Abstract
Background and aims
Hypertension is associated with increased clinical and subclinical coronary artery disease (CAD); however, the relationship between blood pressure and coronary plaque volume is unclear. We examined the effect of systolic blood pressure (SBP) and diastolic blood pressure (DBP) on coronary artery plaque volume.
Methods
285 subjects with stable CAD on statin therapy underwent coronary computed tomographic angiography to measure volume of fatty, fibrous, noncalcified, calcified and total coronary plaque.
Results
Mean (SD) age was 63.1 (7.7); mean (SD) LDL-C, 78.7 mg/dL (28.5). Compared to the highest DBP tertile (>76 mmHg), those in the lowest DBP tertile (≤ 68 mmHg) had lower volumes of fatty: 10.0 vs. 7.7 mm3/mm, (p trend=0.042), fibrous: 19.6 vs. 13.8 mm3/mm (p trend = 0.011), non-calcified: 29.7 vs. 22.5 mm3/mm (p trend=0.017) and total plaque: 37.8 vs. 25.1 mm3/mm (p trend=0.010) whereas there was no relationship with SBP tertiles. Similarly, when examined as a continuous variable, higher DBP was a significant independent predictor of higher plaque volume after multivariate adjustment: for every 1 mmHg increase in DBP, fibrous plaque increased 0.128 mm3/mm (p=0.022), noncalcified plaque increased 0.176 mm3/mm (p=0.045), calcified plaque increased 0.096 mm3/mm (p=0.001) and total plaque increased 0.249 mm3/mm (p=0.019) whereas SBP ranging from 95 to 154 mmHg did not predict plaque volume.
Conclusions
Level of DBP predicts coronary plaque with a DBP tertile ≤ 68 mmHg associated with the least amount of coronary plaque in subjects with LDL-C < 80 mg/dL.
Keywords: Diastolic blood pressure, coronary plaque, coronary computed tomographic angiography, systolic blood pressure
1. Introduction
Hypertension is a major risk factor for coronary artery disease (CAD) and is associated with increased cardiovascular morbidity and mortality [1]. Observational studies have demonstrated a direct, graded association between higher systolic blood pressure (SBP) and diastolic blood pressure (DBP) and increased cardiovascular disease (CVD) risk [2,3]. In the Multiple Risk Factor Intervention Trial of over 316,000 men followed for 12 years, strong graded relationships between SBP above 110 mmHg and DBP above 70 mmHg and mortality due to coronary heart disease (CHD) were evident [3]. At every DBP, SBP above 115 mmHg was directly associated with an increasing risk of death from CHD [3]. In a meta-analysis of prospective epidemiologic studies for 1 million adults aged 40 to 70 years, a strong, independent and log-linear association was observed between mortality from CVD and total mortality down to a SBP of at least 115 mmHg [2]. Moreover, each increment of 20 mmHg in SBP or 10 mmHg in DBP was associated with a doubling of the risk of CHD events for blood pressure (BP) of 115/75 to 185/115 mmHg [2]. While the association between hypertension and CHD events is well established, how elevated BP affects coronary plaque volume and characteristics remains a matter of continued investigation. The aim of the current study was to determine the effect of SBP and DBP on overall coronary artery plaque volume and its subtypes measured by coronary computed tomographic angiography (CCTA) in patients with CAD on a stable dose of statin therapy with well-controlled levels of low-density-lipoprotein cholesterol (LDL-C) < 80 mg/dL.
2. Patients and methods
2.1. Study design
This is a cross-sectional analysis at baseline of the Slowing HEART diSease With Lifestyle and Omega-3 Fatty Acids trial. The trial is a randomized, parallel, single-center study of 3.36 g daily of EPA and DHA compared to no EPA and DHA over 30 months. The design has been described previously [4,5]. The protocol was approved by the Beth Israel Deaconess Medical Center Institutional Review Board and all subjects signed an informed consent.
2.2. Study population
Eligible participants were aged 21 to 80 years and had stable, established CAD defined as at least 1 of the following: ≥ 50% stenosis in at least 1 coronary artery at catheterization, previous myocardial infarction (MI) (≥ 6 months prior) or percutaneous coronary intervention (PCI) (≥ 6 months prior), coronary bypass surgery (≥12 months prior), abnormal exercise treadmill test or an area of reversible ischemia on nuclear imaging, pharmacologic stress or stress echocardiography with subsequent revascularization. All subjects were recommended to be on a stable dose of a statin for at least 3 months. Inclusion criteria also included a body mass index (BMI) ≥ 27 kg/m2 or a BMI of 25 to 26.9 with either an increased waist circumference or at least 2 components of the metabolic syndrome which include: triglyceride ≥ 150 mg/dL, high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL if male or < 50 mg/dL if female, glucose ≥ 100 mg/dL or treated hypertension or BP ≥ 130/85 mmHg. Additional inclusion criteria included estimated creatinine clearance as measured by the Cockcroft-Gault equation ≥ 60 ml/min/1.73 m2. Exclusion criteria for CCTA were BMI > 35 kg/m2 (females) or > 40 kg/m2 (males), contraindication to iodinated contrast agents and serum creatinine > 1.5 mg/dL.
2.3. Blood pressure measurements
Subjects were seated quietly for at least 5 minutes in a chair at 60° to 85° with their feet on the floor and the right arm supported at heart level. A cuff bladder encircling at least 80% of the arm was used to ensure accuracy. Measurements of BP were performed using cycling Dinamaps (GE Medical Systems Information Technologies, Inc, Milwaukee, Wis). Two BP readings were obtained at least 30 seconds apart as in the Treatment of Mild Hypertension Study [6]. If there was more than a 5–mmHg difference in SBP between the 2 readings, a third reading was obtained.
2.4. CCTA Imaging protocol
Imaging was performed at Beth Israel Deaconess Medical Center using a 320-row detector scanner (Aquilion ONE, Toshiba Medical Systems, Otawara, Japan) with prospective electrocardiogram gating. The protocol for performance of CCTA, plaque identification and quantification has been previously published [7,8] with image acquisition details and references in Appendix 1 in Supplement 2 of reference 8. Patients were placed in a standard position to enable CT synchronization with the electrocardiogram. Oral or intravenous beta blockade with metoprolol was administered in patients with a heart rate greater than 65 beats per minute to avoid cardiac motion artifacts and assure accurate gated imaging. Sublingual nitroglycerine 0.4 mg was given to all patients just prior to the scan. The starting point of the volume scan and coverage area was cranio-caudally from one centimeter below the tracheal bifurcation to the diaphragm. Prior to the examination, all patients were instructed on quiet breathing and breath holding in order to minimize artifacts during scanning. An intravenous bolus of non-ionic iodinated contrast agent Optiray-350 (70–90 ml) was given at the rate of 4–5 ml per second followed by a bolus of saline. The region of interest was placed over the descending aorta and exposure triggered at 300 Hounsfield units (HU). All patients were imaged at 60–80% of R-R interval using a prospective gating technique. Scanning parameters were determined based upon patient’s weight, height and BMI values. Transaxial images were reconstructed with 0.5 mm slice thickness.
2.5. Coronary plaque identification and quantification
CCTA images underwent 3-dimensional reconstruction for coronary segment plaque volume analysis using semiautomated software (SUREPlaque, version 6.3.2, Vital Images, Minnetonka, MN, USA) [9–12]. Analysis was performed for all patients by using standard axial, maximum intensity and multiplanar reformats. The readers had access to scroll through axial images, to interactively perform multiplanar reconstructions, maximum intensity projections, as well as curved multiplanar reformats for both data sets. Using sculpt tool and various window levels, the main coronary vessels were exposed. The probe feature was then used to quantify plaque in the four major coronary vessels: right coronary artery, left main artery, left anterior descending artery and left circumflex artery. Stents and distal segments with diameter less than 2 mm were not included in analysis, the latter due to limited spatial resolution [13]. Coronary plaque characteristics (fibrous, fatty, and calcified) were analyzed in all patients. Representative images have been previously published (Supplement 2 in reference 8). Segments with prior revascularization or significant calcification causing calcium-bloom artifact were excluded. Noncalcified plaque, the sum of fatty and fibrous plaque, was based on HU densities of fatty (−100 to 49 HU) and fibrous (50 to 150 HU). Calcified plaque was > 150 HU. Plaque volumes were indexed to the length of the plaque lesion; indexed plaque volume was defined as plaque volume (mm3) divided by artery segment length (mm). The coronary plaque thresholds were based on HU density ranges as reported and validated by others comparing CCTA to intravascular ultrasound [10,12–18] and histopathological comparisons with CCTA [19,20]. Thresholds for coronary plaque quantification were preset in the Vitrea analysis tool before performing the analysis. The software analysis tool provides color coding for lumen and the different plaque components and automatically generates total volume and percentage of different plaque components. Calcified plaque usually causes partial volume artifact in quantifying fibrous and fatty plaque. To avoid calcium blooming artifact, manual adjustments were done by redrawing contours in those particular segments. All analyses were performed by two independent readers (blinded to patient identifiers) to assess the inter-observer and tool reproducibility. The average of both readings was used for final analysis.
2.6. Statistical analysis
Categorical variables were expressed as counts and percentages. Normality tests were conducted using the Shapiro-Wilk test. Continuous variables were reported as the mean and SD for normally distributed variables or median and interquartile range [IQR] for non-normally distributed variables. Plaque volumes were not normally distributed and, therefore, were reported as median [IQR].
Plaque volume was stratified by tertile of SBP and DBP. Proportions according to tertiles were compared using a Chi-square test. A p value for trend was measured for plaque volumes across tertiles of SBP and DBP using linear regression. SBP and DBP were also examined as continuous variables. We examined the association between SBP or DBP and volume of plaque subtypes using linear regression. A backward multivariate linear regression was used to adjust for confounding effects. Variables associated with plaque volume with a p <0.1 in the univariate analysis were included in the fully adjusted backward regression models. These variables included age, sex, BMI, history of MI, history of PCI, coronary artery bypass surgery or hypertension, HbA1c, LDL-C, HDL-C, triglyceride, creatinine clearance and medication use (statin, aspirin, angiotensin converting enzyme inhibitor [ACE-I], angiotensin receptor blocker [ARB], hydrochlorothiazide, furosemide, calcium channel blocker and beta blocker). A 2-sided p value <0.05 was considered statistically significant. Data analyses were performed using SPSS 20.0 (IBM Corp. Armonk, NY).
3. Results
A total of 285 subjects underwent a baseline CCTA evaluation and were included in this analysis. Mean (SD) age was 63.1 (7.7), 18.2% were female, and 240 (84.2%) subjects had a diagnosis of hypertension. In the total group, 272 (95.4%) were on statins with a mean (SD) LDL-C level of 78.7 mg/dL (28.5) and median [IQR] triglyceride level of 117 mg/dL [79, 167].
Baseline characteristics according to systolic and diastolic BP tertiles are shown in Table 1 and Table 2, respectively. Those in the highest tertile of SBP were significantly more likely to be older, have a higher BMI and history of hypertension and use calcium channel blockers whereas those in the lowest tertile of SBP were more likely to have a history of MI. As shown in Supplementary Table 1, those with MI were more aggressively treated to a lower SBP compared to those without MI (121 mmHg vs 126 mmHg, respectively; p=0.002), a finding accounting for the higher prevalence of subjects with MI in the lowest tertile of SBP. In regards to DBP, those in the highest DBP tertile were significantly more likely to be male and less likely to have diabetes or be on a statin or furosemide. A significantly higher percent were receiving any antihypertensive drug in the lowest diastolic BP tertile. There were no current smokers.
Table 1.
Baseline characteristics according to tertiles of systolic blood pressure.
| Characteristics | 1st Tertile (≤ 118 mmHg) (n = 95) | 2nd Tertile (119–130 mmHg) (n = 95) | 3rd Tertile (> 130 mmHg) (n = 95) | p for trend |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, mean±SD, y | 61.7±7.6 | 62.8±8.6 | 64.8±6.6 | 0.007 |
| Male sex, No. (%) | 78 (82.1) | 79 (83.2) | 76 (80.0) | 0.708 |
| Inclusion criteria (may have more than one), No. (%) | ||||
| History of MI | 56 (58.9) | 36 (37.9) | 38 (40.0) | 0.009 |
| History of PCI | 62 (65.3) | 58 (61.7) | 56 (58.9) | 0.372 |
| History of CABG | 22 (23.2) | 22 (23.4) | 24 (25.3) | 0.735 |
| Cardiovascular risk factors, No. (%) | ||||
| Hypertension | 66 (69.5) | 89 (93.7) | 85 (89.5) | <0.001 |
| Diabetes | 26 (27.4) | 26 (27.4) | 30 (31.6) | 0.523 |
| Anthropometric and blood pressure, mean±SD | ||||
| Weight, kg | 90.1±14.8 | 90.1±13.8 | 92.8±14.3 | 0.208 |
| Body mass index, kg/m2* | 30.4±3.9 | 30.2±3.1 | 31.3±3.7 | 0.078 |
| Waist circumference, cm | 105.8±10.8 | 105.5±9.6 | 108.6±10.8 | 0.069 |
| Systolic BP, mmHg | 108.9±7.4 | 123.9±3.4 | 139.9±8.2 | NA |
| Diastolic BP, mmHg | 66.4±6.7 | 73.7±7.2 | 78.8±9.9 | <0.001 |
| Pulse pressure, mmHg | 42.5±6.7 | 50.1±7.6 | 61.1±11.4 | <0.001 |
| Complete blood count, mean±SD | ||||
| WBC, 109 cells/L | 6.6±1.7 | 6.7±2.7 | 6.6±1.8 | 0.986 |
| Monocytes, cells/μL | 517.6±158.6 | 512.7±146.4 | 548.6±184.3 | 0.193 |
| Neutrophils, cells/μL | 4193.9±1454.4 | 4109.3±1448.9 | 4233.1±1604.2 | 0.857 |
| Lymphocytes, cells/μL | 1701.2±554.6 | 1894.6±2140.4 | 1672.0±589.0 | 0.879 |
| Platelets, cells/μL | 194.8±52.8 | 187.8±49.2 | 195.2±51.2 | 0.959 |
| Lipids, mean±SD | ||||
| Total cholesterol, mg/dL a | 151.2±32.8 | 147.3±35.9 | 158.9±39.5 | 0.143 |
| Triglyceride, median [IQR], mg/dL b | 116.5 [77.0,166.0] | 122.0 [79.0,167.0] | 112.0 [84.0,168.0] | 0.880 |
| HDL-C, mg/dL a | 46.0±13.0 | 46.6±12.0 | 48.7±17.3 | 0.195 |
| LDL-C, mg/dL a | 78.5±25.7 | 73.7±25.7 | 83.9±32.7 | 0.193 |
| Biochemical profile, mean±SD | ||||
| Glucose, mg/dL c | 107.8±41.0 | 107.1±31.6 | 106.8±31.3 | 0.847 |
| HbA1c, % (mmol/mol) | 6.2±1.1 | 6.2±0.9 | 6.2±.8 | 0.850 |
| Creatinine clearance (mL/min) | 103.0±29.1 | 100.3±24.7 | 100.4±27.9 | 0.512 |
| hs-CRP | 0.8 [0.3, 2.6] | 0.7 [0.4, 1.8] | 0.9 [0.5, 2.6] | 0.728 |
| Medication, No. (%) | ||||
| Statin | 92 (96.8) | 91 (95.8) | 89 (93.7) | 0.299 |
| Aspirin | 92 (96.8) | 89 (93.7) | 92 (96.8) | 1.000 |
| ACE-I | 49 (51.6) | 50 (52.6) | 58 (61.1) | 0.191 |
| ARB | 15 (15.8) | 19 (20.0) | 17 (17.9) | 0.706 |
| Hydrochlorothiazide | 15 (15.8) | 22 (23.2) | 19 (20.0) | 0.467 |
| Furosemide | 7 (7.4) | 11 (11.6) | 7 (7.4) | 1.000 |
| Calcium channel blocker | 16 (16.8) | 20 (21.1) | 32 (33.7) | 0.006 |
| Beta blockers | 69 (72.6) | 71 (74.7) | 66 (69.5) | 0.628 |
| On any antihypertensive medication, No. (%) | 88 (92.6) | 92 (96.8) | 87 (91.6) | 0.288 |
ACE-I = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; BP = blood pressure; CABG = coronary artery bypass grafting; HbA1c = hemoglobin A1c; HDL-C = high density lipoprotein cholesterol; hs-CRP = high-sensitivity C-reactive protein; LDL-C = low density lipoprotein cholesterol; MI = myocardial infarction; PCI = percutaneous coronary intervention; WBC = white blood cell count.
To convert to SI unit (mmol/L), multiply by 0.0259
To convert to SI unit (mmol/L), multiply by 0.0113
To convert to SI unit (mmol/L), multiply by 0.0555
Table 2.
Baseline characteristics according to tertiles of diastolic blood pressure.
| Characteristics | 1st Tertile (≤ 68 mmHg) (n=97) | 2nd Tertile (69–76 mmHg) (n=93) | 3rd Tertile (> 76 mmHg) (n=95) | p for trend |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, mean±SD, y | 63.5±8.1 | 63.7±7.8 | 62.1±7.3 | 0.204 |
| Male sex, No. (%) | 70 (72.2) | 81 (87.1) | 82 (86.3) | 0.011 |
| Inclusion criteria (may have more than one), No. (%) | ||||
| History of MI | 48 (49.5) | 44 (47.3) | 38 (40.0) | 0.189 |
| History of PCI | 55 (56.7) | 63 (67.7) | 58 (61.7) | 0.471 |
| History of CABG | 22 (22.7) | 28 (30.1) | 18 (19.1) | 0.579 |
| Cardiovascular risk factors, No. (%) | ||||
| Hypertension | 79 (81.4) | 78 (83.9) | 83 (87.4) | 0.262 |
| Diabetes | 40 (41.2) | 22 (23.7) | 20 (21.1) | 0.002 |
| Anthropometric and blood pressure, mean±SD | ||||
| Weight, kg | 88.8±14.6 | 90.5±15.1 | 93.7±12.9 | 0.019 |
| Body mass index, kg/m2* | 30.6±3.7 | 30.3±3.5 | 31.0±3.6 | 0.417 |
| Waist circumference, cm | 105.9±10.7 | 106.5±10.9 | 107.5±9.8 | 0.290 |
| Systolic BP, mmHg | 115.0±13.6 | 125.1±11.9 | 132.7±11.4 | <0.001 |
| Diastolic BP, mmHg | 63.3±4.4 | 72.3±2.2 | 83.6±6.1 | NA |
| Pulse pressure, mmHg | 51.8±12.9 | 52.9±12.0 | 49.1±9.7 | 0.122 |
| Complete blood count, mean±SD | ||||
| WBC, 109 cells/L | 6.8±1.8 | 6.8±2.8 | 6.4±1.6 | 0.240 |
| Monocytes, cells/μL | 532.6±175.7 | 511.4±145.6 | 534.5±169.5 | 0.941 |
| Neutrophils, cells/μL | 4291.6±1570.9 | 4249.0±1534.6 | 3994.8±1385.1 | 0.172 |
| Lymphocytes, cells/μL | 1722.4±619.9 | 1877.2±2157.3 | 1671.6±543.2 | 0.795 |
| Platelets, cells/μL | 192.8±50.2 | 187.5±52.0 | 197.3±50.9 | 0.547 |
| Lipids, mean±SD | ||||
| Total cholesterol, mg/dL a | 150.0±38.7 | 148.6±30.9 | 158.8±38.3 | 0.095 |
| Triglyceride, median [IQR], mg/dL b | 117.0 [79.0, 167.5] | 111.0 [77.0, 161.0] | 124.0 [83.0, 175.0] | 0.970 |
| HDL-C, mg/dL a | 46.2±14.1 | 47.8±15.0 | 47.3±13.9 | 0.617 |
| LDL-C, mg/dL a | 76.5±29.8 | 75.4±22.7 | 84.1±31.4 | 0.065 |
| Biochemical profile, mean±SD | ||||
| Glucose, mg/dL c | 112.4±38.2 | 106.4±39.9 | 102.7±23.6 | 0.052 |
| HbA1c, % (mmol/mol) | 6.3±1.1 | 6.1±1.0 | 6.1±.8 | 0.063 |
| Creatinine clearance (mL/min) | 99.0±31.4 | 102.2±27.1 | 102.6±22.5 | 0.363 |
| hs-CRP | 0.9 [0.4, 3.2] | 0.6 [0.4, 1.5] | 0.7 [0.3, 2.3] | 0.529 |
| Medication, No. (%) | ||||
| Statin | 95 (97.9) | 91 (97.8) | 86 (90.5) | 0.014 |
| Aspirin | 94 (96.9) | 85 (91.4) | 94 (98.9) | 0.493 |
| ACE-I | 56 (57.7) | 49 (52.7) | 52 (54.7) | 0.675 |
| ARB | 19 (19.6) | 16 (17.2) | 16 (16.8) | 0.620 |
| Hydrochlorothiazide | 19 (19.6) | 22 (23.7) | 15 (15.8) | 0.514 |
| Furosemide | 14 (14.4) | 7 (7.5) | 4 (4.2) | 0.012 |
| Calcium channel blocker | 22 (22.7) | 23 (24.7) | 23 (24.2) | 0.803 |
| Beta blockers | 78 (80.4) | 63 (67.7) | 65 (68.4) | 0.063 |
| On any antihypertensive medication, No. (%) | 96 (99.0) | 85 (91.4) | 86 (90.5) | 0.030 |
ACE-I = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; BP = blood pressure; CABG = coronary artery bypass grafting; HbA1c = hemoglobin A1c; HDL-C = high density lipoprotein cholesterol; LDL-C = low density lipoprotein cholesterol; MI = myocardial infarction; PCI = percutaneous coronary intervention; WBC = white blood cell count.
To convert to SI unit (mmol/L), multiply by 0.0259
To convert to SI unit (mmol/L), multiply by 0.0113
To convert to SI unit (mmol/L), multiply by 0.0555
Table 3 shows median plaque volumes according to systolic and diastolic BP tertiles. The Intra-observer and Inter-observer agreement indexes for coronary plaque measurements were 0.99 and 0.98, respectively, showing excellent correlation between readings. When examined by tertiles of SBP, there was no significant difference in any of the plaque subtypes. Of note, SBP (mean ± SD) ranged from 108.9±7.4 in the lowest SBP tertile to 139.9±8.2 mmHg in the highest SBP tertile (Table 1). In contrast, when examined by tertiles of DBP, the higher the tertile, the higher the plaque volume. For example, compared to subjects in the highest DBP tertile (> 76 mmHg ), subjects in the lowest DBP tertile (≤ 68 mmHg) had lower volumes of fatty: 10.0 vs. 7.7 mm3/mm segment length, respectively, (p for trend=0.042), fibrous plaque: 19.6 vs. 13.8 mm3/mm segment length, respectively, (p for trend=0.011), noncalcified: 29.7 vs. 22.5 mm3/mm segment length, respectively, (p for trend=0.017), calcified plaque: 5.4 vs. 4.2 mm3/mm segment length, respectively, (p for trend=0.054) and total plaque: 37.8 vs. 25.1 mm3/mm segment length, respectively, (p for trend=0.010).
Table 3.
Comparison of indexed plaque volume components stratified by tertiles of systolic and diastolic blood pressure
| Systolic blood pressure tertiles | ||||
| Plaque type a | 1st Tertile (≤ 118 mmHg) Median [IQR] | 2nd Tertile (119–130 mmHg) Median [IQR] | 3rd Tertile (> 130 mmHg) Median [IQR] | p value for trend |
| Fatty | 9.3 [5.3,13.7] | 8.8 [4.8, 14.0] | 9.2 [4.9, 14.3] | 0.812 |
| Fibrous | 16.5 [9.4, 24.3] | 15.1 [8.3, 23.6] | 17.1 [9.6, 24.8] | 0.758 |
| Noncalcified | 25.4 [15.0, 35.9] | 23.7 [13.6, 37.5] | 26.9 [14.3, 38.9] | 0.775 |
| Calcified | 4.6 [2.1, 6.8] | 3.9 [1.0, 8.8] | 4.2 [2.0, 8.6] | 0.690 |
| Total | 30.0 [18.1, 43.8] | 29.3 [15.1, 45.8] | 30.9 [17.9, 46.0] | 0.645 |
| Diastolic blood pressure tertiles | ||||
| Plaque type a | 1st Tertile (≤ 68 mmHg) Median [IQR] | 2nd Tertile (69–76 mmHg) Median [IQR] | 3rd Tertile (> 76 mmHg) Median [IQR] | p value for trend |
| Fatty | 7.7 [5.1,12.9] | 9.0 [4.4,13.7] | 10.0 [6.1,14.9] | 0.042 |
| Fibrous | 13.8 [9.0,20.9] | 16.5 [8.3,24.5] | 19.6 [10.1,25.8] | 0.011 |
| Noncalcified | 22.5 [14.3,33.5] | 25.0 [12.9,37.2] | 29.7 [16.4,39.6] | 0.017 |
| Calcified | 4.2 [2.1,7.3] | 3.6 [1.3,6.7] | 5.4 [1.8,9.7] | 0.054 |
| Total | 25.1 [17.2,42.0] | 30.6 [16.9,43.8] | 37.8 [20.1,47.0] | 0.010 |
Plaque volume expressed as mm3 per mm artery segment length
When examined as a continuous variable, in both the univariate and fully adjusted regression models, SBP did not predict the volume of any of the plaque subtypes or total plaque volume (Table 4). The SBP ranged from 95 mmHg to 154 mmHg for 95% of the subjects. On the other hand, DBP predicted volume of all plaque components in the univariate regression model (Table 4). After multivariate adjustment for subject characteristics (Table 4), higher DBP was a significant independent predictor of plaque subtypes: for every 1 mmHg increase in DBP, fibrous plaque increased 0.128 mm3/mm (95% CI: 0.019 to 0.237: p=0.022), noncalcified plaque increased 0.176 mm3/mm (95% CI: 0.004 to 0.348; p=0.045), calcified plaque increased 0.096 mm3/mm (95% CI: 0.039 to 0.152; p=0.001) and total plaque increased 0.249 mm3/mm (95% CI: 0.041 to 0.457; p=0.019). The fully adjusted models for SBP and DBP for total plaque volume are shown in Table 5. Of note, LDL-C did not predict plaque volume in the univariate or multivariate models for either SBP or DBP. The p-values for LDL-C in the univariate model were 0.557 for fatty plaque, 0.594 for fibrous plaque, 0.576 for noncalcified plaque, 0.453 for calcified plaque and 0.603 for total plaque. There was no association between pulse pressure and volume of any of the plaque subtypes.
Table 4.
Systolic and diastolic blood pressures as predictors of plaque volume as a continuous variable.
| Systolic blood pressure | ||||||
| Univariable | Multivariable (fully adjusted) | |||||
| β | 95% CI | p | β | 95% CI | p | |
| Fatty plaque | 0.007 | (−0.041 to 0.054) | 0.781 | 0.010 | (−0.032 to 0.052) | 0.636 |
| Fibrous plaque | 0.022 | (−0.059 to 0.103) | 0.598 | 0.030 | (−0.042 to 0.102) | 0.408 |
| Noncalcified plaque | 0.028 | (−0.099 to 0.155) | 0.660 | 0.040 | (−0.072 to 0.152) | 0.478 |
| Calcified plaque | 0.006 | (−0.033 to 0.044) | 0.764 | 0.003 | (−0.034 to 0.041) | 0.857 |
| Total plaque | 0.045 | (−0.108 to 0.198) | 0.564 | 0.052 | (−0.084 to 0.188) | 0.451 |
| Diastolic blood pressure | ||||||
| Univariable | Multivariable (fully adjusted) | |||||
| β | 95% CI | p | β | 95% CI | p | |
| Fatty plaque | 0.089 | (0.018 to 0.159) | 0.014 | 0.029 | (−0.036 to 0.094) | 0.381 |
| Fibrous plaque | 0.192 | (0.072 to 0.312) | 0.002 | 0.128 | (0.019 to 0.237) | 0.022 |
| Noncalcified plaque | 0.281 | (0.092 to 0.469) | 0.004 | 0.176 | (0.004 to 0.348) | 0.045 |
| Calcified plaque | 0.080 | (0.023 to 0.137) | 0.006 | 0.096 | (0.039 to 0.152) | 0.001 |
| Total Plaque | 0.371 | (0.145 to 0.598) | 0.001 | 0.249 | (0.041 to 0.457) | 0.019 |
Table 5.
Fully adjusted models for systolic and diastolic blood pressure for total plaque volume.
| Systolic blood pressure | |||
| β | p value | 95% C.I. | |
| Constant | 53.644 | <0.001 | (25.735 to 81.552) |
| Systolic blood pressure | 0.052 | 0.451 | (−0.084 to 0.188) |
| Age | −0.283 | 0.033 | (−0.543 to −0.024) |
| Gender | −0.782 | 0.786 | (−6.443 to 4.879) |
| History of CABG | −16.665 | <0.001 | (−21.326 to −12.004) |
| Weight | 0.224 | 0.005 | (0.067 to 0.382) |
| Neutrophils | −0.002 | 0.021 | (−0.003 to 0.000) |
| HbA1c | −3.352 | 0.001 | (−5.406 to −1.297) |
| On hydrochlorothiazide | 4.728 | 0.059 | (−0.177 to 9.632) |
| Diastolic blood pressure | |||
| β | p value | 95% C.I. | |
| Constant | 50.740 | 0.001 | (19.823 to 81.656) |
| Diastolic blood pressure | 0.249 | 0.019 | (0.041 to 0.457) |
| Age | −0.139 | 0.305 | (−0.404 to 0.127) |
| Gender | 0.492 | 0.864 | (−5.164 to 6.149) |
| History of myocardial infarction | −3.989 | 0.042 | (−7.836 to −0.142) |
| History of CABG | −16.248 | <0.001 | (−20.797 to −11.699) |
| Hypertension | −6.349 | 0.022 | (−11.788 to −0.910) |
| Weight | 0.451 | 0.001 | (0.192 to 0.710) |
| Waist circumference | −0.362 | 0.032 | (−0.693 to −0.031) |
| Neutrophils | −0.001 | 0.078 | (−0.002 to 0.000) |
| HbA1c | −2.543 | 0.015 | (−4.594 to −0.493) |
| On Hydrochlorothiazide | 6.198 | 0.014 | (1.269 to 11.128) |
CABG = coronary artery bypass grafting; HbA1c = hemoglobin A1c
The analysis was repeated for men only. Supplementary Tables 2 and 3 describe similar results for the tertile analysis (Supplementary Table 2) and multivariate analysis (Supplementary Table 3) as shown for the total group of men and women in Tables 3 and 4.
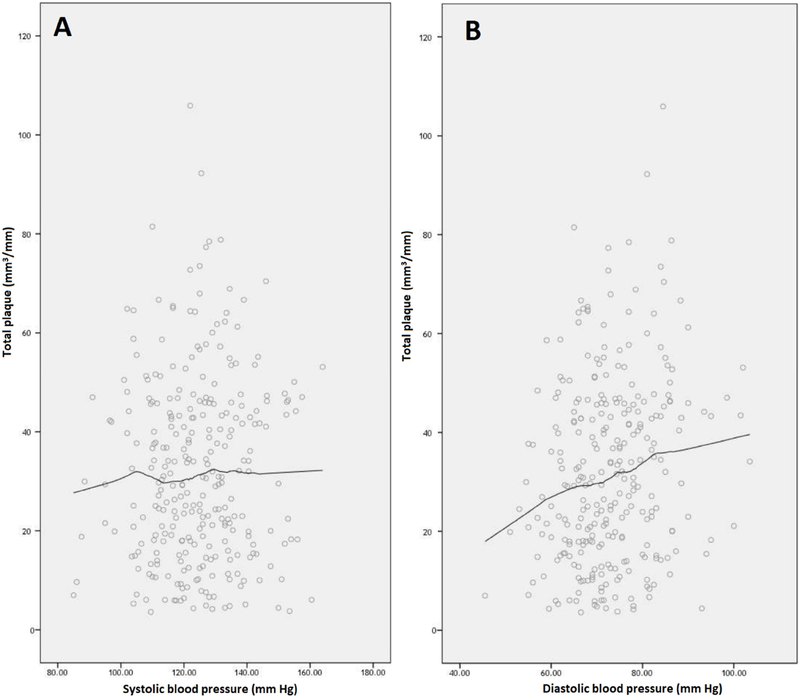
To determine if a DBP < 68 mmHg, which is the lowest tertile, provided additional benefit on plaque volume, SBP and DBP were plotted versus plaque volume. Locally weighted scatterplot smoothing was used to generate non-linear regression lines. For DBP, Fig. 1A shows that plaque volume continued to decrease as DBP decreased well below 68 mmHg. In contrast, the graph for SBP (Fig. 1B) was flat. Supplementary Figures 1 to 4 show similar findings for fatty, fibrous, noncalcified and calcified plaque. These graphs illustrate a dramatic difference in the effect of DBP versus SBP on plaque volume.
Fig. 1.
Blood pressure.
Systolic blood pressure (A) and diastolic blood pressure (B) graphed versus indexed total plaque volume (mm3/mm).
4. Discussion
In the current study, our results using CCTA show a graded increase in coronary plaque volume as DBP tertile increased above 68 mmHg in patients with stable CAD whereas plaque volume did not differ by tertile of SBP. Similar significant results were observed for DBP when examined as a continuous variable and fully adjusted for subject characteristics including LDL-C, again demonstrating a strong direct association between DBP and all plaque components while SBP in the range of 95 mmHg to 154 mmHg (for 95% of the subjects) was not associated with plaque volume. Our findings suggest that level of DBP is an important factor in determining plaque volume whereas SBP in the range in the current study is not. A DBP tertile ≤ 68 mmHg was associated with the least amount of coronary plaque volume in these subjects with well controlled LDL-C < 80 mg/dL. Moreover, plaque volume continued to decrease for all plaque types as DBP decreased well below 68 mmHg. In contrast, the graph for SBP was flat. These results illustrate a dramatic difference in the effect of DBP vs SBP on plaque volume. SBP was well-controlled in the majority of our subjects. These findings suggest that in the setting of well-controlled SBP, DBP remains critically important in affecting plaque volume.
The reason for our finding of a graded, direct relationship between DBP and plaque volume, but not between SBP, is unclear. A significantly higher percent were receiving any antihypertensive drug in the lowest DBP tertile, a finding accounting for the lower DBP. Whether the type of antihypertensive treatment affects plaque volume is unknown; however, the only difference in type of antihypertensive medication was use of furosemide. There is no current evidence to support that furosemide may affect plaque volume. Subjects in the lowest tertile for DBP also had a significantly higher prevalence of diabetes. The reason for this difference is unclear but could perhaps be related to more aggressive treatment of blood pressure in subjects with diabetes. Another potential explanation is through the effect of diabetes on arterial stiffness. Diabetes leads to an increase in arterial stiffness which in turn lowers diastolic blood pressure [21]. Diabetes also causes capillary rarefaction in the small arteries and increases the reflected pulse wave causing a wider pulse pressure and thus lower DBP.
Another explanation that could be hypothesized to account for differences in effect of SBP versus DBP could be due to the fact that flow in the coronary artery occurs mainly during diastole. Higher DBP may be associated with increased coronary flow and alteration in shear stress leading to an increased number of areas with non-laminar flow and the activation of inflammatory pathways leading to plaque formation and growth [22]. Endothelial shear stress can be estimated from CCTA using computational fluid dynamics with sophisticated software [23–25]; therefore, future studies could examine this hypothesis further.
Several prior studies have examined the relationship between BP and volume of coronary plaque. In a cross-sectional study using electron beam computed tomography (EBCT) of subjects with an average age of 40 years in the Rochester Family Heart Study, after adjustment for sex and age, ambulatory systolic and diastolic BP levels were predictive of coronary artery calcification (CAC); however, after additional adjustment for office BP, only the ambulatory DBP level was an independent predictor of CAC whereas SBP was not [26]. Moreover, hypertension was the most important independent risk factor for the presence of CAC and more important than diabetes and hyperlipidemia. In the Muscatine study of 384 subjects ages 20–34 years, in multivariate analysis, DBP independently predicted CAC at ages 29 to 37 years whereas SBP and LDL-C did not predict [27]. In a study of 330 patients examined with intravascular ultrasound, baseline DBP independently predicted an increase in % atheroma volume at 1-year follow-up in the culprit artery [28]. The authors concluded that lowering DBP may retard progression of atherosclerosis and thus reduce CVD events. In a cross-sectional analysis of 100 patients with stable angina using intravascular ultrasound, Iwata et al. [29] reported that DBP predicted plaque volume, but LDL-C was a better predictor. Of note, mean LDL-C levels were 107 ± 30 mg/dL in their study. These findings are in contrast to ours where DBP was a better predictor than LDL-C. LDL-C may not have predicted in our study due to the fact that LDL-C levels were < 80 mg/dL (mean [SD] 78.7±28.5 mg/dL). These low LDL-C levels allowed us to examine factors contributing to plaque volume independently of cholesterol. Our study also differs from that of Iwata et al. [29] in that we used CCTA whereas they used intravascular ultrasound. A limitation of intravascular ultrasound is that it is limited to examining the culprit artery in patients with symptomatic CAD who are undergoing invasive cardiac catheterization in most studies. Therefore, intravascular ultrasound does not provide information on the entire coronary tree whereas CCTA does. To our knowledge, the current study is the first to report the effect of systolic vs. diastolic BP on coronary plaque volume measured by CCTA in all coronary arteries.
Because a J-shaped relation between BP and CVD events has been observed in the past, the threshold for DBP has been widely debated. In the Systolic Blood Pressure Intervention Trial (SPRINT), subjects with hypertension were randomized to an intensive strategy of lowering SBP < 120 mmHg versus a standard strategy of lowering SBP < 140 mmHg [30]. Those achieving a SBP < 120 mmHg in the intensively treated arm had a significantly lower rate of CVD events and all-cause mortality compared to those with SBP < 140 mmHg in the standard treatment arm [30]. In the intensive treatment arm, the DBP was lowered from a mean of 78.2 mmHg to a mean of 68.7 mmHg, a BP similar to the lowest DBP tertile in our analysis which showed the least amount of coronary plaque volume. In SPRINT, a diastolic threshold of < 55 mmHg was associated with increased cardiovascular events in both patients with and without cardiovascular disease [31]. The hazard ratios (95% CI) of DBP < 55 mmHg versus 55 to 90 mmHg were 1.68 (1.16–2.43, p=0.006) and 1.52 (0.99–2.34), p=0.06 in those without and with CVD, respectively [31]. Thus, the SPRINT results identify a DBP threshold – 55 mmHg – below which increased risk of CVD occurs. Concern has been raised about an increased incidence of dizziness, falls, hypotension and syncope with aggressive BP lowering. In the SPRINT trial, those patients achieving a SBP < 120 mmHg (mean DBP of 68 mmHg ) had similar outcome measures as assessed by the Physical Component Summary and Mental Component Summary of the Veterans RAND 12-Item Health Survey, the Patient Health Questionnaire 9-item depression scale, patient-reported satisfaction with their BP care and BP medications, and adherence to BP medications as compared to those who received standard treatment (target BP < 140 mmHg ) including among those with decreased physical or cognitive function [32]. Therefore, in addition to being associated with fewer CVD events, the BP levels in the intensive treatment arm in SPRINT were well-tolerated [32]. Thus, the SPRINT data suggest that lowering SBP to 120 mmHg, with a DBP down to 55 mmHg, may be safely achieved. In our study, those in the lowest DBP tertile had no dizziness, falls, hypotension or syncope. Taken together with the SPRINT data, DBP between 55 to 68 mmHg may be safely achieved and may be associated with the least amount of coronary plaque.
Several studies assessing coronary plaque subtypes with CCTA have shown that higher volume of noncalcified plaque and total plaque are associated with higher rates of cardiac death, MI and coronary revascularization [33] and higher rates of acute coronary syndrome [34,35]. Furthermore, evidence from intravascular ultrasound studies shows that progression of plaque atheroma volume is independently associated with higher rates of a composite of cardiac death, MI and coronary revascularization (p <0.002) and regression is associated with fewer events [36]. Since plaque volume has been shown to be associated with cardiovascular mortality, our findings further the field by demonstrating a potential mechanism - lower coronary plaque volume - by which DBP reduction lowers cardiovascular mortality. Thus, lowering DBP to 68 mmHg, the mean level for those with SBP < 120 mmHg which showed the most optimal outcome in SPRINT, may be beneficial in preventing CVD events due to lower plaque volume and prevention of plaque rupture. Taken together, these findings suggest that plaque composition and volume predict CVD events and support the potential clinical importance of lower amounts of coronary plaque at DBP ≤ 68 mmHg.
Limitations of the study include the small number of subjects; thus, the results are hypothesis generating. Our subjects have clinical coronary artery disease; therefore, the results may be limited to this population.
In conclusion, a graded increase in coronary plaque volume occurred as DBP tertile increased whereas plaque volume did not differ by SBP tertile. Similar results were observed when DBP and SBP were examined as continuous variables. Our findings suggest that level of DBP is an important factor in determining plaque volume whereas SBP in the range in the current study is not. Therefore, one would predict that maintaining a DBP in the lowest tertile (≤ 68 mmHg ) would limit coronary plaque formation the most in those with LDL-C < 80 mg/dL.
Supplementary Material
Highlights.
Coronary plaque volume was measured by coronary computed tomographic angiography
Coronary plaque volume was higher at higher levels of diastolic blood pressure
In contrast, systolic blood pressure did not predict coronary plaque volume
Diastolic blood pressure ≤ 68 mm Hg had the lowest coronary plaque volume
Reduction of diastolic blood pressure to ≤ 68 mm Hg may prevent plaque progression
Acknowledgments
We thank the study subjects for their participation.
Financial support
This work was supported by a National Heart, Lung, and Blood Institute (NHLBI) Specialized Centers of Clinically Oriented Research (SCCOR) program grant to Dr. Welty: P50 HL083813 and supported by the Harvard Clinical and Translational Science Center Award, NIH UL1 TR001102.
Footnotes
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 131 (2015) e29–322. [DOI] [PubMed] [Google Scholar]
- [2].Lewington S, Clarke R, Qizilbash N, Peto R, Collins R: Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 360 (2002) 1903–1913. [DOI] [PubMed] [Google Scholar]
- [3].Neaton JD, Wentworth D. For the Multiple Risk Factor Intervention Trial research group. Serum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease overall findings and differences by age for 316099 white men. Arch Intern Med. 152 (1992) 56–64. [PubMed] [Google Scholar]
- [4].Elajami TK, Alfaddagh A, Lakshminarayan D, Soliman M, Chandnani M, Welty FK. Eicosapentaenoic and docosahexaenoic acids attenuate progression of albuminuria in patients with type 2 diabetes and coronary artery disease. J Am Heart Assoc. 6 (7) (2017). pii: e004740. doi: 10.1161/JAHA.116.004740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Alfaddagh A, Elajami T, Ashfaque H, Saleh M, Bistrian BR, Welty FK. Effect of Eicosapentaenoic and Docosahexaenoic Acids Added to Statin Therapy on Coronary Artery Plaque in Patients with Coronary Artery Disease: A Randomized Clinical Trial. J Am Heart Assoc. 6 (12) (2017) pii: e006981. doi: 10.1161/JAHA.117.006981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liebson PR, Grandits G, Prineas R, et al. Echocardiographic correlates of left ventricular structure among 844 mildly hypertensive men and women in the Treatment of Mild Hypertension Study (TOMHS). Circulation. 87 (1993) 476–486. [DOI] [PubMed] [Google Scholar]
- [7].Khosa F, Khan AN, Nasir K, et al. Comparison of coronary plaque subtypes in male and female patients using 320-row MDCTA. Atherosclerosis. 226 (2013) 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hauser TH, Salastekar N, Schaefer EJ, et al. Targeting Inflammation Using Salsalate in Cardiovascular Disease (TINSAL-CVD) study team. Effect of targeting inflammation with salsalate: The TINSAL-CVD randomized clinical trial on progression of coronary plaque in overweight and obese patients using statins. JAMA Cardiol. 1 (2016) 413–423. [DOI] [PubMed] [Google Scholar]
- [9].Rinehart S, Vazquez G, Qian Z, Murrieta L, Christian K, Voros S. Quantitative measurements of coronary arterial stenosis, plaque geometry, and composition are highly reproducible with a standardized coronary arterial computed tomographic approach in high-quality CT datasets. J Cardiovasc Comput Tomogr. 5 (2011) 35–43. [DOI] [PubMed] [Google Scholar]
- [10].Brodoefel H, Burgstahler C, Sabir A, et al. Coronary plaque quantification by voxel analysis: dual-source MDCT angiography versus intravascular sonography. Am J Roentgenol. 192 (2009) W84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Voros S, Rinehart S, Qian Z, et al. Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. J Am Coll Cardiol. 4 (2011) 537–548. [DOI] [PubMed] [Google Scholar]
- [12].Brodoefel H, Burgstahler C, Heuschmid M, et al. Accuracy of dual-source CT in the characterisation of non-calcified plaque: use of a colour-coded analysis compared with virtual histology intravascular ultrasound. Br J Radiol. 82 (2009) 805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Knez A, Becker CR, Leber A, et al. Usefulness of multislice spiral computed tomography angiography for determination of coronary artery stenoses. Am J Cardiol. 88 (2001) 1191–1194. [DOI] [PubMed] [Google Scholar]
- [14].Leber AW, Knez A, Becker A, et al. Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques: a comparative study with intracoronary ultrasound. J Am Coll Cardiol. 43 (2004) 1241–1247. [DOI] [PubMed] [Google Scholar]
- [15].Schroeder S, Kopp AF, Baumbach A, et al. Noninvasive detection and evaluation of atherosclerotic coronary plaques with multislice computed tomography. J Am Coll Cardiol. 37 (2001) 1430–1435. [DOI] [PubMed] [Google Scholar]
- [16].Iriart X, Brunot S, Coste P, et al. Early characterization of atherosclerotic coronary plaques with multidetector computed tomography in patients with acute coronary syndrome: a comparative study with intravascular ultrasound. Eur Radiol. 17 (2007) 2581–2588. [DOI] [PubMed] [Google Scholar]
- [17].Pohle K, Achenbach S, Macneill B, et al. Characterization of non-calcified coronary atherosclerotic plaque by multi-detector row CT: comparison to IVUS. Atherosclerosis. 190 (2007) 174–180. [DOI] [PubMed] [Google Scholar]
- [18].Sun J, Zhang Z, Lu B, et al. Identification and quantification of coronary atherosclerotic plaques: a comparison of 64-MDCT and intravascular ultrasound. AJR Am J Roentgenol. 190 (2008) 748–754. [DOI] [PubMed] [Google Scholar]
- [19].Estes JM, Quist WC, Lo Gerfo FW, Costello P. Noninvasive characterization of plaque morphology using helical computed tomography. J Cardiovasc Surg (Torino). 39 (1998) 527–534. [PubMed] [Google Scholar]
- [20].Becker CR, Nikolaou K, Muders M, et al. Ex vivo coronary atherosclerotic plaque characterization with multi-detector-row CT. Eur Radiol. 13 (2003) 2094–2098. [DOI] [PubMed] [Google Scholar]
- [21].Smulyan H, Lieber A, Safar ME. Hypertension, Diabetes Type II, and Their Association: Role of Arterial Stiffness. Am. J. Hypertens. 29 (1) (2016) 5–13. [DOI] [PubMed] [Google Scholar]
- [22].Nigro P, Abe J, Berk BC. Flow shear stress and atherosclerosis: a matter of site specificity. Antioxid. Redox Signal. 15 (2011) 1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Choi G, Lee JM, Kim HJ, et al.Coronary Artery Axial Plaque Stress and its Relationship With Lesion Geometry: Application of Computational Fluid Dynamics to Coronary CT Angiography. JACC Cardiovasc. Imaging 8(10) (2015)1156–66. doi: 10.1016/j.jcmg.2015.04.024. Epub 2015 Sep 9. [DOI] [PubMed] [Google Scholar]
- [24].Chaichana T, Sun Z, Jewkes J. Investigation of the haemodynamic environment of bifurcation plaques within the left coronary artery in realistic patient models based on CT images. Australas Phys Eng Sci Med. 35 (2) (2012) 231–236. [DOI] [PubMed] [Google Scholar]
- [25].Katranas SA, Kelekis AL, Antoniadis AP et al. , Non-invasive assessment of endothelial shear stress and coronary stiffness using multislice computed tomography. Int J Cardiol 152 (2) (2011):281–284. doi: 10.1016/j.ijcard.2011.08.032. Epub 2011 Sep 6. [DOI] [PubMed] [Google Scholar]
- [26].Turner ST, Bielak LF, Narayana AK, Sheedy PF, Schwartz GL, Peyser PA. Ambulatory blood pressure and coronary artery calcification in middle-aged and younger adults. Am J Hypertens. 15 (2002) 518–524. [DOI] [PubMed] [Google Scholar]
- [27].Mahoney LT, Burns TL, Stanford W, et al. Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: the Muscatine Study. J Am Coll Cardiol. 27 (1996) 277–284. [DOI] [PubMed] [Google Scholar]
- [28].García-García HM, Klauss V, Gonzalo N, et al. Relationship between cardiovascular risk factors and biomarkers with necrotic core and atheroma size: a serial intravascular ultrasound radiofrequency data analysis. Int J Cardiovasc Imaging. 28 (2012) 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Iwata A, Miura S, Mori K, Kawamura A, Nishikawa H, Saku K. Associations between metabolic factors and coronary plaque growth or arterial remodeling as assessed by intravascular ultrasound in patients with stable angina. Hypertens Res. 31 (2008) 1879–1886. [DOI] [PubMed] [Google Scholar]
- [30].Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. SPRINT Research Group. N Engl J Med. 373 (2015) 2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Khan NA, Rabkin SW, Zhao Y, et al. Effect of Lowering Diastolic Pressure in Patients With and Without Cardiovascular Disease: Analysis of the SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension. 71 (5) (2018) 840–847. doi: 10.1161/HYPERTENSIONAHA.117.10177. Epub 2018 Mar 26. [DOI] [PubMed] [Google Scholar]
- [32].Berlowitz DR, Foy CG, Kazis LE, et al. SPRINT research group. Effect of intensive blood-pressure treatment on patient-reported outcomes. N Engl J Med. 377 (2017) 733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nadjiri J, Hausleiter J, Jähnichen C, et al. Incremental prognostic value of quantitative plaque assessment in coronary CT angiography during 5 years of follow up. J Cardiovasc Comput Tomogr. 10 (2016) 97–104. [DOI] [PubMed] [Google Scholar]
- [34].Versteylen MO, Kietselaer BL, Dagnelie PC, et al. Additive value of semiautomated quantification of coronary artery disease using cardiac computed tomographic angiography to predict future acute coronary syndrome. J Am Coll Cardiol 61 (2013) 2296–2305. [DOI] [PubMed] [Google Scholar]
- [35].Motoyama S, Sarai M, Harigaya H,et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 54 (2009) 49–57. [DOI] [PubMed] [Google Scholar]
- [36].Nicholls SJ, Hsu A, Wolski K, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol. 55 (2010) 2399–2407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.