Supplemental Digital Content is available in the text.
Keywords: capillaries, coronavirus disease 2019, hypoxia, inflammation, microcirculation, sublingual
OBJECTIVES:
In this study, we hypothesized that coronavirus disease 2019 patients exhibit sublingual microcirculatory alterations caused by inflammation, coagulopathy, and hypoxemia.
DESIGN:
Multicenter case-controlled study.
SETTING:
Two ICUs in The Netherlands and one in Switzerland.
PATIENTS:
Thirty-four critically ill coronavirus disease 2019 patients were compared with 33 healthy volunteers.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
The microcirculatory parameters quantified included total vessel density (mm × mm–2), functional capillary density (mm × mm–2), proportion of perfused vessels (%), capillary hematocrit (%), the ratio of capillary hematocrit to systemic hematocrit, and capillary RBC velocity (μm × s–1). The number of leukocytes in capillary-postcapillary venule units per 4-second image sequence (4 s–1) and capillary RBC microaggregates (4 s–1) was measured. In comparison with healthy volunteers, the microcirculation of coronavirus disease 2019 patients showed increases in total vessel density (22.8 ± sd 5.1 vs 19.9 ± 3.3; p < 0.0001) and functional capillary density (22.2 ± 4.8 vs 18.8 ± 3.1; p < 0.002), proportion of perfused vessel (97.6 ± 2.1 vs 94.6 ± 6.5; p < 0.01), RBC velocity (362 ± 48 vs 306 ± 53; p < 0.0001), capillary hematocrit (5.3 ± 1.3 vs 4.7 ± 0.8; p < 0.01), and capillary-hematocrit-to-systemic-hematocrit ratio (0.18 ± 0.0 vs 0.11 ± 0.0; p < 0.0001). These effects were present in coronavirus disease 2019 patients with Sequential Organ Failure Assessment scores less than 10 but not in patients with Sequential Organ Failure Assessment scores greater than or equal to 10. The numbers of leukocytes (17.6 ± 6.7 vs 5.2 ± 2.3; p < 0.0001) and RBC microaggregates (0.90 ± 1.12 vs 0.06 ± 0.24; p < 0.0001) was higher in the microcirculation of the coronavirus disease 2019 patients. Receiver-operating-characteristics analysis of the microcirculatory parameters identified the number of microcirculatory leukocytes and the capillary-hematocrit-to-systemic-hematocrit ratio as the most sensitive parameters distinguishing coronavirus disease 2019 patients from healthy volunteers.
CONCLUSIONS:
The response of the microcirculation to coronavirus disease 2019-induced hypoxemia seems to be to increase its oxygen-extraction capacity by increasing RBC availability. Inflammation and hypercoagulation are apparent in the microcirculation by increased numbers of leukocytes and RBC microaggregates.
The coronavirus disease 2019 (COVID-19) epidemic has brought a wide range of challenges in intensive care medicine related to the understanding of its pathophysiology, pathogenesis, immunology, and therapeutic resolution. Unresolved COVID-19 leads to acute respiratory distress syndrome (ARDS)-induced hypoxemia associated with a state of hyperinflammation and procoagulation requiring intensive care admission for invasive mechanical ventilation as well as virus induced organ damage (1, 2). COVID-19-induced ARDS, however, differs markedly in its phenotype in comparison with the better understood bacterial sepsis-induced ARDS although there may be important similarities (3).
Endothelial cell dysfunction has been suggested to be a central unifying event in the pathogenesis of sepsis in general and in COVID-19 in particular (4–8). Such endothelial cell injury may cause microcirculatory dysfunction, which could underlie the deterioration of oxygen transport seen in COVID-19. The importance to investigate the nature of microcirculatory alterations induced by COVID-19 has been suggested (1, 9–11), although as yet not adequately investigated.
Handheld vital microscopes used to investigate the sublingual microcirculation of patients has identified microcirculatory alterations as a key event in the states of critical illness (12). These have shown a high sensitivity and specificity for identifying the underlying pathophysiology and for predicting outcome in septic shock patients independent of changes in systemic hemodynamic variables (13–15). Recently, we developed and clinically validated an automatic image analysis platform for quantitative analysis of microcirculatory hemodynamic parameters (16, 17) and developed methodologies to measure microcirculatory leukocyte-endothelial interactions from sublingual microcirculatory images (18, 19).
With these tools, we undertook a multicenter microcirculation study in COVID-19 ICU patients. We formulated three hypotheses: 1) there are microcirculatory alterations in response to COVID-19-induced ARDS, 2) there is an increased presence of leukocytes in the microcirculation in response to inflammation, and 3) there are RBC microthrombi due to coagulopathy present in the microcirculation.
MATERIALS AND METHODS
Inclusion of Patients
About 38 intensive care patients with ARDS associated with COVID-19 were included in this study. The diagnosis of ARDS was confirmed according to the Berlin criteria (20).
Sublingual microcirculation measurements were carried out in patients following optimization of ventilatory and hemodynamic settings in centers including the Department of Intensive Care, Erasmus Medical Center, Rotterdam, The Netherlands; the Department of Intensive Care, University Hospital of Zurich, Zürich, Switzerland; and Haga Hospital, The Hague, The Netherlands.
A group of 41 healthy volunteers (University Hospital of Zurich) who participated in a previous study (21) were included as a control group.
Microcirculatory Measurements
Sublingual microcirculatory measurements were performed using handheld vital microscopes based on incident dark field microscopy imaging (22) (Braedius Medical, Huizen, The Netherlands) and automatically analyzed by using full-frame analysis of the MicroTools automatic software (Supplemental Table 1, http://links.lww.com/CCM/G161; and Supplemental Fig. 1A, http://links.lww.com/CCM/G162 [legend, http://links.lww.com/CCM/G170]) (16,17) (Active Medical, Leiden, The Netherlands). Microcirculatory leucocytes and RBC microaggregates were analyzed using spacetime diagrams (18, 19). These microcirculatory methodologies are described in Supplemental text A (http://links.lww.com/CCM/G160).
Statistics
Microcirculatory hemodynamics were compared between the COVID-19 patients and the volunteers using linear mixed-model analysis (23, 24), a description of which can be found in Supplemental text B (http://links.lww.com/CCM/G163).
Ethics Approval
In Rotterdam and The Hague, the microcirculation measurements were approved by the ethics protocol MEC2018-1572. In Zürich, the ethics protocol was approved under BASEC-ID2020–00646.
RESULTS
Sublingual microcirculation measurements were collected at the three institutions with 38 COVID-19 patients and compared with a cohort of 41 healthy volunteers from a previous study (25). For each patient, a measurement was made at the discretion of the intensivist at a time point where ventilatory and circulatory parameters were optimally adjusted. Four patients and eight volunteers did not meet the quality criteria and were excluded from the study. The number of recordings that met the quality inclusion criteria for measurements is summarized in Supplemental Table 2 (http://links.lww.com/CCM/G164).
Clinical Demographics
All patients included in the study had a confirmed severe acute respiratory syndrome coronavirus 2 infection with moderate-to-severe ARDS and were invasively mechanically ventilated. The mean age of the COVID-19 patients was 59.18 ± 11.7 years. About 27% of the patients died in intensive care. The characteristics, physiologic status, management, and outcome of the patients and the physiologic properties of the volunteers can be found in Supplemental Table 3 (http://links.lww.com/CCM/G165).
Systemic and Microhemodynamics
The mean arterial pressure was similar between the COVID-19 patients and the healthy volunteers but their heart rate (HR) was significantly higher (Fig. 1, C and D). The systemic hemoglobin concentration and systemic hematocrit (sHct) were significantly lower in COVID-19 patients than in volunteers (Fig. 1, A and B).
Figure 1.
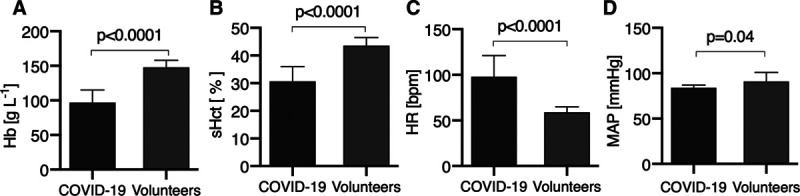
The coronavirus disease 2019 (COVID-19) patients had a lower hemoglobin (Hb) (A) and systemic hematocrit (sHct) (B) and a higher heart rate (HR) (C) than volunteers. D, The mean arterial pressure (MAP) in the COVID-19 patients and volunteers was similar.
The COVID-19 patients were considered as hypoxemic, as indicated by their Pao2 values (69 ± 12 mm Hg) and mean oxygenation index (Pao2/Fio2 = 149 ± 65 mm Hg). Other clinical variables can be found in Supplemental Table 3 (http://links.lww.com/CCM/G165).
Quantitative analysis of images to identify alterations in microcirculatory hemodynamics was carried out using MicroTools (Supplemental Table 1, http://links.lww.com/CCM/G161) (Fig. 2). The COVID-19 patients showed a higher total vessel density (TVD) and functional capillary density (FCD) than the volunteers (Fig. 2B). The proportion of perfused vessel (PPV) in the COVID-19 patients and volunteers, however, was similarly close to 100% (Fig. 2C).
Figure 2.
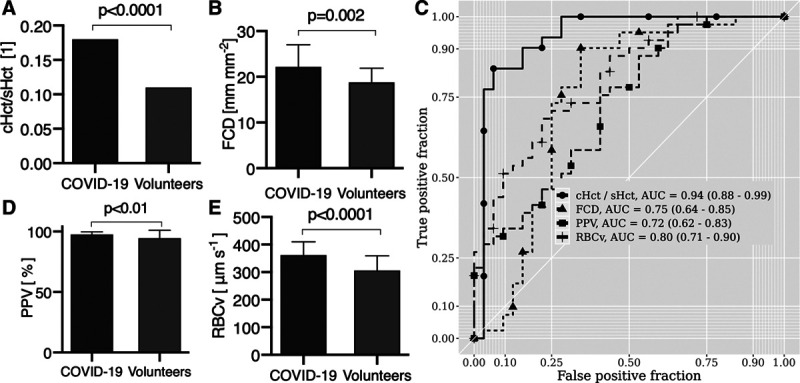
Analysis of the microhemodynamics of the coronavirus disease 2019 (COVID-19) patients in comparison with those of the volunteers. COVID-19 patients had higher ratio capillary-to-systemic hematocrit (cHct/sHct) ratio (A) than in volunteers and higher functional capillary density (FCD) (B) than the volunteers. C, There was no evidence of plugged vessels in the COVID-19 patients as evidenced by a proportion of perfused vessel (PPV) > 94% in both groups. D, RBC velocity (RBCv) was significantly higher in COVID-19 patients than in the volunteers. E, Receiver-operating-characteristic curve analysis of the different microcirculatory hemodynamic parameters identifies cHct/sHct and RBCv as the parameters with the highest specificity and sensitivity for distinguishing the COVID-19 patients from the healthy volunteers. AUC = area under the curve.
An important finding was that, despite a lower sHct, capillary hematocrit (cHct) was higher in the COVID-19 patients than in volunteers (Supplemental Table 4, http://links.lww.com/CCM/G166), resulting in a significantly higher cHct/sHct ratio in the COVID-19 patients than in the volunteers indicating a shift of the RBC mass from the systemic circulation to the microcirculation (Fig. 2A). Furthermore, COVID-19 patients exhibited significantly higher RBC velocity (RBCv) than the volunteers (Fig. 2D).
Receiver-operating-curve (ROC) analysis was used to identify microcirculatory hemodynamic parameters with the highest specificity and sensitivity for distinguishing COVID-19 patients from healthy volunteers. Results showed that RBCv and cHct/sHct were the most sensitive parameters in identifying COVID-19 patients (Fig. 2E).
Systemic Inflammation and Leukocytes in the Microcirculation
COVID-19 patients showed inflammatory activation with elevated C-reactive protein (176.9 ± 115.7 mg × L–1) and procalcitonin (median of 0.9 [0.3–2.6] μg × L–1) levels. In a portion of the patients, interleukin-6 (n = 20) was measured higher than reference values. Furthermore, a significant increase in leukocyte count was found in drawn blood samples (13.16 ± 5.09 × 109 L–1) (Supplemental Table 3, http://links.lww.com/CCM/G165). No such measurements were performed in volunteers.
Spacetime-diagram analysis (18) (Fig. 3B) of capillary-postcapillary venule units (Supplemental Fig. 1B, http://links.lww.com/CCM/G162 [legend, http://links.lww.com/CCM/G170]; and Fig. 3A) showed a significantly higher microcirculatory leukocyte numbers in COVID-19 patients than in volunteers (for moving images, see Supplemental Video 1, http://links.lww.com/CCM/G167; Supplemental Video 2, http://links.lww.com/CCM/G168; and Supplemental Video 3, http://links.lww.com/CCM/G169 [legend, http://links.lww.com/CCM/G170]) (Fig. 3C).
Figure 3.
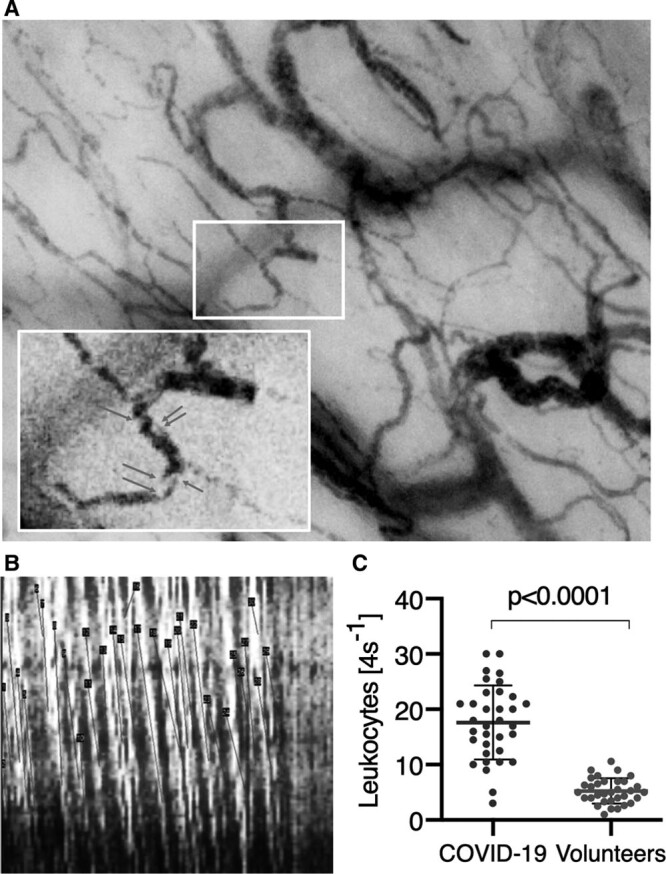
Microcirculatory leukocytes in coronavirus disease 2019 (COVID-19) patients. A, Examples of leukocytes present in the microcirculation are shown. B, Presence of leukocytes can be seen in a space time diagram as slanted white lines as they travel down the length of a capillary (y-axis) over time (x-axis). The number of such white lines per 4-s clip is used to calculate the number of leukocytes. The dark slanted lines measure the presence of the RBC lines. The slopes of these lines are used to calculate RBC velocities. C, The total number of leukocytes measured in capillary-postcapillary venule units shows that there are significantly more microcirculatory leukocytes in the COVID-19 patients than in the volunteers.
Coagulation and Microaggregates in the Microcirculation
The hypercoagulable state of COVID-19 patients was reflected by raised d-dimer concentration levels (2.5 [1.2–4.5) mg × L–1). In the microcirculation of 22 of the 34 COVID-19 patients, RBC microaggregates quantified using spacetime-diagram analysis (Fig. 4D) were observed (Fig. 4C; for moving images, see Supplemental Video 1, http://links.lww.com/CCM/G167; Supplemental Video 2, http://links.lww.com/CCM/G168; and Supplemental Video 3, http://links.lww.com/CCM/G169 [legend, http://links.lww.com/CCM/G170]). Microaggregates were found in only two of the 33 volunteers. In three patients, microaggregates obstructing capillary flow were observed. No such obstructions were observed in the volunteers.
Figure 4.
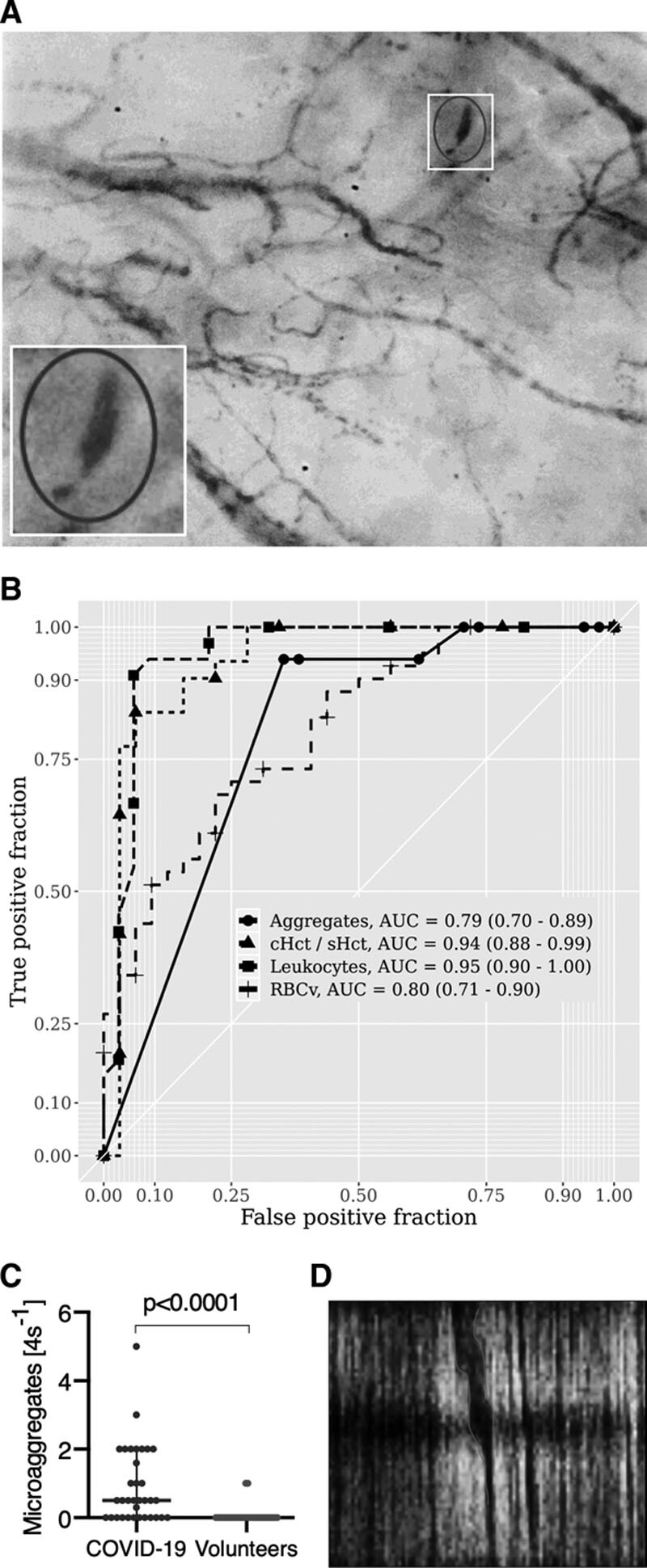
Microaggregates in coronavirus disease 2019 (COVID-19) patients. A, An example of a plugged vessel with microaggregates. B, Receiver-operating-characteristic curve analysis of the number of leukocytes, RBC microaggregate, the capillary-to-systemic hematocrit (cHct/sHct) ratio, and RBC velocity (RBCv) show that the number of leukocytes, cHct/sHct, and RBCv show the highest sensitivity and specificity for distinguishing COVID-19 patients from volunteers. C, The raised number of microaggregates measured in the image segments of the COVID-19 patients was much higher than that found in the volunteers. D, Presence of an RBC microaggregate as a dark thick band in the space time diagram indicative of RBC clumping in this capillary vessel. AUC = area under the curve.
ROC curve analysis comparing COVID-19 patients with healthy volunteers identified RBCv and cHct/sHct ratio in combination with raised leukocyte and RBC microaggregate numbers as the microcirculatory parameters with the highest specificity and sensitivity for distinguishing COVID-19 patients from the volunteers (Fig. 4B).
To investigate whether these microcirculatory alterations were related to the clinical variables, we investigated the dependency of microcirculatory alterations on Pao2/Fio2 ratio (≥ 150 vs < 150 mm Hg), on days from ICU admission to inclusion (≤ 7 vs > 7d ICU), and on Sequential Organ Failure Assessment (SOFA) scores (< 10 vs ≥ 10). No dependency of microcirculatory alterations was found on Pao2/Fio2 or on ICU days (data not shown). However, we found that the ability of the microcirculation to increase its cHct/sHct ratio and FCD was only present in patients whose SOFA scores was less than 10, whereas those patients with higher SOFA did not exhibit this microcirculatory adaptive response associated with increased oxygen-extraction capacity. In addition, the patients with higher SOFA scores exhibited higher levels of leukocytes and microaggregates (Table 1).
TABLE 1.
Functional Parameters of the Microcirculation in Critically Ill Coronavirus Disease 2019 Patients Are Dependent on the Severity of Disease
| Microcirculatory Parameters | COVID-19 Patients | Healthy Volunteers (n = 33 Patients) | pDISEASE STATE | pSOFA | ||
|---|---|---|---|---|---|---|
| Overall (n = 34 Patients) | COVID-19 Patients, SOFA < 10 (n = 22 Patients) | COVID-19 Patients, SOFA ≥ 10 (n = 12 Patients) | ||||
| Total vessel density (mm mm–2) | 22.8 ± 5.1 | 23.6 ± 5.3 | 20.8 ± 4.2 | 20.0 ± 3.3 | < 0.001 | 0.02 |
| Functional capillary density (mm mm–2) | 22.2 ± 4.8 | 22.95 ± 4.96 | 20.31 ± 3.98 | 18.9 ± 3.2 | < 0.0001 | 0.02 |
| Proportion of perfused vessels (%) | 97.6 ± 2.1 | 0.98 ± 0.02 | 0.98 ± 0.02 | 0.95 ± 0.06 | 0.02 | 0.94 |
| RBC velocity (μm s–1) | 362 ± 48 | 356 ± 50 | 376 ± 40 | 306 ± 53 | < 0.0001 | 0.29 |
| Capillary hematocrit (%) | 5.3 ± 1.3 | 5.5 ± 1.3 | 4.8 ± 0.8 | 4.7 ± 0.8 | < 0.01 | 0.02 |
| Capillary-to-systemic hematocrit ratio (1) | 0.18 ± 0.0 | 0.18 ± 0.04 | 0.17 ± 0.04 | 0.11 ±0.0 | < 0.0001 | 0.57 |
| Leukocytes (4 s–1) | 17.6 ± 6.7 | 16.5 ± 6.2 | 19.6 ± 7.5 | 5.2 ±2.3 | < 0.0001 | 0.09 |
| Microaggregates (4 s–1) | 0.90 ± 1.12 | 0.80 ± 0.87 | 1.12 ± 1.51 | 0.00 ± 0.00 | < 0.0001 | 0.28 |
SOFA = Sequential Organ Failure Assessment score.
Data are presented as the mean ± sd. p values are given for linear mixed-model analysis with disease state and SOFA subgroups as fixed effects and individual per-patient random slopes as random effects. Overall differences between the coronavirus disease 2019 patients and healthy volunteers are represented by pDISEASE STATE and were evaluated using a likelihood ratio test of the full model versus a partial model disregarding disease state as the effect in question. Subgroup analysis to determine individual effects of patients with high or low SOFA scores is represented by pSOFA and was calculated using Satterthwaite approximation.
DISCUSSION
This multicenter study reports, to our knowledge, the first direct observation of recruitment of the microcirculation (increased vessel and FCD), increased capillary Hct, and large numbers microcirculatory of leukocytes and RBC aggregates as a result of the microhemodynamic response to hypoxemia, hyperinflammation, and hypercoagulation in the sublingual microcirculation of COVID-19 patients. The COVID-19 patients showed elevated values of TVD, FCD, RBCv, cHct, and cHct/sHct ratio in the presence of almost 100% perfused vessel density in comparison with the microcirculatory parameters of the volunteers. ROC curve analysis identified that a combination of cHct/sHct ratio, RBCv, and leukocyte count provided the most sensitive and specific parameters for distinguishing COVID-19 patients from healthy volunteers.
The increase in FCD, cHct, and cHct/sHct ratio found in the COVID-19 patients is consistent with the action of a microcirculatory compensatory mechanism to increase oxygen extraction in reaction to the COVID-19-induced hypoxemia in the presence of hyperinflammatory and hypercoagulatory states. Despite the presence of RBC microaggregates in the COVID-19 patients, a little to no obstructions of capillaries associated with a reduction in FCD such as seen in bacterial septic patients were observed (13–15). Systemic hemodynamic variables showed normal blood pressure values with slightly elevated lactate levels, but with high HR values and low systemic hemoglobin and Hct values despite the presence of ARDS with clear signs of a hypercoagulation state and raised systemic leukocyte counts. Of specific interest was the finding that the most severely ill patients with SOFA scores exceeding 10 had lost the ability of the microcirculation to increase its microcirculatory oxygen-extraction capacity by increasing its FCD and cHct as an adaptive response to hypoxemia. In addition, the patients with the higher SOFA scores also had higher levels of microcirculatory leukocytes and microaggregates in comparison with the COVID-19 patients with SOFA score was less than 10.
The increased TVD, FCD, cHct, and cHct/sHct, in the presence of an almost 100% perfused vessel density, were an important characteristic finding regarding the microcirculatory response to COVID-19. Under resting physiologic conditions, 30% of microvessels are not filled with RBCs and can be recruited under conditions of enhanced oxygen requirements. Our finding of an elevated FCD, increased RBCv, and a shift in RBCs from the systemic circulation to the microcirculation suggests the presence of an activated microcirculatory reserve capacity in response to hypoxemia, which can be identified by sublingual application of topical nitroglycerine (21). This physiologic compensatory reaction to increase the oxygen-extraction capacity by decreasing diffusion distances between the capillaries (increased TVD) and increasing convection of the RBCs seems to be intact in COVID-19 patients with SOFA scores less than 10 but not in patients with SOFA greater than or equal to 10. Similar microcirculatory compensatory mechanisms have been reported in high-altitude microcirculation studies in the Himalayas, where hypoxia also increased sublingual (25) and labial frenulum (26) microcirculatory TVD. The present findings of increases in TVD, RBCv, cHct, and cHct/sHct-ratio have previously also been shown to occur in early animal experiments studying the response of the microcirculation to hypoxia (27, 28). At high altitude, subjects have a surprising ability to cope with quite low levels of oxygen (arterial oxygen pressures of 24 mm Hg at the summit of Mount Everest) (29). The similar ability of COVID-19 patients to cope with low levels of arterial Po2 has even led to the use of the term “happy hypoxia” to describe this phenomenon (30). That such low levels of oxygen that can be tolerated in COVID-19-induced ARDS could be explained by our findings of an intact microcirculatory regulatory and respiratory system that is able to increase the oxygen-extraction capacity of the microcirculation by decreasing diffusion distances between the capillaries (increased TVD) and increasing convection of the RBCs.
COVID-19 patients were fluid-positive and had low levels of systemic hemoglobin and Hct. Although we interpreted these effects to be in response to hypoxia, they could also have been caused by iatrogenic hemodilution. However, if these low systemic hemoglobin values were due to hemodilution, a parallel decrease in TVD would have been expected as shown in cardiac surgery patients undergoing hemodilution (31).
The compensatory response of the microcirculation to hypoxemia found here in COVID-19 sepsis has not been described in earlier sublingual microcirculatory studies in conventional sepsis. Here, without exception, TVD, PPV, FCD, and microcirculatory flow were found to be reduced, explaining the oxygen-extraction deficit characteristic of these forms of sepsis (13, 14, 32). In a recent sublingual microcirculatory study in septic ARDS patients having 24-hour Pao2/Fio2 ratios (173 mm Hg) (33) similar to those in our COVID-19 ARDS patients (149 mm Hg), a 25% reduced microcirculatory flow and a reduced PPV were found. This is in contrast to our COVID-19 ARDS patients who showed increased microcirculatory flow as well as increased FCD with 100% perfused vessels. In addition, the microcirculatory response to COVID-19 may also be different in other types of viral sepsis. This is suggested by an earlier sublingual microcirculation study in H1N1-induced viral sepsis that demonstrated reduced microcirculatory RBC flow and PPV (34), unlike the COVID-19-induced viral sepsis described in this study.
This is the first report of the finding of large numbers of leukocytes in the microcirculation of COVID-19 patients. The presence of microcirculatory leukocytes and RBC microaggregates is in parallel with increased levels of leukocytes, enhanced levels of prothrombotic markers, and raised inflammatory biomarkers obtained from blood samples. In contrast to the microcirculatory function parameters that indicate adaptation to hypoxemia, these changes are most likely related to virus-induced inflammation and hypercoagulability. In a previous brief communication reporting sublingual microcirculation in a series of 12 COVID-19 patients, an inverse relation between TVD and d-dimer values was found, an effect that we did not find (35). This study also found a reduced perfused vessel density in the COVID-19 patients, an effect we also did not find.
Microcirculatory RBC aggregates are a rare occurrence with only a case study being reported, where these were elicited by the administration of protamine following cardiac surgery (19). Those such RBC microaggregates can obstruct capillaries in COVID-19 were seen in only three cases in the present cohort.
Limitations in our study are several-fold. It may be that the sublingual microcirculatory alterations do not reflect that of other organs such as the lung. This could be quite likely as the lung microcirculation is probably more compromised than the sublingual microcirculation, explaining the reduced oxygen-extraction capacity of the lung causing arterial hypoxemia. In addition, other mechanisms than an adaptive response of the microcirculation to hypoxemia could explain the observed increase in FCD and cHct seen in the COVID patients. Those such alterations could instead be associated with microcirculatory damage associated with COVID-19 infections cannot be excluded. For example, capillary leak of plasma associated with vascular barrier dysfunction could also result in an increase in cHct. However, there was not much evidence of tissue edema in our patients such as a high positive fluid balance or high central venous pressure. An increase in FCD could also be caused by an increase in blood viscosity because of increased number of leukocytes. However, such an effect could not explain the increase in cHct observed. The observation that in high SOFA score patients, the increases in FCD and cHct are absent that would support our explanation that the microcirculatory response to COVID-19 seen in the lower SOFA score patients may be the result of the presence of an intact compensatory response to hypoxemia. A further limitation of our study may be that a more appropriate control group would have been a group of COVID-19-negative patients suffering from ARDS. However, at the time of the measurements, due to the overwhelming presence of COVID-19 patients in our ICUs, we did not have such a control group.
A further limitation of our study may be the heterogeneity regarding the timing (days since diagnosis/ICU admission) of measurement. Additionally, biomarker investigations aimed at endothelial dysfunction and glycocalyx degradation may have provided additional information about the effects of inflammation on endothelial cell function. In our study, we had hoped to identify abnormal leukocyte kinetics indicative of such endothelial injury (4) as we had identified in the states of inflammation during cardiac surgery and sepsis (19, 32). However, the high RBCv in the microcirculation precluded the observation of such kinetics.
CONCLUSIONS
This study identified several microcirculatory alterations associated with the inflammatory response and hypoxemia found in COVID-19 patients. The increase in oxygen-extraction capacity of the microcirculation of the COVID-19 patients by its increase in its FCD in parallel with an increase in cHct was similar to that which occurs in healthy individuals during exposure to hypoxia at high altitude. Our results also showed that the microcirculation exhibited increases in leukocytes and microaggregates in parallel with those in the systemic circulation. In summary, quantitative measurement of the response of the microcirculation to COVID-19 may provide an additional patient-friendly noninvasive measurement directed at understanding the physiologic response of the patient to COVID-19 and monitoring the response to therapy. Future studies should be conducted to investigate the response of the microcirculation to new therapies such as anti-inflammatory and anticoagulant therapies, especially in the COVID-19 patients with high SOFA scores.
ACKNOWLEDGMENTS
We thank the colleagues, who have helped in the care of the patients and helped us to obtain the needed data for this study under difficult circumstances, for their dedication and help in this study:
From the Department of Intensive Care Erasmus Medical Center, we thank Peter Somhorst for his help with the measurements of our patients.
From the Department of Intensive Care Zurich University Hospital, we thank the Zurich ICU-COVID research group for the help in our investigation. University Hospital of Zurich, Institute for Intensive Care: Jan Bartussek; Phillip Buehler; Dorothea Monika Heuberger; Martina Anna Maibach; Schuepbach Reto Andreas; Department of Infectious Diseases and Hospital Epidemiology, University Hospital of Zurich: Silvio Brugger; Srikanth Mairpady Shambat; Annelies Zinkernagel.
From the Department of Intensive Care, Haga Hospital, The Hague: We thank the intensivists Rémon Baak, Sanjeev Grewal, Tim Jansen, Mirelle Koeman, Jeroen Ludikhuize, Iwan Meynaar, Ralph Nowitzky, Thomas Ottens, and Ilse Purmer. We also thank the research nurse Lettie van den Berg and the Circulation Practitioner Linda van Dorsten for their support of our investigation.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Drs. Favaron, Ince, and Hilty contributed equally.
Dr. Ince has received honoraria and independent research grants from Fresenius-Kabi, Bad Homburg, Germany; La Jolla Pharmaceutical, La Jolla, CA; and Cytosorbents Monmouth, NJ. He has developed sidestream dark field imaging, which is the handheld video microscope and is listed as the inventor on related patents commercialized by MicroVision Medical (MVM) under a license from the Academic Medical Center. He receives no royalties or benefits from this license. He has been a consultant for MVM in the past but has not been involved with this company for more than 5 years now and holds no shares of stock. Braedius Medical, a company owned by a relative of Dr. Ince, has developed and designed the incident dark field device used in this study. Dr. Ince has no financial relationship with Braedius Medical of any sort and has never owned shares, or received consultancy or speaker fees from Braedius Medical. The MicroTools software is being developed by Dr. Hilty and owned by Active Medical BV Leiden, The Netherlands, of which Drs. Ince and Hilty are shareholders. Active Medical runs an Internet site called microcirculationacademy.org, which offers educational courses and services related to clinical microcirculation. Dr. Ince’s institution received funding from La Jolla Pharmaceuticals and Cytosorbents Monmouth, and he received funding from Fresenius-Kabi. Drs. Ince and Hilty disclosed that the MicroTools software that was used for analysis of the images in the current study is owned by Active Medical, of which Drs. Ince and Hilty own shares. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Li T, Lu H, Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020; 9:687–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Endeman H, van der Zee P, van Genderen ME, et al. Progressive respiratory failure in COVID-19: A hypothesis. Lancet Infect Dis. 2020; 20:1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gattinoni L, Coppola S, Cressoni M, et al. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020; 201:1299–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joffre J, Hellman J, Ince C, et al. Endothelial responses in sepsis. Am J Respir Crit Care Med. 2020; 202:361–370 [DOI] [PubMed] [Google Scholar]
- 5.Pons S, Fodil S, Azoulay E, et al. The vascular endothelium: The cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020; 24:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020; 220:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020; 395:1417–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020; 383:120–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martini R. The compelling arguments for the need of microvascular investigation in COVID-19 critical patients. Clin Hemorheol Microcirc. 2020; 75:27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colantuoni A, Martini R, Caprari P, et al. COVID-19 sepsis and microcirculation dysfunction. Front Physiol. 2020; 11:747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried JA, Ramasubbu K, Bhatt R, et al. The variety of cardiovascular presentations of COVID-19. Circulation. 2020; 141:1930–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ince C, Boerma EC, Cecconi M, et al. Cardiovascular Dynamics Section of the ESICM Second consensus on the assessment of sublingual microcirculation in critically ill patients: Results from a task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2018; 44:281–299 [DOI] [PubMed] [Google Scholar]
- 13.Edul VS, Enrico C, Laviolle B, et al. Quantitative assessment of the microcirculation in healthy volunteers and in patients with septic shock. Crit Care Med. 2012; 40:1443–1448 [DOI] [PubMed] [Google Scholar]
- 14.De Backer D, Donadello K, Sakr Y, et al. Microcirculatory alterations in patients with severe sepsis: Impact of time of assessment and relationship with outcome. Crit Care Med. 2013; 41:791–799 [DOI] [PubMed] [Google Scholar]
- 15.Massey MJ, Hou PC, Filbin M, et al. ProCESS Investigators Microcirculatory perfusion disturbances in septic shock: Results from the ProCESS trial. Crit Care. 2018; 22:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilty MP, Guerci P, Ince Y, et al. MicroTools enables automated quantification of capillary density and red blood cell velocity in handheld vital microscopy. Commun Biol. 2019; 2:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilty MP, Akin S, Boerma C, et al. Automated algorithm analysis of sublingual microcirculation in an international multicentral database identifies alterations associated with disease and mechanism of resuscitation. Crit Care Med. 2020; 48:e864–e875 [DOI] [PubMed] [Google Scholar]
- 18.Uz Z, van Gulik TM, Aydemirli MD, et al. Identification and quantification of human microcirculatory leukocytes using handheld video microscopes at the bedside. J Appl Physiol (1985). 2018; 124:1550–1557 [DOI] [PubMed] [Google Scholar]
- 19.Uz Z, Milstein DMJ, Ince C, et al. Circulating microaggregates during cardiac surgery precedes postoperative stroke. J Thromb Thrombolysis. 2017; 44:14–18 [DOI] [PubMed] [Google Scholar]
- 20.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012; 307:2526–2533 [DOI] [PubMed] [Google Scholar]
- 21.Hilty MP, Pichler J, Ergin B, et al. Assessment of endothelial cell function and physiological microcirculatory reserve by video microscopy using a topical acetylcholine and nitroglycerin challenge. Intensive Care Med Exp. 2017; 5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aykut G, Veenstra G, Scorcella C, et al. Cytocam-IDF (incident dark field illumination) imaging for bedside monitoring of the microcirculation. Intensive Care Med Exp. 2015; 3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bates D, Mächler M, Bolker B, et al. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015; 67:1–48 [Google Scholar]
- 24.Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang. 2008; 59:390–412 [Google Scholar]
- 25.Hilty MP, Merz TM, Hefti U, et al. Recruitment of non-perfused sublingual capillaries increases microcirculatory oxygen extraction capacity throughout ascent to 7126 m. J Physiol. 2019; 597:2623–2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilbert-Kawai E, Coppel J, Phillip H, et al. Changes in labial capillary density on ascent to and descent from high altitude. F1000Res. 2016; 5:2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher AJ, Schrader NW, Klitzman B. Effects of chronic hypoxia on capillary flow and hematocrit in rat skeletal muscle. Am J Physiol. 1992; 262:H1877–H1883 [DOI] [PubMed] [Google Scholar]
- 28.Parthasarathi K, Lipowsky HH. Capillary recruitment in response to tissue hypoxia and its dependence on red blood cell deformability. Am J Physiol. 1999; 277:H2145–H2157 [DOI] [PubMed] [Google Scholar]
- 29.Grocott MP, Martin DS, Levett DZ, et al. Caudwell Xtreme Everest Research Group Arterial blood gases and oxygen content in climbers on Mount Everest. N Engl J Med. 2009; 360:140–149 [DOI] [PubMed] [Google Scholar]
- 30.Couzin-Frankel J. The mystery of the pandemic’s ‘happy hypoxia.’ Science. 2020; 368:455–456 [DOI] [PubMed] [Google Scholar]
- 31.Atasever B, van der Kuil M, Boer C, et al. Red blood cell transfusion compared with gelatin solution and no infusion after cardiac surgery: Effect on microvascular perfusion, vascular density, hemoglobin, and oxygen saturation. Transfusion. 2012; 52:2452–2458 [DOI] [PubMed] [Google Scholar]
- 32.Fabian-Jessing BK, Massey MJ, Filbin MR, et al. ProCESS Investigators In vivo quantification of rolling and adhered leukocytes in human sepsis. Crit Care. 2018; 22:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ospina-Tascón GA, Bautista DF, Madriñán HJ, et al. Microcirculatory dysfunction and dead-space ventilation in early ARDS: A hypothesis-generating observational study. Ann Intensive Care. 2020; 10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salgado DR, Ortiz JA, Favory R, et al. Microcirculatory abnormalities in patients with severe influenza A (H1N1) infection. Can J Anaesth. 2010; 57:940–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Damiani E, Carsetti A, Casarotta E, et al. Microvascular alterations in patients with SARS-COV-2 severe pneumonia. Ann Intensive Care. 2020; 10:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


