Abstract
OBJECTIVE:
To evaluate elagolix, an oral gonadotropin-releasing hormone receptor antagonist, alone or with add-back therapy, in premenopausal women with heavy menstrual bleeding (greater than 80 mL per month) associated with uterine leiomyomas.
METHODS:
This double-blind, randomized, placebo-controlled, parallel-group study evaluated efficacy and safety of elagolix in cohorts 1 (300 mg twice daily) and 2 (600 mg daily) with four arms per cohort: placebo, elagolix alone, elagolix with 0.5 mg estradiol/0.1 norethindrone acetate, and elagolix with 1.0 mg estradiol/0.5 mg norethindrone acetate. A sample size of 65 per group was planned to compare elagolix with add-back to placebo on the primary end point: the percentage of women who had less than 80 mL menstrual blood loss and 50% or greater reduction in menstrual blood loss from baseline to the last 28 days of treatment. Safety assessments included changes in bone mineral density.
RESULTS:
From April 8, 2013, to December 8, 2015, 571 women were enrolled, 567 were randomized and treated (cohort 15259; cohort 25308), and 80% and 75% completed treatment, respectively. Participants had a mean±SD age of 43±5 years (cohort 2, 42±5 years), and 70% were black (cohort 2, 74%). Primary end point responder rates in cohort 1 (cohort 2) were 92% (90%) for elagolix alone, 85% (73%) for elagolix with 0.5 mg estradiol/0.1 mg norethindrone acetate, 79% (82%) for elagolix with 1.0 mg estradiol/0.5 mg norethindrone acetate, and 27% (32%) for placebo (all P<.001 vs placebo). Elagolix groups had significant decreases compared with placebo in lumbar spine bone mineral density, which was attenuated by adding 1.0 mg estradiol/0.5 mg norethindrone acetate.
CONCLUSION:
Elagolix with and without add-back significantly reduced menstrual blood loss in women with uterine leiomyomas. Add-back therapy reduced hypoestrogenic effects on bone mineral density.
Uterine leiomyomas are benign, estrogen- and progesterone-dependent neoplasms of the uterus and affect up to 70% of women by the age of 50 years.1 –5 The primary risk factors for uterine leiomyomas are age, race, premenopausal status, time since childbirth, and higher body mass index.6–8The incidence and prevalence of uterine leiomyomas are highest for black women.1,2,7,8
The primary symptom associated with uterine leiomyomas is heavy or prolonged menstrual bleeding, which can lead to anemia.1,9,10 Other symptoms include pelvic pain and pressure and urinary and gastrointestinal symptoms.1 Leiomyomas are also associated with infertility and pregnancy complications.11 Overall, the combined effects of these symptoms often negatively affect quality of life.9,12–14 Uterine leiomyomas are primarily managed by surgery to remove the leiomyomas or uterus or with other treatment options such as nonsteroidal antiinflammatory drugs, hormonal contraceptives, and gonadotropin-releasing hormone agonists, which are limited to short-term efficacy.15,16 The expense, adverse effects of surgery, and high burden of disease for women leave an unmet need for long-term, safe, and effective medical therapies.4,9
Elagolix is an oral, nonpeptide, gonadotropin-releasing hormone-releasing receptor antagonist, which reduces serum estradiol and progesterone concentrations in women.17–19 The objective of the present study was to evaluate the safety and efficacy of elagolix with or without hormone add-back therapy in the management of heavy menstrual bleeding in premenopausal women with uterine leiomyomas and assess the effect of hormone add-back therapy20 on reducing hypoestrogenic effects associated with elagolix alone.
MATERIALS AND METHODS
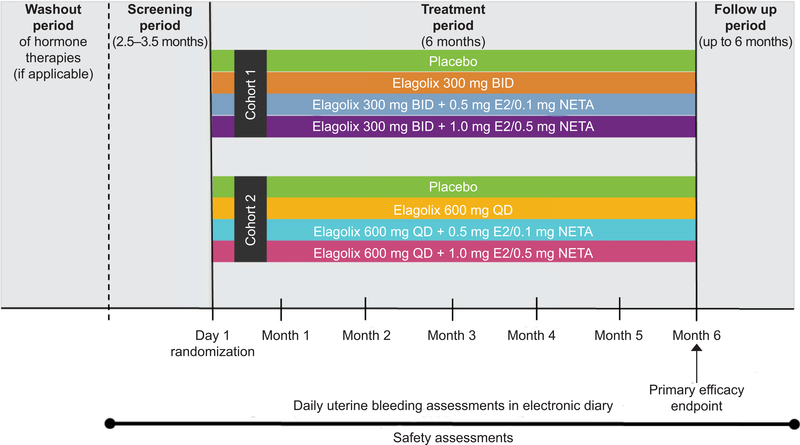
This was a randomized, double-blind, placebo-controlled, two-cohort, phase 2b study conducted in 86 sites in the United States, Puerto Rico, Canada, Chile, and the United Kingdom (Fig. 1). Study sites and principal investigators for each cohort are listed in Appendices 1 and 2, available online at http://links.lww.com/AOG/B162. The study included a washout of hormonal therapies (if applicable), screening period, 6-month treatment period, and up to 6-month posttreatment period.
Fig. 1.
Study design. BID, twice daily; E2, estradiol; NETA, norethindrone acetate; QD, daily.
The study was designed to recruit two cohorts, each to compare placebo with elagolix alone, elagolix with 0.5 mg estradiol/0.1 mg norethindrone acetate, or 1.0 mg estradiol/0.5 mg norethindrone acetate add-back therapy. Cohort 1, including 300 mg elagolix twice daily, was fully recruited first. Subsequently, recruitment for cohort 2 commenced, which included the 600-mg elagolix once daily dose. Enrollment was conducted from April 8, 2013, to December 8, 2015. Women were randomized within 10 days of the start of menstruation by means of an interactive response system in a one:one:one:one ratio to receive either placebo, elagolix alone, elagolix with 0.5 mg estradiol/0.1 mg norethindrone acetate, or elagolix with 1.0 mg estradiol/0.5 mg norethindrone acetate (see Appendix 3, available online at http://links.lww.com/AOG/B162, for protocol requirements). The sponsor, investigators, and participants were blinded to the treatment groups.
This trial was conducted in accordance with International Conference on Harmonisation guidelines and applicable regulations and ethical principles of the Declaration of Helsinki. The study protocols were approved by the Schulman institutional review board for central sites and by the institutions’ ethics committee for all other study sites in all participating countries. All women provided written, informed consent.
Nonpregnant, premenopausal women between ages 18 and 51 years at the time of screening with menstrual blood loss of greater than 80 mL during two or greater separate menstrual cycles during the screening period21,22 and regular menstrual cycles (less than 38 days) were eligible. Participants had a diagnosis of uterine leiomyomas with one or greater leiomyoma (3 cm or greater diameter) or multiple small leiomyomas (total uterine volume, 200–2,500 cm3, inclusive). Participants who also had focal or diffuse nondominant adenomyosis were included. Women were excluded if they had a persistent (greater than two cycles) or complex (greater than 3.5 cm diameter) ovarian cyst, endometrioma, malignancy, pelvic inflammatory disease, history of osteoporosis, history of hysterectomy or bilateral oophorectomy, or a Z-score at or below −1.5 of the lumbar spine, total hip, or femoral neck.
The primary end point was the percentage of women who had menstrual blood loss volume of less than 80 mL at the final month and a 50% or greater reduction in menstrual blood loss volume from baseline to the final month. These two measures were also included separately as secondary end points. Other secondary end points included the percentage of women with amenorrhea and suppression of bleeding, percentage of women who had a 1-g/dL or greater increase in hemoglobin concentration, and mean change from baseline in hemoglobin concentration, leiomyoma and uterine volume, and Uterine Fibroid Symptom and Health Related Quality of Life Questionnaire score.
Menstrual blood loss volume was objectively quantified from collected sanitary products using the alkaline hematin method for each cycle during screening and each 28-day interval for treatment months 4, 5, and 6.22,23 Briefly, the alkaline hematin method pummels used sanitary products using sodium hydroxide, which leads to the conversion of hemoglobin to alkaline hematin. Hematin absorbance was then measured by photometric techniques against calibration curves. When compared with the woman’s serum hemoglobin, the volume of blood loss in the sanitary products was determined. Additionally, the presence of menstrual bleeding and intensity were both recorded daily from screening through 3 months posttreatment in an electronic diary (e-diary) in which bleeding intensity was rated using the Mansfield-Voda-Jorgenson Menstrual Bleeding Scale. Uterine bleeding was also recorded at study visits months 1–6 on the Uterine Bleeding Questionnaire.
The presence of leiomyomas, uterine volume, total leiomyoma volume (up to three largest leiomyomas), endometrial thickness, and potential presence of ovarian or uterine pathology were assessed or measured by pelvic ultrasonography at screening, baseline (day 1), months 3 and 6, or the time of premature discontinuation, and read centrally with blindness to the treatment. The 4-week recall version of the Uterine Fibroid Symptom and Health Related Quality of Life Questionnaire was used to assess disease-specific quality of life on the Symptom Severity Scale and Health-Related Quality of Life total score at baseline; months 1, 3, and 6; or time of premature discontinuation. Higher Symptom Severity Scale total scores indicate worse symptoms, and higher Health Related Quality of Life total scores indicate better quality of life.24,25
Treatment-emergent adverse events were monitored, recording the onset, investigator-rated severity (mild, moderate, or severe), and relationship to the study drug (reasonable possibility or no reasonable possibility). Adverse events were considered serious if they were life-threatening, required medical–surgical intervention or hospitalization to prevent a serious outcome, or resulted in persistent disability or death. Bone mineral density was assessed by dual-energy x-ray absorptiometry scans of the lumbar spine, total hip, and femoral neck during screening, month 6 (or at premature discontinuation), and read centrally. Women were required to have bone mineral density assessed at posttreatment month 6 if their treatment month 6 results compared with baseline were 1) a decrease greater than 1.5% in the lumbar spine or greater than 2.5% in the total hip with less than 5% in both the lumbar spine and total hip or 2) a decrease in the spine or total hip 5% or greater. Additional safety evaluations included endometrial assessments and laboratory measures (see Appendix 3, http://links.lww.com/AOG/B162).
A sample size of 65 per group (N=520) was planned to provide at least 97% power to detect a significant difference between elagolix with add-back and placebo on the primary end point. The analyses of the primary and secondary outcomes did not change once enrollment began. All randomized participants who received 28 or greater days of the study drug were included in the primary efficacy analysis. All randomized participants who took one or greater dose of the study drug were included in secondary efficacy and safety analyses.
For menstrual blood loss volume, baseline was defined as the last qualified menstrual cycle during the screening period before the first study dose date. For all other outcomes, baseline was the last nonmissing measurement collected on the first day of dosing or prior. For menstrual blood loss volume assessments, final month was defined as the last 28 days of treatment, which included women who prematurely discontinued. All statistical tests were two-sided with a significance level of P<.05. SAS 9.3 was used to perform all analyses.
The primary end point was analyzed using a logistic regression model including treatment as the main factor and baseline menstrual blood loss volume as a covariate to compare with placebo. Menstrual blood loss volume was based on alkaline hematin measured volumes; however, if no sanitary products were returned, and bleeding was recorded in the Uterine Bleeding Questionnaire as none, the volume was zero. Otherwise, missing data were imputed with the best linear unbiased predictor derived from the linear mixed model based on quantitative e-diary data. Statistical analyses for secondary end points are summarized in Appendix 3, http://links.lww.com/AOG/B162.
RESULTS
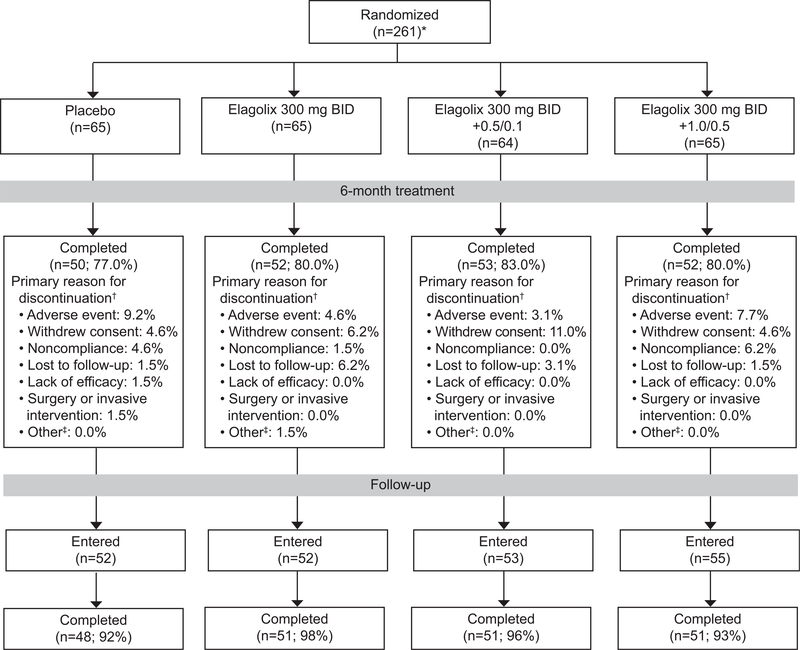
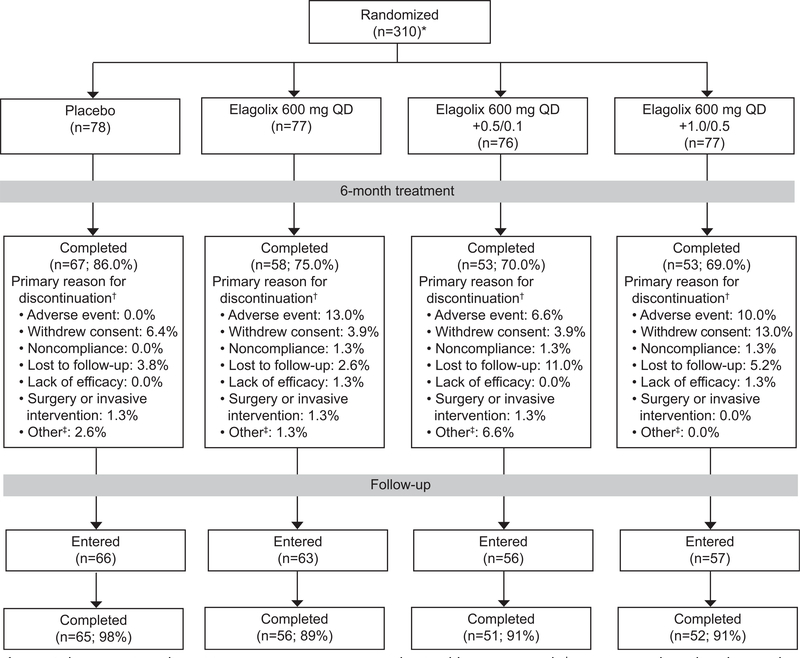
There were 259 women randomized and treated in cohort 1 and 308 in cohort 2; the majority of women (greater than 69%) in each treatment group completed the study (Figs. 2 and 3). Each cohort and treatment groups had similar demographic at baseline (Table 1); women in cohort 1 had a mean±SD age of 43±5 years (cohort 2, 42±5 years), and 70% were black (cohort 2, 74%). At baseline, menstrual blood loss volumes ranged from 81 to 1,598 mL per month and uterine volumes ranged from 72 to 2,928 cm3 across treatment groups.
Fig. 2.
Cohort 1 patient disposition. *Two women were randomized but not treated. †Women may have listed more than one reason for premature discontinuation, but only the primary reasons are included. ‡Other category is a combination of pregnancy, exclusionary medication received, and other categories. BID, twice daily; 0.5/0.1, 0.5 mg estradiol/0.1 mg norethindrone acetate; 1.0/0.5, 1.0 mg estradiol/0.5 mg norethindrone acetate.
Fig. 3.
Cohort 2 patient disposition. *Two women were randomized but not treated. †Women may have listed more than one reason for premature discontinuation, but only the primary reasons are included. ‡Other category is a combination of pregnancy, exclusionary medication received, and other categories. QD, daily; 0.5/0.1, 0.5 mg estradiol/0.1 mg norethindrone acetate; 1.0/0.5, 1.0 mg estradiol/0.5 mg norethindrone acetate.
Table 1.
Baseline Characteristics
| 300 mg Elagolix Twice Daily |
600 mg Elagolix Daily |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Placebo (n=65) | Without Add-Back (n=65) | +0.5 mg E2/0.1 mg NETA (n=64) | +1.0 mg E2/0.5 mg NETA (n=65) | Placebo (n=78) | Without Add-Back (n=77) | +0.5 mg E2/0.1 mg NETA (n=76) | +1.0 mg E2/0.5 mg NETA (n=77) |
| Age (y) | 44 (29–50) | 43 (29–50) | 44 (26–52) | 44 (29–51) | 43 (27–51) | 42 (25–51) | 42 (30–52) | 43 (25–51) |
| Race | ||||||||
| Black | 44 (68) | 41 (63) | 46 (72) | 50 (77) | 61 (78) | 51 (66) | 60 (79) | 55 (72) |
| White | 18 (28) | 21 (32) | 18 (28) | 14 (22) | 17 (22) | 22 (29) | 13 (17) | 18 (24) |
| Other† | 3 (4.6) | 3 (4.6) | 0 | 1 (1.5) | 0 | 4 (5.2) | 3 (3.9) | 3 (3.9) |
| BMI (kg/m2) | 30±4.7 | 30±5.4 | 30±5.6 | 30±5.1 | 30.7±5.1 | 30±5.2 | 31±5.5 | 31±5.7 |
| Menstrual blood loss (mL) | 238±189 | 265±146 | 270±220 | 296±253 | 222±132 | 208±141 | 242±157 | 247±187 |
| Hemoglobin (g/dL) | 11.1±1.6 | 10.5±1.5* | 11.0±1.7 | 10.4±1.7* | 10.9±1.6 | 10.7±1.9 | 10.6±1.9 | 11.0±1.8 |
| Total uterine volume (cm3) | 627±440 | 680±471 | 634±456 | 816±596* | 570±381 | 577±444 | 579±436 | 582±441 |
| Total leiomyoma volume (cm3)‡ | 135±155 | 175±211 | 150±176 | 188±209 | 138±161 | 132±156 | 147±267 | 149±209 |
| UFS-QoL symptom severity score (LS mean±SE) | 62.1±2.5 | 63.1±2.5 | 59.8±2.5 | 64.8±2.5 | 65.9±2.4 | 66.3±2.4 | 63.2±2.4 | 62.1±2.4 |
| UFS-QoL HRQL total (LS mean±SE) | 39.4±3.1 | 39.9±3.1 | 43.3±3.1 | 35.9±3.1 | 37.2±2.6 | 34.3±2.6 | 37.4±2.7 | 37.5±2.6 |
| Bone mineral density Z-score, lumbar spine | 0.72±1.25 | 0.73±1.16 | 0.86±1.05 | 0.80±1.20 | 1.09±1.21 | 0.87±1.18 | 0.53±1.07 | 1.16±1.22 |
| Total hip | 0.56±0.98 | 0.32±0.73 | 0.57±0.91 | 0.28±1.08 | 0.52±0.95 | 0.40±0.96 | 0.61±1.06 | 0.62±0.97 |
| Femoral neck | 0.47±0.97 | 0.27±0.74 | 0.30±0.94 | 0.32±1.16 | 0.44±1.01 | 0.37±0.99 | 0.46±0.90 | 0.50±1.04 |
E2, estradiol; NETA, norethindrone acetate; BMI, body mass index; UFS-QOL, Fibroid Symptom and Health Related Quality of Life; LS, least square; SE, standard error; HRQL, Health Related Quality of Life.
Data are median (range), n (%), or mean±SD unless otherwise specified. Significant differences between each elagolix group and placebo for menstrual blood loss, hemoglobin concentration, total uterine, and leiomyoma volumes at baseline were tested using an analysis of variance model with treatment as the main effect and indicated for
P<.05.
Other category includes Asian, Native American and Alaska Native, Native Hawaiian or other Pacific islander, and multiracial.
Volume of three or less largest leiomyomas.
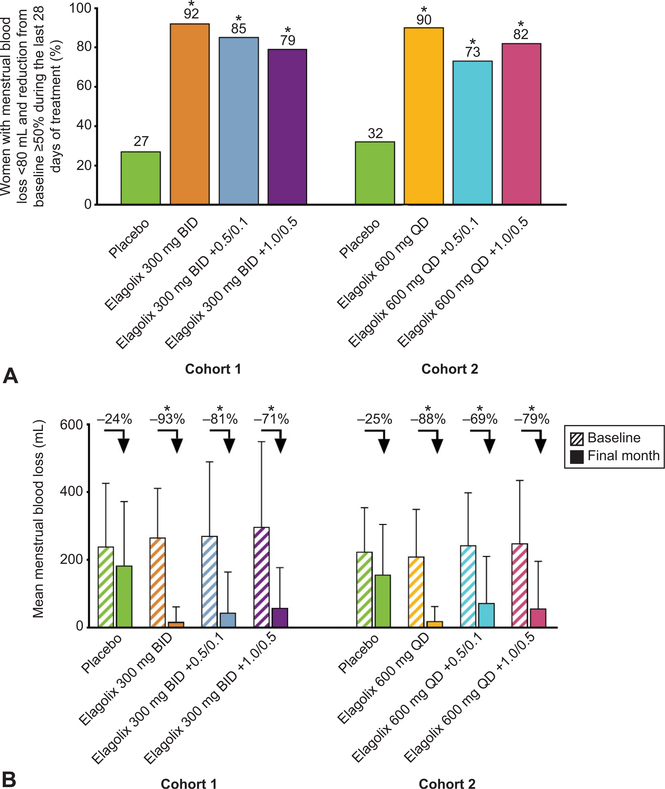
Compared with placebo, elagolix with and without add-back therapy led to significantly more women attaining the primary outcome (percentage of women achieving menstrual blood loss less than 80 mL at the final month and menstrual blood loss volume reduction of 50% or greater from baseline to the final month) (Fig. 4A) and for each outcome separately (Table 2). The mean percent change from baseline to final visit in menstrual blood loss ranged from −71% to −93% in the 300-mg elagolix twice-daily groups compared with −24% with placebo; results with 600 mg elagolix daily were similar (Fig. 4B). The primary menstrual blood loss outcome was also significant for all comparisons at both the second and third last 28 days of treatment, which includes all other months alkaline hematin was collected (Table 2).
Fig. 4.
Percentage of women who met the composite primary end point (A) and mean menstrual blood loss at baseline and final month (B). Menstrual blood loss was measured from sanitary products by the alkaline hematin method. A. *Statistical significance vs placebo is indicated for P<.001. B. Arrows indicate the mean percent change from baseline to final month in menstrual blood loss. *Significance vs placebo is indicated for P<.001. BID, twice daily; QD, daily; 0.5/0.1, 0.5 mg estradiol/0.1 mg norethindrone acetate; 1.0/0.5, 1.0 mg estradiol/0.5 mg norethindrone acetate.
Table 2.
Secondary Efficacy Outcomes at Final Visit
| 300 mg Elagolix Twice Daily |
600 mg Elagolix Daily |
|||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n=64) | Without Add-Back (n=62) | +0.5 mg E2/0.1 mg NETA (n=61) | +1.0 mg E2/0.5 mg NETA (n=62) | Placebo (n=76) | Without Add-Back (n=71) | +0.5 mg E2/0.1 mg NETA (n=73) | +1.0 mg E2/0.5 mg NETA (n=76) | |
| Women who achieved outcome at final visit | ||||||||
| Menstrual blood loss less than 80 mL | 33% (21/64) | 92% *** (57/62) | 89%*** (54/61) | 79%*** (49/62) | 37% (28/76) | 92%*** (65/71) | 73%*** (53/73) | 86%*** (65/76) |
| Reduction from baseline in menstrual blood loss 50% or greater | 31% (20/64) | 94%*** (58/62) | 87%*** (53/61) | 82%*** (51/62) | 36% (27/76) | 90%*** (64/71) | 79%*** (58/73) | 86%*** (65/76) |
| Amenorrhea† | 1.6% (1/61) | 56%*** (32/57) | 33%*** (19/57) | 28%*** (17/60) | 1.3% (1/75) | 51%*** (34/67) | 18%*** (11/63) | 23%*** (15/66) |
| Suppression of bleeding‡ | 1.6% (1/61) | 75%*** (43/57) | 53%*** (30/57) | 43%*** (26/60) | 2.7% (2/75) | 67%*** (45/67) | 32%*** (20/63) | 35%*** (23/66) |
| Hemoglobin concentration 1-g/dL or greater increase | 30% (19/64) | 80%*** (49/61) | 70%*** (41/59) | 59%** (36/61) | 26% (20/76) | 52%** (37/71) | 51%** (37/72) | 53%** (39/74) |
| Women who achieved outcome at 3rd last 28-d interval (month 4) | ||||||||
| Menstrual blood loss less than 80 mL | 23% (14/61) | 96%*** (54/56) | 91%*** (52/57) | 81%*** (47/58) | 27% (20/74) | 89%*** (59/66) | 76%*** (47/62) | 80%*** (52/65) |
| Reduction from baseline in menstrual blood loss 50% or greater | 30% (18/61) | 98%*** (55/56) | 89%*** (51/57) | 91%*** (53/58) | 31% (23/74) | 86%*** (57/66) | 81%*** (50/62) | 78%*** (51/65) |
| Women who achieved outcome at 2nd last 28-d interval (month 5) | ||||||||
| Menstrual blood loss less than 80 mL | 19% (12/62) | 95%*** (55/58) | 90%*** (53/59) | 85%*** (51/60) | 24% (18/76) | 87%*** (59/68) | 69%*** (44/64) | 80%*** (56/70) |
| Reduction from baseline in menstrual blood loss 50% or greater | 11% (7/62) | 95%*** (55/58) | 92%*** (54/59) | 90%*** (54/60) | 28% (21/76) | 88%*** (60/68) | 75%*** (48/64) | 86%*** (60/70) |
| Least squares mean percent change from baseline to final visit | ||||||||
| Hemoglobin concentration (SE) | 7.6±2.0 | 19.3±2.0*** | 20.1±2.1*** | 15.0±2.0** | 3.9±1.7 | 15.8±1.7*** | 11.6±1.7*** | 12.6±1.7*** |
| Mean percentage change from baseline to final visit | ||||||||
| Total leiomyoma volume§ | 4.6±48.6 | −39.6±28.7*** | −24.0±29.9*** | −12.9±46.2* | 0.1±28.8 | −36.4±30.1*** | −16.6±32.7** | −1.6±42.8 |
| Total uterine volume | 15.9±38.0 | −31.5±31.4*** | −22.0±28.5*** | −11.8±22.6*** | 11.6±25.4 | −26.6±28.3*** | −11.5±25.3*** | −6.7±21.8*** |
| Mean score change from baseline to final visit | ||||||||
| UFS-QoL symptom severity scale total score|| | −17.9 (2.6) | −45.6 (2.7)*** | −38.8 (2.7)*** | −36.5 (2.6)*** | −21.9 (2.6) | −44.2 (2.7)*** | −36.2 (2.7)*** | −32.6 (2.6)** |
| UFS-QoL HrQl total score|| | 14.7 (2.8) | 44.9 (2.9)*** | 41.2 (2.9)*** | 36.0 (2.9)*** | 24.8 (3.0) | 43.5 (3.1)*** | 36.3 (3.1)** | 34.7 (3.0)* |
E2, estradiol; NETA, norethindrone acetate; SE, standard error; UFS-QoL, Uterine Fibroid Symptom and Health Related Quality of Life Questionnaire; HRQL, Health Related Quality of Life.
Data are % (n/N) or mean±SD unless otherwise specified.
Statistical significance vs placebo is denoted for
P≤.05
P≤.01
P≤.001.
0 days of bleeding or spotting based on observed alkaline hematin data and imputed e-diary data during last 56 days of treatment.
0 days of bleeding (spotting allowed) based on observed alkaline hematin data and imputed e-diary data during last 56 days of treatment.
Volume of three or less largest leiomyomas.
Improvement of uterine leiomyoma symptom severity score is measured as a negative mean change from baseline to final visit UFS-QoL Symptom Severity Scale total score. Improvement of quality-of-life assessment is indicated by a positive mean change from baseline to final visit HRQL total score vs placebo.
A significantly higher percentage of elagolix-treated women, with or without add-back therapy, had amenorrhea, suppression of bleeding, or both during the last 56 days of treatment compared with placebo (Table 2). All elagolix groups had a significant increase in their hemoglobin concentration from baseline to the final visit compared with placebo, and a significantly higher percentage of women in each elagolix treatment group had more than a 1-g/dL increase compared with placebo (Table 2).
The majority of the women who were amenorrheic on entering the posttreatment period returned to menses during posttreatment month 1 or 2 (month 1 [month 2]: 41% [48%] 300 mg elagolix twice daily, 35% [65%] 300 mg elagolix twice daily with 0.5 mg estradiol/0.1 mg norethindrone acetate, and 80% [13%] 300 mg elagolix twice daily with 1.0 mg estradiol/0.5 mg norethindrone acetate; 48% [52%] 600 mg elagolix daily, 64% [27%] 600 mg elagolix daily with 0.5 mg estradiol/0.1 mg norethindrone acetate, and 64% [36%] 600 mg elagolix daily with 1.0 mg estradiol/0.5 mg norethindrone acetate therapy). Less than 4% of women in each group had their first menses 56 days posttreatment or later.
All elagolix groups had a significant decrease in total leiomyoma volume from baseline to the final visit compared with placebo, except for 600 mg elagolix daily with 1.0 mg estradiol/0.5 mg norethindrone acetate (Table 2). All elagolix groups had a significant decrease in total uterine volume from baseline to the final visit compared with placebo, which was greatest for each elagolix-alone group (Table 2). All elagolix groups had significant improvement from baseline to the final visit in uterine leiomyoma symptom severity (Uterine Fibroid Symptom and Health Related Quality of Life Symptom Severity Scale total score) and health-related quality of life (Health-Related Quality of Life total score) compared with placebo (Table 2).
The majority of women in each treatment group had at least one adverse event, regardless of relatedness to treatment (Table 3), and less than 10% in each treatment group had a serious adverse event. Hot flush was the most frequently reported adverse event in the elagolix groups without add-back therapy (Table 3), and the majority of reported hot flushes had a maximal severity of mild or moderate. Women treated with either dose of elagolix with 1.0 mg estradiol/0.5 mg norethindrone acetate had no severe hot flushes. The percentage of women in each elagolix group who discontinued as a result of hot flush ranged from 0% to 4%; women treated with either dose of elagolix with 1.0 mg estradiol/0.5 mg norethindrone acetate had no discontinuations resulting from hot flush (Table 3).
Table 3.
Summary of Treatment-Emergent Adverse Events
| 300 mg Elagolix Twice Daily |
600 mg Elagolix Daily |
|||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n=65) | Without Add-Back (n=65) | +0.5 mg E2/0.1 mg NETA (n=64) | +1.0 mg E2/0.5 mg NETA (n=65) | Placebo (n=78) | Without Add-Back (n=77) | +0.5 mg E2/0.1 mg NETA (n=76) | +1.0 mg E2/0.5 mg NETA (n=77) | |
| Any AE | 47 (72) | 52 (80) | 47 (73) | 48 (74) | 53 (68) | 67 (87) | 56 (74) | 55 (71) |
| Any serious AE | 6 (9.2) | 3 (4.6) | 3 (4.7) | 1 (1.5) | 1 (1.3) | 5 (6.5) | 3 (3.9) | 4 (5.2) |
| Any discontinuation resulting from AE | 7 (11) | 4 (6.2) | 2 (3.1) | 6 (9.2) | 1 (1.3) | 11 (14) | 7 (9.2) | 9 (12) |
| AEs occurring in 5% or greater of women* | ||||||||
| Hot flush | 2 (3.1) | 29 (45) | 16 (25) | 7 (11) | 4 (5.1) | 38 (49) | 14 (18) | 11 (14) |
| Headache | 6 (9.2) | 8 (12) | 9 (14) | 13 (20) | 8 (10) | 13(17) | 11 (15) | 14 (18) |
| Nausea | 6 (9.2) | 4 (6.2) | 4 (6.3) | 12 (19) | 3 (3.8) | 10 (13) | 12 (16) | 20 (26) |
| Insomnia | 1 (1.5) | 7 (11) | 5 (7.8) | 0 | † | † | † | † |
| Urinary tract infection | 2 (3.1) | 2 (3.1) | 5 (7.8) | 4 (6.2) | † | † | † | † |
| Back pain | 6 (9.2) | 5 (7.7) | 2 (3.1) | 3 (4.6) | 2 (2.6) | 6 (7.8) | 6 (7.9) | 7 (9.1) |
| Fatigue | 2 (3.1) | 3 (4.6) | 4 (6.3) | 3 (4.6) | † | † | † | † |
| Menorrhagia | 2 (3.1) | 3 (4.6) | 3 (4.7) | 4 (6.2) | † | † | † | † |
| Pain in extremity | 2 (3.1) | 4 (6.2) | 2 (3.1) | 4 (6.2) | † | † | † | † |
| Anemia | † | † | † | † | 7 (9.0) | 4 (5.2) | 6 (7.9) | 5 (6.5) |
| Diarrhea | † | † | † | † | 4 (5.1) | 5 (6.5) | 3 (3.9) | 4 (5.2) |
| Dizziness | † | † | † | † | 3 (3.8) | 3 (3.9) | 4 (5.3) | 5 (6.5) |
| Vomiting | † | † | † | † | 4 (5.1) | 4 (5.2) | 3 (3.9) | 5 (6.5) |
| Maximum hot flush severity | ||||||||
| Mild | 1 | 18 | 11 | 5 | 2 | 22 | 12 | 11 |
| Moderate | 1 | 9 | 4 | 2 | 1 | 14 | 2 | 0 |
| Severe | 0 | 2 | 1 | 0 | 1 | 2 | 0 | 0 |
| Discontinuation as a result of hot flush | 0 | 2 (3.1) | 1 (1.6) | 0 | 0 | 3 (3.9) | 0 | 0 |
E2, estradiol; NETA, norethindrone acetate; AE, adverse event.
Data are n (%) or n.
There was no statistical comparison between treatment groups.
MedDRA preferred terms in descending order for elagolix overall in cohort 1, then cohort 2.
Preferred terms with an incidence below 5%.
A single pregnancy occurred during the trial; a 33-year-old woman in the 600-mg elagolix without add-back therapy group was diagnosed with a pregnancy and discontinued the study drug on day 2 of treatment. She elected to terminate the pregnancy.
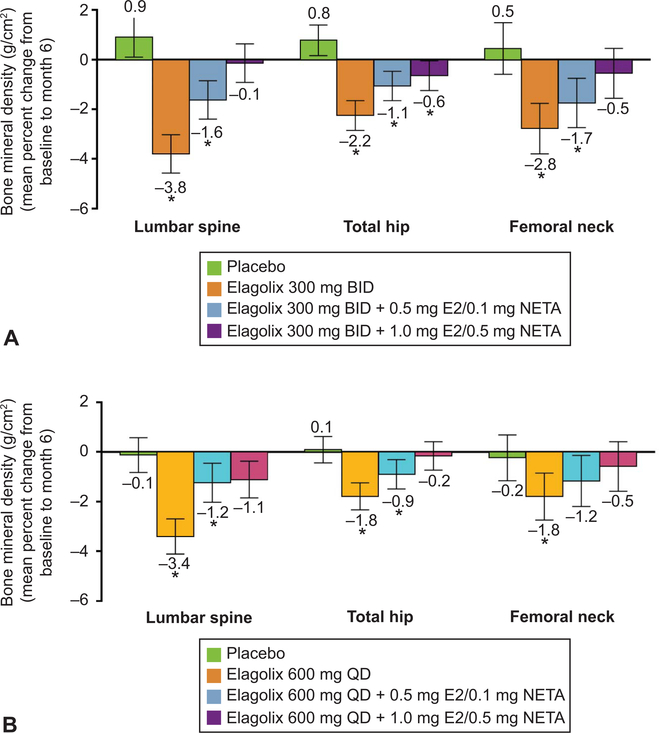
Women in the elagolix groups without or with 0.5 mg estradiol/0.1 mg norethindrone acetate had significant decreases in bone mineral density compared with placebo in the mean percent change from baseline to month 6 measured at the lumbar spine, total hip, and femoral neck in both cohorts, whereas women treated with elagolix with 1.0 mg estradiol/0.5 mg norethindrone acetate did not have significant changes compared with placebo in the lumbar spine or femoral neck (Fig. 5).
Fig. 5.
Mean percent change from baseline to month 6 in bone mineral density. Cohort 1 (A) and cohort 2 (B). *Significance vs placebo is indicated for P≤.05 using observed data. Error bars indicate 95% CI. BID, twice daily; E2, estradiol; NETA, norethindrone acetate; QD, daily.
Exploratory analyses of biomarkers for bone resorption (C-terminal collagen telopeptide) and formation (procollagen type 1 N-terminal propeptide) showed that elagolix groups without add-back had significant increases from baseline to month 6 in bone resorption and formation biomarker concentrations compared with placebo and elagolix with add-back groups (Appendix 4, available online at http://links.lww.com/AOG/B162); however, elagolix groups with add-back did not have significant increases compared with placebo. At posttreatment month 6, none of the elagolix groups had significant differences from placebo in the mean change from baseline in bone resorption and formation biomarker concentrations.
Elagolix at 300 mg twice daily and 600-mg daily treatment resulted in the median estradiol concentration of 12 pg/mL through month 6 and progesterone concentrations remained low (Appendix 5, available online at http://links.lww.com/AOG/B162). Elagolix with 0.5 mg estradiol/0.1 mg norethindrone acetate and 1.0 mg estradiol/0.5 mg norethindrone acetate resulted in numerically higher estradiol concentrations than elagolix alone. The median estradiol concentrations were 30 and 61 pg/mL for the 300-mg elagolix twice-daily with 0.5 mg estradiol/0.1 mg norethindrone acetate and with 1.0 mg estradiol/0.5 mg norethindrone acetate groups, respectively, and 34 and 66 pg/mL for the 600-mg elagolix daily with 0. 5 mg estradiol/0.1 mg norethindrone acetate and with 1.0 mg estradiol/0.5 mg norethindrone acetate groups.
The 300-mg twice-daily elagolix with 1.0 mg estradiol/0.5 mg norethindrone acetate group did not have significant mean percent changes from baseline to month 6 compared with placebo in triglycerides and low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol (Appendix 6, available online at http://links.lww.com/AOG/B162). All other elagolix groups had significant differences compared with placebo in serum lipid outcomes (Appendix 6, http://links.lww.com/AOG/B162). Mean percent changes from baseline to month 6 in the LDL:HDL ratio for all elagolix groups, except for 300 mg twice daily with 1.0 mg estradiol/0.5 mg norethindrone acetate, were significant compared with placebo (Appendix 6, http://links.lww.com/AOG/B162); less than 6% of women in each elagolix group had a LDL:HDL ratio greater than 4 after 6 months of treatment. There were no discontinuations resulting from increased cholesterol or triglyceride concentrations. All serum lipid parameter, which had increased during the treatment period, returned to baseline or toward baseline levels by posttreatment month 3 (Appendix 6, http://links.lww.com/AOG/B162).
Elagolix groups did not have statistically significant mean increases in liver function tests (aspartate and alanine aminotransferases and total bilirubin) from baseline to month 6 compared with placebo (Appendix 7, available online at http://links.lww.com/AOG/B162). Across all elagolix groups (n=397), eight elagolix-treated women had aspartate, alanine aminotransferase, or both levels three or greater times the upper limit of normal without elevations of bilirubin, once per participant, during the treatment period. For the four women who remained on treatment, the elevations resolved within 34 days; for the two women who had the elevations within 1 day of the last day of the treatment period, the elevations resolved in 14 and 59 days; and for the remaining two women who prematurely discontinued as a result of the elevations, it resolved 21 and 73 days after stopping treatment. Elagolix groups did not have significant changes from baseline to month 6 in glucose compared with placebo.
The 300-mg elagolix twice-daily with 0.5 mg estradiol/0.1 mg norethindrone acetate and 600-mg elagolix daily groups had a significant decrease from baseline to month 6 in endometrial thickness compared with placebo; all other groups had no significant changes compared with placebo (Appendix 8, available online at http://links.lww.com/AOG/B162). There were no cases of endometrial hyperplasia or malignancy after 6 months of treatment, as determined by histologic evaluation of endometrial tissue obtained by biopsy.
DISCUSSION
Elagolix treatment with and without add-back therapy showed superiority to placebo in significantly reducing menstrual bleeding, increasing hemoglobin concentration, and reducing leiomyoma and uterine volumes in women with heavy menstrual bleeding associated with uterine leiomyomas. These results were associated with improvements in quality of life and confirmed the phase 2a study efficacy results for the 600-mg total daily dose of elagolix in improving heavy menstrual bleeding with statistical comparison with placebo for two dosing regimens.
Elagolix with add-back therapy showed an acceptable safety profile in women with heavy menstrual bleeding associated with uterine leiomyomas. There were no adverse effects on the endometrium. There were changes in lipids; however, the LDL-to-HDL ratio was minimally affected as a result of the increase in both LDL and HDL. Although hot flush was the most commonly reported adverse event by elagolix-treated women, none of the women in the elagolix with 1.0 mg estradiol/0.5 mg norethindrone acetate groups and less than 4% in the other elagolix groups discontinued as a result of hot flushes. Elagolix-treated women had decreases in bone mineral density, which were attenuated with add-back therapy.
Elagolix is not associated with a flare in hyperestrogenic symptoms; as such, fewer than five women in the elagolix-alone groups had a maximal severity of severe for their hot flush adverse events. The incidence of hot flushes and decrease in bone mineral density in elagolix groups were consistent with its mechanism of action, because they are associated with relatively low estradiol levels. Women treated with elagolix had lower levels of estradiol compared with placebo. Based on posttreatment data in elagolix-treated women with endometriosis, the hypoestrogenic effects of elagolix on bone mineral density and lipids are reversible.26
The 300-mg twice-daily and 600-mg daily elagolix doses had similar efficacy profiles with statistical significance compared with placebo on bleeding and uterine volume outcomes. Their safety profiles were somewhat different: the 300-mg twice-daily dose had fewer women who had nausea (6.2% vs 13%) and fewer who prematurely discontinued as a result of an adverse event (4.6% vs 13%). Although the total daily dose for the two regimens of elagolix are the same, elagolix exhibits greater than dose proportional increases in exposures with 600 mg daily.17,18 The safety and efficacy profile of the 600-mg daily dose may be acceptable; however, the 300-mg twice-daily dose has been chosen for phase 3 studies based on its overall pharmacokinetic, safety and efficacy profile.
Hormone add-back therapy was included in this study to prevent bone mineral decreases and hot flushes resulting from elagolix treatment. All elagolix with add-back therapy groups had numerically higher levels of plasma estradiol than elagolix alone; however, the higher add-back dose (1.0 mg estradiol/0.5 mg norethindrone acetate) best attenuated the effect of elagolix on bone mineral density. Exploratory analyses also showed an increase in bone turnover markers with elagolix treatment, which the add-back therapy prevented. Although add-back therapy reduced bone mineral loss, the effect of elagolix alone on total leiomyoma volume was attenuated by the addition of add-back therapy.
A strength of this study was the objective quantification by a central reader of menstrual blood loss volume using the alkaline hematin method, which quantifies blood loss from sanitary products.23 Other methods include the pictorial blood loss assessment chart27 and bleeding journals,28 which are semiquantitative and subjective.29 The randomized, placebo-controlled study design is also a strength of the study.
Women with dominant adenomyosis or polyps greater than 1 cm were not included, and thus, the safety and efficacy of elagolix in those patients could not be assessed. Large (greater than 1 cm) polyps were excluded because they are a well-established cause of abnormal uterine bleeding (independent of uterine leiomyomas) and it is recommended to remove these when they are 1 cm or greater. Menstrual blood loss measured by the alkaline hematin method was collected and analyzed for months 4 through 6, and therefore effects of elagolix on menstrual blood loss volume in the first 3 months of treatment could not be assessed. Menstrual blood loss measurements were also limited by variability because approximately 30% of women in the placebo groups met the primary end point. This effect has been observed in other studies17,30 and could be the result of lower adherence of women in the placebo groups to sanitary product collection compared with active treatment groups.30 Posttreatment bone mineral density assessment was limited because women were not required to have their bone mineral density assessed in the posttreatment period unless their lumbar spine or total hip bone mineral density decreased by 5% or greater from baseline to month 6.
This randomized, placebo-controlled phase 2b study confirmed the efficacy and safety of the 600-mg total daily dose of elagolix in women with heavy menstrual bleeding associated with uterine leiomyomas. Hormone add-back therapy attenuated hypoestrogenic effects of elagolix on bone mineral density while maintaining the efficacy on reducing heaving menstrual bleeding in women with uterine leiomyomas.
Supplementary Material
Acknowledgments
Financial Disclosure
Dr. Carr has been a study investigator and received research support from AbbVie and Agile Therapeutics. He also served on the Repros Therapeutics Data and Safety Monitoring Board. Dr. Stewart has been a consultant for AbbVie, Allergan, Astellas, Bayer, Gynesonics, Myovant, and Welltwigs. She has received research support from the National Institutes for Health (HS023418) and holds a patent for Methods and Compounds for Treatment of Abnormal Uterine Bleeding (US 6440445). Dr. Archer has received research support from AbbVie, TherapeuticsMD, Bayer HealthCare, Endoceutics, Glenmark, Shionogi, Symbio, and Radius. He has been a consultant to AbbVie, TherapeuticsMD, Bayer HealthCare, Endoceutics, Agile Pharmaceuticals, Exeltis/CHEMO France, and TEVA/HR Pharma. Dr. Al-Hendy has been a consultant to Abbvie, Bayer, Allergan, and MD Stem Cells. He has received research support, National Institute of Health (R01 ES 028615–01, R01HD 087417, R01 HD 094378, R01 HD 094380), and holds a patent for methods for novel diagnostics and therapeutics for uterine sarcoma (US Patent No. 9,790,562 B2). Dr. Bradley has been an advisor to Medtronic, AbbVie, Allergan, PCORI, Bayer, Boston Scientific, and Female Health. She has been a speaker for Bayer, Smith & Nephew, and Karl Storz and served on the Data Safety and Monitoring Board of Gynesonics. She received research support from Bayer and royalties as author/editor for Elsevier, Wolters Kluwer, and UpToDate. Dr. Watts has been a speaker for Amgen, Radius, and Shire and has been a consultant to AbbVie, Amgen, Radius, and Sanofi. He is the owner of OsteoDynamics. Dr. Diamond has received research support from Abbvie Inc, Bayer Healthcare, and ObsEva. He is a stockholder and serves on the Board of Directors of Advanced Reproductive Care. Dr. Gao is a former employee of AbbVie Inc and a current employee of Gilead Sciences. Drs. Owens, Chwalisz, Duan, Soliman, and Dufek are employees with stock/stock options in AbbVie, Inc. Dr. Simon has received research support from AbbVie Inc, Actavis, Agile Therapeutics, Bayer Healthcare, New England Research Institute, Novo Nordisk, Palatin Technologies, Symbio Research, and TherapeuticsMD. He has been a speaker for Amgen, Eisai, Merck, Noven Pharmaceuticals, Novo Nordisk, and Shionogi. He has been an advisor and/or consultant for AbbVie Inc, AMAG Pharmaceuticals, Amgen, Apotex, Ascend Therapeutics, JDS Therapeutics, Merck & Co, Noven Pharmaceuticals, Novo Nordisk, Nuelle, Perrigo Company, Radius Health, Regeneron Pharmaceutical, Sanofi SA, Sermonix Pharmaceuticals, Shionogi, Sprout Pharmaceuticals, Symbiotec Pharmalab, and TherapeuticsMD. He is a stockholder in Sermonix Pharmaceuticals.
The authors thank all of the trial investigators and the women who participated in this clinical trial. Jane Rodgers, PhD, and Jeanie K. Meckes, PhD, of AbbVie Inc., provided medical writing assistance.
Footnotes
AbbVie Inc funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication.
CLINICAL TRIAL REGISTRATION: ClinicalTrials.gov, NCT01817530; EU Clinical Trial Register, 2013–000082-37.
REFERENCES
- 1.Stewart EA, Laughlin-Tommaso SK, Catherino WH, Lalitkumar S, Gupta D, Vollenhoven B. Uterine fibroids. Nat Rev Dis Primers 2016;2:16043. [DOI] [PubMed] [Google Scholar]
- 2.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188:100–7. [DOI] [PubMed] [Google Scholar]
- 3.Valladares F, Frías I, Báez D, García C, López FJ, Fraser JD, et al. Characterization of estrogen receptors alpha and beta in uterine leiomyoma cells. Fertil Steril 2006;86:1736–43. [DOI] [PubMed] [Google Scholar]
- 4.Dvorská D, Braný D, Danková Z, Halašová E, Višňovský J. Molecular and clinical attributes of uterine leiomyomas. Tumour Biol 2017;39:1010428317710226. [DOI] [PubMed] [Google Scholar]
- 5.Carr BR, Marshburn PB, Weatherall PT, Bradshaw KD, Breslau NA, Byrd W, et al. An evaluation of the effect of gonadotropin-releasing hormone analogs and medroxyprogesterone acetate on uterine leiomyomata volume by magnetic resonance imaging: a prospective, randomized, double blind, placebo-controlled, crossover trial. J Clin Endocrinol Metab 1993;76:1217–23. [DOI] [PubMed] [Google Scholar]
- 6.Takeda T, Sakata M, Isobe A, Miyake A, Nishimoto F, Ota Y, et al. Relationship between metabolic syndrome and uterine leiomyomas: a case-control study. Gynecol Obstet Invest 2008;66:14–7. [DOI] [PubMed] [Google Scholar]
- 7.Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG 2017; 124:1501–12. [DOI] [PubMed] [Google Scholar]
- 8.Pavone D, Clemenza S, Sorbi F, Fambrini M, Petraglia F. Epidemiology and risk factors of uterine fibroids. Best Pract Res Clin Obstet Gynaecol 2018;46:3–11. [DOI] [PubMed] [Google Scholar]
- 9.Al-Hendy A, Myers ER, Stewart E. Uterine fibroids: burden and unmet medical need. Semin Reprod Med 2017;35:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munro MG, Critchley HO, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol 2011; 113:3–13. [DOI] [PubMed] [Google Scholar]
- 11.Levy G, Hill MJ, Beall S, Zarek SM, Segars JH, Catherino WH. Leiomyoma: genetics, assisted reproduction, pregnancy and therapeutic advances. J Assist Reprod Genet 2012;29:703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart EA, Nicholson WK, Bradley L, Borah BJ. The burden of uterine fibroids for African-American women: results of a national survey. J Womens Health (Larchmt) 2013;22:807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Downes E, Sikirica V, Gilabert-Estelles J, Bolge SC, Dodd SL, Maroulis C, et al. The burden of uterine fibroids in five European countries. Eur J Obstet Gynecol Reprod Biol 2010;152: 96–102. [DOI] [PubMed] [Google Scholar]
- 14.Borah BJ, Nicholson WK, Bradley L, Stewart EA. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol 2013;209:319.e1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merrill RM. Hysterectomy surveillance in the United States, 1997 through 2005. Med Sci Monit 2008;14:CR24–31. [PubMed] [Google Scholar]
- 16.Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update 2016;22:665–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Archer DF, Stewart EA, Jain RI, Feldman RA, Lukes AS, North JD, et al. Elagolix for the management of heavy menstrual bleeding associated with uterine fibroids: results from a phase 2a proof-of-concept study. Fertil Steril 2017;108:152–60.e4. [DOI] [PubMed] [Google Scholar]
- 18.Ng J, Chwalisz K, Carter DC, Klein CE. Dose-dependent suppression of gonadotropins and ovarian hormones by elagolix in healthy premenopausal women. J Clin Endocrinol Metab 2017; 102:1683–91. [DOI] [PubMed] [Google Scholar]
- 19.Struthers RS, Nicholls AJ, Grundy J, Chen T, Jimenez R, Yen SS, et al. Suppression of gonadotropins and estradiol in premenopausal women by oral administration of the nonpeptide gonadotropin-releasing hormone antagonist elagolix. J Clin Endocrinol Metab 2009;94:545–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marjoribanks J, Farquhar C, Roberts H, Lethaby A, Lee J. Long-term hormone therapy for perimenopausal and postmenopausal women. The Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No.: CD004143. DOI: 10.1002/14651858.CD004143.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallberg L, Högdahl AM, Nilsson L, Rybo G. Menstrual blood loss—a population study. Variation at different ages and attempts to define normality. Acta Obstet Gynecol Scand 1966; 45:320–51. [DOI] [PubMed] [Google Scholar]
- 22.Hallberg L, Nilsson L. Determination of menstrual blood loss. Scand J Clin Lab Invest 1964;16:244–8. [PubMed] [Google Scholar]
- 23.Magnay JL, Schönicke G, Nevatte TM, O’Brien S, Junge W. Validation of a rapid alkaline hematin technique to measure menstrual blood loss on feminine towels containing superabsorbent polymers. Fertil Steril 2011;96:394–8. [DOI] [PubMed] [Google Scholar]
- 24.Coyne KS, Soliman AM, Margolis MK, Thompson CL, Chwalisz K. Validation of the 4 week recall version of the Uterine Fibroid Symptom and Health-related Quality of Life (UFS-QOL) Questionnaire. Curr Med Res Opin 2017;33:193–200. [DOI] [PubMed] [Google Scholar]
- 25.Spies JB, Coyne K, Guaou Guaou N, Boyle D, Skyrnarz-Murphy K, Gonzalves SM. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol 2002;99:290–300. [DOI] [PubMed] [Google Scholar]
- 26.Surrey E, Taylor H, Giudice L, Lessey BA, Abrao MS, Archer DF, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol 2018;132:147–60. [DOI] [PubMed] [Google Scholar]
- 27.Higham JM, O’Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol 1990; 97:734–9. [DOI] [PubMed] [Google Scholar]
- 28.Mansfield PK, Voda A, Allison G. Validating a pencil-and-paper measure of perimenopausal menstrual blood loss. Womens Health Issues 2004;14:242–7. [DOI] [PubMed] [Google Scholar]
- 29.Reid PC, Coker A, Coltart R. Assessment of menstrual blood loss using a pictorial chart: a validation study. BJOG 2000;107: 320–2. [DOI] [PubMed] [Google Scholar]
- 30.Fraser IS, Parke S, Mellinger U, Machlitt A, Serrani M, Jensen J. Effective treatment of heavy and/or prolonged menstrual bleeding without organic cause: pooled analysis of two multinational, randomised, double-blind, placebo-controlled trials of oestradiol valerate and dienogest. Eur J Contracept Reprod Health Care 2011;16:258–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.