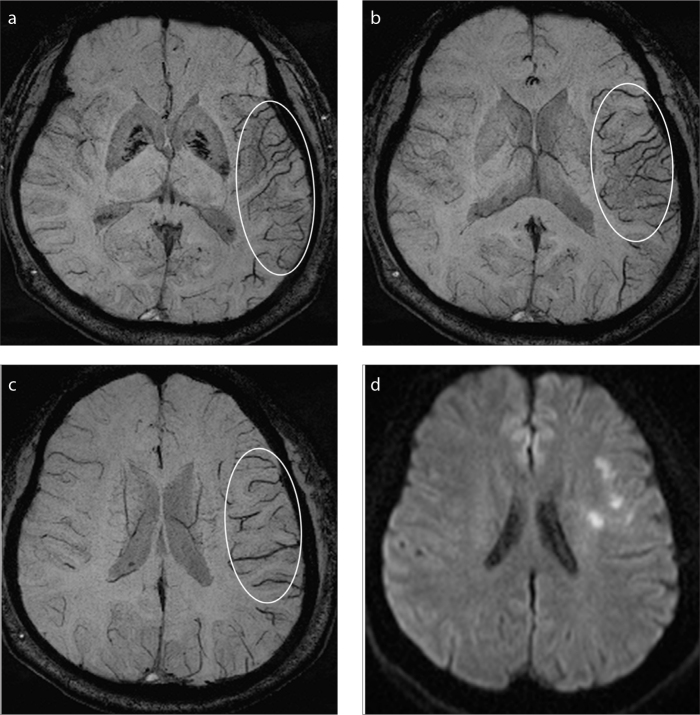
Figure 2. a–d.
A representative case with only prominent cortical veins (PCV). Axial minimum intensity projection images of susceptibility-weighted imaging (SWI) (a–c) show prominent vessel sign (PVS) ASPECTS 7 indicating extensive PVS without prominent medullary veins. Axial diffusion-weighted imaging (DWI) (d) reveals the ischemic infarctions are much smaller than the range of PVS, indicating SWI-DWI mismatch.
Figure 3.
Comparison of cases with only prominent cortical veins (PCV) and those with both PCV and prominent medullary veins (PMV).
Table 4.
Comparison of demographic characteristics and clinical presentation between patients with and without extensive PVS
| Characteristics | Extensive PVS (n=30) | Non-extensive PVS (n=17) | p | |
|---|---|---|---|---|
| Baseline | Age (years) | 54.2±11.7 | 62.8±10.1 | 0.013 |
| Male sex | 83.3% (25/30) | 70.6% (12/17) | 0.460 | |
| Onset-to-MRI time (min) | 268.2±104.4 | 222.3±67.8 | 0.163 | |
| NIHSS | 8 (4, 12) | 4 (2, 7) | 0.013 | |
| Volume 1 (mL) | 10.0 (4.1, 35.1) | 2.67 (0.7, 11.1) | 0.019 | |
| ICA/MCA TIMI 0–1 | 86.7% (26/30) | 82.4% (14/17) | 0.692 | |
| SVS positive | 56.7% (17/30) | 64.7% (11/17) | 0.589 | |
| SVS length (mm) | 15.8±7.7 | 13.8±3.9 | 0.366 | |
| Reperfusion therapy | 60.0% (18/30) | 35.3% (6/17) | 0.104 | |
| 7-day follow-up | NIHSS (n=45) | 4 (1, 9) | 2 (1, 7) | 0.263 |
| 7-day good outcome (n=46) | 44.8% (13/29) | 58.8% (10/17) | 0.359 | |
| Volume 2 (mL) (n=46) | 34.1 (10.2, 88.7) | 21.5 (7.3, 51.2) | 0.153 | |
| Infarct growth (mL) (n=46) | 15.8 (1.5, 46.7) | 12.8 (2.2, 30.1) | 0.596 | |
| HT (PH1+PH2) | 23.3% (7/30) | 18.8% (3/16) | 1.000 | |
| ICA/MCA TIMI 2–3 | 66.7% (20/30) | 64.7% (11/17) | 0.892 |
Data are presented as mean±SD, median (interquartile range), or percentage (number/total).
PVS, prominent vessel sign; MRI, magnetic resonance imaging; NIHSS, National Institutes of Health Stroke Scale; ICA, internal carotid artery; MCA, middle cerebral artery; TIMI, thrombolysis in myocardial infarction; SVS, susceptibility vessel sign; HT, hemorrhagic transformation; PH1, parenchymal hematoma type 1; PH2, parenchymal hematoma type 2.
Univariate analysis showed that patients with early poor outcome had longer onset time at baseline (p = 0.043), lower proportion of intracranial internal carotid artery (ICA)/MCA TIMI 2–3 at 7 days (p = 0.005), and larger infarct volumes (p < 0.001) and larger infarct growth (p < 0.001) by day 7 (Table 5). Multivariate regression analysis showed that onset time at baseline (p = 0.013) and infarct growth in 7 days (p = 0.014) were independent predictors of early poor clinical outcome (Table 6).
Table 5.
Univariate analysis of the baseline and follow-up variables according to the early outcome
| Characteristics | mRS scores ≤2 | mRS scores >2 | p | |
|---|---|---|---|---|
| Baseline | Age (years) | 57.7±12.2 | 58.0±10.5 | 0.918 |
|
| ||||
| Male sex | 78.3% (18/23) | 78.3% (18/23) | 1.000 | |
|
| ||||
| Risk factors | ||||
| Atrial fibrillation | 21.7% (5/23) | 8.7% (2/23) | 0.414 | |
| TIA before stroke | 8.7% (2/23) | 13.0% (3/23) | 1.000 | |
| Hypertension | 52.2% (12/23) | 43.5% (10/23) | 0.555 | |
| Diabetes | 8.7% (2/23) | 4.4% (1/23) | 1.000 | |
| Hyperlipidemia | 17.4% (4/23) | 8.7% (2/23) | 0.665 | |
|
| ||||
| Onset-to-MRI time (min) | 224.7±94.9 | 281.6±90.5 | 0.043 | |
|
| ||||
| NIHSS | 4 (2, 11) | 6 (4, 10) | 0.171 | |
|
| ||||
| Volume 1 (mL) | 7.5 (2.7, 19.5) | 5.1 (1.4, 38.9) | 0.965 | |
|
| ||||
| ICA/MCA TIMI 0–1 | 78.3% (18/23) | 91.3% (21/23) | 0.414 | |
|
| ||||
| Extensive PVS | 56.5% (13/23) | 69.6% (16/23) | 0.359 | |
|
| ||||
| Cases with both PCV and PMV | 56.5% (13/23) | 78.3% (18/23) | 0.116 | |
|
| ||||
| SVS positive | 56.5% (13/23) | 60.9% (14/23) | 0.765 | |
|
| ||||
| SVS length (mm) | 14.4±7.1 | 15.3±6.4 | 0.831 | |
|
| ||||
| Reperfusion therapy | 56.5% (13/23) | 43.5% (10/23) | 0.376 | |
|
| ||||
| 7-day follow-up | Volume 2 (mL) | 11.9 (4.8, 32.2) | 62.2 (31.6, 107.2) | <0.001 |
|
| ||||
| Infarct growth (mL) | 2.6 (−0.5, 15.0) | 37.1 (19.6, 86.6) | <0.001 | |
|
| ||||
| HT (PH1+PH2) | 13.6% (3/22) | 30.4% (7/23) | 0.284 | |
|
| ||||
| ICA/MCA TIMI 2–3 | 87.0% (20/23) | 47.8% (11/23) | 0.005 | |
Data are presented as mean±SD, median (interquartile range), or percentage (number/total).
mRS, modified Rankin Scale; TIA, transient ischemic attack; MRI, magnetic resonance imaging; NIHSS, National Institutes of Health Stroke Scale; ICA, internal carotid artery; MCA, middle cerebral artery; TIMI, thrombolysis in myocardial infarction; PVS, prominent vessel sign; PCV, prominent cortical veins; PMV, prominent medullary veins; SVS, susceptibility vessel sign; HT, hemorrhagic transformation; PH1, parenchymal hematoma type 1; PH2, parenchymal hematoma type 2.
Table 6.
Multivariate analysis for prediction of the early poor outcome
| Characteristics | p | OR | 95% CI | p of model |
|---|---|---|---|---|
| Onset-to-MRI time (min) | 0.013 | 1.026 | 1.006–1.047 | <0.001 |
| Volume 2 (mL) | 0.764 | 1.011 | 0.942–1.085 | |
| Infarct growth (mL) | 0.014 | 1.351 | 1.063–1.718 | |
| Follow-up ICA/MCA TIMI 2–3 | 0.488 | 3.218 | 0.118–87.751 |
OR, odds ratio; CI, confidence interval; MRI, magnetic resonance imaging; ICA, internal carotid artery; MCA, middle cerebral artery; TIMI, thrombolysis in myocardial infarction.
Discussion
The presence of PVS on SWI in AIS patients has been previously described, with PVS incidence varying from 6.8% to 64% depending on different inclusion criteria (4, 5, 11). PVS has been proven to be associated with presence of arterial occlusion (12) or severe stenosis (4). In our study, PVS with ICA/MCA TIMI 0–1 was found in 85% of the patients (40/47), which was consistent with the literature. In order to analyze the PVS more comprehensively, we recruited all PVS positive cases regardless of their arterial status.
We found that patients with extensive PVS had higher NIHSS scores and larger infarction volumes at baseline than those without extensive PVS, similar to previous reports (5, 13). In general, PVS on SWI reflects a state of misery perfusion in AIS that usually leads to a relatively large in infarct volume. Therefore, extensive PVS within the MCA territory implies a poor early stage outcome (11). Although extensive PVS indicated larger infarction volume at baseline, it did not predict poor early outcome in our results. This may be because of several reasons such as different inclusion criteria, different PVS ASPECTS classification, and infarct volume assessment. The time of stroke onset in Chen et al. (11) was between 7 and 60 hours, which is mostly beyond the reperfusion window. Furthermore, their total PVS ASPECTS score was 10 (PVS in M1, M2, M3, M4, M5, M6, I, C, L, or IC). During clinical work, PVS on SWI frequently appeared in deep white matter, but not in the caudate, lentiform nucleus, and internal capsule (7). Thus, we divided the MCA territory into eight areas including one deep white-matter area and seven cortical areas without caudate, lentiform nucleus, and internal capsule. Lastly, Chen et al. (11) assessed the infarct volume based on the ASPECTS topographic system, but we compared the infarct volumes quantitatively. Park et al. (14) showed that extensive PVS on SWI in AIS patients is associated with lower initial NIHSS scores, smaller diffusion lesion volume, and better collateral flow, which are contrary to our results. Park et al. (14) evaluated collateral flow according to the distal hyperintense vessels on FLAIR and vessels on post-contrast TOF MRA source images, which are still controversial and not gold-standard methods in the evaluation of the collateral flow.
Recent reports have compared SWI-ASPECTS to mean transit time (MTT)-ASPECTS, demonstrating that they are correlated (15, 16). Therefore, it can be extrapolated from the concept of diffusion-perfusion mismatch that SWI-DWI mismatch may represent an acute ischemic penumbra with misery perfusion. Other studies demonstrated that SWI-DWI mismatch was associated with smaller infarct volume and good outcome (17). Payabvash et al. (17) included patients without PVS in their study and found SWI-DWI mismatch in 29.3% of their cases; on the other hand, we included only cases with positive PVS, and determined SWI-DWI mismatch in 97.9% of them. This indicates that the SWI-DWI mismatch may overestimate the penumbra tissue just as in traditional perfusion imaging.
Several authors (13, 18, 19) have reported the relation between deep medullary veins and the outcome, but the PCV were not mentioned at all. In the current study, there were 68.1% cases with PMV, always coincident with PCV, so we compared the cases with PCV alone and those with both PCV and PMV. We found that cases with both PCV and PMV had higher admission NIHSS, larger infarct volumes at both baseline and 7 days, and larger infarct growth at 7 days than did those with PCV alone. Additionally, larger infarct growth at 7 days was associated with early poor outcome. Hence, the PMV may be more suitable to predict the early outcome than the PCV. Besides an increased ratio of deoxyhemoglobin to oxyhemoglobin caused by an uncoupling between the oxygen supply and demand within the hypoperfused tissue, another possible mechanism underlying PVS is an increased venous volume due to vasodilatation induced by regional ischemia, which may result in the difference between PCV and PMV in assessing the prognosis of AIS. In addition, Payabvash et al. (12) found that PCV were independent predictors of arterial occlusion, whereas PMV were more strongly associated with larger infarct volumes, which was consistent with our results. Besides the majority of PVS patients with arterial occlusion or severe stenosis, 14.9% of the patients (7/47) had MCA TIMI 2–3 at baseline in the current study. Potentially pre-existing extracranial ICA occlusions leading to PVS have been reported (20). Since extracranial ICA or common carotid artery occlusions were not imaged, the reason for the presence of PVS with mild MCA stenosis or normal MCA is still uncertain.
In previous studies, most of the patients were beyond the reperfusion window. In this study, the time from stroke onset to MRI examination was less than 8 hours, and reperfusion therapy was administered within an extended window time of up to 9 hours after symptom onset, which could offer more patients an opportunity to undergo reperfusion/recanalization treatment, in line with other studies (21, 22). Until now, treatment protocols for AIS within the time window mainly depended on whether an arterial occlusion was present. Evaluation for the presence of PMV on SWI may provide a noninvasive estimation of blood oxygen demand and would be an effective supplement to reflect the severity of stroke. Therefore, as indicated by our results that patients with PMV tended to have poorer outcomes, reperfusion therapy may be required more urgently for the AIS patients with PMV, regardless of whether the affected artery has occlusion. This is especially important for convincing the AIS patients and/or their kin because signed informed consents should be obtained before reperfusion therapy.
In addition, deep medullary veins provide drainage for the basal ganglia and white matter of the cerebral hemispheres. We compared the cases with and without basal ganglia and deep white-matter infarction, but no significant difference was found. Hence, PMV were associated with the severity of stroke, but not with the location of stroke. Because PMV were only found in strokes within the MCA territory, it remains uncertain whether the value of PCV in the anterior cerebral artery and posterior cerebral artery territory are similar to that in the MCA territory.
Some limitations of our study merit consideration. A small number of patients was included. PWI images were obtained at baseline but not evaluated, because the majority of cases had mismatched SWI-DWI in our analyses.
In conclusion, we showed that the patients with extensive PVS had higher NIHSS scores and larger infarction volumes at baseline; the cases with both PCV and PMV had higher admission NIHSS, larger infarct volumes at both baseline and 7 days, and larger infarct growth at 7 days than did those with PCV alone. Hence, the PMV sign may be more suitable than the PCV sign for prediction of early poor outcome, and reperfusion therapy may be more urgently required for AIS patients with PMV.
Main points.
Symptoms were more severe in cases with both prominent cortical veins (PCV) and prominent medullary veins (PMV) than those with only PCV at admission.
Infarct volume and infarct growth in cases with both PCV and PMV were larger than those with PCV alone.
PMV may be more suitable than PCV for prediction of the early poor outcome, and reperfusion therapy may be more urgently required for acute ischemic stroke patients with PMV.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Hsu CC, Kwan GNC, Hapugoda S, Craigie M, Watkins TW, Haacke EM. Susceptibility weighted imaging in acute cerebral ischemia: review of emerging technical concepts and clinical applications. Neuroradiol J. 2017;30:109–119. doi: 10.1177/1971400917690166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halefoglu AM, Yousem DM. Susceptibility weighted imaging: Clinical applications and future directions. World J Radiol. 2018;10:30–45. doi: 10.4329/wjr.v10.i4.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hermier M, Nighoghossian N. Contribution of susceptibility-weighted imaging to acute stroke assessment. Stroke. 2004;35:1989–1994. doi: 10.1161/01.STR.0000133341.74387.96. [DOI] [PubMed] [Google Scholar]
- 4.Mundiyanapurath S, Ringleb PA, Diatschuk S, et al. Cortical vessel sign on susceptibility weighted imaging reveals clinically relevant hypoperfusion in internal carotid artery stenosis. Eur J Radiol. 2016;85:534–539. doi: 10.1016/j.ejrad.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Shi T, Chen B, Lin G, Xu Y, Geng Y. Prominent hypointense vessel sign on susceptibility-weighted imaging is associated with clinical outcome in acute ischaemic stroke. Eur Neurol. 2018;79:231–239. doi: 10.1159/000488587. [DOI] [PubMed] [Google Scholar]
- 6.Kufner A, Nolte CH, Galinovic I, et al. Smoking-thrombolysis paradox: recanalization and reperfusion rates after intravenous tissue plasminogen activator in smokers with ischemic stroke. Stroke. 2013;44:407–413. doi: 10.1161/STROKEAHA.112.662148. [DOI] [PubMed] [Google Scholar]
- 7.Park MG, Yeom JA, Baik SK, Park KP. Total mismatch of diffusion-weighted imaging and susceptibility-weighted imaging in patients with acute cerebral ischemia. J Neuroradiol. 2017;44:308–312. doi: 10.1016/j.neurad.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Rovira A, Orellana P, Alvarez-Sabin J, et al. Hyperacute ischemic stroke: middle cerebral artery susceptibility sign at echo-planar gradient-echo MR imaging. Radiology. 2004;232:466–473. doi: 10.1148/radiol.2322030273. [DOI] [PubMed] [Google Scholar]
- 9.Fiebach JB, Al-Rawi Y, Wintermark M, et al. Vascular occlusion enables selecting acute ischemic stroke patients for treatment with desmoteplase. Stroke. 2012;43:1561–1566. doi: 10.1161/STROKEAHA.111.642322. [DOI] [PubMed] [Google Scholar]
- 10.Leigh R, Christensen S, Campbell BC, et al. Pretreatment blood-brain barrier disruption and post-endovascular intracranial hemorrhage. Neurology. 2016;87:263–269. doi: 10.1212/WNL.0000000000002862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen CY, Chen CI, Tsai FY, Tsai PH, Chan WP. Prominent vessel sign on susceptibility-weighted imaging in acute stroke: prediction of infarct growth and clinical outcome. PLoS One. 2015;10:e0131118. doi: 10.1371/journal.pone.0131118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Payabvash S, Benson JC, Taleb S, et al. Prominent cortical and medullary veins on susceptibility-weighted images of acute ischaemic stroke. Br J Radiol. 2016;89:20160714. doi: 10.1259/bjr.20160714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu X, Yuan L, Jackson A, et al. Prominence of medullary veins on susceptibility-weighted images provides prognostic information in patients with subacute stroke. AJNR Am J Neuroradiol. 2016;37:423–429. doi: 10.3174/ajnr.A4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park MG, Yang TI, Oh SJ, Baik SK, Kang YH, Park KP. Multiple hypointense vessels on susceptibility-weighted imaging in acute ischemic stroke: surrogate marker of oxygen extraction fraction in penumbra? Cerebrovasc Dis. 2014;38:254–261. doi: 10.1159/000367709. [DOI] [PubMed] [Google Scholar]
- 15.Luo S, Yang L, Wang L. Comparison of susceptibility-weighted and perfusion-weighted magnetic resonance imaging in the detection of penumbra in acute ischemic stroke. J Neuroradiol. 2015;42:255–260. doi: 10.1016/j.neurad.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Mashhood A, Kim P, Almaguel F, McWilliams G, Jacobson JP. Cerebral misery perfusion on susceptibility weighted imaging in acute carotid dissection. J Radiol Case Rep. 2016;10:1–6. doi: 10.3941/jrcr.v10i10.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Payabvash S, Taleb S, Benson JC, et al. Susceptibility-diffusion mismatch in middle cerebral artery territory acute ischemic stroke: clinical and imaging implications. Acta Radiol. 2017;58:876–882. doi: 10.1177/0284185116675658. [DOI] [PubMed] [Google Scholar]
- 18.Duan Y, Xu Z, Li H, Cai X, Chang C, Yang B. Prominent deep medullary veins: a predictive biomarker for stroke risk from transient ischemic attack? Acta Radiol. 2018;59:606–611. doi: 10.1177/0284185117726813. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Zhang S, Chen Q, Ding W, Campbell BCV, Lou M. Ipsilateral prominent thalamostriate vein on susceptibility-weighted imaging predicts poor outcome after intravenous thrombolysis in acute ischemic stroke. AJNR Am J Neuroradiol. 2017;38:875–881. doi: 10.3174/ajnr.A5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng B, Schroder N, Forkert ND, et al. Hypointense vessels detected by susceptibility-weighted imaging identifies tissue at risk of infarction in anterior circulation stroke. J Neuroimaging. 2017;27:414–420. doi: 10.1111/jon.12417. [DOI] [PubMed] [Google Scholar]
- 21.Bhaskar S, Stanwell P, Cordato D, Attia J, Levi C. Reperfusion therapy in acute ischemic stroke: dawn of a new era? BMC Neurol. 2018;18:8. doi: 10.1186/s12883-017-1007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]