Abstract
Motor abnormalities (e.g., dyskinesia, psychomotor slowing, neurological soft signs) are core features of schizophrenia that occur independent of drug treatment and are associated with the genetic vulnerability and pathophysiology for the illness. Among this list, psychomotor slowing in particular is one of the most consistently observed and robust findings in the field. Critically, psychomotor slowing may serve as a uniquely promising endophenotype and/or biomarker for schizophrenia considering it is frequently observed in those with genetic vulnerability for the illness, predicts transition in subjects at high-risk for the disorder, and is associated with symptoms and recovery in patients. The purpose of the present review is to provide an overview of the history of psychomotor slowing in psychosis, discuss its possible neural underpinnings, and review the current literature supporting slowing as a putative endophenotype and/or biomarker for the illness. This review summarizes substantial evidence from a diverse array of methodologies and research designs that supports the notion that psychomotor slowing not only reflects genetic vulnerability, but is also sensitive to disease processes and the pathophysiology of the illness. Furthermore, there are unique deficits across the cognitive (prefix “psycho”) and motor execution (root word “motor”) aspects of slowing, with cognitive processes such as planning and response selection being particularly affected. These findings suggest that psychomotor slowing may serve as a promising endophenotype and biomarker for schizophrenia that may prove useful for identifying individuals at greatest risk and tracking the course of the illness and recovery.
Keywords: Psychomotor, Slowing, Catatonia, Psychosis, Schizophrenia, Endophenotype, Biomarker, Clinical High-Risk
The current understanding of the trajectory and pathophysiology of schizophrenia spectrum disorders has advanced considerably. However, attempts to improve early identification and prevention in at-risk populations, and to predict treatment response and clinical outcomes in schizophrenia remain promising but limited (Klosterkotter, Schultze-Lutter, Bechdolf, & Ruhrmann, 2011; Seidman & Nordentoft, 2015). This has led to efforts to identify endophenotypes that reflect the genetic risk for the illness, as well as biomarkers sensitive to disease progression and treatment response (Weickert, Weickert, Pillai, & Buckley, 2013). Endophenotypes are a specific type of biomarker that reflect abnormal biochemical, neurophysiological, neuroanatomical, cognitive, and neuropsychological characteristics associated with the genetic vulnerability for an illness that must also be state-independent and present within affected families at a higher rate than in the general population (Gottesman & Gould, 2003). In contrast, a biomarker for schizophrenia is any objectively measured characteristic that is an indicator of the risk for or presence of the disorder (Goff et al., 2016; Weickert et al., 2013). Broadly, biomarkers are intended to ultimately provide a means for identifying individuals at greatest risk for an illness, as well as track progression and remission. Consistent with a diathesis-stress model of psychosis, endophenotypes reflect the inherent diathesis whereas biomarkers are sensitive to a myriad of stressors and pathophysiological processes that contribute to the onset of the disorder.
Accumulating evidence indicates that motor dysfunction commonly observed in psychosis is associated with genetic vulnerability for the disorder, the severity and progression of the illness, as well as structural and functional abnormalities in motor circuitry across the different stages of psychosis (Dean et al., 2014; Dean et al., 2015; Hirjak, Northoff, Thomann, Kubera, & Wolf, 2018; Kent et al., 2019; Mittal, Bernard, & Northoff, 2017; Mittal, Dean, Pelletier, & Caligiuri, 2011; Mittal et al., 2007; Mittal, Jalbrzikowski, et al., 2011; Mittal et al., 2013; Osborne et al., 2020; Walther & Mittal, 2017). As this abnormal motor circuitry overlaps with neural regions implicated in prominent etiological models of schizophrenia (Cao & Cannon, 2019; Howes & Kapur, 2009), indices of motor dysfunction may serve as promising endophenotype and biomarker candidates in psychosis. In addition, motor dysfunction occurs in the absence and presence of antipsychotic medication (Peralta, Campos, De Jalón, & Cuesta, 2010), indicating that motor abnormalities cannot be accounted for by antipsychotic medication alone.
One motor deficit in particular, psychomotor slowing, has been called “the closest thing to a North-star in schizophrenia research” (Cancro, Sutton, Kerr, & Sugerman, 1971). Psychomotor slowing is an observable and measurable reduction in the initiation, amount, or speed of movement that results from deficits in either the automatic and controlled cognitive processes involved in movement (i.e., the prefix “psycho”), or the direct execution of movement itself (i.e., the root word motor). It can be measured in several ways such as traditional processing and motor speed measures (e.g., Trails Making Test, Digit Symbol Substitution Test, finger tapping), reaction time paradigms, actigraphy, and clinician rated slowing. This slowing of action planning and execution co-occurs with other motor symptoms in psychosis such as spontaneous involuntary movements (e.g., dyskinesias) (Peralta & Cuesta, 2011; Walther & Strik, 2012), and is a core feature of the disorder that is present across the different stages of schizophrenia (i.e., risk populations, first-episode, chronic) (Aas et al., 2014; Fatouros-Bergman, Cervenka, Flyckt, Edman, & Farde, 2014; Kelleher et al., 2013). However, it is currently unclear if psychomotor slowing in psychosis primarily reflects the disorder’s genetic vulnerability (i.e., endophenotype) or broader disease processes (i.e., biomarker) more specifically. Thus, considering the current evidence on psychomotor slowing as it relates to genotype-phenotype pathways, pathophysiology, staging of the disorder, and response to treatment will inform both the development of motor-related biomarkers for psychosis and diathesis-stress models of psychosis more broadly.
The purpose of this review is to provide a summary of the current findings on psychomotor slowing in schizophrenia. We first introduce the conceptual history of psychomotor slowing before discussing the cognitive and motor processes implicated in slowing. Then we review the putative neural underpinnings of psychomotor slowing in schizophrenia before pivoting to a review of the current evidence for psychomotor slowing in schizophrenia, and evidence suggesting it reflects the genetic vulnerability for the disorder (i.e., endophenotypes) and disease mechanisms (i.e., biomarkers).
1. An Introduction to Psychomotor Slowing in Schizophrenia
1.1. History of Psychomotor Slowing in Schizophrenia
Portending modern biomarker work, psychomotor slowing has been considered an important behavioral manifestation of the pathophysiology of psychosis since the earliest phenomenological descriptions of the illness. For example, in 1874, Kahlbaum described various hypokinetic motor signs (slowed and decreased movements) in catatonia, their link to volition and motivation, and suspected abnormal neural activity within the cerebral motor system (Kahlbaum, 1874). Similarly, in his book on psychomotor symptoms, Kleist specifically suspected motor slowing to arise from a “… dissociation between the cerebellar-frontal-system and the sensorimotor system of the central gyri (i.e. primary motor cortex)” (Kleist, 1909, p. 147). Roughly thirty years later, Wernicke introduced the term “psychomotor”, and classified psychomotor slowing as akinetic (reduced movements) and hypothesized that dysconnectivity between neural regions gives rise to akinesias (slowed or lack of movement) observed in psychosis (Beer, 1995; Lanczik & Keil, 1991; Ungvari, 1993; Wernicke, 1906). Furthermore, he separated intrapsychic akinesia (lack of intrinsic motivation/initiative, which affects the whole motor system and compromises self-initiated movements much more than reactive movements) from psychomotor akinesia (negativism, flexibilitas cerea, rigor, and gegenhalten). Therefore, Wernicke considered non-intentional movements without outcome expectations to be psychomotor slowing, which is in contrast to Kraepelin who used the term psychomotor slowing to describe volitional movements as well (Kleist, 1909). Further, whereas Wernicke believed that psychomotor slowing was important for the broader concept of psychosis, Kraepelin (and Bleuler) firmly integrated psychomotor slowing into their clinical conceptualization of schizophrenia (rather than psychotic disorders more broadly). For example, Bleuler observed that “spontaneous movements are… executed slowly and weakly” and even ambulatory and active patients with schizophrenia do so “slowly, tremulously, and feebly” (Bleuler, 1950).
Early twentieth century experimental findings in patients with schizophrenia paralleled early clinical descriptions of psychomotor slowing, pointing to slowed responses across a wide range of task paradigms and sensory modalities (Huston, Shakow, & Riggs, 1937; Nuechterlein, 1977; Saunders & Isaacs, 1929; Wells & Kelley, 1922). Indeed, a great deal of early and mid 20th century work sought to determine the possible cognitive contributions to task-related slowing in patients with schizophrenia (see Nuechterlein, 1977), as well as the utility of slowing for predicting prognosis, symptomatology, and discriminating amongst patient subgroups and other disorders (e.g., depression) (Cancro et al., 1971; Court & Garwoli, 1968; Rosenthal, Lawlor, Zahn, & Shakow, 1960). With the advent of prominent information processing models (Atkinson & Shiffrin, 1968; Broadbent, 1971), explanations for task-related slowing progressed from non-specific attentional deficits and clinical characteristics (e.g., motivation, cooperation, apathy) (Hunt & Cofer, 1944; Rodnick & Shakow, 1940; Shakow, 1963) to also include sensory filtering and short term memory abnormalities (McGhie, 1969; McGhie & Chapman, 1961) and slowed motor execution (Court & Garwoli, 1968; Venables, 1958). This focus on determining the subprocesses that contribute to psychomotor slowing has persisted as a dominant area of research in schizophrenia (see Morrens, Hulstijn, & Sabbe, 2007) with much recent work attempting to identify the specific neural substrates of slowing in psychosis (Barch, Moore, Nee, Manoach, & Luck, 2011; Grillon et al., 2013; Kim et al., 2003; Ojeda et al., 2002; Panagiotaropoulou et al., 2019; Repovs, Csernansky, & Barch, 2011).
1.2. Psychomotor Slowing within the Broader Psychomotor Syndrome of Schizophrenia
Psychomotor slowing exists as a symptom within a broader psychomotor syndrome exhibited in schizophrenia. Other psychomotor symptoms include neurological soft signs, catatonia, and extrapyramidal signs. Neurological soft signs (NSS) are subtle deficits in fine motor coordination, sensory integration, and sequencing of motor actions. Catatonia reflects a cluster of symptoms such as stupor, motor agitation, posturing, and negativism. Extrapyramidal signs include dyskinesias, parkinsonism (e.g., tremor, rigidity, bradykinesia), and dystonia. It is notable that there is considerable overlap across the above-mentioned symptoms that comprise the psychomotor syndrome and often co-occur within an individual (Peralta et al., 2010; Walther & Strik, 2012). Due to this overlap, it is difficult to determine the primary nature of motor slowing given its vast overlap with negative symptoms (avolition), catatonia, and extrapyramidal signs (Docx et al., 2012; McKenna, Lund, Mortimer, & Biggins, 1991; Peralta & Cuesta, 2011), all of which include psychomotor slowing as a characteristic feature of their symptom cluster. This is a particular issue when determining the nature of slowing in self-initiated movements given symptoms such as abulia and apathy are common in psychosis and would themselves result in reduced movement.
Furthermore, consistent with Rogers (1985) “conflict of paradigms”, it is difficult to determine if psychomotor slowing across these different symptom clusters reflects real etiological differences or are due to various investigators attributing the same clinical phenomenon to different etiologies. Because of this, several definitions of psychomotor slowing have emerged that place differing etiological emphasis including volition and deficits in information processing (Morrens, Hulstijn, & Sabbe, 2006). Although there is evidence for each of these models for understanding psychomotor dysfunction, the present review focuses on outward and measurable manifestations of psychomotor slowing, as they are clinically feasible and amenable to endophenotype and biomarker applications. However, it is important to acknowledge that the definition of psychomotor slowing used in the current review is quite broad in that it encompasses a large number of cognitive and motor processes. This broad definition was used in order to accurately reflect the complexity of the various potential underlying sub-processes that may contribute to slowing in psychosis.
1.3. Cognitive and Motor Processes of Psychomotor Slowing
Here, the cognitive and motor sub-processes that comprise psychomotor slowing in schizophrenia are differentiated in order to better elucidate distinct deficits contributing to slowing in schizophrenia. Various automatic and controlled cognitive processes are required to translate perceived sensory information into task-related or volitional (self-initiated) responses. Visuo-motor transformations requires an initial perception of relevant stimuli, these stimuli are then held in working memory until a decision that is consistent with task demands or intended goals is reached, and then a subsequent response is made. Depending on the complexity of the response, planning and sequencing of the movement(s) may also be required. Each of these processes is further facilitated by a number of additional cognitive and motor functions. For example, perception of stimuli requires adequate allocation of attentional resources, decision-making requires cognitive control to resolve task-relevant conflicts and plan sequences of motor actions, and execution of a response requires cognitive processes such as performance monitoring and inhibition of competing motor behaviors.
Depending on the required goal, each process may occur once, such as in a single trial of a choice reaction time paradigm or reaching for a glass, or multiple times such as in the Digit Symbol Substitution Test (DSST) or playing a piano. It is important to note that psychomotor processes involved in traditional paradigms are primarily externally triggered (i.e., those triggered by a stimulus) and are typically measured using choice reaction time paradigms or the performance of processing speed tasks (Trail Making Test [TMT]- A and B, DSST) on writing tablets. In contrast, planned and spontaneous self-initiated movements are made in the absence of a stimulus or cue and thus also involve a strong motivational and volitional component, as well as decisions regarding if and when to carry out an action (Haggard, 2008; Khalighinejad, Schurger, Desantis, Zmigrod, & Haggard, 2018), and are primarily measured via actigraphy. From this framework, the cognitive aspects of psychomotor slowing include deficits involved comparing stimuli to previously learned stimulus-response mappings in working memory (i.e., response selection), decision making, inhibiting competing responses, volition, and motor planning and sequencing. In contrast, the motor aspects of psychomotor slowing involve the relatively automatic processes involved in initiation, coordination, and execution of a response. Thus, slowing across any one or more of these sub-processes would result in the task-related slowing and slowed motor behavior observed across the schizophrenia spectrum.
Although the abovementioned cognitive and motor processes all contribute to effective movement, due to the variety of methods used to assess psychomotor slowing, coupled with the fact that the majority of research examining psychomotor slowing in psychosis is not well differentiated in regards to the sub-processes that contribute to slowing, we employ past approaches in the interest of organization (Jogems-Kosterman, Zitman, Van Hoof, & Hulstijn, 2001). Specifically, we refer to all sub-processes that occur before the initiation (i.e., onset) of movement as cognitive and the sub-processes involved in the initiation, coordination, and execution of movement as motor execution. See Table 1 for the sub-processes subsumed within these domains along with the measures that assess them. Note, the measures listed in Table 1 are not exhaustive and are meant to illustrate the types of paradigms that can be used to assess the various sub-process that may contribute to psychomotor slowing.
Table 1.
Definitions of Organizational Terms and Associated Sub-Processes and Measures
| Terms | Sub-Processes | Measures of Assessment |
|---|---|---|
| General Psychomotor Slowing | ||
| Refers to measures of slowing that only afford a single metric of slowing in psychosis. Thus, these measures do not distinguish between the cognitive and motor sub-processes that contribute to psychomotor slowing. | Depending on the task or action performed, measures of general slowing would include most, if not all, of the cognitive and motor processes implicated in perception, decision/response selection, and motor planning and execution. | TMT- A and B; DSST |
| Cognitive | ||
| Refers to the cognitive sub-processes that occur prior to initiation and execution of movement, as well as the measures that assess those sub-processes. | Response Selection and Motor Planning | Traditional paradigms on writing table; reaction time paradigms; S-LRP |
| Motor Inhibition | Stop Signal | |
| Volition | Actigraphy | |
| Motor Execution | ||
| Refers to the motor sub-processes that occur after the initiation and execution of movement, as well as the measures that assess those sub-processes. | Fine Motor Coordination | Grooved Pegboard Test |
| Motor Speed | Finger Tapping; R-LRP |
Note: TMT = Trail Making Test; DSST = Digit Symbol Substitution Test; S-LRP = stimulus-locked lateralized readiness potential; R-LRP = response-locked lateralized readiness potential.
1.4. Neural Underpinnings Across the Sub-Processes of Psychomotor Processing
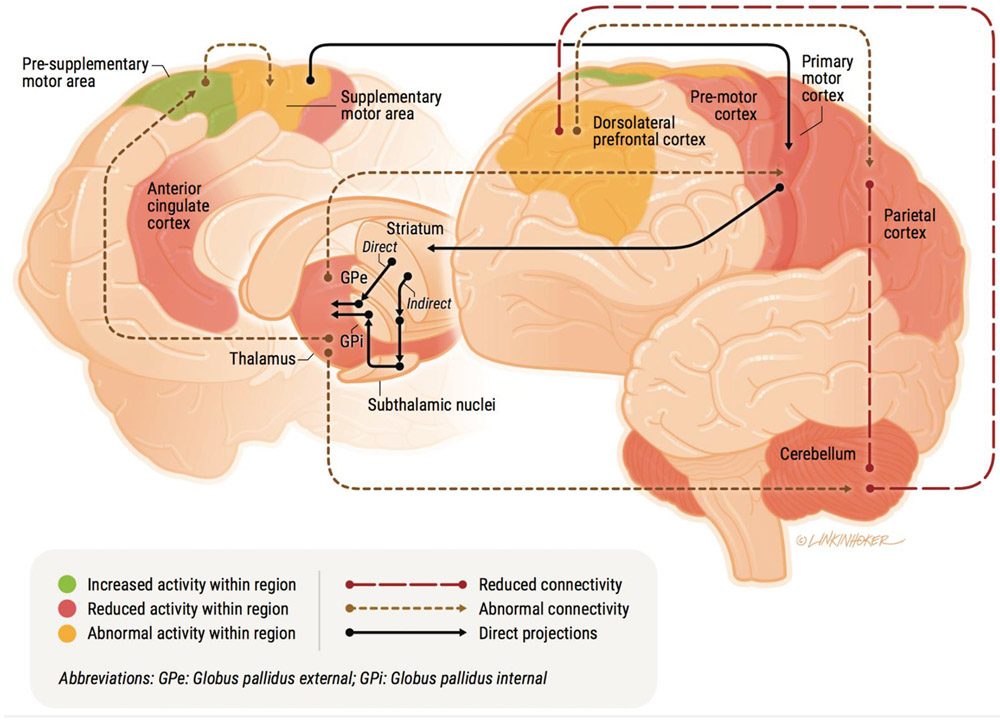
Regarding neural networks and circuitry, both cortico-cortical and cortico-basal ganglia networks have been proposed to subserve the cognitive and motor processes involved in visuo-motor transformations and goal-direct actions (Hélie, Ell, & Ashby, 2015; Rowe, Friston, Frackowiak, & Passingham, 2002; Sharpe et al., 2019). Critically, these same networks are well-evidenced to be altered in schizophrenia and are implicated in prominent etiological models for the illness (Cao & Cannon, 2019; Howes & Kapur, 2009). Because the exact nature of how these networks and circuits functionally translate visual input to motor output is not fully understood, we largely focus on cortico-cortical networks as they relate to cognitive aspects of psychomotor behavior and cortical-striatal circuits as they relate to motor function. Although dividing these networks into distinct functional categories is primarily done to enhance conceptual clarity, it is largely consistent with a body of evidence from research in humans and non-human primates (Gallivan & Culham, 2015). For instance, evidence from neuroimaging work with non-human primates and humans suggests that sensory information is integrated in the parietal cortex and then transformed into representations of movement-related features (e.g., intention to move, movement direction) (Barany, Della-Maggiore, Viswanathan, Cieslak, & Grafton, 2014; Gallivan & Culham, 2015; Gallivan, McLean, Valyear, Pettypiece, & Culham, 2011). Movement-related information in the parietal cortex is then projected to the premotor cortex (PMC), which is implicated in maintaining representations for potential actions and motor planning (Nakayama, Yamagata, & Hoshi, 2016; Svoboda & Li, 2018). It has been suggested that parieto-frontal connectivity may form the fundamental space for the maintenance of potential actions that are ultimately selected by subcortical circuitry to be relayed to the primary motor cortex (M1) for execution (Cisek, 2007; Frank, 2006).
1.4.1. Neural Underpinnings of Psychological Sub-Processes of Psychomotor Processing
Given that both attention to relevant environmental information and working memory involve parieto-frontal networks (Ester, Sprague, & Serences, 2015; Mackey & Curtis, 2017; Rajan et al., 2018; Szczepanski, Konen, & Kastner, 2010), these parieto-frontal networks may mediate the cognitive aspects of psychomotor behaviors. However, it is important to note that, depending on the required behavior, several other regions implicated in cognitive aspects of visuo-motor transformation may be involved. For instance, both the supplementary motor area (SMA) and pre-supplementary motor area (pre-SMA) contribute to planning sequences of motor behaviors (Gompf, Pflug, Laufs, & Kell, 2017; Scholz et al., 2000; Verwey, Lammens, & van Honk, 2002). Similarly, accurate movements require continuous monitoring of performance to ensure that any potential errors in movement are inhibited or corrected. Error monitoring is a cognitive function that relies on anterior cingulate cortex (ACC) activity. Taken together, the cognitive aspects of psychomotor behavior rely on an interconnected, multiregional network involved in processing stimuli, maintaining attention, and accurate motor planning using working memory.
1.4.2. Neural Underpinnings of Motor Sub-Processes of Psychomotor Processing
Ultimately, motor-related information is delivered to the primary motor cortex (M1) where it is relayed to the corticospinal tracts (Gallivan & Culham, 2015; Witham, Fisher, Edgley, & Baker, 2016). Evidence suggests that this process is subserved via cortico-basal ganglia circuitry. Specifically, the basal ganglia consist of several subcortical regions (globus pallidus, striatum, subthalamic nucleus, and substantia nigra) that are involved in two parallel, dopamine-dependent pathways critical for voluntary movement. Both pathways form closed loop circuits that originate in motor cortical neurons in the cortex (e.g., premotor and motor cortex). The direct pathway facilitates planned motor behavior via cortical projections to the striatum which then decreases the tonic inhibition of the internal segment of the globus pallidus (GPi) on thalamus, which increases thalamic projections back to the motor cortex, ultimately causing the execution of movement. In contrast, the indirect pathway suppresses movement via cortical projections to the striatum, which projects to the external segment of the globus pallidus (GPe), which projects to the subthalamic nucleus (STN), which projects to the GPi, increasing its tonic inhibition on the thalamus, which decreases thalamic projections back to the motor cortex, suppressing movement. Taken together, the motor aspects of psychomotor slowing involve the modulation of parieto-frontal networks via cortico-basal ganglia circuitry that selects from a number of cortical motor programs and projects them to M1.
1.5. Proposed Pathophysiological Model of Psychomotor Slowing in Schizophrenia
Determining the neural underpinnings of slowing in psychosis has relevance for the field’s understanding of the pathophysiological mechanisms putatively contributing to development and progression of the illness. Studies utilizing task-based fMRI, resting-state functional connectivity, and fiber tracking in psychosis populations implicate altered functional activity and connectivity across the previously described neural regions and their associated psychomotor processes (see Figure 1). For example, using fMRI, hyper- and hypoactivity and connectivity in parieto-frontal regions has been shown to be associated with psychomotor slowing in reaction time paradigms in patients (Fassbender, Scangos, Lesh, & Carter, 2014; Panagiotaropoulou et al., 2019; Woodward et al., 2009), and has been interpreted as reflecting the cognitive aspects of slowing including response selection, decision making, cognitive control, and working memory (Barch et al., 2011; Grillon et al., 2013; Kim et al., 2003; Ojeda et al., 2002; Panagiotaropoulou et al., 2019; Repovs et al., 2011). Furthermore, findings from fMRI, probabilistic fiber tracking, and arterial spin labeling in patients with clinician-rated slowing (i.e., akinesia, hypokinesia, retarded catatonia) have provided evidence for abnormal functional activity and connectivity across several regions including the pre-SMA, SMA, ACC, and PMC, as well as reductions in white matter integrity from the PFC to the striatum (Bracht et al., 2013; Foucher et al., 2018; Honey et al., 2005; Kaladjian et al., 2007; Payoux et al., 2004; Walther, Schäppi, et al., 2017; Walther, Stegmayer, et al., 2017), which further implicate deficits in performance monitoring, response inhibition, and planning in slowing.
Figure 1:
A conceptual model of psychomotor slowing in psychosis.
Whereas the neural underpinnings of the cognitive components contributing to slowing have received more attention, research examining fine motor execution deficits associated with slowing is more limited. However, resting state functional connectivity between the putamen and SMA is linked to slower motor execution during fine motor tasks in patients, whereas reduced connectivity between caudate and DLPFC was linked to longer planning durations (Viher et al., 2019). In addition, functional neuroimaging investigations of slowing in fine motor control in patients have demonstrated abnormal activity in M1, PMC, SMA, thalamus, basal ganglia, and cerebellum (Kodama et al., 2001; Müller, Röder, Schuierer, & Klein, 2002; Müller, Röder, Schuierer, & Klein, 2002; Singh et al., 2014).
2. Psychomotor Slowing as an Endophenotype and/or Biomarker for Psychosis
In the following sections, the current evidence for general psychomotor slowing (e.g., processing speed) in psychosis is reviewed before turning to research on the distinct cognitive and motor execution components contributing to slowing (see Table 1 for organizational terms). Within each section, findings that provide evidence for slowing being an endophenotype and/or biomarker for schizophrenia are reviewed. Consistent with a Research Domain Criteria approach (RDoC; Insel et al., 2010), we treat behavioral and biological findings of psychomotor slowing as “units of analysis” rather than independent constructs that are easily separable (see Miller, 2010). Thus, relevant behavioral and biological psychomotor findings are discussed together.
2.1. General Psychomotor Slowing in Psychosis
For the purpose of this review, general psychomotor slowing will be defined as slowing on various measures of psychomotor slowing that do not differentiate amongst the various (i.e., cognitive) and motor processes that may be contributing to slowing when completing traditional assessments. It has been suggested that traditional measures of slowed processing speed in schizophrenia, such as the TMT- A and B and DSST, should primarily be considered as measures of higher-order cognitive processes (e.g., working memory, attention, visuospatial skills) rather than indices of psychomotor slowing because the execution of movement is not the principal task component (Morrens et al., 2008). However, given that slowing has been observed in both the cognitive and motor execution processes of these tasks in patients with psychosis (Bervoets et al., 2014; Morrens et al., 2007), and they represent one of the most used neuropsychological assessments in the field, research examining processing speed abnormalities across the schizophrenia spectrum warrants discussion. Consistent with a staging model of schizophrenia, slowed processing speed is present in youth at clinical high-risk for the illness (Kelleher et al., 2013; Niendam et al., 2006), and is well-evidenced in first-episode psychosis and patients with chronic schizophrenia (Aas et al., 2014; Faber et al., 2011; Fatouros-Bergman et al., 2014). Further, evidence from a wide array of robust research suggests that processing speed may be both an endophenotype and biomarker for schizophrenia.
2.1.1. General Psychomotor Slowing Deficits as an Endophenotype for Psychosis
Evidence that psychomotor slowing is an endophenotype for psychosis would include findings demonstrating its association with the genetic liability for the illness. Candidate studies include research designs involving unaffected first-degree relative and twin populations, and findings in individuals with schizotypy or subclinical psychotic-like experiences in the general population. In a well-powered study of 147 patients with schizophrenia and 193 of their unaffected siblings, Egan et al. (2001) found that healthy siblings of individuals with schizophrenia exhibited slower processing speed on the TMT-B than healthy controls which was associated with increased relative risk suggesting that slowed performance is familial and possibly heritable. This finding has been replicated in numerous studies using different psychomotor paradigms (e.g., TMT-A/B, Digit Symbol Coding Test [DST]) highlighting its robustness (see Hou et al., 2016; Keefe et al., 1994; Pogue-Geile, Garrett, Brunke, & Hall, 1991). Further, similar results have been shown using extended pedigree research designs that do not select participants based on a single phenotype (i.e., schizophrenia) and instead randomly recruit large samples of both nuclear and extended families (e.g., first through fifth degree relatives) in order to examine genetic liability for psychosis (Glahn et al., 2007; Glahn et al., 2015). Findings in monozygotic and dizygotic twins both concordant and discordant for schizophrenia have corroborated these family studies by showing that slowed processing speed is moderately genetically linked to schizophrenia (Toulopoulou et al., 2007).
Other evidence for psychomotor slowing as an endophenotype for schizophrenia comes from research on parkinsonian motor abnormalities (e.g., bradykinesia, slowed gait, akinesia) in unaffected first-degree relatives (Koning et al., 2010). Kamis et al. (2015) used transcranial ultrasound to examine the relationship between echogenicity of the basal ganglia and parkinsonian motor impairment in never-treated patients with schizophrenia and their unaffected first-degree relatives. Similar to findings in Parkinson’s disease (Berg, 2011; Bouwmans, Vlaar, Mess, Kessels, & Weber, 2013), both patients with schizophrenia and their relatives exhibited increased echogenicity of the substantia nigra compared to healthy controls and this hyperechogenicity was associated with more severe parkinsonian symptoms in both groups. Molina et al. (2016) replicated this finding in a sample of neuroleptic-naïve schizophrenia patients and unaffected first-degree relatives, suggesting that parkinsonian motor deficits may be a key endophenotype for the illness that is associated with structural basal ganglia abnormalities. In another line of supporting evidence, slowed processing speed has been found in individuals in the general population with psychotic-like experiences (i.e., nonclinical psychotic symptoms such as fleeting hallucinations or mild social apathy) (Martín-Santiago et al., 2016; Simons, Jacobs, Jolles, Van Os, & Krabbendam, 2007). Critically, the severity of psychomotor slowing has been associated with the frequency and distress of both positive and negative psychotic-like experiences (Martín-Santiago et al., 2016). Taken together, although more direct research is required, the presence of psychomotor slowing in unaffected relatives and individuals with psychotic-like experiences in the general population suggests that slowing is not state dependent in psychosis.
2.1.2. General Psychomotor Slowing Deficits as a Biomarker for Psychosis
Evidence that psychomotor slowing is a biomarker for psychosis would include findings demonstrating its association with the emergence, course (i.e., progression or remission), or severity of the illness. Candidate studies include research designs involving birth cohort studies, high-risk and first-episode populations with longitudinal follow-ups, and cross-sectional research examining associations with illness severity and pathophysiology. For instance, slowing in speed of processing at age 8 and declines in speed of processing from 8 to 11 were the strongest predictors of psychotic experiences at age 12 in a large birth cohort study in children (Niarchou et al., 2013). Similarly, children that ultimately develop schizophrenia exhibit a slower rate of development in processing speed (as measured by the DST) compared to typically developing children (Reichenberg et al., 2009), suggesting that deficits in psychomotor slowing may appear early developmentally and provide a useful marker for illness risk. Further, it has been shown that youth at clinical high-risk (CHR) for psychosis also have slowed processing speed that is at an intermediate level between controls and patients (Carrión et al., 2011; Hou et al., 2016; Keefe et al., 2006; Kelleher et al., 2013; Seidman et al., 2010), and that this impairment predicts transition to psychosis (Giuliano et al., 2012; Jahshan, Heaton, Golshan, & Cadenhead, 2010; Seidman et al., 2016). Interestingly, CHR youth with slowing also exhibit resting state functional connectivity abnormalities that parallel those observed in schizophrenia (i.e., increased thalamocortical connectivity to M1) (Dean, Walther, Bernard, & Mittal, 2018).
Consistent with findings in CHR youth, when comparing first-episode psychosis patients that performed within normal limits on a neuropsychological battery to first-episode patients with cognitive impairment, the cognitively normal subgroup performed similarly to controls in several cognitive domains but exhibited deterioration in processing speed (relative to premorbid levels) equivalent to the cognitively impaired subgroup (González-Blanch et al., 2010). This finding suggests that processing speed deterioration may be a core feature of the illness that is present in patients with and without general cognitive impairment. In addition, performance on speed of processing tasks with less reliance on externally guided action (e.g., TMT-A/B, etc.), such as verbal fluency (which requires internally guided action), has been shown to predict recovery and deterioration in first-episode patients (Faber et al., 2011; Jahshan et al., 2010). After initial declines from the prodromal to first-episode stages of the illness, evidence suggests that slowed processing speed may remain relatively stable in chronic schizophrenia (Bonner-Jackson, Grossman, Harrow, & Rosen, 2010; Heilbronner, Samara, Leucht, Falkai, & Schulze, 2016) but is still sensitive to deficits in social and role functioning over time (Sánchez et al., 2009).
Several other lines of converging evidence support the idea that psychomotor slowing is a putative biomarker for the illness. For example, actigraphy has been used to demonstrate that patients with schizophrenia produce less volitional movement compared to healthy controls (Bracht et al., 2013; Walther et al., 2011; Walther, Stegmayer, Horn, Rampa, et al., 2015), which has been interpreted as reflecting overall motor slowing (Sano et al., 2012; Walther, Horn, et al., 2009; Walther, Koschorke, Horn, & Strik, 2009). Further, less baseline volitional motor activity has been shown to predict the trajectory of negative symptoms at future psychotic episodes as well as deterioration across multiple episodes (Walther, Stegmayer, Horn, Rampa, et al., 2015; Walther, Stegmayer, Horn, Razavi, et al., 2015). In fact, preliminary evidence suggests that across episodes, actigraphy patterns remain quite stable (Walther, Stegmayer, Horn, Razavi, et al., 2015). Using kinematic analysis of handwriting and structural MRI, it has been shown that CHR youth exhibit bradykinesia (i.e., slowed movement) when required to scale their velocity across shorter and longer targets distances, and that these deficits are associated with striatal volume and more severe positive and negative symptoms (Dean & Mittal, 2015).
2.2. Distinct Sub-Processes Contributing to Psychomotor Slowing in Schizophrenia
Taken together, there appears to be substantial evidence from a diverse array of methodologies and research designs to support the notion that general psychomotor slowing is not only a core feature of schizophrenia, but is also sensitive to disease processes, as well as the pathophysiology and genetic vulnerability for the disorder. However, the abovementioned findings are limited by their reliance on a single endpoint for quantifying psychomotor slowing (e.g., subjective ratings of motor behavior, time to finish a task) (van Harten, Walther, Kent, Sponheim, & Mittal, 2017). Specifically, the distinct cognitive and motor execution deficits contributing to psychomotor slowing are lost by collapsing across the various processes involved in action planning and motor execution, resulting in difficulty isolating the abnormal processes contributing to psychomotor slowing across the schizophrenia spectrum. Below, we review evidence for deficits in distinct areas of dysfunction in psychomotor slowing, as well as evidence that these deficits reflect endophenotypes or biomarkers for the illness.
Similar with past definitions (Jogems-Kosterman et al., 2001), cognitive processes involved in comparing stimuli to previously learned stimulus-response mappings in working memory (i.e., response selection), decision making, inhibiting competing responses, volition, and motor planning and sequencing are referred to as the planning aspect of psychomotor slowing. In contrast, the processes implicated in the initiation, coordination, and execution of movements are interpreted as reflecting the motor execution sub-processes of psychomotor slowing. Here, evidence for distinct impairments across these processes are reviewed. One of the most common means for delineating the cognitive and motor processes of psychomotor slowing in schizophrenia has been the use of digitizing writing tablets to quantify the time required to complete different aspects of common neuropsychological measures of processing speed (e.g., TMT-A/B, DSST). A common application of this approach is to have participants complete the DSST on a writing tablet and measure the time it takes to match the digits to their corresponding symbols (i.e., planning) and the time spent writing the symbols (i.e., motor). In this and similar psychomotor tasks with writing tablets, impairments in planning have been found consistently, whereas results for motor execution time are more mixed (Bervoets et al., 2014; Jogems-Kosterman et al., 2001; Morrens, Hulstijn, Hecke, Peuskens, & Sabbe, 2006). Specifically, it is important to note that the motor execution deficits are not always present using similar methods (Van Hoof, Jogems-Kosterman, Sabbe, Zitman, & Hulstijn, 1998), or across psychomotor tasks within studies (Jogems-Kosterman et al., 2001; Wölwer & Gaebel, 2002).
Noticeable similarities emerge from event-related potential (ERP) studies of psychomotor slowing and research employing writing tablets. Using the lateralized readiness potential (LRP), a negative-going ERP observed over the motor cortex that indexes motor preparation when making left- versus right-hand motor responses, research has shown that schizophrenia patients exhibit deficits in both the cognitive and basic motor processes involved in simple and choice reaction time experiments (Kappenman et al., 2012; Kappenman et al., 2015; Luck et al., 2009). Specifically, the LRP can be measured in two distinct ways that, when combined with an examination of LRP onset variability, provide unique information about the sub-processes contributing to psychomotor slowing (see Kappenman et al., 2015). For example, greater variability in the interval between the presentation of the stimulus and the onset of the LRP will result in reduced LRP amplitude that is the result of greater difficulty with selecting and planning a response. In contrast, greater variability in the interval between the onset of the LRP and the response will result in reduced LRP amplitude that is the result of greater difficulty executing a response. When comparing speeded to unspeeded responses, findings suggest that patients with schizophrenia were not able to modulate their speed across conditions and exhibited greater variability and reduced LRP in the interval between the stimulus and LRP onset, but not the interval between the LRP onset and response, suggesting that slowing is likely due to difficulties in response selection and planning rather than motor execution (Kappenman et al., 2015). However, deficits in more basic motor execution processes have also been observed in patients with schizophrenia (Kappenman et al., 2012). Thus, similar to the above-mentioned writing tablet studies, findings are inconsistent regarding whether the psychomotor slowing is primarily due to the cognitive or motor components of psychomotor processes. For example, Luck et al. (2009) used the latency of the P300, an ERP index of the time required to perceive and categorize a stimulus, and the LRP to examine if psychomotor slowing on a simple reaction time task is largely due to deficits in stimulus categorization, response selection, or more basic motor processes. Findings generally indicated that patients with schizophrenia were equally as fast as controls when evaluating simple stimuli (i.e., no P3 latency group differences) and slowing was primarily the result of impairments in response selection rather than motor execution, as evidenced by longer latencies between stimulus onset and motor preparation rather than the interval between preparation and response. These findings are largely consistent with results from other studies using the LRP to examine psychomotor slowing in schizophrenia (Kappenman et al., 2015; Karayanidis et al., 2006; Kieffaber, O'Donnell, Shekhar, & Hetrick, 2007).
Taken together, impaired cognitive processes involved in response selection and motor preparation are consistently found in schizophrenia, whereas findings for motor execution are less consistent. Yet this interpretation is confounded due to the majority of the reviewed research using task paradigms that only require simplistic motor responses (e.g., button press, drawing lines or digits) rather than more taxing complex movements typical of everyday goal-directed behavior and fine motor control. Indeed, there is a large body of research suggesting that individuals across the schizophrenia spectrum exhibit slowing on a wide array of fine motor tasks including the grooved pegboard test (Dickinson, Ramsey, & Gold, 2007; Sponheim et al., 2010), finger tapping (Delevoye-Turrell, Wilquin, & Giersch, 2012; Fuller & Jahanshahi, 1999; Gschwandtner et al., 2006), gait (Putzhammer et al., 2004; Putzhammer, Perfahl, Pfeiff, & Hajak, 2005), and handwriting (Tigges et al., 2000). There are several possible reasons for mixed findings that are discussed in the following sections.
2.2.1. Deficits in Distinct Sub-Processes of Psychomotor Slowing as Endophenotypes for Psychosis
Evidence that psychomotor slowing is an endophenotype for psychosis would include findings demonstrating its association with the genetic liability for the illness. Several studies have demonstrated that reaction times are prolonged in unaffected relatives of patients with schizophrenia (Birkett et al., 2007; Meda et al., 2008; Woodward et al., 2009), and there is some evidence to suggest that both the cognitive and motor components are implicated in this slowing. Regarding cognitive processes that may reflect a heritable vulnerability for the disorder, findings from functional MRI studies examining response selection in unaffected relatives suggests that abnormalities in neural regions associated with slowing in patients (See Figure 1) show similar patterns in relatives (Meda et al., 2008; Woodward, Duffy, & Karbasforoushan, 2013; Woodward et al., 2009).
Regarding specific evidence for slowing in motor execution as an endophenotype for psychosis, similar to research in schizophrenia, investigations using fine motor dexterity and motor speed (grooved pegboard, finger tapping) are the most common. For instance, differences have been found between controls and unaffected relatives on basic motor speed tasks (i.e., finger tapping) (Schäppi, Stegmayer, Viher, & Walther, 2018) and meta-analytic evidence suggests the overall effect size for finger tapping is small to moderate (d = .33) (Snitz, MacDonald III, & Carter, 2005). However, more work that examines complex motor execution is needed to better capture the extent that slowing of motor execution reflects the genetic liability for schizophrenia.
2.2.2. Deficits in Distinct Sub-Processes of Psychomotor Slowing as Biomarkers for Psychosis
Evidence that psychomotor slowing is a biomarker for psychosis would include longitudinal and cross-sectional research designs demonstrating its association with the emergence, course (i.e., progression or remission), or severity of the illness. In a study by Grootens et al. (2009), psychomotor slowing in recent-onset schizophrenia was examined using a writing tablet and the DSST along with a series of figure copying tasks that varied in complexity and found that patients with recent-onset schizophrenia were impaired in the planning phase of figure copying and that slowing increased with complexity. Notably, the observed slowing in planning processes in recent-onset schizophrenia were similar to that in chronic schizophrenia but less pronounced. Indeed, the slower planning on figure copying is associated with the severity of both positive (Jogems-Kosterman et al., 2001), and particularly negative symptoms (Bervoets et al., 2014; Jogems-Kosterman, Hulstijn, Wezenberg, & Hoof, 2006; Jogems-Kosterman et al., 2001). Further, several studies suggest that deficits across distinct sub-processes of psychomotor slowing may be limited to patients with pronounced negative symptoms (Jogems-Kosterman, Hulstijn, Wezenberg, & van Hoof, 2006; Jogems-Kosterman et al., 2001), and may be sensitive to specific domains of negative symptoms such as apathy (Bervoets et al., 2014).
Regarding motor execution, evidence suggests increased movement slowing in chronic stages of schizophrenia and patients on neuroleptic medication. For example, patients with chronic schizophrenia performed significantly worse on measures of fine motor dexterity and motor speed compared to those with recent-onset psychosis (Sponheim et al., 2010), which is consistent with the finding that fine motor control deteriorates as the illness progresses (Gold, Arndt, Nopoulos, O’Leary, & Andreasen, 1999; Kurtz, Seltzer, Ferrand, & Wexler, 2005). Notably, there is also evidence that motor dexterity differentiates CHR youth that convert to formal psychosis from non-converters (Hawkins et al., 2008). Lastly, meta-analytic findings suggest that both measures of processing speed (e.g., TMT-A/B, DSST) and motor dexterity (finger tapping, grooved pegboard) are sensitive to neuroleptic medication (Woodward, Purdon, Meltzer, & Zald, 2005), suggesting that it may be possible that both the cognitive and motor components of psychomotor slowing can be used to monitor treatment effects. Taken together, characterizing the individual cognitive and motor contributions to slowing serves to inform the field’s understanding of the specific deficits, and thus specific associations with the genetic vulnerability and pathophysiological mechanisms, underlying psychomotor slowing in schizophrenia. Further, sub-processes involved in planning responses seems to be particularly associated with negative symptoms in psychosis.
3. Conclusion
Evidence suggests that psychomotor slowing is present in unaffected first-degree relatives and twins, and is also associated with the frequency of psychotic-like experiences in the general population, indicating that psychomotor slowing may be a key endophenotype for schizophrenia. At the same time, general psychomotor slowing in psychosis also reflects a dose-dependent relationship across the different stages of the illness, with deficits becoming progressively worse from the prodromal stage to chronic schizophrenia. Furthermore, performance on various psychomotor tasks has been shown to predict recovery and deterioration in patients with schizophrenia, and was associated with both symptoms and relevant structural and functional abnormalities across the schizophrenia spectrum, indicating that psychomotor slowing may be a key biomarker for schizophrenia. Taken together, there appears to be substantial evidence from a diverse array of methodologies and research designs to support the notion that general psychomotor slowing is not only associated with the genetic vulnerability for the disorder, but is also a core feature of schizophrenia that is sensitive to disease processes and its pathophysiology.
Regarding distinct deficits across the “psychological” and motor components of psychomotor slowing, the reviewed evidence supports the conclusion that, although motor slowing is often observed, impairments in cognitive processes such as response selection and planning are the most consistent finding in the literature. This may be due to most research utilizing rather simple motor responses. Although the cognitive components of psychomotor slowing have received less attention in genetic vulnerability research in psychosis, similar findings regarding response selection and fine-motor dexterity have been demonstrated in unaffected relatives. Slowed planning was also demonstrated to be associated with both positive and negative symptoms. Further, performance on fine-motor dexterity tasks differentiated CHR youth that convert to psychosis from non-converters, is sensitive to antipsychotic medication, and is worse in chronic than recent onset schizophrenia. Taken together, there appears to be robust evidence that psychomotor slowing is both sensitive to the genetic vulnerability for schizophrenia (endophenotype) and disease processes (biomarker).
Future work on psychomotor slowing in schizophrenia should continue to disentangle the cognitive and motor components of slowing with a particular focus on using more complex motor movements. This will aid in determining the extent that the speed and fluency of a response contributes to slowing. Consistent with this goal, it will be critical for future research to focus on identifying the specific cognitive and motor processes that contribute to slowing. In regards to work on endophenotypes and biomarker research, more molecular genetic studies are needed to identify potential candidate genes associated with the various cognitive and motor processes contributing to psychomotor deficits. Similarly, neuroimaging studies optimized to differentiate the neural networks involved in distinct sub-processes of slowing and their association with the progression of the illness will help with biomarker development efforts. Together, this work would inform diathesis-stress models of psychosis and contribute to ongoing efforts to determine the etiology and development of schizophrenia.
Acknowledgements
Financial Support: This work was supported by the National Institutes of Health (V.A.M., grant numbers R01MH094650, R21/R33MH103231; K.J.O, grant number T32NS047987; S.A.S., V.A.M., S.W., grant number R01 MH118741; V.A.M., M.G., grant number R21 MH119677).
References
- Aas M, Dazzan P, Mondelli V, Melle I, Murray RM, & Pariante CM (2014). A systematic review of cognitive function in first-episode psychosis, including a discussion on childhood trauma, stress, and inflammation. Frontiers in psychiatry, 4, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RC, & Shiffrin RM (1968). Human memory: A proposed system and its control processes Psychology of learning and motivation (Vol. 2, pp. 89–195): Elsevier. [Google Scholar]
- Barany DA, Della-Maggiore V, Viswanathan S, Cieslak M, & Grafton ST (2014). Feature interactions enable decoding of sensorimotor transformations for goal-directed movement. Journal of Neuroscience, 34(20), 6860–6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Moore H, Nee DE, Manoach DS, & Luck SJ (2011). CNTRICS imaging biomarkers selection: Working memory. Schizophrenia Bulletin, 38(1), 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer MD (1995). Psychosis: from mental disorder to disease concept. History of Psychiatry, 6(22), 177–200. [DOI] [PubMed] [Google Scholar]
- Berg D (2011). Substantia nigra hyperechogenicity is a risk marker of Parkinson’s disease: yes. Journal of Neural Transmission, 118(4), 613–619. [DOI] [PubMed] [Google Scholar]
- Bervoets C, Docx L, Sabbe B, Vermeylen S, Van Den Bossche MJ, Morsel A, & Morrens M (2014). The nature of the relationship of psychomotor slowing with negative symptomatology in schizophrenia. Cognitive Neuropsychiatry, 19(1), 36–46. [DOI] [PubMed] [Google Scholar]
- Birkett P, Sigmundsson T, Sharma T, Toulopoulou T, Griffiths T, Reveley A, & Murray R (2007). Reaction time and sustained attention in schizophrenia and its genetic predisposition. Schizophrenia Research, 95(1-3), 76–85. [DOI] [PubMed] [Google Scholar]
- Bleuler E (1950). Dementia praecox or the group of schizophrenias. [PubMed] [Google Scholar]
- Bonner-Jackson A, Grossman LS, Harrow M, & Rosen C (2010). Neurocognition in schizophrenia: a 20-year multi–follow-up of the course of processing speed and stored knowledge. Comprehensive Psychiatry, 51(5), 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmans AE, Vlaar AM, Mess WH, Kessels A, & Weber WE (2013). Specificity and sensitivity of transcranial sonography of the substantia nigra in the diagnosis of Parkinson's disease: prospective cohort study in 196 patients. BMJ open, 3(4), e002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T, Schnell S, Federspiel A, Razavi N, Horn H, Strik W, … Walther S (2013). Altered cortico-basal ganglia motor pathways reflect reduced volitional motor activity in schizophrenia. Schizophrenia Research, 143(2-3), 269–276. [DOI] [PubMed] [Google Scholar]
- Broadbent DE (1971). Decision and stress. [Google Scholar]
- Cancro R, Sutton S, Kerr J, & Sugerman AA (1971). Reaction time and prognosis in acute schizophrenia. Journal of Nervous and Mental Disease. [DOI] [PubMed] [Google Scholar]
- Cao H, & Cannon TD (2019). Cerebellar Dysfunction and Schizophrenia: From “Cognitive Dysmetria” to a Potential Therapeutic Target: Am Psychiatric Assoc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrión RE, Goldberg TE, McLaughlin D, Auther AM, Correll CU, & Cornblatt BA (2011). Impact of neurocognition on social and role functioning in individuals at clinical high risk for psychosis. American Journal of Psychiatry, 168(8), 806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P (2007). Cortical mechanisms of action selection: the affordance competition hypothesis. Philosophical Transactions of the Royal Society B: Biological Sciences, 362(1485), 1585–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court J, & Garwoli E (1968). Schizophrenic Performance on a Reaction-Time Task with increasing Levels of Complexity. British Journal of Social and Clinical Psychology, 7(3), 216–223. [DOI] [PubMed] [Google Scholar]
- Dean DJ, Bernard JA, Orr JM, Pelletier-Baldelli A, Gupta T, Carol EE, & Mittal VA (2014). Cerebellar morphology and procedural learning impairment in neuroleptic-naive youth at ultrahigh risk of psychosis. Clinical Psychological Science, 2(2), 152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DJ, Kent JS, Bernard JA, Orr JM, Gupta T, Pelletier-Baldelli A, … Mittal VA (2015). Increased postural sway predicts negative symptom progression in youth at ultrahigh risk for psychosis. Schizophrenia Research, 162(1-3), 86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DJ, & Mittal VA (2015). Spontaneous parkinsonisms and striatal impairment in neuroleptic free youth at ultrahigh risk for psychosis. NPJ schizophrenia, 1, 14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DJ, Walther S, Bernard JA, & Mittal VA (2018). Motor clusters reveal differences in risk for psychosis, cognitive functioning, and thalamocortical connectivity: evidence for vulnerability subtypes. Clinical psychological science, 6(5), 721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delevoye-Turrell Y, Wilquin H, & Giersch A (2012). A ticking clock for the production of sequential actions: Where does the problem lie in schizophrenia? Schizophrenia Research, 135(1-3), 51–54. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, & Gold JM (2007). Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Archives of General Psychiatry, 64(5), 532–542. [DOI] [PubMed] [Google Scholar]
- Docx L, Morrens M, Bervoets C, Hulstijn W, Fransen E, De Hert M, … Sabbe B (2012). Parsing the components of the psychomotor syndrome in schizophrenia. Acta Psychiatrica Scandinavica, 126(4), 256–265. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Gscheidle T, Weirich M, Rawlings R, Hyde TM, … Weinberger DR (2001). Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biological Psychiatry, 50(2), 98–107. [DOI] [PubMed] [Google Scholar]
- Ester EF, Sprague TC, & Serences JT (2015). Parietal and frontal cortex encode stimulus-specific mnemonic representations during visual working memory. Neuron, 87(4), 893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber G, Smid HG, Van Gool AR, Wunderink L, Wiersma D, & Van den Bosch RJ (2011). Neurocognition and recovery in first episode psychosis. Psychiatry Research, 188(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Scangos K, Lesh TA, & Carter CS (2014). RT distributional analysis of cognitive-control-related brain activity in first-episode schizophrenia. Cognitive, Affective, & Behavioral Neuroscience, 14(1), 175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatouros-Bergman H, Cervenka S, Flyckt L, Edman G, & Farde L (2014). Meta-analysis of cognitive performance in drug-naïve patients with schizophrenia. Schizophrenia Research, 158(1-3), 156–162. [DOI] [PubMed] [Google Scholar]
- Foucher JR, Zhang YF, Roser M, Lamy J, De Sousa PL, Weibel S, … Berna F (2018). A double dissociation between two psychotic phenotypes: periodic catatonia and cataphasia. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 86, 363–369. [DOI] [PubMed] [Google Scholar]
- Frank MJ (2006). Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Networks, 19(8), 1120–1136. [DOI] [PubMed] [Google Scholar]
- Fuller R, & Jahanshahi M (1999). Concurrent performance of motor tasks and processing capacity in patients with schizophrenia. Journal of Neurology, Neurosurgery and Psychiatry, 66(5), 668–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallivan JP, & Culham JC (2015). Neural coding within human brain areas involved in actions. Current Opinion in Neurobiology, 33, 141–149. [DOI] [PubMed] [Google Scholar]
- Gallivan JP, McLean DA, Valyear KF, Pettypiece CE, & Culham JC (2011). Decoding action intentions from preparatory brain activity in human parieto-frontal networks. Journal of Neuroscience, 31(26), 9599–9610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano A, Li H, I Mesholam-Gately R, M Sorenson S, A Woodberry K, & J Seidman L (2012). Neurocognition in the psychosis risk syndrome: a quantitative and qualitative review. Current Pharmaceutical Design, 18(4), 399–415. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Almasy L, Blangero J, Burk GM, Estrada J, Peralta JM, … Nicolini H (2007). Adjudicating neurocognitive endophenotypes for schizophrenia. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 144(2), 242–249. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Williams JT, McKay DR, Knowles EE, Sprooten E, Mathias SR, … Göring HH (2015). Discovering schizophrenia endophenotypes in randomly ascertained pedigrees. Biological Psychiatry, 77(1), 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff DC, Romero K, Paul J, Perez-Rodriguez MM, Crandall D, & Potkin SG (2016). Biomarkers for drug development in early psychosis: current issues and promising directions. European Neuropsychopharmacology, 26(6), 923–937. [DOI] [PubMed] [Google Scholar]
- Gold S, Arndt S, Nopoulos P, O’Leary DS, & Andreasen NC (1999). Longitudinal study of cognitive function in first-episode and recent-onset schizophrenia. American Journal of Psychiatry, 156(9), 1342–1348. [DOI] [PubMed] [Google Scholar]
- Gompf F, Pflug A, Laufs H, & Kell CA (2017). Non-linear Relationship between BOLD Activation and Amplitude of Beta Oscillations in the Supplementary Motor Area during Rhythmic Finger Tapping and Internal Timing. Frontiers in Human Neuroscience, 11, 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, & Gould TD (2003). The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry, 160(4), 636–645. [DOI] [PubMed] [Google Scholar]
- Grillon M-L, Oppenheim C, Varoquaux G, Charbonneau F, Devauchelle A-D, Krebs M-O, … Huron C (2013). Hyperfrontality and hypoconnectivity during refreshing in schizophrenia. Psychiatry Research: Neuroimaging, 211(3), 226–233. [DOI] [PubMed] [Google Scholar]
- Grootens KP, Vermeeren L, Verkes RJ, Buitelaar JK, Sabbe BG, Van Veelen N, … Hulstijn W (2009). Psychomotor planning is deficient in recent-onset schizophrenia. Schizophrenia Research, 107(2-3), 294–302. [DOI] [PubMed] [Google Scholar]
- Gschwandtner U, Pflüger M, Aston J, Drewe M, Stieglitz RD, & Riecher–Rössler A (2006). Fine motor function and neuropsychological deficits in individuals at risk for schizophrenia. European Archives of Psychiatry and Clinical Neuroscience, 256(4), 201–206. [DOI] [PubMed] [Google Scholar]
- Haggard P (2008). Human volition: towards a neuroscience of will. Nature Reviews Neuroscience, 9(12), 934. [DOI] [PubMed] [Google Scholar]
- Hawkins KA, Keefe RS, Christensen BK, Addington J, Woods SW, Callahan J, … Breier A (2008). Neuropsychological course in the prodrome and first episode of psychosis: findings from the PRIME North America Double Blind Treatment Study. Schizophrenia Research, 105(1-3), 1–9. [DOI] [PubMed] [Google Scholar]
- Heilbronner U, Samara M, Leucht S, Falkai P, & Schulze TG (2016). The longitudinal course of schizophrenia across the lifespan: clinical, cognitive, and neurobiological aspects. Harvard Review of Psychiatry, 24(2), 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélie S, Ell SW, & Ashby FG (2015). Learning robust cortico-cortical associations with the basal ganglia: An integrative review. Cortex, 64, 123–135. [DOI] [PubMed] [Google Scholar]
- Hirjak D, Northoff G, Thomann P, Kubera K, & Wolf R (2018). Genuine motor phenomena in schizophrenia: neuronal correlates and pathomechanisms. Der Nervenarzt, 89(1), 27–43. [DOI] [PubMed] [Google Scholar]
- Honey GD, Pomarol-Clotet E, Corlett PR, Honey RA, Mckenna PJ, Bullmore ET, & Fletcher PC (2005). Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain, 128(11), 2597–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C-L, Xiang Y-T, Wang Z-L, Everall I, Tang Y, Yang C, … Jia F-J (2016). Cognitive functioning in individuals at ultra-high risk for psychosis, first-degree relatives of patients with psychosis and patients with first-episode schizophrenia. Schizophrenia Research, 174(1-3), 71–76. [DOI] [PubMed] [Google Scholar]
- Howes OD, & Kapur S (2009). The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophrenia Bulletin, 35(3), 549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J, & Cofer CN (1944). Psychological deficit. [Google Scholar]
- Huston PE, Shakow D, & Riggs LA (1937). Studies of motor function in schizophrenia: II. Reaction time. The Journal of General Psychology, 16(1), 39–82. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, … Wang P (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders: Am Psychiatric Assoc. [DOI] [PubMed] [Google Scholar]
- Jahshan C, Heaton RK, Golshan S, & Cadenhead KS (2010). Course of neurocognitive deficits in the prodrome and first episode of schizophrenia. Neuropsychology, 24(1), 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jogems-Kosterman, Hulstijn, Wezenberg, & Hoof v. (2006). Movement planning deficits in schizophrenia: Failure to inhibit automatic response tendencies. Cognitive Neuropsychiatry, 11(1), 47–64. [DOI] [PubMed] [Google Scholar]
- Jogems-Kosterman B, Hulstijn W, Wezenberg E, & van Hoof J (2006). Movement planning deficits in schizophrenia: Failure to inhibit automatic response tendencies. Cognitive Neuropsychiatry, 11(1), 47–64. [DOI] [PubMed] [Google Scholar]
- Jogems-Kosterman BJM, Zitman F, Van Hoof J, & Hulstijn W (2001). Psychomotor slowing and planning deficits in schizophrenia. Schizophrenia Research, 48(2-3), 317–333. [DOI] [PubMed] [Google Scholar]
- Kahlbaum K (1874). Die katatonie oder das spannungsirresein, eine klinische form psychischer krankheit, 1st Hirschwald: Berlin. [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin J-M, Grimault S, Anton J-L, & Mazzola-Pomietto P (2007). Blunted activation in right ventrolateral prefrontal cortex during motor response inhibition in schizophrenia. Schizophrenia Research, 97(1-3), 184–193. [DOI] [PubMed] [Google Scholar]
- Kamis D, Stratton L, Calvó M, Padilla E, Florenzano N, Guerrero G, … de Erausquin GA (2015). Sex and laterality differences in parkinsonian impairment and transcranial ultrasound in never-treated schizophrenics and their first degree relatives in an Andean population. Schizophrenia Research, 164(1-3), 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappenman ES, Kaiser ST, Robinson BM, Morris SE, Hahn B, Beck VM, … Luck SJ (2012). Response activation impairments in schizophrenia: evidence from the lateralized readiness potential. Psychophysiology, 49(1), 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappenman ES, Luck SJ, Kring AM, Lesh TA, Mangun GR, Niendam T, … Swaab TY (2015). Electrophysiological evidence for impaired control of motor output in schizophrenia. Cerebral Cortex, 26(5), 1891–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayanidis F, Nicholson R, Schall U, Meem L, Fulham R, & Michie PT (2006). Switching between univalent task-sets in schizophrenia: ERP evidence of an anticipatory task-set reconfiguration deficit. Clinical Neurophysiology, 117(10), 2172–2190. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Perkins DO, Gu H, Zipursky RB, Christensen BK, & Lieberman JA (2006). A longitudinal study of neurocognitive function in individuals at-risk for psychosis. Schizophrenia Research, 88(1-3), 26–35. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Silverman JM, Roitman SEL, Harvey PD, Duncan MA, Alroy D, … Mohs RC (1994). Performance of nonpsychotic relatives of schizophrenic patients on cognitive tests. Psychiatry Research, 53(1), 1–12. [DOI] [PubMed] [Google Scholar]
- Kelleher I, Murtagh A, Clarke MC, Murphy J, Rawdon C, & Cannon M (2013). Neurocognitive performance of a community-based sample of young people at putative ultra high risk for psychosis: support for the processing speed hypothesis. Cognitive Neuropsychiatry, 18(1-2), 9–25. [DOI] [PubMed] [Google Scholar]
- Kent JS, Disner SG, Van Voorhis AC, Urošević S, Caligiuri MP, & Sponheim SR (2019). Exploring the Relationship of Transdiagnostic Mood and Psychosis Symptom Domains with Motor Dysfunction. Neuropsychobiology, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalighinejad N, Schurger A, Desantis A, Zmigrod L, & Haggard P (2018). Precursor processes of human self-initiated action. Neuroimage, 165, 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffaber PD, O'Donnell BF, Shekhar A, & Hetrick WP (2007). Event related brain potential evidence for preserved attentional set switching in schizophrenia. Schizophrenia Research, 93(1), 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-J, Kwon JS, Park HJ, Youn T, Kang DH, Kim MS, … Lee MC (2003). Functional disconnection between the prefrontal and parietal cortices during working memory processing in schizophrenia: a [15O] H2O PET study. American Journal of Psychiatry, 160(5), 919–923. [DOI] [PubMed] [Google Scholar]
- Kleist K (1909). Weitere untersuchungen an geisteskranken mit psychomotorischen störungen Weitere untersuchungen an geisteskranken mit psychomotorischen störungen. [Google Scholar]
- Klosterkotter J, Schultze-Lutter F, Bechdolf A, & Ruhrmann S (2011). Prediction and prevention of schizophrenia: what has been achieved and where to go next? World Psychiatry, 10(3), 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S, Fukuzako H, Fukuzako T, Kiura T, Nozoe S, Hashiguchi T, … Nakabeppu Y (2001). Aberrant brain activation following motor skill learning in schizophrenic patients as shown by functional magnetic resonance imaging. Psychological Medicine, 31(6), 1079–1088. [DOI] [PubMed] [Google Scholar]
- Koning JP, Tenback DE, Van Os J, Aleman A, Kahn RS, & van Harten PN (2010). Dyskinesia and parkinsonism in antipsychotic-naive patients with schizophrenia, first-degree relatives and healthy controls: a meta-analysis. Schizophrenia Bulletin, 36(4), 723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz MM, Seltzer JC, Ferrand JL, & Wexler BE (2005). Neurocognitive function in schizophrenia at a 10-year follow-up: a preliminary investigation. CNS spectrums, 10(4), 277–280. [DOI] [PubMed] [Google Scholar]
- Lanczik M, & Keil G (1991). Carl Wernicke's localization theory and its significance for the development of scientific psychiatry. History of psychiatry, 2(6), 171–180. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Kappenman ES, Fuller RL, Robinson B, Summerfelt A, & Gold JM (2009). Impaired response selection in schizophrenia: evidence from the P3 wave and the lateralized readiness potential. Psychophysiology, 46(4), 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey WE, & Curtis CE (2017). Distinct contributions by frontal and parietal cortices support working memory. Scientific Reports, 7(1), 6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Santiago O, Suazo V, Rodríguez-Lorenzana A, de Azúa SR, Valcárcel C, Díez Á, … Molina V (2016). Relationship between subclinical psychotic symptoms and cognitive performance in the general population. Revista de Psiquiatría y Salud Mental (English Edition), 9(2), 78–86. [DOI] [PubMed] [Google Scholar]
- McGhie A (1969). Pathology of attention: Penguin Books. [Google Scholar]
- McGhie A, & Chapman J (1961). Disorders of attention and perception in early schizophrenia. British Journal of Medical Psychology, 34(2), 103–116. [DOI] [PubMed] [Google Scholar]
- McKenna P, Lund C, Mortimer A, & Biggins C (1991). Motor, Volitional and Behavioural Disorders in Schizophrenia: 2: The ‘Conflict of Paradigms' Hypothesis. The British Journal of Psychiatry, 158(3), 328–336. [DOI] [PubMed] [Google Scholar]
- Meda SA, Bhattarai M, Morris NA, Astur RS, Calhoun VD, Mathalon DH, … Pearlson GD (2008). An fMRI study of working memory in first-degree unaffected relatives of schizophrenia patients. Schizophrenia Research, 104(1-3), 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA (2010). Mistreating psychology in the decades of the brain. Perspectives on Psychological Science, 5(6), 716–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Bernard JA, & Northoff G (2017). What can different motor circuits tell us about psychosis? An RDoC perspective. Schizophrenia Bulletin, 43(5), 949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Dean DJ, Pelletier A, & Caligiuri M (2011). Associations between spontaneous movement abnormalities and psychotic-like experiences in the general population. Schizophrenia Research, 132(2-3), 194–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Hasenkamp W, Sanfilipo M, Wieland S, Angrist B, Rotrosen J, & Duncan EJ (2007). Relation of neurological soft signs to psychiatric symptoms in schizophrenia. Schizophrenia Research, 94(1-3), 37–44. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Jalbrzikowski M, Daley M, Roman C, Bearden CE, & Cannon TD (2011). Abnormal movements are associated with poor psychosocial functioning in adolescents at high risk for psychosis. Schizophrenia Research, 130(1-3), 164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Orr JM, Turner JA, Pelletier AL, Dean DJ, Lunsford-Avery J, & Gupta T (2013). Striatal abnormalities and spontaneous dyskinesias in non-clinical psychosis. Schizophrenia Research, 151(1-3), 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JL, González Alemán G, Florenzano N, Padilla E, Calvó M, Guerrero G, … Molina Rangeon B (2016). Prediction of neurocognitive deficits by parkinsonian motor impairment in schizophrenia: a study in neuroleptic-naïve subjects, unaffected first-degree relatives and healthy controls from an indigenous population. Schizophrenia Bulletin, 42(6), 1486–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrens, Hulstijn, Hecke V, Peuskens, & Sabbe. (2006). Sensorimotor and cognitive slowing in schizophrenia as measured by the Symbol Digit Substitution Test. Journal of Psychiatric Research, 40(3), 200–206. [DOI] [PubMed] [Google Scholar]
- Morrens, Hulstijn W, Matton C, Madani Y, Van Bouwel L, Peuskens J, & Sabbe B (2008). Delineating psychomotor slowing from reduced processing speed in schizophrenia. Cognitive Neuropsychiatry, 13(6), 457–471. [DOI] [PubMed] [Google Scholar]
- Morrens M, Hulstijn W, & Sabbe B (2007). Psychomotor slowing in schizophrenia. Schizophrenia Bulletin, 33(4), 1038–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller JL, Röder C, Schuierer G, & Klein HE (2002). Subcortical overactivation in untreated schizophrenic patients: a functional magnetic resonance image finger-tapping study. Psychiatry and Clinical Neurosciences, 56(1), 77–84. [DOI] [PubMed] [Google Scholar]
- Müller JL, Röder CH, Schuierer G, & Klein H (2002). Motor-induced brain activation in cortical, subcortical and cerebellar regions in schizophrenic inpatients. A whole brain fMRI fingertapping study. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 26(3), 421–426. [DOI] [PubMed] [Google Scholar]
- Nakayama Y, Yamagata T, & Hoshi E (2016). Rostrocaudal functional gradient among the pre-dorsal premotor cortex, dorsal premotor cortex and primary motor cortex in goal-directed motor behaviour. European Journal of Neuroscience, 43(12), 1569–1589. [DOI] [PubMed] [Google Scholar]
- Niarchou M, Zammit S, Walters J, Lewis G, Owen MJ, & van den Bree MB (2013). Defective processing speed and nonclinical psychotic experiences in children: longitudinal analyses in a large birth cohort. American Journal of Psychiatry, 170(5), 550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Bearden CE, Johnson JK, McKinley M, Loewy R, O'Brien M, … Cannon TD (2006). Neurocognitive performance and functional disability in the psychosis prodrome. Schizophrenia Research, 84(1), 100–111. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH (1977). Reaction time and attention in schizophrenia: A critical evaluation of the data and theories. Schizophrenia Bulletin, 3(3), 373. [DOI] [PubMed] [Google Scholar]
- Ojeda N, Ortuno F, Arbizu J, Lopez P, Martí-Climent JM, Penuelas I, & Cervera-Enguix S (2002). Functional neuroanatomy of sustained attention in schizophrenia: contribution of parietal cortices. Human Brain Mapping, 17(2), 116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne KJ, Damme KS, Gupta T, Dean DJ, Bernard JA, & Mittal VA (2020). Timing dysfunction and cerebellar resting state functional connectivity abnormalities in youth at clinical high-risk for psychosis. Psychological Medicine, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotaropoulou G, Thrapsanioti E, Pappa E, Grigoras C, Mylonas D, Karavasilis E, … Smyrnis N (2019). Hypo-activity of the dorsolateral prefrontal cortex relates to increased reaction time variability in patients with schizophrenia. NeuroImage: Clinical, 23, 101853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payoux P, Boulanouar K, Sarramon C, Fabre N, Descombes S, Galitsky M, … Manelfe C (2004). Cortical motor activation in akinetic schizophrenic patients: a pilot functional MRI study. Movement Disorders, 19(1), 83–90. [DOI] [PubMed] [Google Scholar]
- Peralta V, Campos MS, De Jalón EG, & Cuesta MJ (2010). Motor behavior abnormalities in drug-naïve patients with schizophrenia spectrum disorders. Movement Disorders, 25(8), 1068–1076. [DOI] [PubMed] [Google Scholar]
- Peralta V, & Cuesta MJ (2011). Neuromotor abnormalities in neuroleptic-naive psychotic patients: antecedents, clinical correlates, and prediction of treatment response. Comprehensive Psychiatry, 52(2), 139–145. [DOI] [PubMed] [Google Scholar]
- Pogue-Geile M, Garrett A, Brunke J, & Hall J (1991). Neuropsychological impairments are increased in siblings of schizophrenic patients. Schizophrenia Research, 4(3), 390. [Google Scholar]
- Putzhammer A, Heindl B, Broll K, Pfeiff L, Perfahl M, & Hajak G (2004). Spatial and temporal parameters of gait disturbances in schizophrenic patients. Schizophrenia Research, 69(2-3), 159–166. [DOI] [PubMed] [Google Scholar]
- Putzhammer A, Perfahl M, Pfeiff L, & Hajak G (2005). Gait disturbances in patients with schizophrenia and adaptation to treadmill walking. Psychiatry and Clinical Neurosciences, 59(3), 303–310. [DOI] [PubMed] [Google Scholar]
- Rajan A, Siegel SN, Liu Y, Bengson J, Mangun GR, & Ding M (2018). Theta Oscillations Index Frontal Decision-Making and Mediate Reciprocal Frontal–Parietal Interactions in Willed Attention. Cerebral Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RS, Murray RM, … Moffitt TE (2009). Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. American Journal of Psychiatry, 167(2), 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovs G, Csernansky JG, & Barch DM (2011). Brain network connectivity in individuals with schizophrenia and their siblings. Biological Psychiatry, 69(10), 967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnick E, & Shakow D (1940). Set in the schizophrenic as measured by a composite reaction time index. American Journal of Psychiatry, 97(1), 214–225. [Google Scholar]
- Rogers D (1985). The motor disorders of severe psychiatric illness: a conflict of paradigms. The British Journal of Psychiatry, 147(3), 221–232. [DOI] [PubMed] [Google Scholar]
- Rosenthal D, Lawlor WG, Zahn TP, & Shakow D (1960). The relationship of some aspects of mental set to degree of schizophrenic disorganization. Journal of Personality. [DOI] [PubMed] [Google Scholar]
- Rowe J, Friston K, Frackowiak R, & Passingham R (2002). Attention to action: specific modulation of corticocortical interactions in humans. Neuroimage, 17(2), 988–998. [PubMed] [Google Scholar]
- Sánchez P, Ojeda N, Peña J, Elizagárate E, Yoller AB, Gutiérrez M, & Ezcurra J (2009). Predictors of longitudinal changes in schizophrenia: the role of processing speed. The Journal of clinical psychiatry. [DOI] [PubMed] [Google Scholar]
- Sano W, Nakamura T, Yoshiuchi K, Kitajima T, Tsuchiya A, Esaki Y, … Iwata N (2012). Enhanced persistency of resting and active periods of locomotor activity in schizophrenia. PloS One, 7(8), e43539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders EB, & Isaacs S (1929). Tests of reaction-time and motor inhibition in the psychoses. American Journal of Psychiatry, 86(1), 79–112. [Google Scholar]
- Schäppi L, Stegmayer K, Viher PV, & Walther S (2018). Distinct associations of Motor Domains in relatives of schizophrenia Patients—Different Pathways to Motor abnormalities in schizophrenia? Frontiers in psychiatry, 9, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz VH, Flaherty A, Kraft E, Keltner J, Kwong K, Chen Y, … Jenkins B (2000). Laterality, somatotopy and reproducibility of the basal ganglia and motor cortex during motor tasks. Brain Research, 879(1-2), 204–215. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, … Walker EF (2010). Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Archives of General Psychiatry, 67(6), 578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, & Nordentoft M (2015). New targets for prevention of schizophrenia: is it time for interventions in the premorbid phase? Schizophrenia Bulletin, 41(4), 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Shapiro DI, Stone WS, Woodberry KA, Ronzio A, Cornblatt BA, … Cannon TD (2016). Association of neurocognition with transition to psychosis: baseline functioning in the second phase of the North American prodrome longitudinal study. JAMA psychiatry, 73(12), 1239–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakow D (1963). Psychological deficit in schizophrenia. Behavioral Science, 8(4), 275–305. [DOI] [PubMed] [Google Scholar]
- Sharpe MJ, Stalnaker T, Schuck NW, Killcross S, Schoenbaum G, & Niv Y (2019). An integrated model of action selection: distinct modes of cortical control of striatal decision making. Annual Review of Psychology, 70, 53–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons C, Jacobs N, Jolles J, Van Os J, & Krabbendam L (2007). Subclinical psychotic experiences and cognitive functioning as a bivariate phenotype for genetic studies in the general population. Schizophrenia Research, 92(1-3), 24–31. [DOI] [PubMed] [Google Scholar]
- Singh S, Goyal S, Modi S, Kumar P, Singh N, Bhatia T, … Khushu S (2014). Motor function deficits in schizophrenia: an fMRI and VBM study. Neuroradiology, 56(5), 413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, MacDonald III AW, & Carter CS (2005). Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponheim S, Jung R, Seidman L, Mesholam-Gately R, Manoach D, O’Leary D, … Schulz S (2010). Cognitive deficits in recent-onset and chronic schizophrenia. Journal of Psychiatric Research, 44(7), 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda K, & Li N (2018). Neural mechanisms of movement planning: motor cortex and beyond. Current Opinion in Neurobiology, 49, 33–41. [DOI] [PubMed] [Google Scholar]
- Szczepanski SM, Konen CS, & Kastner S (2010). Mechanisms of spatial attention control in frontal and parietal cortex. Journal of Neuroscience, 30(1), 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges P, Mergl R, Frodl T, Meisenzahl EM, Gallinat J, Schröter A, … Hegerl U (2000). Digitized analysis of abnormal hand–motor performance in schizophrenic patients. Schizophrenia Research, 45(1-2), 133–143. [DOI] [PubMed] [Google Scholar]
- Toulopoulou T, Picchioni M, Rijsdijk F, Hua-Hall M, Ettinger U, Sham P, & Murray R (2007). Substantial genetic overlap between neurocognition and schizophrenia: genetic modeling in twin samples. Archives of General Psychiatry, 64(12), 1348–1355. [DOI] [PubMed] [Google Scholar]
- Ungvari GS (1993). The Wernicke-Kleist-Leonhard school of psychiatry. Biological Psychiatry, 34(11), 749–752. [DOI] [PubMed] [Google Scholar]
- van Harten PN, Walther S, Kent JS, Sponheim SR, & Mittal VA (2017). The clinical and prognostic value of motor abnormalities in psychosis, and the importance of instrumental assessment. Neuroscience and Biobehavioral Reviews, 80, 476–487. [DOI] [PubMed] [Google Scholar]
- Van Hoof J, Jogems-Kosterman B, Sabbe B, Zitman FG, & Hulstijn W (1998). Differentiation of cognitive and motor slowing in the Digit Symbol Test (DST): differences between depression and schizophrenia. Journal of Psychiatric Research, 32(2), 99–103. [DOI] [PubMed] [Google Scholar]
- Venables P (1958). Stimulus complexity as a determinant of the reaction time of schizophrenics. Canadian Journal of Psychology/Revue canadienne de psychologie, 12(3), 187. [DOI] [PubMed] [Google Scholar]
- Verwey WB, Lammens R, & van Honk J (2002). On the role of the SMA in the discrete sequence production task: a TMS study. Neuropsychologia, 40(8), 1268–1276. [DOI] [PubMed] [Google Scholar]
- Viher PV, Docx L, Van WH, Parizel PM, Sabbe B, Federspiel A, … Morrens M (2019). Aberrant fronto-striatal connectivity and fine motor function in schizophrenia. Psychiatry research. Neuroimaging, 288, 44–50. [DOI] [PubMed] [Google Scholar]
- Walther S, Federspiel A, Horn H, Razavi N, Wiest R, Dierks T, … Müller TJ (2011). Alterations of white matter integrity related to motor activity in schizophrenia. Neurobiology of Disease, 42(3), 276–283. [DOI] [PubMed] [Google Scholar]
- Walther S, Horn H, Razavi N, Koschorke P, Müller TJ, & Strik W (2009). Quantitative motor activity differentiates schizophrenia subtypes. Neuropsychobiology, 60(2), 80–86. [DOI] [PubMed] [Google Scholar]
- Walther S, Koschorke P, Horn H, & Strik W (2009). Objectively measured motor activity in schizophrenia challenges the validity of expert ratings. Psychiatry Research, 169(3), 187–190. [DOI] [PubMed] [Google Scholar]
- Walther S, & Mittal VA (2017). Motor system pathology in psychosis. Current psychiatry reports, 19(12), 97. [DOI] [PubMed] [Google Scholar]
- Walther S, Schäppi L, Federspiel A, Bohlhalter S, Wiest R, Strik W, & Stegmayer K (2017). Resting-state hyperperfusion of the supplementary motor area in catatonia. Schizophrenia Bulletin, 43(5), 972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, & Viher PV (2017). Aberrant hyperconnectivity in the motor system at rest is linked to motor abnormalities in schizophrenia spectrum disorders. Schizophrenia Bulletin, 43(5), 982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S, Stegmayer K, Horn H, Rampa L, Razavi N, Müller TJ, & Strik W (2015). The longitudinal course of gross motor activity in schizophrenia–within and between episodes. Frontiers in psychiatry, 6, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S, Stegmayer K, Horn H, Razavi N, Müller TJ, & Strik W (2015). Physical activity in schizophrenia is higher in the first episode than in subsequent ones. Frontiers in psychiatry, 5, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S, & Strik W (2012). Motor symptoms and schizophrenia. Neuropsychobiology, 66(2), 77–92. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Weickert TW, Pillai A, & Buckley PF (2013). Biomarkers in schizophrenia: a brief conceptual consideration. Disease Markers, 35(1), 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells F, & Kelley C (1922). The simple reaction in psychosis. American Journal of Psychiatry, 79(1), 53–59. [Google Scholar]
- Wernicke C (1906). Grundriss der Psychiatrie in klinischen Vorlesungen: Thieme. [Google Scholar]
- Witham CL, Fisher KM, Edgley SA, & Baker SN (2016). Corticospinal inputs to primate motoneurons innervating the forelimb from two divisions of primary motor cortex and area 3a. Journal of Neuroscience, 36(9), 2605–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölwer W, & Gaebel W (2002). Impaired Trail-Making Test-B performance in patients with acute schizophrenia is related to inefficient sequencing of planning and acting. Journal of Psychiatric Research, 36(6), 407–416. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Duffy B, & Karbasforoushan H (2013). Prefrontal cortex activity during response selection predicts processing speed impairment in schizophrenia. Journal of the International Neuropsychological Society, 19(7), 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Purdon SE, Meltzer HY, & Zald DH (2005). A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. International Journal of Neuropsychopharmacology, 8(3), 457–472. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Waldie B, Rogers B, Tibbo P, Seres P, & Purdon SE (2009). Abnormal prefrontal cortical activity and connectivity during response selection in first episode psychosis, chronic schizophrenia, and unaffected siblings of individuals with schizophrenia. Schizophrenia Research, 109(1-3), 182–190. [DOI] [PubMed] [Google Scholar]