Abstract
The menopause transition (MT) may be an opportunity for early intervention to prevent rapid bone loss. In order to intervene early, we need to be able to prospectively identify pre- and perimenopausal women who are beginning to lose bone. This study examined whether estradiol (E2), or follicle stimulating hormone (FSH), measured in pre- and perimenopausal women, can predict significant bone loss by the next year. Bone loss was considered significant if BMD decline at the lumbar spine (LS) or femoral neck (FN) from a pre- or early perimenopausal baseline to 1 year after the E2 or FSH measurement was greater than the least detectable change. We used data from 1,559 participants in the Study of Women’s Health Across the Nation and tested E2 and FSH as separate predictors using repeated measures modified Poisson regression. Adjusted for MT stage, age, race/ethnicity, and body mass index, women with lower E2 (and higher FSH) were more likely to lose BMD: At the LS, each halving of E2 and each doubling of FSH were associated with 10% and 39% greater risk of significant bone loss, respectively (p<0.0001 for each). At the FN, each halving of E2 and each doubling of FSH were associated with 12% (p=0.01) and 27% (p<0.001) greater risk of significant bone loss. FSH was more informative than E2 (assessed by the area under the receiver-operator curve) at identifying women who were more vs. less likely to begin losing bone, especially at the LS. Prediction was better when hormones were measured in pre- or early perimenopause than in late perimenopause. Tracking within-individual change in either hormone did not predict onset of bone loss better than a single measure. We conclude that measuring FSH in the MT can help prospectively identify woman with imminent or ongoing bone loss at the LS.
Keywords: Menopause, estradiol, follicle stimulating hormone, DXA, general population studies
INTRODUCTION
The menopause transition (MT) is a period of rapid bone loss that contributes to a woman’s risk of osteoporosis and fracture in later life. Bone mineral density (BMD) decline, at rates commonly observed during the MT (1), can be associated with irreversible deterioration in bone microarchitecture (2–4), and with increased fracture risk (5–7). Indeed, in some studies, fast BMD decline in midlife is associated with appendicular and vertebral fractures within the first postmenopausal decade (8–10). This suggests that the MT may be an opportune time for early, short-term intervention to prevent rapid BMD decline, and reduce the risk of future fracture (11).
In order to intervene before substantial bone loss has occurred, we first need to be able predict whether a pre- or perimenopausal woman is about to begin losing bone. MT-related BMD decline accelerates approximately 1 year before the final menstrual period (FMP) (1). Currently, however, this time point can only be identified retrospectively, i.e., after ≥12 months of amenorrhea when the FMP date can be assigned (12). By the time the FMP date can be defined, many women will have already been losing bone for the preceding 2 years. Since the rate of BMD decline at the lumbar spine during the MT averages 2.5% per year (1), even a relatively short period of bone loss can be significant. The objective of this study was, therefore, to determine whether markers of ovarian function – estradiol (E2) or follicle stimulating hormone (FSH) – measured in pre- and perimenopause can help prospectively identify the onset of significant bone loss, in advance of substantial BMD decline.
E2 is the major sex steroid hormone in women, and the likely effector estrogen at the estrogen receptor (13, 14). FSH is produced by the anterior pituitary under negative feedback inhibition by estrogen. We considered E2 and FSH as potential predictors of imminent BMD decline because an increase in FSH and decrease in E2 temporally precedes the MT-related acceleration in bone loss (1, 15–17). We thus designed this study to address 2 questions: 1) Can measuring E2 or FSH during pre- (regular menstrual bleeding), early peri- (less predictable bleeding at least once every 3 months), or late perimenopause (less predictable bleeding at least once every 3 to 12 months) help determine if a woman will have significant decline in BMD (from an earlier baseline) by the next 12 months; and 2) Does tracking within-individual change in E2 or FSH improve this determination?
This study was conducted in the Study of Women’s Health Across the Nation (SWAN), a longitudinal cohort study of the MT in a multi-ethnic, community-based cohort of women with annual measurements of E2, FSH, and BMD.
MATERIALS AND METHODS
SWAN is a multi-center, longitudinal study of the MT in a multi-racial/ethnic cohort of ambulatory, community-dwelling women (18). SWAN was initiated in 1996, when participants were aged 42 to 52, and in pre- (no change in menstrual bleeding in the past year) or early perimenopause (less predictable menstrual bleeding at least once every 1 to 3 months in the past year). A total of 3,302 SWAN participants were recruited seven clinical sites: Boston, MA; Chicago, IL; Detroit, MI; Pittsburgh, PA; Los Angeles, CA; Newark, NJ; and Oakland CA. The SWAN Bone Cohort includes 2,417 participants from five sites (excluding the Chicago and Newark sites). Among these women, E2, FSH, and BMD were measured at baseline and at each follow-up visit thereafter. Each clinical site obtained IRB approval, and all participants provided written informed consent.
Study Sample
Of 2,417 SWAN bone cohort participants, 336 women were excluded because they did not have at least two measurements (at baseline visit and at least one follow up visit) of E2 or FSH. The most common reason for exclusion was starting a bone-modifying medication (including sex steroid hormones, oral glucocorticoids, aromatase inhibitors, chemotherapy for breast cancer, and osteoporosis medications [bisphosphonates, selective estrogen receptor modulators, calcitonin, parathyroid hormone]) before the second E2 or FSH measurement. Of the remaining participants, another 507 women were excluded because their first E2 or FSH measurement was not obtained during the early follicular phase (days 2-5) of the menstrual cycle. We lastly excluded 15 women who did not have at least 1 follow-up visit before postmenopause (defined as ≥1 year after the FMP), around which we could determine whether significant bone loss occurred. We could not assess for BMD loss if there was missing baseline or follow-up BMD data, or if a bone-modifying medication was initiated before the second DXA scan. Our analytic sample was thus 1,559 women (Figure 1). Among these participants, a total of 3,618 follow-up visits starting from the first follow-up visit to the last visit before the clinical diagnosis of postmenopause could be made were included in our analyses.
Figure 1. Analysis sample derivation.
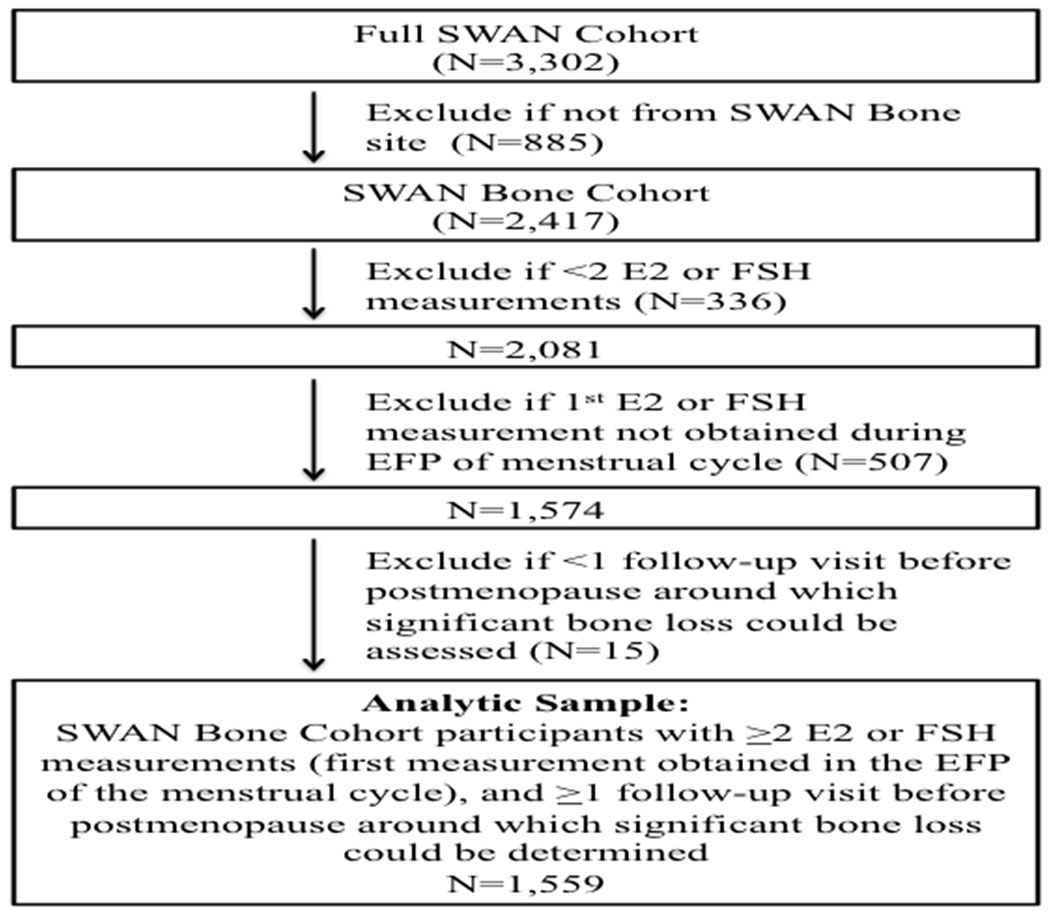
This flow chart shows the derivation of the analysis sample. In order to be included in the study, participants needed to meet the following criteria: 1) be from a SWAN Bone site; 2) have at least 2 E2 or FSH measurements, the first of which was obtained during the early follicular phase (EFP) of the menstrual cycle; and 3) have at least 1 follow-up visit before postmenopause, around which significant bone loss could be assessed.
Predictors
Every effort was made to perform phlebotomy before 10:00 AM during the early follicular phase (between days 2 and 5) of a spontaneous menstrual cycle. If a follicular phase sample could not be obtained after 2 attempts, a random fasting sample was taken within a 90-day window of the anniversary of the baseline visit. Collected specimens were initially stored between −20 to −80 degrees Celsius at individual study sites for up to 30 days, and then shipped to the Central Lab at the University of Michigan (Ann Arbor, MI), and stored at −80 degrees Celsius. Assays were then performed in batch mode. Serum E2 was measured in duplicate with a modified, off-line ACS:180 (E2–6) immunoassay using an ACS:180 automated analyzer (Bayer Diagnostics Corp., Tarrytown, New York). The average between duplicates was recorded in the dataset and used in the analyses in this study. The lower limit of detection was 1.0 pg/ml, and inter- and intraassay coefficients of variation (CV) were 10.6% and 6.4%, respectively. Serum FSH was measured in singlicate with a 2-site chemiluminometric assay (Bayer Diagnostics Corp., Tarrytown, New York). The lower limit of detection was 1.05 mIU/ml, and inter- and intraassay CV were 12.0% and 6.0%, respectively.
Outcomes
BMD at the lumbar spine (LS) and femoral neck (FN) BMD was measured by DXA. At study inception, the Pittsburgh and Oakland sites used the Hologic QDR 2000 machine, and the Boston, Los Angeles, and Michigan sites used the Hologic QDR 4500A model. At follow-up visit 8, Pittsburgh and Oakland upgraded to the 4500A models. To develop cross-calibration regression equations, each site obtained duplicate scans using the old and new hardware in 40 volunteers within a maximum of 90 days. Of the 3,618 observations included in our analyses, only 56 occurred after the machine changes. To determine the short-term in vivo precision error, each study site measured LS and FN BMD twice in 5 women with complete subject repositioning between duplicate scans. Using the root mean square SD approach, the precision error in SWAN was 1.4% at the LS and 2.2% at the FN. An anthropomorphic spine phantom was circulated between sites for cross-site calibration. Standard quality control phantom scans were conducted before each BMD measurement session. If necessary, these were used to adjust for longitudinal machine drift.
For each follow-up visit N, we calculated the percentage decline in LS and FN BMD from SWAN baseline to follow-up visit N+1. Significant BMD decline was defined as loss of BMD that exceeded the site-specific least significant change (LSC). LSC is the amount of change that is considered statistically significant using a 2-sided Type I error (alpha) of 5%, given the measure’s precision error (coefficient of variation, CV). The LSC (which is 2.8 times the measurement’s CV) is thus, 3.9% for LS BMD and 6.2% for FN BMD.
Covariates
Body mass index (BMI) was calculated from weight and height measurements [BMI = weight in kilograms/(height in meters)2]. Clinical MT stage was determined using menstrual bleeding patterns. Premenopause was defined as no change in menstrual regularity in the past year. Early perimenopause was defined as less predictable menstrual bleeding at least once every 3 months. Late perimenopause was defined as less predictable menstrual bleeding at least once every 3-12 months.
Statistical Analysis
We generated descriptive statistics for all variables and assessed the distributions of continuous variables. E2 and FSH had skewed distributions and were thus log transformed to base 2 for all analyses.
In our first set of analyses, we assessed whether a one-time measurement of E2 or FSH could predict imminent bone loss by the next year. We used repeated measures, modified Poisson regression with E2 or FSH measured at each follow-up visit N as primary predictor, and significant bone loss (yes vs. no) at the LS or FN from SWAN baseline to follow-up visit N+1 (yes/no) as the dependent variable (Figure 2A). E2 and FSH were tested in separate models. Models were adjusted for MT stage (pre- vs. early peri- vs. late perimenopause) and relevant clinical covariates (age, race/ethnicity, BMI, SWAN study site, and whether follow-up E2 or FSH more measured during the early follicular phase of the menstrual cycle).
Figure 2. Visual representation of analyses.
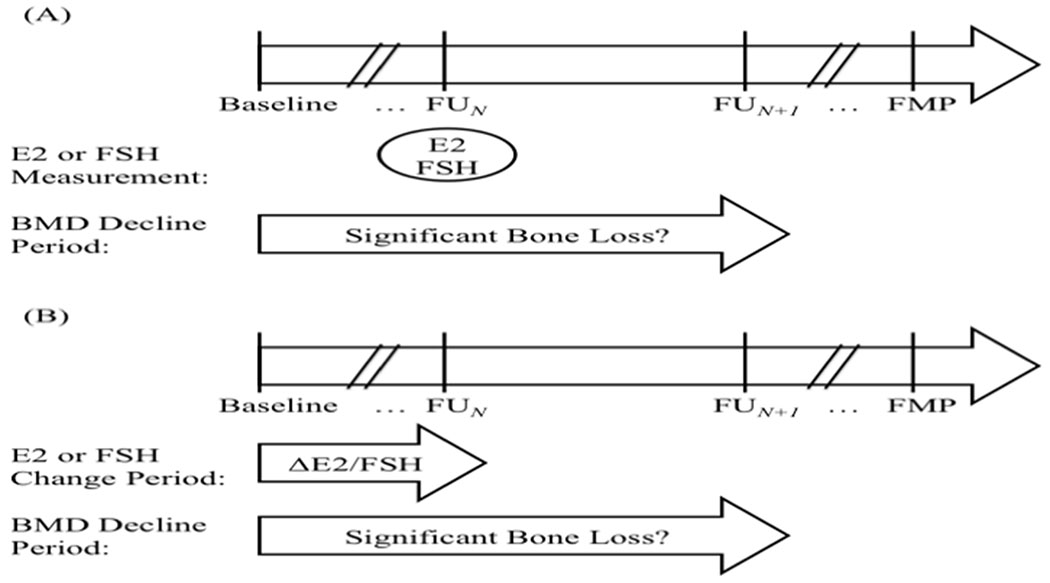
Analyses included 1,559 women from whom there were a total of 3,618 follow-up visits (starting from the first follow-up visit to the last follow-up before the clinical diagnosis of postmenopause could be made). (A) The first set of analyses examined whether single measures of E2 or FSH could predict imminent MT-related bone loss. The primary predictors were E2 or FSH (tested in separate models) measured at each follow-up visit N. The dependent variable was significant bone loss (categorical outcome, yes vs. no) from SWAN baseline to follow-up visit N+1. Significant bone loss was defined as an annualized rate of decrease in BMD that was greater than the site-specific (LS vs. FN) least specific change. (B) The second set of analyses examined whether within-individual change in E2 or FSH could predict imminent MT-related bone loss. The primary predictors were within individual change in E2 or FSH (tested in separate models) from SWAN baseline to each follow-up visit N. The dependent variable was significant bone loss (categorical outcome, yes vs. no) from SWAN baseline to follow-up visit N+1.
In our second set of analyses, we examined the ability of within-individual change in E2 or FSH to predict imminent bone loss by the next year of the second hormone measurement. We again used repeated measures, modified Poisson regression, this time with change in log-transformed E2 or FSH from SWAN baseline to each follow-up visit N as primary predictor, and significant bone loss at the LS or FN from SWAN baseline to follow-up visit N+1 (yes/no) as dependent variable (Figure 2B). Models were adjusted for MT stage, and relevant clinical covariates as above. Time-varying covariates were obtained at the time of the second hormone measurement.
In both sets of analyses, we compared the abilities of E2 and FSH to discriminate women who were more likely from those less likely to be losing bone, using the area under the receiver operating characteristic curve (AUC) metric (estimated using logistic regression) (19). We also tested for interactions of each hormone with race/ethnicity, MT stage, and whether the hormone was measured during the early follicular phase (EFP, days 2-5) of the menstrual cycle to see if the strength of each hormone’s association (effect size) differed by those factors.
Lastly we conducted three sets of sensitivity analyses. First, we examined whether excess weight loss or weight gain affected the associations of E2 and FSH with significant bone loss. Specifically, we excluded observations for which change in weight from the baseline visit to the exposure visit was in the bottom 5% or top 5% of the population distribution. (i.e., weight loss > 5.8 kg or weight gain >9.2 kg). Second, the SWAN protocol for cross-calibration following a DXA hardware change did not meet the International Society for Clinical Densitometry’s (ISCD’s) current recommendation to obtain duplicate scans on old and new machines within 60 days (20). To determine if this affected our findings, we excluded the 56 (out of 3,618 [approximately 1.5%]) observations that occurred after the Pittsburgh and Oakland machine upgrades. Third, SWAN’s DXA precision estimates did not meet the ISCD’s current recommendation to obtain triplicate or duplicate scans in 15 or 30 subjects (20). We thus conducted sensitivity analyses using the ISCD’s limit of acceptable LSC thresholds (5.3% at the LS and 6.9% at the FN) as alternative definitions for significant bone loss.
RESULTS
Participant Characteristics: Study Baseline
This study included 1,559 SWAN participants. Half were white, 25% Black, 11% Chinese, and 14% Japanese. At study baseline, 58% were premenopausal, and 42% were in early perimenopause. Mean BMD values at the LS and FN were 1.071 and 0.837 g/cm2, respectively. E2 and FSH had skewed distributions, with median E2 being 52.5 pg/ml (interquartile range [IQR] 32.8, 82.1), and median FSH being 15.1 mIU/ml (IQR 11.1, 23.3) (Table 1).
Table 1.
Descriptive statistics for analytic sample at study baseline; Study of Women’s Health Across the Nation (SWAN)
| Descriptive statistic Na=1,559 |
|
|---|---|
| Age (years)b | 46.1 (2.6) |
| Race/ethnicityc | |
| Black | 383 (25%) |
| Chinese | 171 (11%) |
| Japanese | 222 (14%) |
| White | 783 (50%) |
| Body mass index (kg/m2)b | 27.2 (7.8) |
| Menopause transition stagec | |
| Premenopause | 907 (58%) |
| Early Perimenopause | 652 (42%) |
| Hormone predictorsd | |
| Estradiol (pg/ml) | 52.5 (32.8, 82.1) |
| Follicle stimulating hormone (mIU/ml) | 15.1 (11.1, 23.3) |
| Bone mineral densityb | |
| Lumbar spine (g/cm2) | 1.071 (0.1) |
| Femoral neck (g/cm2) | 0.837 (0.1) |
N=1,559 participants. All participants were pre- or early perimenopausal at SWAN baseline.
Continuous variables with normal distributions expressed as mean (standard deviation).
Categorical variables expressed as count (proportion).
Continuous variables with skewed distributions expressed as median (interquartile range).
Participant Characteristics: Repeated Measures
Among the 1,559 participants, a total of 3,618 follow-up visits after SWAN baseline and before the first postmenopausal visit (defined as ≥1 year after the FMP) were included in our analyses. Eleven percent of these follow-up visits occurred during premenopause, 75% in early perimenopause, and 14% in late perimenopause. Median E2 was similar during pre- (40.1 pg/ml) and early perimenopause (44.9 pg/ml), but was significantly lower in late perimenopause (21.7 pg/ml) (p<0.001 for comparison of late perimenopause vs. pre- or early perimenopause). Analogously, median FSH was similar in pre- (15.5 mIU/ml) and early perimenopause (18.9 mIU/ml), but was significantly higher in late perimenopause (83.6 mIU/ml) (p<0.001 for comparison of late perimenopause vs. pre- or early perimenopause) (Table 2).
Table 2.
Descriptive statistics for analytic sample across all follow-up visits by menopause transition stagea; Study of Women’s Health Across the Nation (SWAN)
| Number of observationsb: | Premenopause 399 |
Early Perimenopause 2,715 |
Late Perimenopause 504 |
|---|---|---|---|
| Age (years)c | 48.3 (2.4) | 48.6 (2.9) | 51.8 (2.4) |
| Body mass index (kg/m2) c | 26.7 (6.6) | 27.2 (6.6) | 28.1 (7.1) |
| Absolute level of hormone level at follow-up visit Nd | |||
| Estradiol (pg/ml) | 40.1 (25.6, 66.5) | 44.9 (26.6, 88.3) | 21.7 (13.7, 55.3) |
| Follicle stimulating hormone (mIU/ml) | 15.5 (12.1, 24.0) | 18.9 (12.0, 36.0) | 83.6 (50.6, 114.0) |
| Change in hormone level from SWAN baseline to follow-up visit Nd | |||
| Estradiol (pg/ml) | −7.9 (−28.4, +7.8) | −4.9 (−29.5, +27.1) | −18.9 (−61.3, +0.4) |
| Follicle stimulating hormone (mIU/ml) | +2.8 (−1.2, +9.0) | +4.3 (−1.8, +19.5) | +58.5 (+23.8, +90.5) |
| Annualized change in bone mineral density from SWAN baseline to follow-up visit N+1c | |||
| Lumbar spine (g/cm2*year) | −0.4 (1.4) | −0.9 (1.4) | −1.5 (1.5) |
| Femoral neck (g/cm2*year) | −0.4 (1.3) | −0.7 (1.4) | −1.3 (1.4) |
| Significant bone loss (yes vs. no) from SWAN baseline to follow-up visit N+1d | |||
| Lumbar spine | 15 (3.8%) | 312 (11.6%) | 190 (38.4%) |
| Femoral neck | 8 (2.0%) | 121 (4.5%) | 50 (10.1%) |
All follow-up visits after SWAN baseline for each participant transitioned to postmenopause.
Number of visits across all participants in each menopause transition stage.
Continuous variables with normal distributions expressed as mean (standard deviation).
Continuous variables with skewed distributions expressed as median (interquartile range).
Categorical variables expressed as count (percentage).
BMD decreased at a higher rate in early perimenopause (0.9% per year [LS]; 0.7% per year [FN]) compared to premenopause (0.4% per year [LS]; 0.4% per year [FN]) (p<0.001), and in late perimenopause (1.5% per year [LS]; 1.3% per year [FN]) compared to early perimenopause (p<0.001). As a consequence, the proportion of observations that were associated with significant bone loss was lowest in premenopause and greatest in late perimenopause (Table 2). The risk of imminent bone loss at the LS was 2.1-fold greater in early peri- vs. premenopausal women (risk ratio [RR] 2.1, p=0.008), after accounting for clinical covariates (age, BMI, race/ethnicity, and SWAN study site). Similarly, risk of imminent bone loss at the LS and FN was 2.1-fold greater in late peri- vs. early perimenopausal women (risk ratio [RR] 2.1, p<0.0001).
Single Measure of E2 or FSH as Predictor of Imminent Bone Loss
In repeated measures modified Poisson regression, after adjusting for MT stage (pre- vs. early peri- vs. late perimenopause) and clinical covariates (age, BMI [at the time of E2 measurement], race/ethnicity, SWAN study site, and whether E2 was measured during the EFP of the menstrual cycle), lower E2 was associated with greater risk of imminent bone loss at both the LS and FN. With each 50% decrement in E2, risk of significant bone loss was 10% and 12% greater at the LS (p<0.0001) and FN (p=0.01), respectively. The ability of E2 (combined with MT stage and clinical covariates) to identify women with imminent bone loss, as assessed by the model AUC, was 0.756 for the LS (compared to 0.752 for MT stage and clinical covariates alone, p=0.07) and 0.740 for the FN (compared to 0.735 for MT stage and clinical covariates alone, p=0.01) (Table 3).
Table 3.
Associations of estradiol (E2) and follicle stimulating hormone (FSH), single measures and within-woman change, with significant bone loss by the next yeara
| Relative Risk (RR) of Significant Bone Loss By the Next Year (Per 50% decrement (halving) of E2, per 100% increment (doubling) of FSH) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Lumbar Spine | Femoral Neck | |||||||
| RR (95% CI) | p-valueb | AUC | p-valuec | RR (95% CI) | p-valueb | AUC | p-valuec | |
| Single Measures | ||||||||
| E2 | 1.10 (1.06, 1.15) | <0.0001 | 0.756 | 0.07 | 1.12 (1.02, 1.23) | 0.01 | 0.740 | 0.1 |
| FSH | 1.39 (1.30, 1.49) | <0.0001 | 0.782 | <0.0001 | 1.27 (1.11, 1.44) | <0.001 | 0.751 | 0.02 |
| Within-individual Change | ||||||||
| E2 | 1.09 (1.04, 1.12) | <0.0001 | 0.759 | 0.04 | 1.09 (1.00, 1.19) | 0.05 | 0.745 | 0.1 |
| FSH | 1.17 (1.10, 1.24) | <0.0001 | 0.757 | 0.04 | 1.06 (0.94, 1.19) | 0.3 | 0.739 | 0.8 |
| Covariates only model | N/A | N/A | 0.752 | N/A | N/A | N/A | 0.735 | N/A |
Associations estimated using modified Poisson regression on repeated measures from all follow-up visits up to the last visit before postmenopause (1 year after the FMP). Separate models were run for each hormone predictor level and within-woman change. Bone loss considered significant if decrease in bone mineral density (from SWAN baseline to the follow-up visit around 1 year after the hormone measurement) was greater than the site-specific least significant change (3.9% for the lumbar spine and 6.2% for the femoral neck). All models included the following covariates: menopause transition stage, age [years], race/ethnicity, clinical site, body mass index [kg/cm2], and whether samples were collected during the early follicular phase of the menstrual cycle [yes/no]). The area under the receiver operator curves (AUC) for each model was estimated using logistic regression to assess the model’s ability to discriminate between women who were more vs. less likely to have significant bone loss in the next year.
p-value for hormone predictor
p-value for AUC of model containing hormone predictor with covariates compared to model with covariates only
Higher FSH was also associated with greater risk of imminent bone loss at both the LS and FN, adjusted for the same covariates. For each two-fold increment in FSH, risk of significant bone loss at the LS and FN was 39% and 27% greater (p<0.0001 for both sites), respectively. When combined with MT stage and clinical covariates, the ability of FSH to identify women with imminent bone loss (as assessed by AUC) was 0.782 (p<0.0001 compared to MT stage and clinical covariates alone) at the LS and 0.751 (p=0.02 compared to MT stage and clinical covariates alone) at the FN (Table 3).
Within-woman change in E2 or FSH as Predictor of Imminent Bone Loss
Greater within-individual declines in E2 and greater increases in FSH were associated with greater risk of imminent bone loss at the LS, but not the FN, after adjusting for MT stage (pre- vs. early peri- vs. late perimenopause) and clinical covariates. Similarly, the AUCs for the hormone-plus-covariates models were significantly higher than the AUCs for the covariates-only models for the LS, but not the FN (Table 3).
Single Measure of FSH as Predictors of Imminent Bone Loss, Stratified Analyses
Because identification of women with significant bone loss was greatest for single measures of FSH (i.e., the model AUC was greatest), and obtaining single measures of FSH is more practical than checking within-individual change E2 or FSH, our remaining analyses focused on one-time measures of FSH. We further characterized the association of FSH with imminent bone loss by examining whether the association was modified by race/ethnicity, MT stage or timing of hormone measurements within the menstrual cycle. Formal interaction testing confirmed that the ability of FSH to predict significant bone loss was similar during pre- and early perimenopause (interaction p=0.8 [LS]; interaction p=0.4 [FN]), but was different between early perimenopause vs. late perimenopause (interaction p=0.03 [LS]; interaction p=0.04 [FN]). FSH prediction was not modified by race/ethnicity or whether the hormone level was measured during the early follicular phase of the menstrual cycle.
In analyses stratified by MT stage (pre- and early perimenopause in one stratum, late postmenopause in a second stratum), predictions were better earlier in the MT. During pre- and early perimenopause (stratum 1), each two-fold increment in FSH was associated with 45% and 22% greater risk of significant bone loss at the LS (p<0.001) and FN (p=0.01), respectively, after accounting for MT stage (pre- vs. early perimenopause) and clinical covariates. The AUC for FSH plus MT stage and clinical covariates to predict bone loss at the LS was 0.777 (compared to 0.732 for MT stage and clinical covariates alone, p<0.0001), and 0.732 at the FN (compared to 0.732 for MT stage and clinical covariates alone, p=0.8) (Table 4). During late perimenopause (stratum 2), each two-fold increment in FSH was associated with 21% and 71% greater risk of significant bone loss at the LS (p=0.001) and FN (p=0.001), respectively. As in pre- and early perimenopause, discrimination for imminent bone loss was greater with FSH plus MT stage and covariates compared to MT stage and covariates alone at the LS (AUC 0.725 vs. 0.642, p<0.0001), but not the FN (AUC 0.621 vs. 0.603, p=0.4) (Table 4). Table 5 reports the sensitivity and specificity of various FSH thresholds for imminent bone loss.
Table 4.
Associations of FSH with significant bone lossa by the next year; stratified by menopause transition (MT) stage
| MT Stage | Relative Risk (RR) of Significant Bone Loss By the Next Year (per two-fold increment of FSH) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Lumbar Spine | Femoral Neck | |||||||
| RR (95% CI) | p-valueb | AUC | p-valuec | RR (95% CI) | p-valueb | AUC | p-valuec | |
| Pre- and Early Perimenopause | ||||||||
| FSH | 1.46 (1.34, 1.59) | <0.0001 | 0.777 | <0.0001 | 1.22 (1.04, 1.43) | 0.01 | 0.732 | 0.8 |
| Covariates only model | N/A | N/A | 0.732 | N/A | N/A | N/A | 0.732 | N/A |
| Late Perimenopause | ||||||||
| FSH | 1.21 (1.09, 1.36) | 0.001 | 0.725 | <0.0001 | 1.71 (1.23, 2.37) | 0.001 | 0.621 | 0.4 |
| Covariates only model | N/A | N/A | 0.642 | N/A | N/A | N/A | 0.603 | N/A |
Associations estimated using modified Poisson regression on repeated measures from all follow-up visits up to the last visit before postmenopause (1 year after the FMP). Bone loss considered significant if decrease in bone mineral density (from SWAN baseline to the follow-up visit around 1 year after FSH measurement) was greater than the site-specific least significant change (3.9% for the lumbar spine and 6.2% for the femoral neck). All models included the following covariates: age [years], race/ethnicity, clinical site, body mass index [kg/cm2]. In the pre- and early perimenopause stratum, models also included a flag for pre- vs. early perimenopause], and a flag for whether samples were collected during the early follicular phase of the menstrual cycle [yes/no]). The area under the receiver operator curves (AUC) for each model was estimated using logistic regression to assess the model’s ability to discriminate between women who were more vs. less likely to have significant bone loss in the next year.
p-value for hormone predictor
p-value for AUC of model containing hormone predictor with covariates compared to model with covariates only (all comparisons made within each MT stage stratum)
Table 5.
Sensitivity and specificity of various FSH thresholds for significant bone loss by the next year
| Sensitivity and Specificitya of Various FSH Thresholds for Significant Bone Loss By the Next Year | ||||
|---|---|---|---|---|
| Pre- and Early Perimenopause | Lumbar Spine | Femoral Neck | ||
| Sensitivity (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | |
| FSH ≥8 mIU/ml | 97.8 (95.7, 99.0) | 3.6 (3.0, 4.3) | 96.8 (92.6, 98.9) | 3.5 (2.9, 4.2) |
| FSH ≥16 mIU/ml | 77.0 (72.3, 81.2) | 46.9 (45.1, 48.6) | 66.9 (58.9, 74.2) | 45.1 (43.3, 46.8) |
| FSH ≥32 mIU/ml | 45.2 (40.0, 50.5) | 83.4 (82.0, 84.7) | 31.2 (24.0%, 39.1) | 81.0 (79.6, 82.3) |
| FSH ≥64 mIU/ml | 16.7 (13.0, 20.9) | 96.8 (96.2, 97.4) | 11.0 (6.6, 17.1) | 95.7 (94.9, 96.4) |
| Late Perimenopause | Lumbar Spine | Femoral Neck | ||
| Sensitivity (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | |
| FSH ≥8 mIU/ml | 100.0 (98.1, 100.0) | 2.3 (0.9, 4.7) | 100.0 (92.9, 100.0) | 1.6 (0.6, 3.2) |
| FSH ≥16 mIU/ml | 95.8 (91.9, 98.2) | 11.5 (8.1, 15.6) | 98.0 (89.4, 99.9) | 9.5 (6.9, 12.6) |
| FSH ≥32 mIU/ml | 88.9 (83.6, 93.0) | 30.5 (25.4, 36.0) | 86.0 (73.3, 94.2) | 24.1 (20.2, 28.4) |
| FSH ≥64 mIU/ml | 41.6 (34.5, 48.9) | 72.1 (66.7, 77.1) | 52.0 (37.4, 66.3) | 69.1 (64.6, 73.4) |
Sensitivity and specificity reported as %
Sensitivity Analyses
We performed three sets of sensitivity analyses. First, we excluded observations for which change in weight from SWAN baseline to the exposure follow-up visit was in the bottom 5% or top 5% of the population distribution. Second, we excluded observations that occurred after the DXA machine upgrade at the Pittsburgh and Oakland sites. Third, we used the ISCD’s limit of acceptable LSC thresholds at the LS or FN as alternative definitions for significant bone loss. For each set of sensitivity analyses, the associations of E2 and FSH with significant bone loss were similar to the primary analyses in both unstratified and stratified models (data not shown).
DISCUSSION
This study had 2 objectives. The first was to determine if E2 or FSH, measured once early in the MT, could predict if there will be significant MT-related bone loss by the next year. The second was to determine if within-individual change in E2 or FSH was superior at this prediction compared to one-time measures of these hormones. We report that single measures of both E2 and FSH predict imminent bone loss by the next year at the LS and FN, independent of MT stage and clinical covariates. When combined with these covariates, FSH was better than E2 at identifying women who were more vs. less likely to begin losing significant bone, based on the AUC metric. Tracking within-individual change in E2 and FSH did not afford superior prediction of significant bone loss by the following year.
Plausibly, FSH may offer superior prediction of significant bone loss because it is a better marker of average estrogen-mediated bioactivity than is E2 (21). Although osteoclasts and osteoblasts are target cells of E2 (22–26), circulating E2 levels may not accurately reflect the amount of E2 that enters these cells to carry out its biological function. In contrast, FSH is produced by the anterior pituitary gland under feedback inhibition by E2. The amount of circulating FSH is thus a direct reflection of E2-mediated bioactivity at the level of the target cell (i.e., the pituitary). This rationale is analogous to why thyroid stimulating hormone (TSH) is considered a better marker of thyroid hormone status than either thyroxine (T4) or triiodothyronine (T3) (27). Adding FSH to clinical covariates increases the AUC for predicting bone loss by the next year at the LS (from 0.732 to 0.777 in pre- and early perimenopause and from 0.642 to 0.725 in late perimenopause).
Our second main finding was that tracking within-individual change in E2 or FSH was not better than using single measures of these hormones for identifying women who more likely to lose significant BMD by the next year. We hypothesize that this is due to unavoidable measurement error. Because E2 and FSH values fluctuate markedly throughout the menstrual cycle, serial measures of these hormones should be obtained at the same point in the menstrual cycle (15, 28). This becomes less feasible as menstrual cycles became increasingly irregular in perimenopause. In fact, while 100% of SWAN visits in premenopause occurred during the EFP (dates 2-5 of the menstrual cycle), only 57% and 6% of visits in early peri- and late perimenopause, respectively, occurred during the EFP.
Our third key finding is that one-time measures of both E2 and FSH were stronger predictors of significant bone loss by the next year at the LS than at the FN. We suspect that this is attributable to the lesser BMD decline at the FN site during the MT, compounded by the larger CV of FN BMD measures. In our study sample, the mean annual rate of decline in FN BMD during late perimenopause (when MT-related decline is fastest) was lower than the SWAN CV.
Strengths of this study include its multi-racial/ethnic composition; longitudinal study design with repeated measures of BMD, E2, and FSH; and careful documentation of the FMP. However, our study has several limitations that warrant mention. First, while we tried to collect serum samples during the EFP of the menstrual cycle, this was not always possible, especially in the late perimenopausal visits. Since E2 and FSH values vary markedly during the menstrual cycle, tracking within-individual change in measurements obtained at different time points introduces measurement error. Second, SWAN protocols (initiated ~20 years ago) for computing cross-calibrations following a DXA hardware change and for calculating the in vivo precision error of DXA scans do not meet the current ISCD recommendations (20). To address this, we conducted sensitivity analyses that: 1) excluded the 56 (out of 3,618) observations that occurred after the machine changes at the Pittsburgh and Oakland sites; and 2) used the ISCD’s limit of acceptable LSC thresholds at the LS or FN as alternative thresholds for significant bone loss. Results from sensitivity analyses were essentially unchanged from primary analyses. Third, while many studies suggest that BMD decline, at rates commonly observed during the MT (1), is a risk factor for fracture (5–9, 29–31), and fast BMD decline in midlife is associated with appendicular and vertebral fractures (8, 9, 31), the relative contributions of peak bone mass vs. BMD loss to fracture risk have not been established. For example, the risk associated with BMD loss may depend on starting BMD (30) and fracture site (e.g., vertebral vs. hip) (32), but at least one study reported that peak bone mass and BMD loss are equally important (31).
In conclusion, both lower E2 and greater FSH values, measured once during pre- or perimenopause, were associated with greater risk of imminent bone loss, independent of relevant clinical risk factors, especially at the LS. However, FSH was better than E2 at identifying women who were more likely to lose significant BMD bye next year, and tracking within-individual change in E2 or FSH was not better than using one-time measures. Future studies will test FSH in combination with clinical covariates and other biomarkers (e.g., anti-Mullerian hormone or bone turnover markers) to develop models that can prospectively identify women who are about to begin losing bone. This, in turn, will enable us to test whether early, time-limited interventions can prevent MT-related bone loss, and ultimately whether this reduces the risk of future fracture.
ACKNOWLEDGMENTS
SUPPORTING GRANTS/FELLOWSHIPS
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH. Additional support for this project provided by NIA through P30-AG028748; UCLA Claude Pepper Older Adults Independence Center (PI: Reuben) funded by the National Institute of Aging (5P30AG028748); NIH/NCATS UCLA CTSI Grant Number UL1TR000124. Dr. Shieh was supported by the UCLA Specialty Training and Advanced Research Program and the Iris Cantor-UCLA Women’s Health Center Executive Advisory Board.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Chhanda Dutta 2016- present; Winifred Rossi 2012–2016; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
SUPPLEMENTAL DATA
No
DISCLOSURE STATEMENT
AS, GAG, JAC, CKG, CC and ASK have nothing to disclose.
REFERENCES
- 1.Greendale G, Sowers M, Han W, Huang M, Finkelstein J, Crandall C, et al. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: Results from the Study of Women’s Health Across the Nation (SWAN). J Bone Miner Res. 2012;27(1):111–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Recker R, Lappe J, Davies K, Heaney R. Bone remodeling increases substantially in the years after menopause and remains increased in older osteoporosis patients J Bone Miner Res. 2004;19(10):1628–33. [DOI] [PubMed] [Google Scholar]
- 3.Akhter M, Lappe J, Davies K, Recker R. Transmenopausal changes in the trabecular bone structure. Bone. 2007;41(1):111–6. [DOI] [PubMed] [Google Scholar]
- 4.Cooper D, Thomas C, Clement J, Turinsky A, Sensen C, Hallgrímsson B. Age-dependent change in the 3D structure of cortical porosity at the human femoral midshaft. Bone. 2007;40(4):957–65. [DOI] [PubMed] [Google Scholar]
- 5.Berger C, Langsetmo L, Joseph L, Hanley D, Davison K, Josse R, et al. Association between change in BMD and fragility fracture in women and men. J Bone Miner Res. 2009;24(2):361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen T, Center J, Eisman J. Femoral neck bone loss predicts fracture risk independent of baseline BMD. J Bone Miner Res. 2005;20(7):1195–201. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed L, Emaus N, Berntsen G, Bjørnerem A, Fønnebø V, Jørgensen L, et al. Bone loss and the risk of non-vertebral fractures in women and men: the Tromsø study. Osteoporos Int. 2010;21(9):15031511. [DOI] [PubMed] [Google Scholar]
- 8.Riggs BL, Wahner HW, Dunn WL, Mazess RB, Offord KP, Melton LJ 3rd. Differential changes in bone mineral density of the appendicular and axial skeleton with aging: relationship to spinal osteoporosis. J Clin Invest. 1981;67(2):328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riggs BL, Wahner HW, Seeman E, Offord KP, Dunn WL, Mazess RB, et al. Changes in bone mineral density of the proximal femur and spine with aging. Differences between the postmenopausal and senile osteoporosis syndromes. J Clin Invest. 1982;70(4):716–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29(6):517–22. [DOI] [PubMed] [Google Scholar]
- 11.Zaidi M, Turner C, Canalis E, Pacifici R, Sun L, Iqbal J, et al. Bone loss or lost bone: rationale and recommendations for the diagnosis and treatment of early postmenopausal bone loss. Curr Osteoporos Rep. 2009;7(4):118–26. [DOI] [PubMed] [Google Scholar]
- 12.Greendale G, Ishii S, Huang M, Karlamangla A. Predicting the timeline to the final menstrual period: the study of women’s health across the nation. J Clin Endocrinol Metab. 2013;98(4):1483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grodin J, Siiteri P, MacDonald P. Source of estrogen production in postmenopausal women. J Clin Endocrinol Metab. 1973;36(2):207–14. [DOI] [PubMed] [Google Scholar]
- 14.Ettinger B, Pressman A, Sklarin P, Bauer D, Cauley J, Cummings S. Associations between low levels of serum estradiol, bone density, and fractures among elderly women: the study of osteoporotic fractures. J Clin Endocrinol Metab. 1998;83(7):2239–43. [DOI] [PubMed] [Google Scholar]
- 15.Tepper P, Randolph J Jr, McConnell D, Crawford S, El Khoudary S, Joffe H, et al. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among women in the Study of Women’s Health across the Nation (SWAN). J Clin Endocrinol Metab. 2012;97(8):2872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sowers M, Zheng H, Greendale G, Neer R, Cauley J, Ellis J, et al. Changes in bone resorption across the menopause transition: effects of reproductive hormones, body size, and ethnicity. J Clin Endocrinol Metab. 2013;98(7):2854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slemenda C, Longcope C, Peacock M, Hui S, Johnston C. Sex steroids, bone mass, and bone loss. A prospective study of pre-, peri-, and postmenopausal women. J Clin Invest. 1996;97(1):14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sowers M, Crawford S, Sternfeld B, Morganstein D, Gold E, Greendale G, et al. SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo R, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego: Academic Press; 2000. p. 175–88. [Google Scholar]
- 19.Cleves M From the help desk: Comparing areas under receiver operating characteristic curves from two or more probit or logit models. The Stata Journal. 2002;2(3):301–13. [Google Scholar]
- 20.The International Society for Clinical Densitometry. 2019 ISCD Official Positions – Adult 2019. [Available from: https://www.iscd.org/official-positions/2019-iscd-official-positions-adult/.
- 21.Crandall C, Tseng C, Karlamangla A, Finkelstein J, Randolph JJ, Thurston R, et al. Serum sex steroid levels and longitudinal changes in bone density in relation to the final menstrual period. J Clin Endocrinol Metab. 2013;98(4):E654–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almeida M, Laurent MR, Dubois V, Claessens F, O’Brien CA, Bouillon R, et al. Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiological reviews. 2017;97(1):135–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khosla S, Oursler M, Monroe D. Estrogen and the skeleton. Trends Endocrinol Metab. 2012;23(11):576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khosla S, Melton LJ 3rd, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83(7):2266–74. [DOI] [PubMed] [Google Scholar]
- 25.Centrella M, McCarthy TL. Estrogen receptor dependent gene expression by osteoblasts - direct, indirect, circumspect, and speculative effects. Steroids. 2012;77(3):174–84. [DOI] [PubMed] [Google Scholar]
- 26.Nicks KM, Fowler TW, Akel NS, Perrien DS, Suva LJ, Gaddy D. Bone turnover across the menopause transition : The role of gonadal inhibins. Annals of the New York Academy of Sciences. 2010;1192:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hennessey JV, Garber JR, Woeber KA, Cobin R, Klein I. AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY POSITION STATEMENT ON THYROID DYSFUNCTION CASE FINDING. Endocr Pract. 2016;22(2):262–70. [DOI] [PubMed] [Google Scholar]
- 28.Randolph JJ, Zheng H, Sowers M, Crandall C, Crawford S, Gold E, et al. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab. 2011;96(3):746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hansen MA, Overgaard K, Riis BJ, Christiansen C. Role of peak bone mass and bone loss in postmenopausal osteoporosis: 12 year study. BMJ (Clinical research ed) 1991;303(6808):961–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hui SL, Slemenda CW, Johnston CC Jr. The contribution of bone loss to postmenopausal osteoporosis. Osteoporos Int. 1990;1(1):30–4. [DOI] [PubMed] [Google Scholar]
- 31.Riis BJ, Hansen MA, Jensen AM, Overgaard K, Christiansen C. Low bone mass and fast rate of bone loss at menopause: equal risk factors for future fracture: a 15-year follow-up study. Bone. 1996;19(1):9–12. [DOI] [PubMed] [Google Scholar]
- 32.Tabensky A, Duan Y, Edmonds J, Seeman E. The contribution of reduced peak accrual of bone and age-related bone loss to osteoporosis at the spine and hip: insights from the daughters of women with vertebral or hip fractures. J Bone Miner Res. 2001;16(6):1101–7. [DOI] [PubMed] [Google Scholar]


