Supplemental Digital Content is available in the text.
Keywords: acute kidney injury, insulin-like growth factor binding protein 7, tissue inhibitor of metalloproteinases-2
OBJECTIVES:
Although early recognition of sepsis is vital to improving outcomes, the diagnosis may be missed or delayed in many patients. Acute kidney injury is one of the most common organ failures in patients with sepsis but may not be apparent on presentation. Novel biomarkers for acute kidney injury might improve organ failure recognition and facilitate earlier sepsis care.
DESIGN:
Retrospective, international, Sapphire study.
SETTING:
Academic Medical Center.
PATIENTS:
Adults admitted to the ICU without evidence of acute kidney injury at time of enrollment.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
We stratified patients enrolled in the Sapphire study into three groups—those with a clinical diagnosis of sepsis (n = 216), those with infection without sepsis (n = 120), and those without infection (n = 387) at enrollment. We then examined 30-day mortality stratified by acute kidney injury within each group. Finally, we determined the operating characteristics for kidney stress markers (tissue inhibitor of metalloproteinases-2) × (insulin-like growth factor binding protein 7) for prediction of acute kidney injury as a sepsis-defining organ failure in patients with infection without a clinical diagnosis of sepsis at enrollment. Combining all groups, 30-day mortality was 23% for patients who developed stage 2–3 acute kidney injury within the first 3 days compared with 14% without stage 2–3 acute kidney injury. However, this difference was greatest in the infection without sepsis group (34% vs 11%; odds ratio, 4.09; 95% CI, 1.53–11.12; p = 0.005). Using a (tissue inhibitor of metalloproteinases-2) × (insulin-like growth factor binding protein 7) cutoff of 2.0 units, 14 patients (11.7%), in the infection/no sepsis group, tested positive of which 10 (71.4%) developed stage 2–3 acute kidney injury. The positive test result occurred a median of 19 hours (interquartile range, 0.8–34.0 hr) before acute kidney injury manifested by serum creatinine or urine output. Similar results were obtained using a cutoff of 1.0 for any stage of acute kidney injury.
CONCLUSIONS:
Use of the urinary (tissue inhibitor of metalloproteinases-2) × (insulin-like growth factor binding protein 7) test could identify acute kidney injury in patients with infection, possibly helping to detect sepsis, nearly a day before acute kidney injury is apparent by clinical criteria.
Early recognition of sepsis is a key to improving outcomes. Timely antibiotics and source control, recognition and reversal of shock, and provision of organ support are cornerstones of sepsis management. These actions are different from those taken in patients with infection without sepsis. For this reason, sepsis care protocols have been developed to capture these cornerstones of treatment and are implemented at many, if not most, hospitals.
A pragmatic definition of sepsis has been developed in which infection-associated organ dysfunction is the central tenant (1). Implicit in this definition is the notion that clinicians can identify organ dysfunction as well as its relationship to infection. The three most common organ dysfunctions in sepsis are cardiovascular (i.e., shock), renal (i.e., acute kidney injury [AKI]), and respiratory, each occurring in 50% or more of patients (2). For previously healthy adults, shock and hypoxemia are usually easy to recognize, and other organ failures such as mental status changes can produce obvious and dramatic symptoms as well. However, early detection of AKI can be difficult since it does not cause symptoms and changes in serum creatinine, and urine output can be delayed relative to organ damage. In some patients, occult (normotensive) shock may occur, but arterial lactate levels can be used to identify these patients. Similarly, oxygen saturations or arterial blood gases can help detect respiratory failure. Importantly, even in patients with septic shock, AKI is associated with a substantial increased risk of death—27.7% for stage 2–3 AKI versus 6.2% for patients without AKI (3).
Biomarkers to detect AKI prior to clinical/laboratory indicators of kidney dysfunction are now routinely available. The NephroCheck Test (Astute Medical, San Diego, CA) measures the product of two biomarkers, tissue inhibitor of metalloproteinases (TIMP)–2 and insulin-like growth factor binding protein (IGFBP) 7. At a cutoff of 2.0 (ng/mL)2/1,000, the test has a specificity of 95% to predict Kidney disease improving global outcomes (KDIGO) stage 2–3 AKI in the next 12 hours (4). We have previously shown that the test performs well in patients with sepsis with an area under the receiver operating characteristics curve of 0.84 (5). Thus, urinary (TIMP-2) × (IGFBP7) might serve as a means to identify occult sepsis (i.e., not clinically apparent) by identifying AKI in patients with infection.
METHODS
Study Design
We conducted our analysis using a previously published cohort of critically ill patients (Sapphire) in which urinary (TIMP-2) × (IGFBP7) was measured and complete details are reported elsewhere (6). Briefly, Sapphire included 728 adult subjects with critical illness defined as cardiac or respiratory failure and without evidence of AKI at enrollment. After excluding five patients enrolled under consent from a legally authorized representative who later died prior to reconsent, we analyzed 723 subjects. The primary endpoint was moderate-to-severe AKI (KDIGO stage 2–3) within 12 hours of sample collection. Data were collected by the investigators and analyzed by independent statisticians not directly affiliated with the study. The study protocol was approved by the Western Institutional Review Board (Olympia, WA) and also by the institutional review board or ethics committee of each study site, as required. All patients (or authorized representatives) provided written informed consent. We defined infection and sepsis based on the clinical diagnosis assigned by the treating physicians at enrolling sites at the time of enrollment. Antibiotics were prescribed for 98% of patients with infection without sepsis. AKI was defined and staged using full KDIGO criteria (both serum creatinine and urine output) (7) based on interpolation (linear) across each hour to provide precise timing for AKI manifestation. The design, execution, and reporting of this study meet the Strengthening the Reporting of Observational Studies in Epidemiology (8) and the Standards for Reporting of Diagnostic Accuracy criteria (9).
Measurements
Paired urine and serum samples for biomarker and creatinine assessment, respectively, were obtained at the time of enrollment and within 24 hours of ICU admission, flash-frozen, stored at less than or equal to –70°C, and thawed immediately prior to analysis. TIMP-2 and IGFBP7 concentrations were measured by immunoassay with the NephroCheck Test on the Astute140 Meter (Astute Medical, San Diego, CA) by technicians blinded to clinical data. The TIMP-2 and IGFBP7 concentrations in ng/mL were multiplied and divided by 1,000 to obtain the composite (TIMP-2) × (IGFBP7) results in units of (ng/mL)2/1,000. We assessed severity of illness and organ dysfunction/failure with the Acute Physiology and Chronic Health Evaluation (APACHE)–III (10) and Sequential Organ Failure Assessment (SOFA) (11) scores. Nonrenal APACHE-III and SOFA scores were calculated by subtracting the renal components from these scores.
Statistics
We stratified patients into three groups—those with a clinical diagnosis of sepsis (n = 216), those with infection without a clinical diagnosis of sepsis (n = 120), and those without infection (n = 387) at enrollment. All patients had either respiratory or cardiac dysfunction or both; thus, in the infection without sepsis group, organ failure was not attributed to infection. AKI status was determined within the first 3 days after enrollment, and mortality was assessed for up to 9 months from enrollment. We compared 30-day mortality rates between patients with or without AKI for each infection group by using a logistic regression model. Rates are expressed as proportions, and time to event is reported as median with interquartile ranges (IQRs) among patients with events. Mortality out to 9 months was plotted using Kaplan-Meier curves stratified by both urinary (TIMP-2) × (IGFBP7) and AKI. Differences between strata were analyzed using the log-rank test. Hazard ratios for 9-month mortality by urinary (TIMP-2) × (IGFBP7) and AKI strata were determined using Cox proportional hazards analysis. For comparisons of baseline characteristics across the three infection groups or between pairs of groups, categorical variables were analyzed using Fisher exact test and continuous variables using Kruskal-Wallis test across three groups and Wilcoxon rank-sum test for pairwise comparisons. A p value of less than 0.05 was considered to indicate statistical significance. All reported p values are two sided. All analyses were performed using R 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline Characteristics
Of the 723 patients enrolled in Sapphire, 216 had a clinical diagnosis of sepsis at enrollment, whereas 120 had infection as a primary or secondary diagnosis but without a clinical diagnosis of sepsis. The remainder (n = 387) had neither infection nor sepsis at enrollment. Baseline characteristics for the cohort as a whole, as well as broken out by these three categories, can be found in Table 1 (with additional analysis provided in Supplemental Table 1, http://links.lww.com/CCM/G112). Compared with patients with a diagnosis of sepsis at enrollment, those with infection only had lower severity of illness by APACHE and SOFA but were otherwise comparable.
TABLE 1.
Baseline Characteristics
| Characteristics | All Patients | No Infection | Infection No Sepsis | Sepsis | pa |
|---|---|---|---|---|---|
| n | 723 | 387 | 120 | 216 | |
| Male, n (%) | 444 (61.4) | 256 (66.1) | 74 (61.7) | 114 (52.8) | 0.005 |
| Age, median (interquartile range) | 64 (53–73) | 64 (53–72) | 61 (52–73) | 66 (54–74) | 0.207 |
| Race, n (%) | 0.006 | ||||
| White | 568 (78.6) | 312 (80.6) | 92 (76.7) | 164 (75.9) | |
| Black | 87 (12) | 50 (12.9) | 18 (15) | 19 (8.8) | |
| Other/unknown | 68 (9.4) | 25 (6.5) | 10 (8.3) | 33 (15.3) | |
| Medical history, n (%) | |||||
| Chronic kidney disease | 65 (9) | 41 (10.6) | 9 (7.5) | 15 (6.9) | 0.288 |
| Diabetes | 210 (29) | 116 (30) | 29 (24.2) | 65 (30.1) | 0.445 |
| Congestive heart failure | 121 (17.3) | 77 (20.6) | 12 (10.3) | 32 (15.3) | 0.023 |
| Coronary artery disease | 215 (30.3) | 146 (38.6) | 24 (20.2) | 45 (21.2) | < 0.001 |
| Hypertension | 429 (59.3) | 239 (61.8) | 56 (46.7) | 134 (62) | 0.009 |
| Chronic obstructive pulmonary disease | 146 (20.2) | 81 (20.9) | 27 (22.5) | 38 (17.6) | 0.482 |
| Cancer | 187 (26.2) | 88 (23) | 30 (25.4) | 69 (32.2) | 0.050 |
| Reason for ICU admission, n (%) | |||||
| Cardiovascular | 239 (33.1) | 154 (39.8) | 33 (27.5) | 52 (24.1) | < 0.001 |
| Cerebrovascular | 70 (9.7) | 46 (11.9) | 13 (10.8) | 11 (5.1) | 0.017 |
| Sepsis | 135 (18.7) | 0 (0) | 0 (0) | 135 (62.5) | < 0.001 |
| Respiratory | 308 (42.6) | 116 (30) | 73 (60.8) | 119 (55.1) | < 0.001 |
| Trauma | 55 (7.6) | 49 (12.7) | 4 (3.3) | 2 (0.9) | < 0.001 |
| Surgery | 247 (34.2) | 180 (46.5) | 18 (15) | 49 (22.7) | < 0.001 |
| Other | 126 (17.4) | 47 (12.1) | 33 (27.5) | 46 (21.3) | < 0.001 |
| Enrollment serum creatinine (mg/dL), median (interquartile range) | 0.8 (0.7–1.1) | 0.9 (0.7–1.1) | 0.8 (0.6–1.1) | 0.8 (0.6–1.1) | 0.147 |
| Nonrenal APACHE-III, median (interquartile range) | 60 (43–82) | 57 (41–80) | 59 (42–73) | 63 (48–87) | 0.008 |
| APACHE-III, median (interquartile range) | 69 (51–91) | 67 (50–90) | 66 (51–82) | 75 (55–96) | 0.006 |
| Nonrenal SOFA, median (interquartile range) | 7 (5–10) | 7 (5–10) | 7 (4–9.25) | 8 (5.75–10) | 0.035 |
| SOFA, median (interquartile range) | 8 (5–10) | 8 (5–10) | 7 (4.75–10) | 8 (6–11) | 0.033 |
APACHE = Acute Physiology and Chronic Health Evaluation, SOFA = Sequential Organ Failure Assessment.
aComparisons were made across the three subgroups.
AKI Rates and Outcomes
Across the entire cohort, 175 patients (24.2%) developed stage 2–3 AKI within the first 3 days after enrollment. Mortality at 30 days was 23% for patients who developed stage 2–3 AKI compared with 14% without stage 2–3 AKI (p = 0.003). Comparison of AKI rates and outcomes across the three groups are shown in Table 2. Patients with infection with or without sepsis had similar rates of stage 2–3 AKI (27.3% vs 26.7%) and 30-day mortality (20.4% vs 17.5%). However, patients with infection without a diagnosis of sepsis had a larger differential effect on mortality from stage 2–3 AKI (34% vs 11%; odds ratio [OR] 4.09; 95% CI, 1.53–11.12; p = 0.005) compared with (27% vs 18%; OR, 1.71; 95% CI, 0.84–3.44; p = 0.13) for patients with sepsis and (17% vs 12%; OR, 1.44; 95% CI, 0.72–2.75; p = 0.29) for patients without infection (Fig. 1).
TABLE 2.
Outcomes
| Outcomes | All Patients, n (%) | No Infection, n (%) | Infection No Sepsis, n (%) | Sepsis, n (%) | pa |
|---|---|---|---|---|---|
| n | 723 | 387 | 120 | 216 | |
| Any AKI within 3 d | 413 (57.1) | 218 (56.3) | 67 (55.8) | 128 (59.3) | 0.742 |
| Stage 2–3 AKI within 3 d | 175 (24.2) | 84 (21.7) | 32 (26.7) | 59 (27.3) | 0.229 |
| Renal replacement therapy within 30 d | 48 (6.6) | 20 (5.2) | 8 (6.7) | 20 (9.3) | 0.158 |
| Death within 30 d | 116 (16) | 51 (13.2) | 21 (17.5) | 44 (20.4) | 0.060 |
| Major adverse kidney events at 30 d | 155 (21.4) | 68 (17.6) | 27 (22.5) | 60 (27.8) | 0.014 |
AKI = acute kidney injury.
aComparisons were made across the three subgroups.
Figure 1.
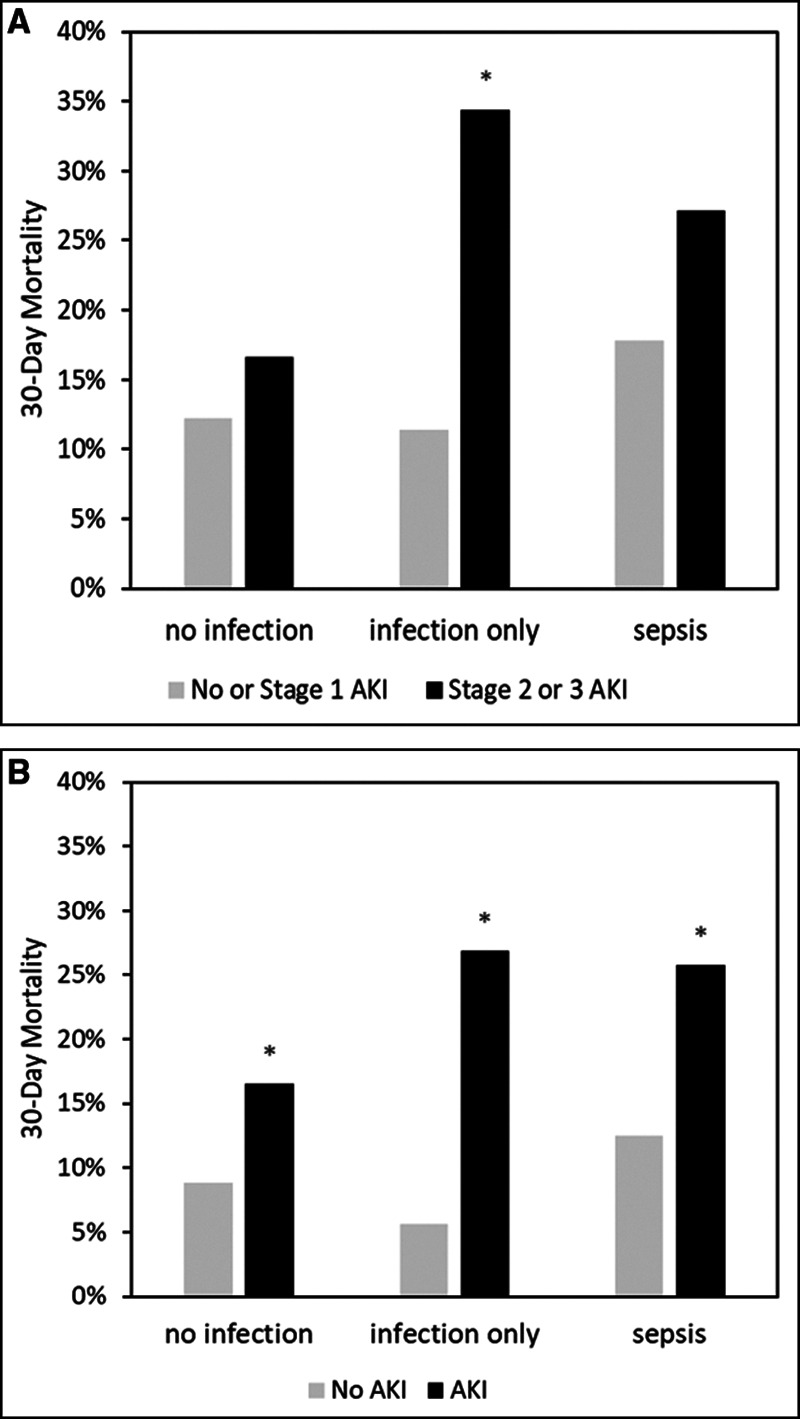
Thirty-day mortality by acute kidney injury (AKI) status. A, Shows mortality for stage 2–3 AKI versus no-AKI, and stage 1 stratified by no infection, infection only, and sepsis. B, Shows mortality for stage 1–3 AKI versus no-AKI stratified by the same three patient groups. *p < 0.05.
Biomarker Results
Using a urinary (TIMP-2) × (IGFBP7) cutoff of 2.0, 14 patients (11.7%) in the infection/no sepsis group tested positive, of which 10 (71.4%) developed stage 2–3 AKI. The positive test result occurred a median of 19 hours (IQR, 0.8–34.0 hr) before stage 2–3 AKI manifested by serum creatinine or urine output. However, this timing is based on retrospective interpolation of creatinine and urine output values such that the actual time for clinical diagnosis was likely much later. To explore this further, we excluded three patients who would have already met clinical criteria for stage 2–3 AKI at the time of enrollment based on these extrapolated values. For the remaining patients, the median lead time was actually 31 hours (IQR, 19–40 hr). We also repeated this analysis using a 1.0 cutoff for urinary (TIMP-2) × (IGFBP7) to detect any stage of AKI (not just stage 2–3). We found that 24 patients (20%) tested positive, of which 18 (75%) developed AKI (six were stage 1).
Long-Term Survival by Biomarkers and AKI Status
Finally, we examined mortality out to 9 months stratified by both urinary (TIMP-2) × (IGFBP7) and AKI. Figure 2A shows results for the 2.0 cutoff and stage 2–3 AKI, and Figure 2B shows the 1.0 cutoff and stage 1–3 AKI. Higher mortality was seen for patients with biomarker-positive AKI compared with biomarker-negative AKI in both analyses. The hazard ratio (95% CI) for 9-month mortality was 2.32 (1.46–3.67) for patients with stage 2–3 AKI and (TIMP-2) × (IGFBP7) greater than 2 relative to patients with no or stage 1 AKI and (TIMP-2) × (IGFBP7) less than or equal to 2. Similarly, the hazard ratio (95% CI) was 2.44 (1.68–3.54) for patients with any AKI and (TIMP-2) × (IGFBP7) greater than 1 relative to patients with no-AKI and (TIMP-2) × (IGFBP7) less than or equal to 1 (ng/mL)2/1,000.
Figure 2.
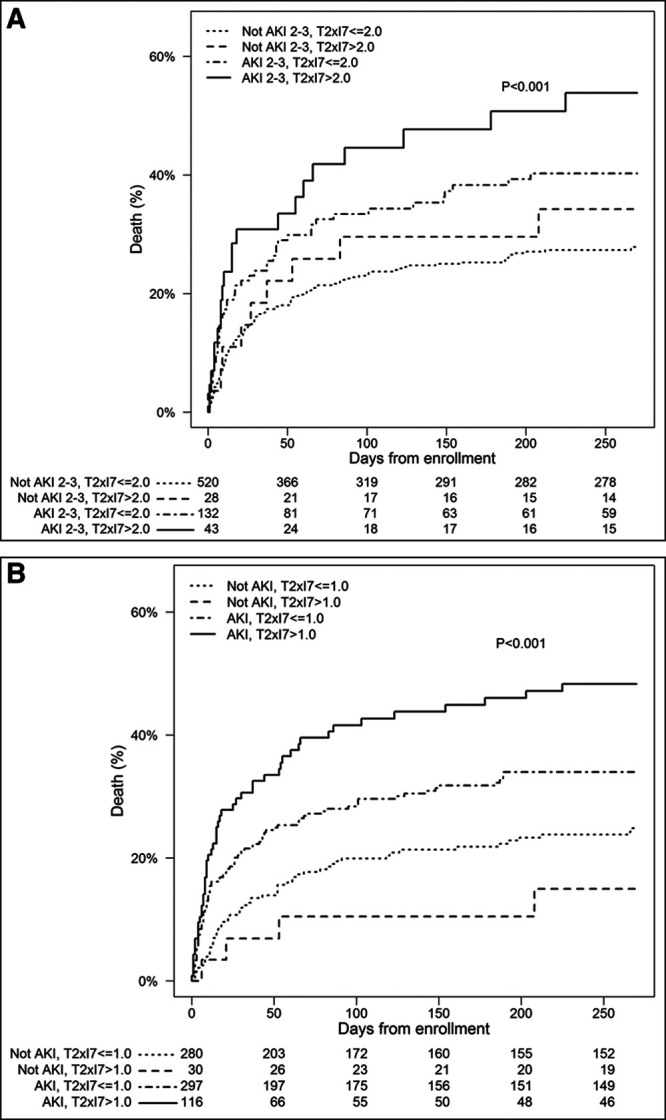
Mortality curves out to 9 mo stratified by the both urinary tissue inhibitor of metalloproteinases-2 (TIMP-2) × insulin-like growth factor binding protein 7 (IGFBP7) and acute kidney injury (AKI). A, Shows results for the 2.0 cutoff and stage 2–3 AKI. Using no-AKI or stage 1 and (TIMP-2) × (IGFBP7) less than or equal to 2 as reference, the individual hazard ratios (95% CI) for each group were as follows: 1.27 (0.64–2.49), p = 0.49 for no-AKI/stage 1 and (TIMP-2) × (IGFBP7) greater than 2; 1.61 (1.16–2.24), p = 0.005 for stage 2–3 AKI and (TIMP-2) × (IGFBP7) less than or equal to 2; and 2.32 (1.46–3.67), p < 0.001 stage 2–3 AKI and (TIMP-2) × (IGFBP7) greater than 2 (ng/mL)2/1,000. B, Shows the 1.0 cutoff and stage 1–3 AKI. Using no-AKI and (TIMP-2) × (IGFBP7) less than or equal to 1 as reference, the individual hazard ratios (95% CI) for each group were as follows: 0.56 (0.20–1.54), p = 0.26 for no-AKI and (TIMP-2) × (IGFBP7) greater than 1; 1.55 (1.12–2.14), p = 0.008 for AKI and (TIMP-2) × (IGFBP7) less than or equal to 1; and 2.44 (1.68–3.54), p < 0.001 for AKI and (TIMP-2) × (IGFBP7) greater than 1 (ng/mL)2/1,000.
DISCUSSION
Despite no specific therapy, sepsis mortality rates have steadily decreased in recent years (12). Presumably, this is due to better recognition and prompt therapy with antibiotics and reversal of shock. Delay of appropriate therapy, even by a few hours, can dramatically increase mortality (13). As such many hospitals have instituted sepsis protocols that are designed to deliver broader spectrum antibiotics quickly, screen for shock (lactate), and admit to the ICU for close monitoring for organ dysfunction. The current definition of sepsis, Sepsis-3, is pragmatic requiring infection with related organ failure (1). AKI is one of the most common organ failures and yet can be difficult to identify early. These difficulties stem from both delayed clinical manifestations (serum creatinine and urine output changes) as well as from challenges to recognition (e.g., lack of baseline creatinine, incomplete urine output data). Importantly, AKI is strongly associated with sepsis mortality. In the Protocolized Care for Early Septic Shock (ProCESS) trial (14), hospital mortality truncated at 60 days was 6.2% for patients without AKI, whereas it was 16.8% for patients with maximum AKI stage 1, and 27.7% for stage 2–3 AKI (p = 0.0001) (3). We observed similar results here in that 30-day mortality was 27% in patients with sepsis and stage 2–3 AKI. Surprisingly, in patients with infection and no clinical diagnosis of sepsis, 30-day mortality was 34% for patients with stage 2–3 AKI compared with 11% without stage 2–3 AKI. Of course, these patients likely “had” sepsis (infection plus AKI) even if it could not be identified at enrollment.
Many authors have lamented the difficulty associated with screening for sepsis among hospitalized patients. Pooled results for 23 studies including more than 150,000 non-ICU patients illustrate that quick SOFA has poor sensitivity for sepsis mortality (51%) and organ failure (47%) (15). Numerous studies have shown that urinary (TIMP-2) × (IGFBP7) is sensitive and specific for AKI including for patients with sepsis (5, 16–18). Many patients with infection are admitted to hospital, and, in the absence of shock or respiratory failure, few are admitted to ICU. However, AKI can have significant consequences in such patients. Murugan et al (19) found that AKI occurred in a third of patients admitted to hospital with community-acquired pneumonia (only 16% to the ICU), and yet, mortality at 1 year was three-fold higher in patients with stage 2–3 AKI. Our patients in this study were all admitted to ICU, and yet, an additional 8% of patients with infection could be diagnosed with sepsis without waiting for AKI to manifest by changes in urine output or serum creatinine. These patients could therefore have been treated for sepsis much earlier, potentially improving outcome. These treatments could have included broader spectrum, faster initiation of IV antibiotics, greater emphasis on source control (e.g., more diagnostic imaging), more use of functional hemodynamic monitoring, etc.
The downside to this approach is that for every four positive tests, one would be a false positive. These patients would receive more expensive care, including more ICU admissions and more invasive monitoring. They might also receive antibiotics generally reserved for patients with sepsis. These measures would increase costs and risks for these patients, even if only slightly. However, these risks need to be balanced against missing sepsis in nearly one of 10 cases.
Importantly, we can envision clinical implementation of these findings in a number of settings. First, sepsis can be challenging to diagnose in the presence of organ failures that appear to be due to the primary disease or condition (e.g., shock in a patient following cardiac surgery or hypoxia in a patient with an exacerbation of chronic lung disease). Thus, patients with infection who are in the ICU for another reason are at particularly high risk for sepsis, and screening for AKI might be very useful. Patients like this are represented in our study. Second, patients being admitted to the hospital outside the ICU are not as closely monitored. Kidney function in particular is not well tracked outside the ICU where Foley catheters are actively discouraged, and serum creatinine is rarely measured more than once daily. In these patients, measures of AKI biomarkers might also be helpful. However, we did not include these types of patients in our study, and because the risk for AKI will be lower, false-positive rates will increase. Thus, we recommend further study in patients outside the ICU or similar high-risk groups. A third category of patients might be those being evaluated in the emergency department with infection and under consideration for admission. Diagnosing organ failure in this population would significantly alter the care plan. However, this group might include very low-risk patients, and thus care needs to be taken to select patients for testing. A recent study in patients not restricted to infection (20) used three criteria to assess high risk for AKI: 1) critical illness, 2) two or more systemic inflammatory response syndrome criteria (21), or 3) triaged for immediate or highly urgent treatment based on the manchester triage system (22). Using these criteria, the event rate for stage 2–3 AKI among patients with urinary (TIMP-2) × (IGFBP7) greater than 0.3 was more than 30%.
Finally, across our entire cohort, we found important differences in 9-month survival when patients with AKI tested positive with urinary (TIMP-2) × (IGFBP7) compared with when AKI occurred without a positive biomarker. Both patients with stage 1 with (TIMP-2) × (IGFBP7) greater than 1 and stage 2–3 with (TIMP-2) × (IGFBP7) greater than 2.0 exhibited worse survival compared with biomarker-negative AKI at each respective stage. These results are consistent with those reported recently by Joannidis et al (23) in which biomarker-positive stage 1 AKI behaved like stage 2–3 AKI in terms of outcomes such as death, dialysis, and persistent stage 3.
The primary study was not designed to test the hypotheses we sought to address with this secondary analysis. As such, there are important limitations. For example, patients in the “infection only” group may have developed AKI for reasons other than sepsis—such as from a nephrotoxic drug. Although this is possible, it seems unlikely given the strong association with mortality. Second, we do not know when or if patients in the infection group were diagnosed with sepsis, only that it was not recorded in the medical record at the time of enrollment. The clinical team may have made the diagnosis based on other criteria, or they may never have diagnosed it. Similarly, we did not collect detailed information on the care these patients received such as the use of functional hemodynamic monitoring, so we cannot comment on whether care would have changed if the diagnosis of sepsis was made sooner. Nevertheless, we can say that for these patients, the biomarker correctly identified AKI several hours before it could be diagnosed using standard criteria.
In conclusion, urinary (TIMP-2) × (IGFBP7) could identify AKI as a sepsis-defining organ failure in patients with infection several hours before clinical diagnosis with less than 30% of positive test results being false positives.
Supplementary Material
Footnotes
A complete list of Sapphire Investigators is provided in the Appendix (Supplemental Digital Content 1, http://links.lww.com/CCM/G111).
Clinical Trial Registration: NCT01209169.
The parent study was designed by Drs. Kellum, Kampf, McPherson and Chawla and was conducted by Drs. Artigas, Gunnerson, Honore, Nguyen, Rimmel,o Shapiro, and Vincent. Dr. Kellum conceived of the current study and drafted the article. Dr. Shi performed the statistical analysis. Drs. Kellum, Kampf, Kwan, and McPherson interpreted the results, and all authors reviewed and revised the article. Dr. Kellum takes responsibility for the content of the article, including the data and analysis.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
The study sponsor (Astute Medical) was primarily responsible for design of the parent study and data collection; the current study was designed and interpreted by the authors.
Dr. Kellum’s institution received funding from Astute Medical and bioMerieux. Drs. Kellum, Rimmelé, Shi, and Chawla received funding from Astute Medical. Drs. Kampf, Kwan, and McPherson are employees of Astute Medical/bioMerieux; Drs. Kellum, Rimmelé, Shi, and Chawla are paid consultants of Astute Medical/bioMerieux; and Dr. Kellum has received grant support from Astute Medical/bioMerieux apart from the current work. Dr. Artigas’s institution received funding from Lilly Foundation, and he received funding from Grifols, Fisher & Paykel. Drs. Kampf, Kwan, and McPherson received funding from Astute Medical (employee). Drs. Nguyen’s and Shapiro’s institutions received funding from Astute Medical. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: For the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016; 315:762–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent JL, Sakr Y, Sprung CL, et al. Sepsis Occurrence in Acutely Ill Patients Investigators Sepsis in European intensive care units: Results of the SOAP study. Crit Care Med. 2006; 34:344–353 [DOI] [PubMed] [Google Scholar]
- 3.Kellum JA, Chawla LS, Keener C, et al. ProCESS and ProGReSS-AKI Investigators The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am J Respir Crit Care Med. 2016; 193:281–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bihorac A, Chawla LS, Shaw AD, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014; 189:932–939 [DOI] [PubMed] [Google Scholar]
- 5.Honore PM, Nguyen HB, Gong M, et al. Sapphire and Topaz Investigators Urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 for risk stratification of acute kidney injury in patients with sepsis. Crit Care Med. 2016; 44:1851–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013; 17:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) Work Group KDIGO clinical practice guildeline for acute kidney injury. Kidney Int Suppl. 2012; 2:1–138 [Google Scholar]
- 8.von Elm E, Altman DG, Egger M, et al. STROBE Initiative The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med. 2007; 147:573–577 [DOI] [PubMed] [Google Scholar]
- 9.Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD Group STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015; 351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991; 100:1619–1636 [DOI] [PubMed] [Google Scholar]
- 11.Vincent JL, Moreno R, Takala J, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. 1996; 22:707–710 [DOI] [PubMed] [Google Scholar]
- 12.Landoni G, Bove T, Székely A, et al. Reducing mortality in acute kidney injury patients: Systematic review and international web-based survey. J Cardiothorac Vasc Anesth. 2013; 27:1384–1398 [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006; 34:1589–1596 [DOI] [PubMed] [Google Scholar]
- 14.Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014; 370:1683–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song JU, Sin CK, Park HK, et al. Performance of the quick sequential (sepsis-related) organ failure assessment score as a prognostic tool in infected patients outside the intensive care unit: A systematic review and meta-analysis. Crit Care. 2018; 22:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maizel J, Daubin D, Vong LV, et al. Urinary TIMP2 and IGFBP7 identifies high risk patients of short-term progression from mild and moderate to severe acute kidney injury during septic shock: A prospective cohort study. Dis Markers. 2019; 2019:3471215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuartero M, Ballús J, Sabater J, et al. Cell-cycle arrest biomarkers in urine to predict acute kidney injury in septic and non-septic critically ill patients. Ann Intensive Care. 2017; 7:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godi I, De Rosa S, Martino F, et al. Urinary [TIMP-2] × [IGFBP7] and serum procalcitonin to predict and assess the risk for short-term outcomes in septic and non-septic critically ill patients. Ann Intensive Care. 2020; 10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murugan R, Karajala-Subramanyam V, Lee M, et al. Genetic and Inflammatory Markers of Sepsis (GenIMS) Investigators Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010; 77:527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schanz M, Wasser C, Allgaeuer S, et al. Urinary [TIMP-2]·[IGFBP7]-guided randomized controlled intervention trial to prevent acute kidney injury in the emergency department. Nephrol Dial Transplant. 2019; 34:1902–1909 [DOI] [PubMed] [Google Scholar]
- 21.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee. American College of chest physicians/society of critical care medicine. Chest. 1992; 101:1644–1655 [DOI] [PubMed] [Google Scholar]
- 22.Mackway-Jones K, Marsden J, Windle J. 2014, Emergency Triage: Hoboken, NJ, John Wiley & Sons [Google Scholar]
- 23.Joannidis M, Forni LG, Haase M, et al. Sapphire Investigators Use of cell cycle arrest biomarkers in conjunction with classical markers of acute kidney injury. Crit Care Med. 2019; 47:e820–e826 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


