Abstract
Objective
The aim of this study was to analyze the clinical, radiologic, and biological features associated with human herpesvirus 6 (HHV-6) encephalitis in immunocompetent and immunocompromised hosts to establish which clinical settings should prompt HHV-6 testing.
Methods
We performed a retrospective research in the virology database of Fondazione IRCCS Policlinico San Matteo (Pavia, Italy) for all patients who tested positive for HHV-6 DNA in the CSF and/or in blood from January 2008 to September 2018 and separately assessed the number of patients meeting the criteria for HHV-6 encephalitis in the group of immunocompetent and immunocompromised hosts.
Results
Of the 926 patients tested for HHV-6 during the period of interest, 45 met the study criteria. Among immunocompetent hosts (n = 17), HHV-6 encephalitis was diagnosed to 4 infants or children presenting with seizures or mild encephalopathy during primary HHV-6 infection (CSF/blood replication ratio <<1 in all cases). Among immunocompromised hosts (n = 28), HHV-6 encephalitis was diagnosed to 7 adolescents/adults with hematologic conditions presenting with altered mental status (7/7), seizures (3/7), vigilance impairment (3/7), behavioral changes (2/7), hyponatremia (2/7), and anterograde amnesia (1/7). Initial brain MRI was altered only in 2 patients, but 6 of the 7 had a CSF/blood replication ratio >1.
Conclusions
The detection of a CSF/blood replication ratio >1 represented a specific feature of immunocompromised patients with HHV-6 encephalitis and could be of special help to establish a diagnosis of HHV-6 encephalitis in hematopoietic stem cell transplant recipients lacking radiologic evidence of limbic involvement.
Human herpesvirus 6 (HHV-6) is a ubiquitous herpesvirus that commonly infects children younger than 3 years.1 Primary infection sometimes causes exanthema subitum, a common exanthematic disease among infants that may be accompanied by neurologic manifestations such as febrile seizures and encephalitis.2–4
The ability of HHV-6 to cause encephalitis in older children and adults remains debated. Although, indeed, the literature reports some cases of HHV-6 encephalitis in immunocompetent adults, in those cases, the detection of HHV-6 DNA in the CSF more likely reflects chromosomal integration rather than active CNS infection.5 About 1% of healthy individuals harbor in fact sequences of HHV-6 DNA integrated in their genome,6 highlighting the need to be extremely careful in the interpretation of the results of PCR testing.
Similarly to other herpesviruses, HHV-6 is able to establish lifelong latency in the host following primary infection and reactivate in the event of immune suppression.1 HHV-6 reactivation is especially common following hematopoietic stem cell transplantation (HSCT), resulting in delayed engraftment,7 fever, rash, hepatitis, pneumonitis, and encephalitis.1 HHV-6 encephalitis is typical of the early posttransplantation period and has been classically described as a limbic encephalitis.8 Nonetheless, some reports suggest that the spectrum of neurologic manifestations associated with HHV-6 in HSCT recipients might be much broader.9
The lack of clarity on the phenotypes associated with HHV-6 encephalitis in immunocompetent and immunocompromised individuals has hampered to reach a consensus on the criteria that should guide HHV-6 testing and interpretation in immunocompetent and immunocompromised hosts, with a high risk of misdiagnosis and a waste of economic resources. In this study, we analyzed a large cohort of patients who tested positive for HHV-6 in the CSF and/or in blood with the aim to define the phenotypes associated with HHV-6 encephalitis in immunocompetent and immunocompromised individuals and pinpoint which clinical settings should prompt HHV-6 testing.
Methods
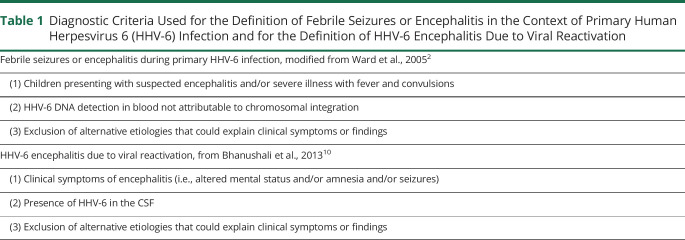
We performed a retrospective research in the virology database of Fondazione IRCCS Policlinico San Matteo (Pavia, Italy) for all patients tested for HHV-6 DNA in the CSF using standard PCR assays from January 2008 to September 2018. The medical records from all patients who tested positive for HHV-6 DNA in the CSF and/or in whole blood during the period of interest were reviewed by a trained team of physicians. Patients with insufficient clinical information, patients without neurologic symptoms or tested more than 15 days after neurologic symptom onset were excluded from the study. We separately assessed the number of patients meeting the criteria for febrile seizures/encephalitis in the context of primary HHV-6 infection2 and for HHV-6 encephalitis due to viral reactivation10 (table 1) in the group of immunocompetent and immunocompromised hosts. Hair follicle testing was performed in all patients who tested positive for HHV-6 DNA in whole blood and CSF and who had viral loads in blood exceeding 106 copies/mL.11 Patients who tested positive for HHV-6 DNA on hair follicles were considered as having chromosomally integrated HHV-6 (ciHHV-6).11
Table 1.
Diagnostic Criteria Used for the Definition of Febrile Seizures or Encephalitis in the Context of Primary Human Herpesvirus 6 (HHV-6) Infection and for the Definition of HHV-6 Encephalitis Due to Viral Reactivation
Standard Protocol Approvals, Registrations, and Patient Consents
Ethic approval was obtained from local institutional review boards.
Statistical Analyses
Statistical analyses were performed using the software R, version 3.6.1. Descriptive statistics were used to summarize quantitative data. The distribution of categorical variables between groups was assessed using the Fisher test. The established threshold for statistical significance was p = 0.05.
Data Availability
Additional data can be made available on request to the authors.
Results
Of the 926 patients who had their CSF tested for HHV-6 DNA during the period of interest, 45 met the study criteria (figure e-1, links.lww.com/NXI/A384). Thirty patients were children/adolescents (aged <18 years) (30/45, 67%), and 15 were adults (aged ≥18 years) (15/45, 33%). Seventeen patients were immunocompetent (17/45, 38%) and 28 patients immunocompromised (28/45, 62%). The main clinical characteristics in the whole cohort (n = 45), in the group of immunocompetent (n = 17), and in the group of immunocompromised (n = 28) patients are reported in table e-1.
Immunocompetent Hosts
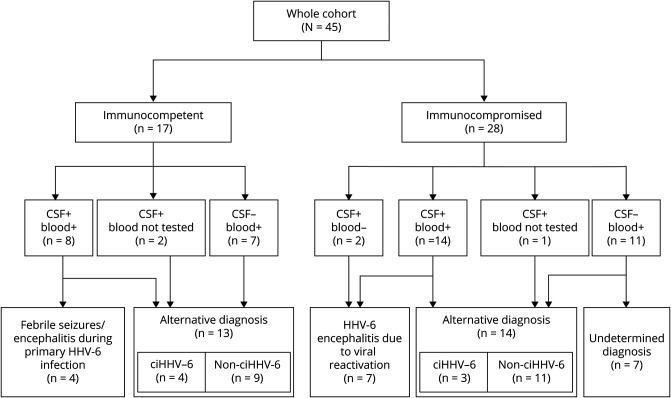
The median age in the group of immunocompetent patients was 2.7 years (range: 0.0–77.0 years). Fever and rash were present in 9 (9/17, 53%) and 3 (3/17, 18%) patients, respectively. Neurologic symptoms included altered mental status (6/17, 35%), seizures (3/17, 18%), and focal deficits (8/17, 47%). Eight patients tested positive for HHV-6 DNA in both CSF and blood (8/17, 47%), 2 patients tested positive in the CSF but were not tested in blood (2/17, 12%), and 7 patients tested positive only in blood (7/17, 41%). Regardless of HHV-6 positivity in the CSF, all immunocompetent individuals had a CSF/blood replication ratio <1. Final diagnosis was of febrile seizures/encephalitis in the context of primary HHV-6 infection in 4 patients (4/17, 24%) and other condition unrelated to HHV-6 in the remaining 13 cases (13/17, 76%) (figure 1).
Figure 1. Flowchart Diagram Illustrating Final Diagnoses Depending on the Immunologic Status of the Host and the Compartment(s) of Viral Replication.
ciHHV-6 = chromosomally integrated human herpesvirus 6.
Febrile Seizures/Encephalitis in the Context of Primary HHV-6 Infection
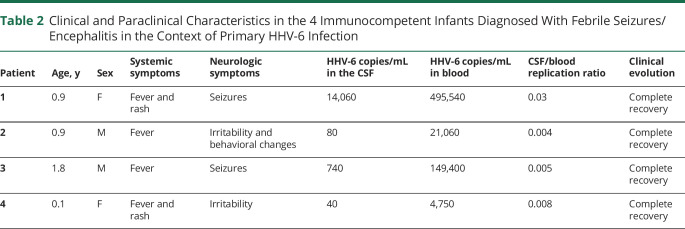
The main clinical and biological features in the 4 immunocompetent individuals meeting the diagnostic criteria for febrile seizures/encephalitis during primary HHV-6 infection are summarized in table 2. All 4 patients were infants or children younger than 2 years presenting with fever, with or without rash. Neurologic manifestations included seizures in 2 patients and irritability in other 2. All 4 patients tested positive for HHV-6 in both blood and CSF, with a median viral load of 410 copies/mL in the CSF (range: 40–14,060 copies/mL) and 85,230 copies/mL in blood (range: 4,750–495,540 copies/mL), resulting in a CSF/blood replication ratio <<1 in all cases. CSF pleocytosis was present in 1 case of 3. A single patient underwent brain MRI (patient 1 in table 2), which showed normal findings. None of the patients received specific antiviral treatment. All of them recovered completely within a week from hospital admission.
Table 2.
Clinical and Paraclinical Characteristics in the 4 Immunocompetent Infants Diagnosed With Febrile Seizures/Encephalitis in the Context of Primary HHV-6 Infection
Alternative Diagnoses
Alternative diagnoses in the 13 immunocompetent patients who did not meet the criteria for febrile seizures or encephalitis during primary HHV-6 infection included post/para-infectious neurologic syndromes (n = 6), MS (n = 1), aseptic meningitis (n = 1), Listeria monocytogenes meningoencephalitis (n = 1), febrile convulsions (n = 1), primary CNS lymphoma (n = 1), cranial mononeuritis (n = 1), and bacterial spondylodiscitis (n = 1).
In 4 patients, HHV-6 DNA detection in blood and CSF reflected chromosomal integration. Patients with ciHHV-6 had positive PCR results in both the CSF and blood, with viral loads in the order of magnitude of millions copies of HHV-6 DNA in blood (median: 6,630,575 copies/mL, range: 1,081,400–7,700,000 copies/mL) and thousands copies in the CSF (median: 6,640 copies/mL, range: 2,640–18,520 copies/mL). The median viral load in the 9 patients without ciHHV-6 was instead of 3,330 copies/mL in blood (range: 300–33,930) and 0 copies/mL in the CSF (range: 0–40). Individual patient data for patients in this group are reported in table e-2 (links.lww.com/NXI/A384).
Immunocompromised Hosts
The median age in the group of immunocompromised patients was 14.3 years (range: 2.0–88.6 years). The underlying condition of immune suppression was represented by a hematologic disorder in 27 of the 28 cases. Patients in this group had been heavily treated, having previously received high-dose chemotherapy (25/27, 93%), monoclonal antibodies (9/17, 53%), and hematopoietic stem cell transplantation (23/27, 85%). The remaining patient was a solid organ transplant recipient who was still receiving immune suppressants for the prevention of graft vs host disease. Fever was an accompanying feature in 4 cases (4/28, 14%) and rash in 2 (2/28, 7%). Neurologic symptoms included altered mental status (17/28, 61%), seizures (11/28, 39%), and focal deficits (5/28, 18%). Fourteen patients tested positive for HHV-6 DNA in both CSF and blood (14/28, 50%), 2 patients tested positive only in the CSF (2/28, 7%), 1 patient tested positive in the CSF but blood was not investigated (1/28, 4%), and 11 patients tested positive only in blood (11/28, 39%). Final diagnosis was of HHV-6 encephalitis due to viral reactivation in 7 patients (7/28, 25%), other condition unrelated to HHV-6 in 14 patients (14/28, 50%), and remained undetermined in 7 cases (7/28, 25%) (figure 1).
HHV-6 Encephalitis Due to Viral Reactivation
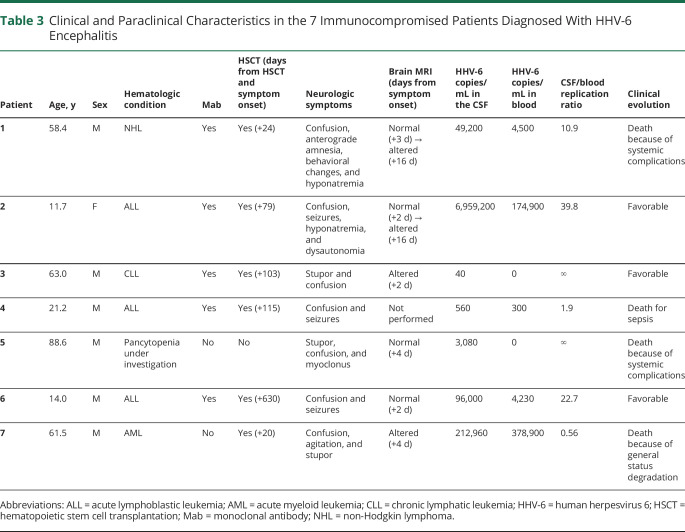
Table 3 summarizes the main clinical and paraclinical features in the 7 immunocompromised patients who met the criteria for HHV-6 encephalitis due to viral reactivation. Five patients were adults, and 2 were adolescents. All patients were affected with hematologic conditions, and 6 of the 7 had received allogeneic HSCT (median delay from HSCT to neurologic symptoms: +91 days, range: 20–630). A single patient had systemic symptoms including fever, pruritus, and rash (patient 7 in table 3).
Table 3.
Clinical and Paraclinical Characteristics in the 7 Immunocompromised Patients Diagnosed With HHV-6 Encephalitis
All 7 patients presented with altered mental status, which was associated with seizures (3/7, 43%), vigilance impairment (3/7, 43%), behavioral changes (2/7, 29%), hyponatremia (2/7, 29%), anterograde amnesia (1/7, 14%), and/or dysautonomia (1/7, 14%). CSF pleocytosis was detected in only half of cases (2/4, 50%) (patients 1 and 3 in table 3). Initial brain MRI, performed from 2 to 4 days after symptom onset, was altered in only 2 patients of 6 (2/6, 33%) (patients 3 and 7 in table 3). However, a second brain MRI, performed a median of 2 weeks after the first, disclosed radiologic alterations compatible with HHV-6 encephalitis in 2 additional patients who initially had normal findings (figure 2).
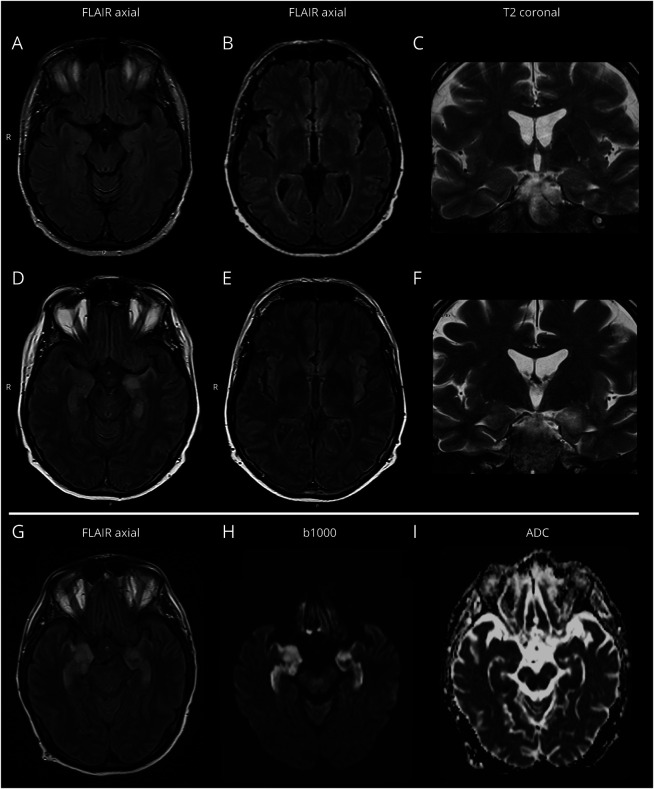
Figure 2. MRI Findings in 2 HSCT Transplant Recipients Developing HHV-6 Encephalitis.
Panels A–F (patient 1 in table 4): on initial brain MRI, performed 3 days after symptom onset, no abnormal findings were detected on axial FLAIR (panels A and B) and coronal T2-weighted (panel C) images, though in the presence of motion artifacts. At control MRI, performed 16 days after symptom onset, a quite marked hyperintensity was evident at the level of the left temporo-mesial region, with swelling of the amygdala and the ipsilateral insular cortex on axial FLAIR (panels D and E) and coronal T2-weighted (panel F) images. No diffusivity restriction or gadolinium enhancement was evident (not shown). Panels G–I (patient 7 in table 4): brain MRI performed 4 days after neurologic symptom onset showed bilateral hyperintensity of temporo-mesial structures, prevalent on the right side, on axial FLAIR images (panel G), with a correspondent b1000 hypersignal (panel H) and some areas of true low ADC signal (panel I) but predominant T2 shine through effect. FLAIR = fluid-attenuated inversion recovery; HHV-6 = human herpesvirus 6; HSCT = hematopoietic stem cell transplantation.
HHV-6 replication was limited to the CSF in 2 patients, whereas the remaining 5 also tested positive in blood. The median viral load in the CSF was 49,200 copies/mL (range: 40–6,959,200 copies/mL), and the median viral load in blood was 4,230 copies/mL (range: 0–378,900 copies/mL). The CSF/blood replication ratio was >1 in all patients except for the one showing systemic symptoms of HHV-6 infection (fever, rash, and pruritus), in whom HHV-6 viral load prevailed in blood.
All patients received IV ganciclovir, with or without associated foscarnet, except for a single patient who died before receiving antiviral treatment (patient 5 in table 3). A total of 4 patients died during the acute phase as a consequence of intervening systemic complications or general status deterioration (4/7, 57%). The remaining 3 patients survived the infection but experienced mild to moderate cognitive sequelae (3/7, 43%).
Alternative Diagnoses
Fourteen immunocompromised patients received alternative diagnoses, including posterior reversible encephalopathy syndrome (n = 2), CNS localizations of their underlying hematologic malignancies (n = 4), cytomegalovirus infection (n = 2), metabolic encephalopathy (n = 2), ischemia (n = 2), inflammatory encephalomyelitis (n = 1) and migraine (n = 1). All patients in this group had HHV-6 viral loads largely prevailing in blood (CSF/blood replication ratio <1).
In 3 patients, HHV-6 DNA detection in blood and CSF reflected chromosomal integration. Patients with ciHHV-6 had viral loads in the order of magnitude of millions copies in blood (median: 572,220 copies/mL, range: 497,200–8,650,000 copies/mL) and thousands copies in the CSF (median: 53,240 copies/mL, range: 1,360–141,140 copies/mL). The median HHV-6 viral load in the 11 remaining patients was of 6,175 copies/mL in blood (range: 100–365,400 copies/mL) and 60 copies/mL in the CSF (range: 0–20,000 copies/mL).
Undetermined Diagnoses
Seven immunocompromised patients remained without a definite etiologic diagnosis despite a comprehensive work-up that included an extensive research for infectious, neoplastic, metabolic, vascular, and autoimmune etiologies. The clinical and paraclinical characteristics of these 7 patients are summarized in table 4. Four patients were children, and 3 were adults. All patients had an underlying hematologic disorder, 6 of the 7 having received allogeneic HSCT. All patients tested positive for HHV-6 only in blood (median: 9,180 copies/mL, range: 400–126,000 copies/mL). Two patients with stupor or seizures arising in a context of fever were diagnosed with systemic HHV-6 reactivation, for which they received IV ganciclovir that led to full recovery (patients 1 and 2 in table 4). Four patients presenting with seizures, encephalopathy, or cerebellar dysfunction had no accompanying fever or rash and spontaneously recovered within few days without specific treatment (patients 3–6 in table 4). The remaining patient (patient 7 in table 4) presented with confusion and anterograde amnesia 1 month after completing HSCT. She had extensive MRI alterations involving temporo-mesial structures and the mammillary bodies of the hypothalamus. HHV-6 viral load was positive in blood (126,000 copies/mL) but undetectable in the CSF. In the inability to distinguish between Wernicke encephalopathy and HHV-6 infection, the patient received both vitamin B1 supplementation and IV foscarnet, but, unfortunately, she died few weeks later because of general status deterioration.
Table 4.
Clinical and Paraclinical Characteristics in the 7 Immunocompromised Patients With HHV-6 Replication Limited to Blood and No Definite Cause Identified for Their Neurologic Symptoms
Discussion
In this study, we reviewed all patients tested for HHV-6 in the CSF at Fondazione IRCCS Policlinico San Matteo during a period of 10 years. Of the 926 patients tested during this period, only 43 tested positive for HHV-6 in the CSF (43/926, 4.6%), and a much lower number received a diagnosis of febrile seizures/encephalitis during primary HHV-6 infection (3/926, 0.4%) or a diagnosis of HHV-6 encephalitis due to viral reactivation (7/926, 0.8%). These proportions confirm that there is ample room for improvement when it comes to choosing which patients to test. Chromosomal integration was detected in nearly 1% of patients tested (7/926, 0.8%), a proportion similar to the one reported in the literature for the general population.6
The strong point of this study was the systematic review of all data concerning viral replication in both CSF and blood, analysis that had never been performed systematically in large cohorts of immunocompetent and immunocompromised hosts and that allowed to confirm the value of the CSF/blood replication ratio for the diagnosis of HHV-6 encephalitis in immunocompromised patients. On the other hand, this study has limitations that are inherent to its retrospective design: clinical presentation and evolution were reconstructed a posteriori from medical records, and data on viral coreplications were interpreted case by case in the light of the immune competence of the host and of individual clinical/paraclinical features. As this distinction is not routinely performed at our laboratory (and could not be performed for the present study due to the exhaustion of most biological samples), in our report, we did not distinguish between HHV-6A and HHV-6B. Although HHV-6A and HHV-6B are now considered as separate species, only HHV-6B has been consistently associated with disease in humans.6 Based on known epidemiology and association with disease, we would thus expect all cases of febrile seizures/encephalitis during primary infection and all cases of encephalitis due to viral reactivation to be related to HHV-6B and cases of chromosomal integration to be distributed between HHV-6B and HHV-6A.6
In immunocompetent patients, the diagnosis of HHV-6 encephalitis was limited to 4 infants younger than 2 years presenting with seizures2,3 or irritability in the context of a symptomatic primary HHV-6 infection. All 5 infants tested positive for HHV-6 in blood and CSF, with viral loads in blood largely exceeding the viral load in the CSF. Based on the observation that HHV-6 viral loads in the CSF of children developing febrile convulsions or encephalopathy during primary infection are generally low and transient,6,12 some authors have hypothesized that neurologic symptoms might result from the indirect effects of cytokine release rather than from direct viral CNS infection.6,13,14 Such pathogenetic mechanism would be consistent with the benign course generally observed in these cases, at least in Caucasian infants.2,6
The observation that, in immunocompetent individuals, HHV-6 encephalitis is limited to primary infection6,15 suggests that testing for HHV-6 should be limited to infants younger than 3 years developing seizures2,3 or encephalopathy4 in a febrile context. All diagnostic tests performed outside these constraints not only represent a waste of economic resources but expose to a high risk of misdiagnosis: presenting high viral loads in both blood and CSF, adult immunocompetent patients with ciHHV-6 might in fact be wrongly diagnosed with HHV-6 encephalitis, delaying diagnosis and treatment for their actual condition. Viral loads in the order of thousands copies in the CSF and millions copies in blood should immediately raise a suspicion of ciHHV-6 and prompt confirmative hair follicle testing.11
Differently from immunocompetent hosts, immunocompromised subjects are at risk of developing HHV-6 encephalitis through a mechanism of viral reactivation.6 HSCT and, especially, the early posttransplantation period is known to be a fertile ground for HHV-6 reactivation.16–18 HHV-6 encephalitis was diagnosed to 7 immunocompromised adolescents or adults in our series having hematologic disorders and a history of allogenic HSCT. In most cases, clinical presentation was dominated by confusion and seizures, whereas more distinctive signs and symptoms such as anterograde amnesia and hyponatremia were present in only a minority of cases.10 Although important to exclude alternative causes of neurologic deterioration, brain MRI showed typical limbic alterations in only two-thirds of cases and, at times, only when performed several days after symptom onset.10 These observations suggest that all HSCT recipients presenting with altered mental status, seizures, or vigilance impairment should have their CSF tested for HHV-6, even in the absence of radiologic correlates of limbic involvement. Testing should then be followed by a cautious interpretation of results. Being prone to periodical HHV-6 reactivations, HSCT recipients may have HHV-6 DNA detected in their blood, or even in their CSF, without it being the cause of neurologic symptoms.19 In this setting, the CSF/blood replication could be a precious tool to guide the clinician in the interpretation of the results of PCR testing. With the exception of a single patient who had systemic symptoms of infection, all immunocompromised patients diagnosed with HHV-6 encephalitis in our series had a CSF/blood replication ratio >1, feature that was exclusive of patients in this group. The CSF/blood replication ratio could thus represent a specific feature to distinguish immunocompromised patients with HHV-6 encephalitis from the remaining immunocompromised patients in whom HHV-6 detection in the CSF represented an incidental finding or reflected chromosomal integration. Being a hallmark of HHV-6 encephalitis in the immunocompromised, the detection of a CSF/blood replication ratio >1 could strengthen the certitude of diagnosis in patients lacking typical clinical and radiologic features or having low viral loads in the CSF, avoiding any delay in the administration of antiviral treatment. Although the role of the CSF/blood replication ratio is not been emphasized in most reports and guidelines, our data strengthen its value for the diagnosis of HHV-6 encephalitis in the immunocompromised so that, in our view, it could be considered a supportive criterion in atypical cases.
The question on whether, in HSCT recipients, HHV-6 replication could be associated with neurologic symptoms, when limited to blood, remains debated. This was the case of few patients in our series, who developed otherwise unexplained neurologic symptoms in a context of systemic HHV-6 reactivation. An original study by Zerr et al.9 has shown an association between early HHV-6 reactivation in blood following HSCT and the development of delirium and cognitive decline. Patients developing neurologic symptoms did not always have a detectable viral load in the CSF,9 suggesting that the latter is either modest and/or transient, and thus difficult to detect, or it is not required to develop neurologic symptoms. Future studies will possibly clarify the nature of these neurologic manifestations and their causal relationship with HHV-6 reactivation, as well as the need for preemptive antiviral treatment.20,21
In conclusion, our study reinforces the evidence that (1) in immunocompetent hosts, CNS involvement from HHV-6 is limited to infants younger than 3 years presenting with primary infection; (2) HSCT recipients can develop HHV-6 encephalitis as a consequence of viral reactivation, although typical clinical imaging features of limbic encephalitis might be lacking, especially when brain MRI is performed shortly after symptoms onset; and (3) the CSF/blood replication ratio can be of great help to interpret the results of PCR testing and establish an accurate diagnosis of HHV-6 encephalitis in HSCT recipients.
Acknowledgment
The authors thank Dr. Katherine N. Ward for her thoughtful comments on this article.
Glossary
- ciHHV-6
chromosomally integrated HHV-6
- HHV-6
human herpesvirus 6
- HSCT
hematopoietic stem cell transplantation
Appendix. Authors
Footnotes
Editorial, page e948
Contributor Information
Giulia Berzero, Email: giulia.berzero@mondino.it.
Giulia Campanini, Email: g.campanini@smatteo.pv.it.
Elisa Vegezzi, Email: elisa.vegezzi@gmail.com.
Matteo Paoletti, Email: matteo.paoletti@mondino.it.
Anna Pichiecchio, Email: anna.pichiecchio@mondino.it.
Anna Maria Simoncelli, Email: a.simoncelli@smatteo.pv.it.
Anna Amelia Colombo, Email: aa.colombo@smatteo.pv.it.
Paolo Bernasconi, Email: p.bernasconi@smatteo.pv.it.
Oscar Borsani, Email: oscar.borsani01@universitadipavia.it.
Angela Di Matteo, Email: a.dimatteo@smatteo.pv.it.
Virginia Rossi, Email: virginia.rossi01@universitadipavia.it.
Thomas Foiadelli, Email: thomas.foiadelli@gmail.com.
Salvatore Savasta, Email: s.savasta@smatteo.pv.it.
Francesca Compagno, Email: f.compagno@smatteo.pv.it.
Marco Zecca, Email: m.zecca@smatteo.pv.it.
Fausto Baldanti, Email: fausto.baldanti@unipv.it.
Study Funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Agut H, Bonnafous P, Gautheret-Dejean A. Update on infections with human herpesviruses 6A, 6B, and 7. Med Mal Infect 2017;47:83–91. [DOI] [PubMed] [Google Scholar]
- 2.Ward KN, Andrews NJ, Verity CM, Miller E, Ross EM. Human herpesviruses-6 and -7 each cause significant neurological morbidity in Britain and Ireland. Arch Dis Child 2005;90:619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward KN, Bryant NJ, Andrews NJ, et al. Risk of serious neurologic disease after immunization of young children in Britain and Ireland. Pediatrics 2007;120:314–321. [DOI] [PubMed] [Google Scholar]
- 4.Yoshikawa T, Ohashi M, Miyake F, et al. Exanthem subitum-associated encephalitis: nationwide survey in Japan. Pediatr Neurol 2009;41:353–358. [DOI] [PubMed] [Google Scholar]
- 5.Ward KN, Leong HN, Thiruchelvam AD, Atkinson CE, Clark DA. Human herpesvirus 6 DNA levels in cerebrospinal fluid due to primary infection differ from those due to chromosomal viral integration and have implications for diagnosis of encephalitis. J Clin Microbiol 2007;45:1298–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward KN. Child and adult forms of human herpesvirus 6 encephalitis: looking back, looking forward. Curr Opin Neurol 2014;27:349–355. [DOI] [PubMed] [Google Scholar]
- 7.Chevallier P, Hebia-Fellah I, Planche L, et al. Human herpes virus 6 infection is a hallmark of cord blood transplant in adults and may participate to delayed engraftment: a comparison with matched unrelated donors as stem cell source. Bone Marrow Transplant 2010;45:1204–1211. [DOI] [PubMed] [Google Scholar]
- 8.Seeley WW, Marty FM, Holmes TM, et al. Post-transplant acute limbic encephalitis: clinical features and relationship to HHV6. Neurology 2007;69:156–165. [DOI] [PubMed] [Google Scholar]
- 9.Zerr DM, Fann JR, Breiger D, et al. HHV-6 reactivation and its effect on delirium and cognitive functioning in hematopoietic cell transplantation recipients. Blood 2011;117:5243–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhanushali MJ, Kranick SM, Freeman AF, et al. Human herpes 6 virus encephalitis complicating allogeneic hematopoietic stem cell transplantation. Neurology 2013;80:1494–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark DA. Clinical and laboratory features of human herpesvirus 6 chromosomal integration. Clin Microbiol Infect 2016;22:333–339. [DOI] [PubMed] [Google Scholar]
- 12.Kawabe S, Ito Y, Ohta R, et al. Comparison of the levels of human herpesvirus 6 (HHV-6) DNA and cytokines in the cerebrospinal fluid and serum of children with HHV-6 encephalopathy. J Med Virol 2010;82:1410–1415. [DOI] [PubMed] [Google Scholar]
- 13.Ichiyama T, Ito Y, Kubota M, Yamazaki T, Nakamura K, Furukawa S. Serum and cerebrospinal fluid levels of cytokines in acute encephalopathy associated with human herpesvirus-6 infection. Brain Dev 2009;31:731–738. [DOI] [PubMed] [Google Scholar]
- 14.Kawamura Y, Yamazaki Y, Ohashi M, Ihira M, Yoshikawa T. Cytokine and chemokine responses in the blood and cerebrospinal fluid of patients with human herpesvirus 6B-associated acute encephalopathy with biphasic seizures and late reduced diffusion. J Med Virol 2014;86:512–518. [DOI] [PubMed] [Google Scholar]
- 15.Agut H. Deciphering the clinical impact of acute human herpesvirus 6 (HHV-6) infections. J Clin Virol 2011;52:164–171. [DOI] [PubMed] [Google Scholar]
- 16.Pruitt AA, Graus F, Rosenfeld MR. Neurological complications of transplantation: part I: hematopoietic cell transplantation. Neurohospitalist 2013;3:24–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zerr DM, Corey L, Kim HW, Huang ML, Nguy L, Boeckh M. Clinical outcomes of human herpesvirus 6 reactivation after hematopoietic stem cell transplantation. Clin Infect Dis 2005;40:932–940. [DOI] [PubMed] [Google Scholar]
- 18.Ogata M, Satou T, Kadota J, et al. Human herpesvirus 6 (HHV-6) reactivation and HHV-6 encephalitis after allogeneic hematopoietic cell transplantation: a multicenter, prospective study. Clin Infect Dis 2013;57:671–681. [DOI] [PubMed] [Google Scholar]
- 19.Hill JA, Boeckh MJ, Sedlak RH, Jerome KR, Zerr DM. Human herpesvirus 6 can be detected in cerebrospinal fluid without associated symptoms after allogeneic hematopoietic cell transplantation. J Clin Virol 2014;61:289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zerr DM. Human herpesvirus 6B in the transplant recipient: when to worry, when to act. J Pediatric Infect Dis Soc 2018;7(suppl 2):S75–S78. [DOI] [PubMed] [Google Scholar]
- 21.Ward KN, Hill JA, Hubacek P, et al. Guidelines from the 2017 European Conference on Infections in Leukaemia for management of HHV-6 infection in patients with hematologic malignancies and after hematopoietic stem cell transplantation. Haematologica 2019;104:2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Additional data can be made available on request to the authors.