Abstract
Background
We compared two serological assays from Roche Diagnostics in individuals with and without COVID-19 vaccination, namely the Elecsys Anti-SARS-CoV-2 assay (detecting antibodies against the nucleocapsid protein of SARS-CoV-2) and the Elecsys Anti-SARS-CoV-2 S assay (detecting antibodies against the spike protein of SARS-CoV-2).
Methods
With both assays, we analyzed 3033 serum samples collected from 2496 patients without COVID-19 vaccination. In addition, we studied 34 healthcare-workers who received two injections of the BNT162b2 COVID-19 vaccine from BioNTech/Pfizer three weeks apart and who had repeatedly determined their antibody response by both assays.
Results
In our cohort of patients without COVID-19 vaccination, 62.9% of all determinations were negative with both Roche assays and 31.5% were positive with both assays. In 5.6% of our cohort, however, there were discordant results with both assays (partly because initially discordant results of the two assays became concordantly positive over time). In the healthcare-workers with the COVID-19 vaccination, the results of the Roche anti-nucleocapsid assay remained negative throughout the observation period of 5 weeks after vaccination. The initially negative antibodies against the spike protein became positive with the Roche assay in all samples two weeks after the initial injection, and the serum concentrations of anti-spike antibodies increased constantly until 4–5 weeks after the initial injection.
Conclusions
Here, we provide information on serological testing with the two Roche assays, which may be important for the application of the two assays in clinical routine. There are differences in the pattern of antibodies in individuals with and without COVID-19 vaccination.
Keywords: Antibody, COVID-19, Laboratory medicine, Serologic testing, Vaccine, Virology
Abbreviations: COI, cut-off index; COVID-19, coronavirus disease 19; CV, coefficient of variation; IQC, internal quality control; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2
1. Introduction
The severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) has been on our minds worldwide for more than a year. Since the beginning of 2020, we know that SARS-CoV-2 is the cause of coronavirus disease 19 (COVID-19) [1], [2]. COVID-19 can have severe courses with the occurrence of COVID-19-associated pneumonia, with the need for intensive medical treatment and with a relatively high mortality [1], [2]. However, there are also mild to asymptomatic courses [2]. In January 2020, the World Health Organization (WHO) declared the outbreak of COVID-19 to be a public health emergency of international concern [1]. In March 2020, the WHO declared COVID-19 as a pandemic [1]. In the course of the last year, COVID-19 has gained more and more importance in health policy worldwide. Recently, various vaccines have become available to prevent COVID-19 [3], [4], [5], [6], [7]. In the member states of the European Union, for example, the vaccination campaign started at the end of December 2020.
The most important cornerstone of laboratory diagnostics is the detection of the pathogen from clinical specimens (e.g., nasopharyngeal swabs, oropharyngeal swabs, bronchoalveolar lavage fluid) by means of molecular testing of SARS-CoV-2 (nucleic acid amplification tests, mostly real-time reverse transcription polymerase chain reaction based molecular tests) [1], [2], [8], [9]. In addition, the possibility of (rapid) antigen detection and of serological testing has become commercially available. In this context, the IFCC interim guidelines on serological testing of antibodies against SARS-CoV-2 were published recently [10]. There is consensus that serological testing can be helpful 1) in diagnosing SARS-CoV-2 infection in symptomatic hospitalized patients (especially if molecular biology testing is repeatedly negative); 2) to detect a previous infection with SARS-CoV-2 in hospitalized and non-hospitalized patients; 3) to estimate the extent of antibody production in a patient; 4) to determine the rate of individuals in certain populations who have already had contact with SARS-CoV-2 (e.g., for prevalence studies, for monitoring development of herd immunity); and possibly also 4) to detect antibody production following COVID-19 vaccination [10], [11], [12], [13].
In this work, we wanted to compare two serological assays from Roche Diagnostics (Rotkreuz, Switzerland) in individuals with and without COVID-19 vaccination, namely the Elecsys Anti-SARS-CoV-2 assay (which can detect antibodies against the nucleocapsid protein of SARS-CoV-2) and the Elecsys Anti-SARS-CoV-2 S assay (which can detect antibodies against the spike protein of SARS-CoV-2). We were interested in how far the results of these two assays differ in vaccinated and non-vaccinated individuals. There are already publications on both Roche assays, but our two research questions have not yet been answered with previous publications [14], [15], [16], [17], [18], [19], [20].
2. Methods
2.1. Study design
This is a retrospective and exploratory study. We wanted to compare SARS-CoV-2 antibody levels in serum against the nucleocapsid protein and the spike protein in two different settings. We intended to use our data generated in routine clinical practice using two commercially available, automated, high-throughput assays. Specifically, we had two aims with this study: 1) We wanted to evaluate the extent of concordant and discordant results of the presence of antibodies against the nucleocapsid protein and spike protein in individuals without COVID-19 vaccination; and 2) we wanted to know the extent to which serum concentrations of antibodies against the nucleocapsid protein and spike protein differ over time following SARS-CoV-2 vaccination.
Because the present study is a purely retrospective data analysis, we did not consider a referral to the ethics committee necessary. For the data analysis, we used MedCalc 17.2 (MedCalc Software Ltd, Ostend, Belgium).
2.2. Measurement of SARS-CoV-2 antibody concentrations in serum
Since 29/10/2020, we have used two methods simultaneously in clinical routine when a SARS-CoV-2 serology is requested. To detect antibodies against the nucleocapsid protein, we use the Elecsys Anti-SARS-CoV-2 assay (Roche Diagnostics, Rotkreuz, Switzerland); to measure the concentration of antibodies against the spike protein, we use the Elecsys Anti-SARS-CoV-2 S assay (Roche Diagnostics, Rotkreuz, Switzerland). In our clinical routine, both assays run on two Cobas e801 systems (Roche Diagnostics, Rotkreuz, Switzerland). We follow the manufacturer's instructions when performing both tests. For blood-collection we use a serum tube from which both tests are performed (Greiner Bio-One, Kremsmuenster, Austria; CAT Serum Sep Clot Activator, Ref. 454078).
The Elecsys Anti-SARS-CoV-2 assay (Ref. # 09203079190) is a qualitative electrochemiluminescence immuno assay that detects an individual's total immunoglobulin against a recombinant nucleocapsid protein of SARS-CoV-2. This assay produces results as a cut-off index (COI; signal of sample divided by cutoff), where results ≥ 1.00 are reported as reactive/positive. The manufacturer's package insert does not specify an analytical coefficient of variation (CV) for the COI, but the literature indicates that the total CV of this assay is <14% at various concentration levels [14], [15], [17], [20]. We do two different internal quality controls (IQC) from the manufacturer (PreciControl Anti SARS-CoV-2) daily on both Cobas e801 systems in clinical routine. At a mean low IQC of 0.09–0.10 COI (depending on the lot used), we have had a CV < 8% since 29/10/2020; at a mean high IQC of 2.88–3.00 COI (depending on the lot and the analyzer used), we have had a CV < 15% since 29/10/2020.
The Elecsys Anti-SARS-CoV-2 S assay (Ref. # 09289275190) is a quantitative electrochemiluminescence immuno assay that measures the concentration of an individual's total immunoglobulin against a recombinant spike protein (receptor binding domain) of SARS-CoV-2. The measurement range of the assay is from 0.40 U/mL to 250 U/mL. Values lower than the limit of quantification are reported as < 0.4 U/mL on our medical reports. For values > 250 U/mL, our analyzer automatically makes a 1:10 dilution and measures again, so that values up to 2500 U/mL can be reported. For our study, we provide measured values < 0.40 U/mL as 0.39 U/mL and values > 2500 U/mL as 2501 U/mL. Antibody concentrations of <0.80 U/mL are considered negative and of ≥0.80 positive. In the manufacturer's package insert, CV values of <3% are given for different concentrations. The literature indicates that the total CV of this assay is <4% at various concentration levels [19], [20]. We do two different IQCs of the manufacturer (PreciControl Anti SARS-CoV-2 S) daily on both Cobas e801 systems in clinical routine. For the negative IQC we have consistently measured < 0.4 U/mL since 29/10/2020, with a mean value of the positive IQC of 8.8–9.6 U/mL (depending on the lot and the analyzer used) we have had a CV < 8% since 29/10/2020.
2.3. Individuals without COVID-19 vaccination
For the method comparison of the anti-nuclocapsid assay with the anti-spike assay in non-vaccinated individuals, we extracted all serological determinations made in the period from 29/10/2020 to 28/12/2020 in the Department of Clinical Pathology of Bolzano from our Laboratory Information System (LIS) and transferred them to an electronic database (including the corresponding patients’ sex and age). The two dates were chosen because in our laboratory we started on 29/10/2020 to perform both assays simultaneously whenever a SARS-CoV-2 serology was requested in the clinical routine, and because in the Province of Bolzano, South Tyrol, the vaccination of healthcare workers and parts of the elderly population against COVID-19 started on 29/12/2020. We wanted to perform the method comparison in our patients without the influence of the COVID-19 vaccination. There were no exclusion criteria for this evaluation. Thus, we used all results in the mentioned period for the data evaluation, even if repeated serological determinations were made in certain patients in the course of time.
2.4. Individuals with COVID-19 vaccination
Eligible for evaluation of SARS-CoV-2 antibody concentrations in serum over time after COVID-19 vaccination were all employees of the Department of Clinical Pathology of Bolzano who received their first vaccination between 29/12/2020 (start of vaccination in the Province of Bolzano, South Tyrol) and 14/01/2021 (n = 46). Those employees who had a documented COVID-19 infection in the past were excluded from the evaluation (n = 1). Additionally, those staff members were excluded from the evaluation who did not have at least two determinations of SARS-CoV-2 antibody levels in serum by 20/02/2021 (n = 11). For each determination of SARS-CoV-2 antibodies, we always simultaneously measured the antibody concentrations against the nucleocapsid protein and the spike protein.
At the beginning of February 2021, we requested the dates of vaccinations from our laboratory staff. Subsequently, with the informed consent of our staff, we extracted the serology data from our Laboratory Information System (LIS) and transferred it to an electronic database.
The classification of the assignment of the dates of the individual blood collections from the employees with regard to the timeline related to the date of the vaccinations of the employees was determined before data analysis as follows: As “baseline”, all blood draws for the determination of SARS-CoV-2 antibodies against the nucleocapsid protein and the spike protein in a period from a maximum of 7 days before the vaccination to a maximum of 3 days after the first COVID-19 vaccination were to be considered. The time points “1 week after baseline“, “2 weeks after baseline”, “3 weeks after baseline“, “4 weeks after baseline” and “5 weeks after baseline“ refer to 7 days after the first vaccination (allowed range, 4 to 10 days after the first vaccination), 14 days after the first vaccination (allowed range, 11 to 17 days after first vaccination), 21 days after first vaccination (allowed range, 18 to 24 days after first vaccination), 28 days after first vaccination (allowed range, 25 to 31 days after first vaccination), and 35 days after first vaccination (allowed range, 32 to 38 days after first vaccination), respectively.
3. Results
3.1. Individuals without COVID-19 vaccination
From 29/10/2020 to 28/12/2020, we received 3033 serum samples in clinical routines with the order to determine the antibodies against SARS-CoV-2. These 3033 serum samples were from 2496 patients (1273 male and 1223 female) with a median age of 61 years (25th-75th percentiles, 46–78 years; range, <1–100 years). Thus, we compared 3033 results of the anti-nucleocapsid assay with 3033 results of the anti-spike assay. In 332 patients, at least two serial determinations were made with both assays during the study period; in 2164 patients, only one determination was made with both assays.
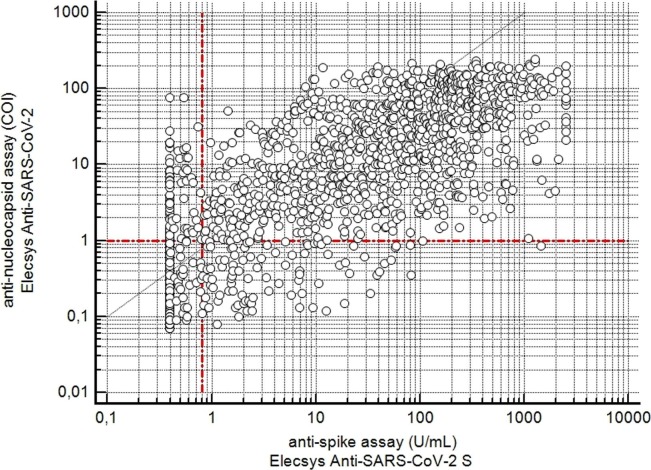
Fig. 1 shows a scatter plot in which the COI values of the anti-nucleocapsid assay were plotted against the concentrations of the anti-spike assay (expressed as U/mL). Table 1 summarizes the results dichotomized according to the cut-off values. About 63% of all determinations were negative with both assays in our cohort and about 32% of all determinations were positive with both assays. In about 6% of our cohort, however, there were discordant results with both assays. In about 3% of all determinations, the result of the anti-nucleocapsid assay was positive and that of the anti-spike assay negative. Conversely, in about 3% of all determinations, the result of the anti-nucleocapsid assay was negative and that of the anti-spike assay was positive.
Fig. 1.
Scatterplot of the values determined with the two assays from 3033 serum samples. The horizontal dotted line indicates the cut off value of the anti-nucleocapsid assay (negative, COI < 1.0; and positive, COI ≥ 1.0). The vertical dotted line indicates the cut off value of the anti-spike assay (negative, <0.80 U/mL; and positive, ≥0.80 U/mL).
Table 1.
Comparison of the results of anti-nucleocapsid assay and the anti-spike assay in individuals without vaccination.
| Anti-spike assay; negative (<0.80 U/mL) | Anti-spike assay; positive (≥0.80 U/mL) | ||
|---|---|---|---|
| Anti-nucleocapsid assay; negative (COI < 1.0) | n = 1907; 62.9% | n = 92; 3.0% | n = 1999; 65.9% |
| Anti-nucleocapsid assay; positive (COI ≥ 1.0) | n = 79; 2.6% | n = 955; 31.5% | n = 1034; 34.1% |
| n = 1986; 65.5% | n = 1047; 34.5% | n = 3033; 100% |
As already described, there were discordant results between the two assays in about 6% of the examinations. Of these 171 discordant results, 52 patients had at least two serial measurements over time, while 104 patients had no serial measurements. The time course of the results for the 52 patients (43 in-patients and 9 out-patients) with discordant results are shown in Table 2 . Using the data from these 52 patients in Table 2, we were able to demonstrate that in 26 patients initially discordant results from the two assays became concordantly positive over time.
Table 2.
Time course of the results in 52 patients, with discordant serologic determinations between the two assays (if there were several time points in a patient for serial blood collections, a maximum of four are listed in the table).
| Patient | Sample | Date | Anti-nucleocapsid assay |
Anti-spike assay |
SARS-CoV-2 RT-PCR‡ |
|||
|---|---|---|---|---|---|---|---|---|
| No. | COI | pos/neg | U/mL | pos/neg | Date | pos/neg | ||
| 1.* | 1 | 30 Oct | 0.09 | neg | 0.39 | neg | 30 Oct | pos |
| 2 | 31. Oct | 0.13 | neg | 0.54 | neg | 23 Nov | pos | |
| 3 | 01 Nov | 0.40 | neg | 1.59 | pos | 02 Dec | neg | |
| 4 | 16 Nov | 5.76 | pos | 224.00 | pos | 04 Dec | neg | |
| 2. | 1 | 31 Oct | 0.08 | neg | 0.39 | neg | 26 Apr | neg |
| 2 | 09 Nov | 0.43 | neg | 0.96 | pos | 26 Oct | pos | |
| 09 Nov | pos | |||||||
| 24 Nov | neg | |||||||
| 3. | 1 | 05 Nov | 0.09 | neg | 0.39 | neg | 05 Nov | neg |
| 2 | 04 Dec | 0.14 | neg | 1.77 | pos | 04 Dec | pos | |
| 11 Dec | pos | |||||||
| 30 Dec | neg | |||||||
| 4. | 1 | 05 Nov | 0.18 | neg | 2.47 | pos | 17 Aug | neg |
| 2 | 19 Nov | 0.16 | neg | 2.24 | pos | 23 Oct | neg | |
| 3 | 04 Dec | 0.16 | neg | 2.10 | pos | 31 Oct | neg | |
| 4 | 17 Dec | 0.15 | neg | 2.03 | pos | 03 Nov | neg | |
| 5.* | 1 | 06 Nov | 1.27 | pos | 0.59 | neg | 04 Nov | pos |
| 2 | 07 Nov | 1.48 | pos | 1.53 | pos | 10 Nov | pos | |
| 6.* | 1 | 06 Nov | 0.13 | neg | 9.32 | pos | 28 Aug | neg |
| 2 | 12 Nov | 5.05 | pos | 454.00 | pos | 04 Nov | pos | |
| 05 Nov | pos | |||||||
| 12 Nov | pos | |||||||
| 7.* | 1 | 06 Nov | 0.26 | neg | 0.96 | neg | 03 Jun | neg |
| 2 | 16 Nov | 35.80 | pos | 661.00 | pos | 06 Nov | pos | |
| 16 Nov | pos | |||||||
| 8.* | 1 | 07 Nov | 6.91 | pos | 0.39 | neg | 07 Nov | neg |
| 2 | 09 Nov | 14.50 | pos | 3.41 | pos | 09 Nov | neg | |
| 9. | 1 | 07 Nov | 0.74 | neg | 0.39 | neg | 03 Aug | neg |
| 2 | 10 Nov | 12.20 | pos | 0.49 | neg | 14 Sept | neg | |
| 26 Oct | pos | |||||||
| 10 Nov | pos | |||||||
| 10.* | 1 | 08 Nov | 12.30 | pos | 0.56 | neg | 08 Nov | pos |
| 2 | 09 Nov | 19.60 | pos | 0.96 | pos | 15 Nov | pos | |
| 22 Nov | pos | |||||||
| 15 Dec | neg | |||||||
| 11. | 1 | 09 Nov | 0.10 | neg | 0.39 | neg | 08 Nov | pos |
| 2 | 16 Nov | 0.48 | neg | 16.60 | pos | 21 Nov | pos | |
| 3 | 23 Nov | 0.56 | neg | 52.40 | pos | |||
| 12. | 1 | 09 Nov | 0.36 | neg | 6.98 | pos | 20 Apr | neg |
| 2 | 04 Dec | 0.30 | neg | 6.78 | pos | 30 Apr | neg | |
| 26 May | neg | |||||||
| 13. | 1 | 09 Nov | 0.09 | neg | 0.39 | neg | 01 Nov | neg |
| 2 | 15 Dec | 0.13 | neg | 0.93 | pos | 03 Nov | neg | |
| 09 Nov | pos | |||||||
| 10 Nov | pos | |||||||
| 14. | 1 | 11 Nov | 0.08 | neg | 0.39 | neg | 11 Nov | pos |
| 2 | 16 Nov | 0.18 | neg | 0.87 | pos | 20 Nov | pos | |
| 27 Nov | pos | |||||||
| 16 Dec | neg | |||||||
| 15. | 1 | 11 Nov | 0.09 | neg | 0.39 | neg | 11 Nov | pos |
| 2 | 18 Nov | 6.28 | pos | 0.39 | neg | 18 Nov | pos | |
| 30 Nov | pos | |||||||
| 07 Dec | pos | |||||||
| 16.* | 1 | 11 Nov | 0.08 | neg | 0.39 | neg | 14 Oct | neg |
| 2 | 27 Nov | 12.30 | pos | 0.59 | neg | 11 Nov | pos | |
| 3 | 03 Dec | 17.10 | pos | 43.90 | pos | 18 Nov | pos | |
| 03 Dec | neg | |||||||
| 17. | 1 | 13 Nov | 27.60 | pos | 0.39 | neg | 21 Mar | neg |
| 2 | 16 Nov | 75.90 | pos | 0.39 | neg | 13 Nov | pos | |
| 20 Nov | pos | |||||||
| 28 Nov | neg | |||||||
| 18. | 1 | 13 Nov | 1.02 | pos | 1.42 | pos | 09 Sep | neg |
| 2 | 16 Nov | 1.06 | pos | 25.10 | pos | 21 Oct | neg | |
| 3 | 18 Nov | 0.86 | neg | 1458.00 | pos | 13 Nov | pos | |
| 21 Nov | pos | |||||||
| 19. | 1 | 13 Nov | 0.09 | neg | 0.39 | neg | 13 Nov | pos |
| 2 | 20 Nov | 1.20 | pos | 0.39 | neg | 21 Nov | pos | |
| 27 Nov | pos | |||||||
| 04 Dec | neg | |||||||
| 20. | 1 | 14 Nov | 1.00 | pos | 0.39 | neg | 13 Nov | pos |
| 2 | 16 Nov | 0.91 | neg | 0.39 | neg | 14 Nov | pos | |
| 21 Nov | pos | |||||||
| 12 Dec | neg | |||||||
| 21.* | 1 | 14 Nov | 0.10 | neg | 0.39 | neg | 11 Nov | pos |
| 2 | 16 Nov | 0.22 | neg | 1.45 | pos | 23 Nov | pos | |
| 3 | 19 Nov | 0.62 | neg | 29.10 | pos | 02 Dec | pos | |
| 4 | 30 Nov | 3.79 | pos | 400.00 | pos | 17 Dec | neg | |
| 22.* | 1 | 15 Nov | 1.99 | pos | 0.41 | neg | 03 Oct | neg |
| 2 | 16 Nov | 3.30 | pos | 0.95 | pos | 09 Nov | pos | |
| 13 Nov | pos | |||||||
| 20 Nov | pos | |||||||
| 23.* | 1 | 15 Nov | 0.09 | neg | 0.39 | neg | 27 Oct | neg |
| 2 | 19 Nov | 0.32 | neg | 2.22 | pos | 09 Nov | pos | |
| 3 | 23 Nov | 17.40 | pos | 60.70 | pos | 15 Nov | pos | |
| 22 Nov | pos | |||||||
| 24.* | 1 | 15 Nov | 15.00 | pos | 0.47 | neg | 11 Aug | neg |
| 2 | 15 Nov | 15.80 | pos | 0.53 | neg | 12 Nov | pos | |
| 3 | 16 Nov | 26.30 | pos | 1.82 | pos | 15 Nov | pos | |
| 4 | 23 Nov | 69.80 | pos | 343.00 | pos | 23 Nov | pos | |
| 25. | 1 | 16 Nov | 4.33 | pos | 598.00 | pos | 15 Oct | pos |
| 2 | 07 Dec | 1.55 | pos | 167.00 | pos | 23 Oct | pos | |
| 3 | 14 Dec | 0.88 | neg | 80.20 | pos | 27 Oct | pos | |
| 4 | 21 Dec | 0.98 | neg | 106.00 | pos | 09 Nov | pos | |
| 26.* | 1 | 17 Nov | 5.76 | pos | 0.65 | neg | 17 Nov | pos |
| 2 | 19 Nov | 10.50 | pos | 1.55 | pos | 26 Nov | pos | |
| 07 Dec | neg | |||||||
| 27.* | 1 | 18 Nov | 1.48 | pos | 0.73 | neg | 30 Jul | neg |
| 2 | 04 Dec | 4.10 | pos | 2.55 | pos | 23 Oct | neg | |
| 31 Oct | pos | |||||||
| 12 Nov | pos | |||||||
| 28. | 1 | 18 Nov | 0.12 | neg | 12.50 | pos | 13 Mar | neg |
| 2 | 14 Dec | 0.50 | neg | 45.70 | pos | 26 Oct | pos | |
| 03 Nov | pos | |||||||
| 16 Nov | neg | |||||||
| 29.* | 1 | 19 Nov | 0.88 | neg | 8.25 | pos | 19 Oct | pos |
| 2 | 09 Dec | 0.90 | neg | 11.10 | pos | 28 Oct | pos | |
| 3 | 17 Dec | 1.02 | pos | 12.80 | pos | 11 Nov | pos | |
| 25 Nov | neg | |||||||
| 30. | 1 | 20 Nov | 0.83 | neg | 0.89 | pos | 20 Nov | pos |
| 2 | 22 Nov | 0.98 | neg | 1.51 | pos | 27 Nov | pos | |
| 28 Nov | pos | |||||||
| 08 Dec | neg | |||||||
| 31. | 1 | 20 Nov | 0.15 | neg | 0.39 | neg | 20 Nov | pos |
| 2 | 23 Nov | 16.80 | pos | 0.39 | neg | 20 Nov | pos | |
| 26 Nov | pos | |||||||
| 07 Dec | neg | |||||||
| 32.* | 1 | 20 Nov | 0.67 | neg | 7.70 | pos | 20 Nov | neg |
| 2 | 24 Nov | 7.27 | pos | 61.50 | pos | 22 Nov | pos | |
| 29 Nov | pos | |||||||
| 30 Nov | pos | |||||||
| 33.* | 1 | 22 Nov | 0.55 | neg | 1.96 | pos | 13 May | neg |
| 2 | 23 Nov | 1.29 | pos | 7.29 | pos | 17 Nov | pos | |
| 3 | 26 Nov | 25.60 | pos | 197.00 | pos | 24 Nov | pos | |
| 17 Dec | neg | |||||||
| 34.* | 1 | 23 Nov | 0.34 | neg | 1.57 | pos | 17 Nov | pos |
| 2 | 28 Nov | 15.20 | pos | 244.00 | pos | 23 Nov | pos | |
| 27 Nov | pos | |||||||
| 10 Dec | pos | |||||||
| 35. | 1 | 24 Nov | 0.09 | neg | 0.39 | neg | 19 Nov | pos |
| 2 | 24 Nov | 1.03 | pos | 0.79 | neg | 24 Nov | pos | |
| 04 Dec | pos | |||||||
| 36. | 1 | 25 Nov | 0.14 | neg | 0.83 | pos | 28 Mar | neg |
| 2 | 26 Nov | 0.19 | neg | 1.19 | pos | 12 Oct | neg | |
| 20 Nov | pos | |||||||
| 27 Nov | pos | |||||||
| 37. | 1 | 25 Nov | 0.28 | neg | 0.39 | neg | 25 Nov | neg |
| 2 | 28 Nov | 8.50 | pos | 0.58 | neg | 28 Nov | pos | |
| 04 Dec | pos | |||||||
| 38. | 1 | 27 Nov | 0.10 | neg | 0.39 | neg | 14 Apr | neg |
| 2 | 30 Nov | 0.92 | neg | 8.20 | pos | 10 Nov | neg | |
| 16 Nov | pos | |||||||
| 28 Nov | pos | |||||||
| 39. | 1 | 27 Nov | 0.10 | neg | 0.39 | neg | 27 Nov | pos |
| 2 | 30 Nov | 3.95 | pos | 0.39 | neg | 04 Dec | pos | |
| 11 Dec | pos | |||||||
| 25 Dec | pos | |||||||
| 40.* | 1 | 27 Nov | 0.08 | neg | 1.12 | pos | 27 Nov | pos |
| 2 | 02 Dec | 15.50 | pos | 50.90 | pos | 02 Dec | pos | |
| 3 | 07 Dec | 41.90 | pos | 461.00 | pos | 14 Dec | pos | |
| 4 | 14 Dec | 51.30 | pos | 453.00 | pos | 21 Dec | neg | |
| 41.* | 1 | 02 Dec | 0.08 | neg | 0.39 | neg | 28 Nov | pos |
| 2 | 07 Dec | 0.32 | neg | 0.90 | pos | 03 Dec | pos | |
| 3 | 14 Dec | 2.36 | pos | 96.60 | pos | 14 Dec | pos | |
| 15 Dec | neg | |||||||
| 42.* | 1 | 04 Dec | 0.20 | neg | 7.26 | pos | 07 May | neg |
| 2 | 07 Dec | 1.90 | pos | 59.90 | pos | 23 Nov | pos | |
| 03 Dec | pos | |||||||
| 43.* | 1 | 04 Dec | 0.09 | neg | 0.39 | neg | 11 Aug | neg |
| 2 | 12 Dec | 0.40 | neg | 6.94 | pos | 24 Nov | neg | |
| 3 | 14 Dec | 2.17 | pos | 115.00 | pos | 04 Dec | pos | |
| 4 | 21 Dec | 30.80 | pos | 1409.00 | pos | 21 Dec | pos | |
| 44.* | 1 | 07 Dec | 0.27 | neg | 1.53 | pos | 07 Dec | pos |
| 2 | 09 Dec | 2.61 | pos | 13.30 | pos | |||
| 45.* | 1 | 08 Dec | 1.19 | pos | 0.39 | neg | 28 Aug | neg |
| 2 | 21. Dec | 15.30 | pos | 135.00 | pos | 07 Dec | pos | |
| 15 Dec | pos | |||||||
| 24 Dec | pos | |||||||
| 46. | 1 | 09 Dec | 0.09 | neg | 0.39 | neg | 08 Dec | pos |
| 2 | 11 Dec | 0.24 | neg | 1.01 | pos | 15 Dec | pos | |
| 22 Dec | pos | |||||||
| 29 Dec | neg | |||||||
| 47. | 1 | 09 Dec | 0.09 | neg | 0.39 | neg | 09 Dec | pos |
| 3 | 14 Dec | 0.70 | neg | 4.09 | pos | 12 Dec | pos | |
| 3 | 21 Dec | 9.78 | pos | 312.00 | pos | 21 Dec | pos | |
| 20 Dec | pos | |||||||
| 48.* | 1 | 11 Dec | 1.45 | pos | 0.43 | neg | 24 Apr | neg |
| 2 | 14 Dec | 7.22 | pos | 7.76 | pos | 18 Nov | neg | |
| 30 Nov | pos | |||||||
| 11 Dec | pos | |||||||
| 49. | 1 | 13 Dec | 0.15 | neg | 18.70 | pos | 07 Dec | pos |
| 2 | 14 Dec | 0.35 | neg | 38.30 | pos | 13 Dec | pos | |
| 20 Dec | pos | |||||||
| 29 Dec | pos | |||||||
| 50.* | 1 | 15 Dec | 0.09 | neg | 0.39 | neg | 15 Dec | pos |
| 2 | 18 Dec | 0.10 | neg | 1.80 | pos | 18 Dec | pos | |
| 3 | 21 Dec | 1.20 | pos | 26.70 | pos | 21 Dec | pos | |
| 4 | 28 Dec | 5.70 | pos | 658.00 | pos | 28 Dec | pos | |
| 51. | 1 | 19 Dec | 0.11 | neg | 0.39 | neg | 28 Nov | neg |
| 2 | 21 Dec | 3.15 | pos | 0.39 | neg | 03 Dec | neg | |
| 10 Dec | pos | |||||||
| 19 Dec | pos | |||||||
| 52.* | 1 | 23 Dec | 31.10 | pos | 0.73 | neg | 20 Dec | pos |
| 2 | 24 Dec | 32.80 | pos | 2.33 | pos | 27 Dec | pos | |
Initially discordant results of the two assays became concordantly positive over time.
In each patient, all medical records of SARS-CoV-2 real-time reverse transcription polymerase chain reaction (RT-PCT) from 01/01/2020 to 31/12/2020 were retrieved from our laboratory information system (LIS). The result of the first SARS-CoV-2 RT-PCR performed in 2020 is given for each patient. If there were several SARS-CoV-2 RT-PCRs performed in a certain patient in 2020, a maximum of four PCR results are listed in the table.
3.2. Individuals with COVID-19 vaccination
During the period 29/12/2020 to 14/01/2020, out of 77 laboratory staff members, 46 were vaccinated against COVID-19 for the first time and 31 were not vaccinated during this period. All vaccinations were administered using the BioNTech/Pfizer's BNT162b2 COVID-19 vaccine [4] in two doses at the dose prescribed by the manufacturer. The second vaccination was administered to each staff member 21 or 22 days after the initial vaccination, i.e. between 19/01/2021 and 04/02/2021. Of the 46 vaccinated staff members, 1 individual had a documented history of COVID-19 infection, so this staff member was excluded from the study. Of the remaining 45 staff members, 5 individuals had no determination of SARS-CoV-2 antibody levels in serum between 27/12/2020 and 20/02/2021, 6 individuals had one determination of SARS-CoV-2 antibody levels in serum between 27/12/2020 and 20/02/2021, and 34 individuals had at least two determinations of SARS-CoV-2 antibody levels in serum between 27/12/2020 and 20/02/2021. For the evaluation of the courses of the SARS-CoV-2 antibody concentrations in the serum of these 34 employees, we were able to use 149 simultaneous measurements of the antibody concentrations against the nucleocapsid protein and the spike protein for the present evaluation.
The 34 participants (10 male and 24 female) had a median age of 50 years (25th-75th percentiles, 46–56 years; range, 24–62 years). The median time between blood sampling for baseline determinations and the first COVID-19 vaccination was 1 day (range, 5 days before first vaccination to 1 day after vaccination). The median time between the first COVID-19 vaccination and the blood draws for the “1 week after baseline“ determinations was 7 days (range, 5 to 9 days after the first vaccination). The median time between the first COVID-19 vaccination and blood sampling for the determinations “2 weeks after baseline” was 14 days (range, 12 to 17 days after the first vaccination). The median time between the first COVID-19 vaccination and blood sampling for the determinations “3 weeks after baseline“ was 21 days (range, 18 to 22 days after the first vaccination). The median time between the first COVID-19 vaccination and blood sampling for the determinations “4 weeks after baseline” was 28 days (range, 26 to 29 days after the first vaccination). The median time between the first COVID-19 vaccination and blood sampling for the determinations “5 weeks after baseline“ was 35 days (range, 32 to 38 days after the first vaccination).
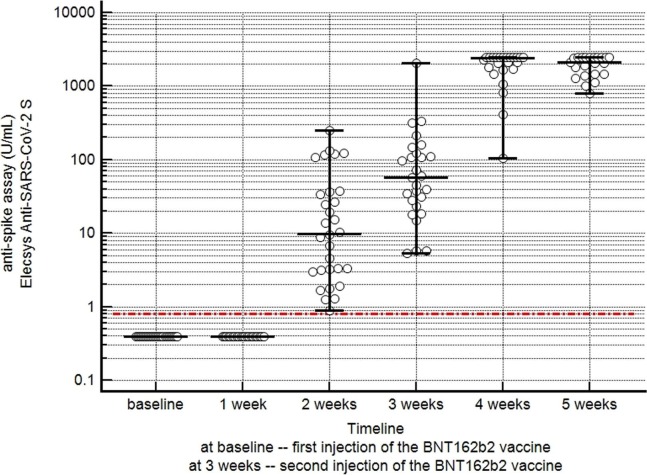
At baseline, all 34 healthcare-workers had negative results with the anti-nucleocapsid assay and with the anti-spike assay. As shown in Table 3 , the results of the anti-nucleocapsid assay remained negative throughout the observation period of 5 weeks after vaccination. The concentrations of antibodies against the spike protein remained negative in all workers during the first two weeks after the first vaccination. From week 3 onwards, the antibody concentrations against the spike protein rose into the positive range of the assay, and then continued to rise steadily until 4–5 weeks after vaccination. The results of the anti-spike assay in vaccinated individuals are summarized in Table 3 and Fig. 2 .
Table 3.
Time course of the results of both assays in 34 individuals with COVID-19 vaccinations (a total of 149 simultaneous measurements of antibody concentrations against the nucleocapsid protein and the spike protein were available).
| Number of serum samples | anti-nucleocapsid assay results | anti-spike assay results | ||
|---|---|---|---|---|
| positive/negative | median U/mL (range) | positive/negative | ||
| At baseline (time of first vaccination) | n = 25 | positive: n = 0 | 0.39 U/mL (0.39–0.39) | positive: n = 0 |
| negative: n = 25 | negative: n = 25 | |||
| 1 week after baseline | n = 21 | positive: n = 0 | 0.39 U/mL (0.39–0.39) | positive: n = 0 |
| negative: n = 21 | negative: n = 21 | |||
| 2 weeks after baseline | n = 30 | positive: n = 0 | 9.90 U/mL (0.90–247) | positive: n = 30 |
| negative: n = 30 | negative: n = 0 | |||
| 3 weeks after baseline (time of second vaccination) | n = 27 | positive: n = 0 | 57.7 U/mL (5.35–2049) | positive: n = 27 |
| negative: n = 27 | negative: n = 0 | |||
| 4 weeks after baseline | n = 24 | positive: n = 0 | 2384 U/mL (106–2501) | positive: n = 24 |
| negative: n = 24 | negative: n = 0 | |||
| 5 weeks after baseline | n = 22 | positive: n = 0 | 2120 U/mL (789–2501) | positive: n = 22 |
| negative: n = 22 | negative: n = 0 | |||
Fig. 2.
Time course of the results of the anti-spike assay in 34 individuals with COVID-19 vaccinations. The horizontal solid lines indicate the median and the whiskers indicate the range of antibody concentrations against the spike protein at the different time points. The horizontal dotted line indicates the cut-off value of the anti-spike assay (negative, <0.80 U/mL; and positive, ≥0.80 U/mL).
4. Discussion
We were able to show in the patient cohort without vaccination that, using the two Roche assays, a large proportion of the determinations of SARS-CoV-2 antibodies against the nucleocapsid protein and the spike protein showed concordant results. In our cohort, however, there were discordant results in about 6% of the cases.
In the patients with discordant results and serial measurements over time, we observed in about half of all cases that initially discordant results of the two assays became concordantly positive over time. We therefore speculate that after contact with SARS-CoV-2, in a certain percentage of cases either the antibodies against the nucleocapsid protein or the antibodies against the spike protein (measured with the Roche assays) may initially become positive, but then both antibodies become detectable with the Roche assays over time. The other cases in our cohort, in which the results of the antibody determination also remained discordant over time, or became discordant, remain obscure with the data from our study.
It would be necessary to conduct prospectively designed studies that systematically clarify the course of the antibodies over a longer period, and which reasons could be responsible for any persistent discordant antibody measurements. Nevertheless, based on our results, we believe that the simultaneous use of the two Roche assays for the detection of antibodies against the nucleocapsid protein and against the spike protein in clinical routine might make sense. The detection of only one of the two antibodies in distinct patients early in time course should result in a higher sensitivity for the detection of previous contact with SARS-CoV-2. However, even this assumption must first be proven by a suitable study.
In the second part of our work, we addressed the question of how the concentrations of antibodies against the nucleocapsid protein and against the spike protein measured with the Roche assays behave after COVID-19 vaccination. We observed that after injection of the BNT162b2 COVID-19 vaccine from BioNTech/Pfizer [4], the antibodies against the nucleocapsid protein remained consistently negative, but the antibodies against the spike protein became positive in all vaccinated individuals.
The initially negative antibodies against the spike protein became positive with the Roche assay in all samples two weeks after the initial injection, and the serum concentrations of anti-spike antibodies increased constantly until 4–5 weeks after the initial injection. We would like to emphasize, however, that our observation can only be related to the Roche assay and the BioNTech/Pfizer vaccine [4]. Other assays or a different vaccine may show different results. Nevertheless, this will certainly be the subject of other future studies (even with a longer follow up period than ours).
We excluded individuals with a history of SARS-CoV-2 infection from our analysis. In this context, we had only one employee who had a mildly symptomatic SARS-CoV-2 infection several months before vaccination. In this employee, at the time of the initial injection of the BNT162b2 COVID-19 mRNA vaccine from BioNTech/Pfizer [4], both the antibodies against the nucleocapsid protein and the antibodies against the spike protein were positive (data not shown). Approximately one to two weeks after the first vaccination, the serum concentration of antibodies against the spike protein increased rapidly and markedly (data not shown). This phenomenon has also been reported by a recent study demonstrating a robust spike antibody response and an increased reactogenicity in seropositive individuals after a single dose of a SARS-CoV-2 mRNA vaccine [21]. It would therefore be interesting for a future study to systematically investigate this phenomenon. In addition, it is still unclear how the antibody concentrations behave in the case of SARS-CoV-2 infection after vaccination but before the onset of full vaccination protection.
In summary, our study has provided information on serological testing with the two Roche assays, which may be important for the application of the two assays in clinical routine. There are differences in the pattern of antibodies in individuals with and without COVID-19 vaccination. The limitation of our study is the retrospective design and the relatively small number of cases in vaccinated individuals. In addition, we do not have any information on neutralizing antibodies in our two cohorts. Nevertheless, a recent study demonstrated that the concentrations of antibodies against the spike protein as measured with the Roche assay correlate well with SARS-CoV-2 neutralization activities [20]. Further studies are needed to systematically investigate the open questions discussed above in the unvaccinated individuals and to confirm our findings in the vaccinated individuals in a larger cohort.
Research funding
None declared.
Employment or leadership
None declared.
Honoraria
None declared.
CRediT authorship contribution statement
Thomas Mueller: Conceptualization, Formal analysis, Writing - original draft.
Declaration of Competing Interest
None declared.
Acknowledgements
I would like to thank Alexandra Palmieri for her utmost dedication in our blood collection center during the COVID-19 pandemic. I would also like to thank Maurizio Tait for his outstanding support during the pandemic and for the implementation of all COVID-19 associated changes to our Cobas system in the last year. Finally, I would like to thank Fabio Rossi for his remarkable achievement in doing the data extraction from our Laboratory Information System (LIS) that was necessary for this study.
References
- 1.Ganesh B., Rajakumar T., Malathi M., Manikandan N., Nagaraj J., Santhakumar A., Elangovan A., Malik Y.S. Epidemiology and pathobiology of SARS-CoV-2 (COVID-19) in comparison with SARS, MERS: An updated overview of current knowledge and future perspectives. Clin. Epidemiol. Glob Health. 2021;10:100694. doi: 10.1016/j.cegh.2020.100694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choudhary J., Dheeman S., Sharma V., Katiyar P., Karn S.K., Sarangi M.K., Chauhan A.K., Verma G., Baliyan N. Insights of Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-2) pandemic: a current review. Biol. Proced. Online. 2021;23(1):5. doi: 10.1186/s12575-020-00141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ophinni Y., Hasibuan A.S., Widhani A., Maria S., Koesnoe S., Yunihastuti E., Karjadi T.H., Rengganis I., Djauzi S. COVID-19 Vaccines: Current Status and Implication for Use in Indonesia. Acta Med Indones. 2020;52(4):388–412. [PubMed] [Google Scholar]
- 4.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Jr, Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T., COVE Study Group Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S., Briner C., Cicconi P., Collins A.M., Colin-Jones R., Cutland C.L., Darton T.C., Dheda K., Duncan C.J.A., Emary K.R.W., Ewer K.J., Fairlie L., Faust S.N., Feng S., Ferreira D.M., Finn A., Goodman A.L., Green C.M., Green C.A., Heath P.T., Hill C., Hill H., Hirsch I., Hodgson S.H.C., Izu A., Jackson S., Jenkin D., Joe C.C.D., Kerridge S., Koen A., Kwatra G., Lazarus R., Lawrie A.M., Lelliott A., Libri V., Lillie P.J., Mallory R., Mendes A.V.A., Milan E.P., Minassian A.M., McGregor A., Morrison H., Mujadidi Y.F., Nana A., O'Reilly P.J., Padayachee S.D., Pittella A., Plested E., Pollock K.M., Ramasamy M.N., Rhead S., Schwarzbold A.V., Singh N., Smith A., Song R., Snape M.D., Sprinz E., Sutherland R.K., Tarrant R., Thomson E.C., Török M.E., Toshner M., Turner D.P.J., Vekemans J., Villafana T.L., Watson M.E.E., Williams C.J., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J. Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., Botikov A.G., Izhaeva F.M., Popova O., Ozharovskaya T.A., Esmagambetov I.B., Favorskaya I.A., Zrelkin D.I., Voronina D.V., Shcherbinin D.N., Semikhin A.S., Simakova Y.V., Tokarskaya E.A., Egorova D.A., Shmarov M.M., Nikitenko N.A., Gushchin V.A., Smolyarchuk E.A., Zyryanov S.K., Borisevich S.V., Naroditsky B.S., Gintsburg A.L. Gam-COVID-Vac Vaccine Trial Group. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;S0140-6736(21):00234-8. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohn M.K., Mancini N., Loh T.P., Wang C.B., Grimmler M., Gramegna M., Yuen K.Y., Mueller R., Koch D., Sethi S., Rawlinson W.D., Clementi M., Erasmus R., Leportier M., Kwon G.C., Menezes M.E., Patru M.M., Singh K., Ferrari M., Najjar O., Horvath A.R., Adeli K., Lippi G. IFCC Interim Guidelines on Molecular Testing of SARS-CoV-2 Infection. Clin. Chem. Lab. Med. 2020;58(12):1993–2000. doi: 10.1515/cclm-2020-1412. [DOI] [PubMed] [Google Scholar]
- 9.Rahbari R., Moradi N., Abdi M. rRT-PCR for SARS-CoV-2: Analytical considerations. Clin. Chim. Acta. 2021;21(516):1–7. doi: 10.1016/j.cca.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohn M.K., Loh T.P., Wang C.B., Mueller R., Koch D., Sethi S., Rawlinson W.D., Clementi M., Erasmus R., Leportier M., Grimmler M., Yuen K.Y., Mancini N., Kwon G.C., Menezes M.E., Patru M.M., Gramegna M., Singh K., Najjar O., Ferrari M., Horvath A.R., Lippi G., Adeli K. and the IFCC Taskforce on COVID-19. IFCC Interim Guidelines on Serological Testing of Antibodies against SARS-CoV-2. Clin. Chem. Lab. Med. 2020;58(12):2001–2008. doi: 10.1515/cclm-2020-1413. [DOI] [PubMed] [Google Scholar]
- 11.Gundlapalli A.V., Salerno R.M., Brooks J.T., Averhoff F., Petersen L.R., McDonald L.C., Iademarco M.F. CDC COVID-19 Response. SARS-CoV-2 Serologic Assay Needs for the Next Phase of the US COVID-19 Pandemic Response. Open Forum Infect. Dis. 2020;8(1):ofaa555. doi: 10.1093/ofid/ofaa555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogl T., Leviatan S., Segal E. SARS-CoV-2 antibody testing for estimating COVID-19 prevalence in the population. Cell Rep. Med. 2021;14:100191. doi: 10.1016/j.xcrm.2021.100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krajewski R., Gołębiowska J., Makuch S., Mazur G., Agrawal S. Update on serologic testing in COVID-19. Clin. Chim. Acta. 2020;510:746–750. doi: 10.1016/j.cca.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Favresse J., Eucher C., Elsen M., Tré-Hardy M., Dogné J.M., Douxfils J. Clinical Performance of the Elecsys Electrochemiluminescent Immunoassay for the Detection of SARS-CoV-2 Total Antibodies. Clin. Chem. 2020;66(8):1104–1106. doi: 10.1093/clinchem/hvaa131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egger M., Bundschuh C., Wiesinger K., Gabriel C., Clodi M., Mueller T., Dieplinger B. Comparison of the Elecsys® Anti-SARS-CoV-2 immunoassay with the EDI™ enzyme linked immunosorbent assays for the detection of SARS-CoV-2 antibodies in human plasma. Clin. Chim. Acta. 2020;509:18–21. doi: 10.1016/j.cca.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang M.S., Hock K.G., Logsdon N.M., Hayes J.E., Gronowski A.M., Anderson N.W., Farnsworth C.W. Clinical Performance of the Roche SARS-CoV-2 Serologic Assay. Clin. Chem. 2020;66(8):1107–1109. doi: 10.1093/clinchem/hvaa132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suhandynata R.T., Hoffman M.A., Kelner M.J., McLawhon R.W., Reed S.L., Fitzgerald R.L. Multi-Platform Comparison of SARS-CoV-2 Serology Assays for the Detection of COVID-19. J. Appl. Lab. Med. 2020;5(6):1324–1336. doi: 10.1093/jalm/jfaa139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suhandynata R.T., Hoffman M.A., Huang D., Tran J.T., Kelner M.J., Reed S.L., McLawhon R.W., Voss J.E., Nemazee D., Fitzgerald R.L. Commercial serology assays predict neutralization activity against SARS-CoV-2. Clin. Chem. 2020:hvaa262. doi: 10.1093/clinchem/hvaa262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins V., Fabros A., Kulasingam V. Quantitative measurement of anti-SARS-CoV-2 antibodies: Analytical and clinical evaluation. J. Clin. Microbiol. 2021 doi: 10.1128/JCM.03149-20. JCM.03149-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.L'Huillier A.G., Meyer B., Andrey D.O., Arm-Vernez I., Baggio S., Didierlaurent A., Eberhardt C.S., Eckerle I., Grasset-Salomon C., Huttner A., Posfay-Barbe K.M., Royo I.S., Pralong J.A., Vuilleumier N., Yerly S., Siegrist C.A., Kaiser L. Geneva Centre for Emerging Viral Diseases. Antibody persistence in the first six months following SARS-CoV-2 infection among hospital workers: a prospective longitudinal study. Clin. Microbiol. Infect. 2021 doi: 10.1016/j.cmi.2021.01.005. S1198-743X(21)00031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.F. Krammer, K. Srivastava, the PARIS team, V. Simon, Robust spike antibody responses and increased reactogenicity in seropositive individuals after a single dose of SARS-CoV-2 mRNA vaccine, medRxiv. 2021 Feb 1. doi: 10.1101/2021.01.29.21250653.