Abstract
Aims
Type 2 diabetes mellitus (T2DM) is a risk factor for severe COVID-19. Our aim was to compare the clinical outcomes of patients with and without T2DM during the first hit of COVID-19 in Istanbul.
Methods
A retrospective population-based study was conducted including all consecutive adult symptomatic COVID-19 cases. Patients were confirmed with rt-PCR; treated and monitored in accordance with standard protocols. The primary endpoints were hospitalization and 30-day mortality.
Results
Of the 93,571 patients, 22.6% had T2DM, with older age and higher BMI. Propensity Score matched evaluation resulted in significantly higher rates of hospitalization (1.5-fold), 30-day mortality (1.6-fold), and pneumonia (1.4-fold). They revealed more severe laboratory deviations, comorbidities, and frequent drug usage than the Non-DM group. In T2DM age, pneumonia, hypertension, obesity, and insulin-based therapies were associated with an increased likelihood of hospitalization; whereas age, male gender, lymphopenia, obesity, and insulin treatment were considerably associated with higher odds of death.
Conclusions
COVID-19 patients with T2DM had worse clinical outcomes with higher hospitalization and 30-day mortality rates than those without diabetes. Compared to most territories of the world, COVID-19 mortality was much lower in Istanbul, which may be associated with accessible healthcare provision and the younger structure of the population.
Keywords: COVID-19, Hospitalization, Mortality, Type 2 diabetes mellitus, Istanbul
Abbreviations: AHD, Antihyperglycemic drugs; ALT, Alanine aminotransferase; ASA, Acetylsalicylic acid; AST, Aspartate aminotransferase; BMI, Body mass index; CAD, Coronary artery disease; CI, Confidence intervals; CKD, Chronic kidney disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; COPD, Chronic obstructive pulmonary disease; COVID-19, Coronavirus disease 2019; CRP, C-reactive protein; CT, Computed tomography; CVD, Cardiovascular diseases (including coronary artery disease, peripheral artery disease, and stroke); eGFR, Estimated glomerular filtration rate; HbA1c, Glycosylated hemoglobin A1c; HDL-chol, High density lipoprotein cholesterol; ICD-10, International Classification of Diseases-10; ICU, Intensive Care Unit; IQR, Interquartile range; LDH, Lactate dehydrogenase; LDL-chol, Low density lipoprotein cholesterol; LMWH, Low molecular weight heparin; Lym, Lymphocytes; MoH, Ministry of Health; Non-DM, Non diabetes mellitus; OR, Odds ratio; PSM, Propensity Score Matching system; rt-PCR, Reverse transcription-polymerase chain reaction; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SPSS, Statistical Package for the Social Sciences; T2DM, Type 2 diabetes mellitus; ULN, Upper limit of normal
1. Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has spread rapidly on a global scale, with over 110 million cases of coronavirus disease (COVID-19) have been diagnosed, and more than 2,4 million people lost worldwide [1], [2]. Turkey is one of the countries affected most by COVID-19; as of Feb 17, 2021, over 2.6 million cumulative confirmed cases were detected, and 27.738 citizens have died [3].
Recent studies have indicated that T2DM was one of the most encountered comorbidities in COVID-19 patients and is also considered an independent risk factor for disease severity and mortality [4], [5]. The prevalence of T2DM in COVID-19 ranges from 5.7% to as high as 68.3% around the world [4], [5], [6], [7]; also hospitalization and mortality rates in COVID-19 patients vary across populations and studies. In a systematic review from China, mortality is estimated at 3–4% [8]. However, reports from several European countries have shown that mortality rates range between 10% and 20% [9], [10]. Furthermore, in the majority of studies, mortality rates were given for hospitalized patients only [11], [12]. Nevertheless, in the literature, there are barely large-scale population studies on the clinical course of COVID-19 patients with T2DM. Yet, reliable population-based estimates of COVID-19 mortality in T2DM patients are essential for supporting clinical experts, providing standards to monitor the epidemic, and reinforcing the performance of health services.
Istanbul is a metropolitan city, with its 15.5 million inhabitants, representing 19% of Turkey’s population [13]. Based on a population-based study in 2010, in Turkey [14] and the data shared by the Turkish Statistical Institution in 2019, the age and sex-standardized prevalence of diabetes in the adult population of Istanbul is estimated as 15.2% (translating to 1.7 million adults) [13], [14]. During the first wave of COVID-19, Istanbul was the epicenter of the pandemic and had nearly two-thirds of all COVID-19 cases identified in Turkey [3]. Considering this fact, we aimed to investigate the clinical characteristics and risk factors of hospitalization and mortality in COVID-19 patients with and without T2DM in Istanbul.
2. Material and methods
2.1. Setting
This retrospective population-based study was included all symptomatic COVID-19 cases with or without T2DM in Istanbul, using the national COVID-19 registry of the Turkish Ministry of Health (MoH). During the study period, a real-time reverse transcription-polymerase chain reaction (rt-PCR) test for SARS-CoV-2 was available for individuals with symptoms consistent with COVID-19, those with a history of traveling abroad or in close contact with a COVID-19 patient in the previous 14 days. All rt-PCR positive results are mandatorily recorded into the national case-based surveillance system, which is integrated with the hospital and laboratory information systems.
This study was approved by the COVID-19 Investigation Review Board under the Bioethics Committee of MoH, which waived the requirement of informed consent due to the retrospective study design and anonymity of the national database (IRB no: 95741342–020:186404/28.10.2020).
2.2. Study population
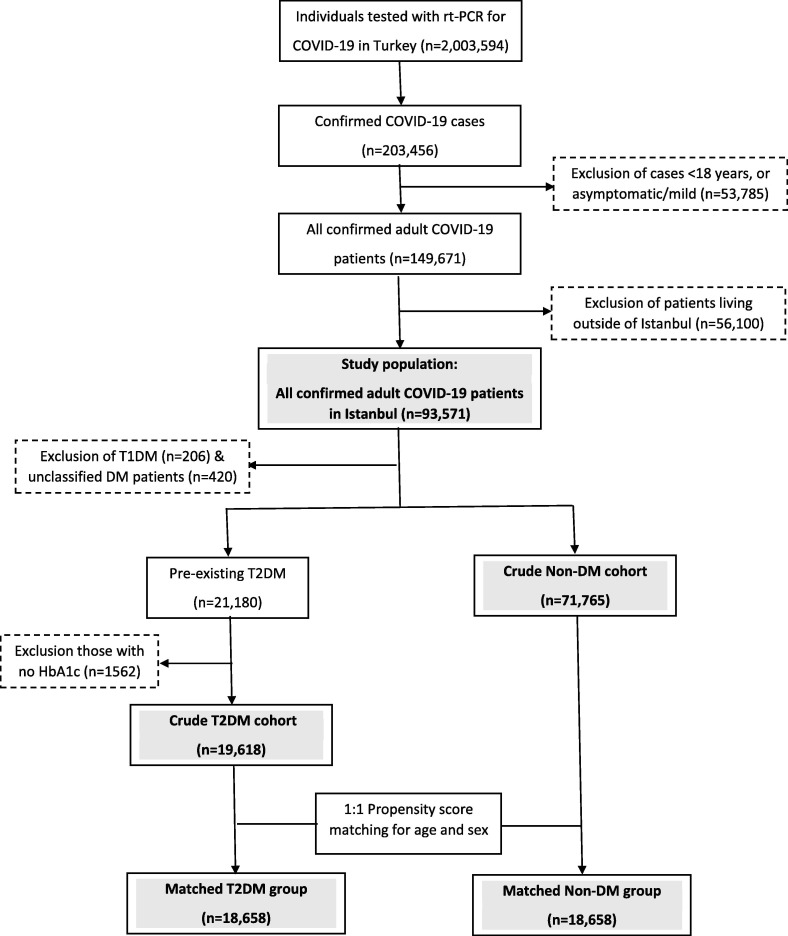
A consort diagram illustrating participant enrollment is provided in Fig. 1 . We included all adult patients to avoid any chance of selection bias. Briefly, from March 11 (date of the first COVID-19 case in Istanbul) to May 30, 2020 (inclusive), of the 203,456 confirmed cases in Turkey, 26.4% (n = 53,785) were excluded as they were < 18 years or asymptomatic/mild (<2 symptoms) cases. Out of 149,671 moderate/severe cases in ambulatory or inpatient settings, 62.5% (n = 93,571) were from Istanbul and formed our study population. After exclusion of patients with type 1 diabetes (n = 206) and unclassified diabetes (including new hyperglycemia, n = 420), the remaining 21,180 T2DM and 71,765 Non-DM patients formed our cohorts. Those with no available HbA1c records (in < 12 months, n = 1562) were also excluded. A sample of 18,658 T2DM patients was matched for age and gender with a non-DM group using the Propensity Score Matching System-PSMS on a 1:1 scale. Finally, the clinical and laboratory data of 37,316 patients were analyzed.
Fig. 1.
Consort diagram of the study participants.
2.3. Data collection
All data were obtained from the national database. Sociodemographic characteristics (age, gender, body mass index-BMI, smoking, and education), information on complications, comorbidities, and medications; and laboratory results including blood glucose, glycosylated hemoglobin A1c-HbA1c, lipids (low-, high-density lipoprotein cholesterol (LDL-chol, HDL-chol), total chol, and triglycerides), creatinine, aminotransferases (ALT, AST), D-dimer, C-reactive protein-CRP, procalcitonin, lymphocyte count, lactate dehydrogenase-LDH, ferritin, and fibrinogen were obtained. Chest computerized tomography-CT results were available in the national database as positive (bilateral distribution of patchy shadows or ground-glass opacities) or negative for COVID-19.
2.4. Definitions
Clinical definitions were explained below. Briefly, smoking was defined as current smoking, higher education as receiving more than formal education (≥9 years). BMI was calculated as the ratio of weight to the square of height (kg/m2). Pre-existing T2DM was identified based on the International Classification of Diseases System-10 (ICD-10) codes or having any HbA1c ≥ 6.5% or prescription of any antihyperglycemic drug-AHD within the last twelve months. Hypertension, dyslipidemia, chronic obstructive pulmonary disease-COPD, asthma, heart failure, coronary artery disease-CAD, peripheral artery disease, and stroke were defined based on the relevant ICD-10 codes. Cardiovascular disease-CVD was composed of CAD, stroke, and peripheral artery disease. Microvascular complications included retinopathy and neuropathy. Obesity was defined as BMI ≥ 30 kg/m2.
Diabetic complications were defined based on the ICD-10 codes; retinopathy as having an intravitreal injection or laser photocoagulation, and neuropathy with the prescription of relevant drugs (i.e., pregabalin, gabapentin, duloxetine, or alpha-lipoic acid). Chronic kidney disease-CKD was described as a reduced estimated glomerular filtration rate (eGFR: <60 mL/min/1.73 m2) based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [15].
Insulin-based regimens were defined as any insulin with/without other AHDs. Renin-angiotensin system (RAS) blocker use was described as receiving any angiotensin-converting enzyme inhibitors or angiotensin receptor blockers or their combination forms.
2.5. Study endpoints
The primary endpoints of the study were hospitalization and 30-day mortality. The secondary outcomes were hospital stay longer than the median duration (7 days), intensive care unit-ICU admission, and ICU stays longer than the median duration (6 days).
2.6. Statistical analysis
All data were analyzed using Statistical Package for Social Sciences v.25 (SPSS Inc. 111 Chicago, IL). Numerical variables were expressed as means (standard deviation-SD) or medians (interquartile range-IQR) and categorical variables as counts and percentages (n, %). Normality of distribution was assessed using the Kolmogorov-Smirnov test, and differences between groups with Chi-square test for categorical variables, student’s t-test, or Mann-Whitney U test, as appropriate.
Univariate analyses were performed to evaluate the potential variables associated with hospitalization or mortality in groups, separately and presented with odds ratio-OR and 95% confidence intervals-CI. Age- and sex-adjusted multivariable logistic regressions were performed to assess the association of clinical characteristics and biochemical results with the study endpoints. Variables with a potential or significant univariate association with the endpoints were included in multiple models set for T2DM and Non-DM, separately. Hosmer-Lemeshow test was used for goodness of fit and Box-Tidwell test to check the linearity of the logit for continuous independent variables. Statistical significance was defined as a two-sided p-value < 0.05.
3. Results
3.1. Overall study population
3.1.1. Principal findings
This study analyzed the demographic and clinical features of 93,571 adult Turkish patients with COVID-19 in Istanbul and identified factors associated with hospitalization and fatality of COVID-19 using the nationwide database. Approximately, 22.6% (n = 21,180) of cases had T2DM.
As presented in Table 1 , the median (IQR) age of the T2DM and Non-DM cohorts was 53 (22) and 38 (20) years, and the proportion of men were 42% and 55%, respectively. Median BMI was higher in T2DM than those without diabetes. Nearly half of T2DM patients had HbA1c over 7%. T2DM cohort had higher median levels of triglycerides, and glucose but lower HDL-chol than the Non-DM cohort. More patients in the T2DM cohort had high levels of AST, D-dimer, CRP, procalcitonin, LDH, and ferritin. In the T2DM cohort, smoking was less prevalent but comorbidities (hypertension, dyslipidemia, obesity, COPD/asthma, heart failure, CAD, peripheral artery disease, stroke, and cancer) were more common, and more patients were using RAS blockers, statins, and acetylsalicylic acid-ASA.
Table 1.
Basic characteristics of patients with and without type 2 diabetes mellitus diagnosed with COVID-19 in Istanbul (before PSM).
| T2DM (n = 21,180) |
Non-DM (n = 71,765) |
Available data (n) (T2DM/Non-DM) |
p | |
|---|---|---|---|---|
| Age, years, median (IQR) | 53 (22) | 38 (20) | 21,180/71,765 | <0.001 |
| Gender, male, n (%) | 8965 (42.3) | 39,471 (55.0) | 21,180/71,765 | <0.001 |
| Follow-up center, n (%) Public hospitals University hospitals Private centers |
15,598 (73.6) 1173 (5.5) 4409 (20.8) |
56,016 (78.1) 2738 (3.8) 13,011 (18.1) |
21,180/71,765 |
<0.001 |
| Education (≥9 years - n, %) | 67.9 (28.4) | 34.2 (39.4) | 2393/8677 | <0.001 |
| BMI, kg/m2, median (IQR) | 30.0 (7.1) | 25.7 (6.6) | 2251/4695 | <0.001 |
| Clinical severity | ||||
| Hospitalization, n (%) | 9468 (44.7) | 15,180 (21.2) | 21,180/71,765 | <0.001 |
| Hospital stay > 7 days, n (%) | 4080 (57.4) | 2689 (55.8) | 7109/4819 | 0.085 |
| ICU admission, n (% of those hospitalized) | 2125 (22.5) | 2197 (14.5) | 9442/15,157 | <0.001 |
| ICU stay > 6 days, n (%) | 1168 (55.1) | 1073 (49.0) | 2119/2191 | <0.001 |
| Death, n (%) | 1451 (6.9) | 1131 (1.6) | 21,179/71,759 | <0.001 |
| Chest CT on admission consistent with COVID-19, n (%) | 6476 (32.1) | 11,637 (17.3) | 20,188/67,230 | <0.001 |
| Laboratory values | ||||
| Glucose, mg/dL, median (IQR) | 125 (76) | 103 (23) | 2157/3039 | <0.001 |
| HbA1c, %, [mmol/mol] median (IQR) | 6.9 (2.3) [52 (2)] | ----- | 8813/---- | ----- |
| HbA1c > 7% [53 mmol/mol], n (%) | 6989 (48.2) | ----- | 14506/---- | ----- |
| Total chol, mg/dL, median (IQR) | 196.0 (64.6) | 194.0 (63.0) | 1320/802 | 0.126 |
| Triglycerides, mg/dL, median (IQR) | 136 (100) | 115.0 (80.7) | 1974/1225 | <0.001 |
| HDL-chol, mg/dL, median (IQR) | 46.9 (17.0) | 49.0 (19.7) | 1465/868 | 0.004 |
| LDL-chol, mg/dL, median (IQR) | 119.5 (55.0) | 118.0 (51.7) | 1549/884 | 0.599 |
| AST, >ULN, n (%) | 632 (23.4) | 831 (20.1) | 2696/4130 | 0.001 |
| ALT, >ULN, n (%) | 586 (21.8) | 944 (22.9) | 2691/4115 | 0.261 |
| D-dimer > ULN, n (%) | 819 (61.1) | 860 (46.6) | 1341/1845 | <0.001 |
| CRP, >ULN, n (%) | 3937 (72.9) | 5844 (62.0) | 5402/9426 | <0.001 |
| Procalcitonin, >ULN, n (%) | 160 (17.3) | 102 (11.8) | 924/861 | 0.001 |
| Lactate dehydrogenase, >ULN, n (%) | 1457 (46.7) | 1566 (36.5) | 3123/4287 | <0.001 |
| Ferritin, >100 ng/mL, n (%) | 1847 (64.3) | 1933 (56.0) | 2871/3451 | <0.001 |
| Fibrinogen, >ULN, n(%) | 306 (79.7) | 347 (74.6) | 384/465 | 0.081 |
| Lymphopenia, Lym # <1000/µL, n (%) | 2387 (20.4) | 4998 (16.6) | 11,684/30,143 | <0.001 |
| Comorbid conditions | ||||
| Smoking (current smoker - n, %) | 2230 (15.5) | 10,845 (20.4) | 14,343/53,230 | <0.001 |
| Hypertension, n (%) | 14,054 (66.4) | 16,328 (22.8) | 21,180/71,765 | <0.001 |
| Dyslipidemia, n (%) | 9245 (43.6) | 4926 (6.9) | 21,180/71,765 | <0.001 |
| COPD/Asthma, n (%) | 6769 (32.0) | 10,666 (14.9) | 21,180/71,765 | <0.001 |
| Obesity, n (%) | 1128 (50.2) | 964 (20.5) | 2249/4694 | <0.001 |
| Heart failure, n (%) | 1676 (7.9) | 868 (1.2) | 21,180/71,765 | <0.001 |
| Coronary artery disease, n (%) | 6205 (29.3) | 4893 (6.8) | 21,180/71,765 | <0.001 |
| Peripheral artery disease, n (%) | 1506 (7.1) | 1373 (1.9) | 21,180/71,765 | <0.001 |
| Stroke, n (%) | 684 (3.2) | 451 (0.6) | 21,180/71,765 | <0.001 |
| Diabetic retinopathy, n (%) | 480 (2.3) | ------ | 21,180/----- | |
| Diabetic neuropathy, n (%) | 2380 (11.2) | ------ | 21,180/------ | |
| Chronic kidney disease, n (%) | 1217 (18.9) | 745 (8.4) | 6442/8873 | <0.001 |
| Cancer, n (%) | 1409 (6.7) | 1576 (2.2) | 21,180/71,765 | <0.001 |
| Treatments | ||||
| Insulin-based regimen, n (%) | 4408 (20.8) | ------ | 21,180/------ | |
| RAS blocker, n (%) | 9569 (45.2) | 7685 (10.7) | 21,180/71,765 | <0.001 |
| Statin, n (%) | 5295 (25.0) | 1677 (2.3) | 21,180/71,765 | <0.001 |
| Acetylsalicylic acid, n (%) | 5905 (27.9) | 4500 (6.3) | 21,180/71,765 | <0.001 |
3.1.2. Endpoint analysis
In the T2DM cohort, the crude rate of hospitalization was 2.1-fold (44.7% vs. 21.2%), and mortality 4.3-fold (6.9% vs. 1.6%) higher than in the Non-DM cohort. Also, patients with T2DM had more severe clinical COVID-19 disease with a significantly higher rate of ICU admission, ICU stays longer than 6 days, and pneumonia. Hospital stays longer than 7 days were also more frequent among patients with T2DM but the difference did not reach statistical significance.
3.2. Propensity matched groups
3.2.1. Principal findings
After matching, the median (IQR) age was 53 (20) years, and male participants were 44%. In T2DM and Non-DM groups median (IQR) BMI was 30.0 (7.1) and 27.1 (6.4) kg/m2, respectively (Table 2 ). The differences in glucose, triglycerides, and HDL-chol levels between the two groups remained significant. There were no differences in education, high level (greater than the upper limit of normal-ULN) AST, and lymphopenia. Higher ALT was more common in the T2DM group. Whereas in the Non-DM group, the median LDL-chol was higher; additionally, more patients had high D-dimer, CRP, LDH, and ferritin levels. In the T2DM group, except for CKD, comorbidities (hypertension, dyslipidemia, obesity, COPD/asthma, heart failure, CAD, peripheral artery disease, stroke, and cancers) were more common, and the use of RAS blocker, statin, and ASA was more frequent than Non-DM group.
Table 2.
Basic characteristics of patients with and without type 2 diabetes mellitus diagnosed with COVID-19 in Istanbul (after PSM).
| T2DM (n = 18,658) |
Non-DM (n = 18,658) |
Available data (n) (T2DM/Non-DM) |
p | |
|---|---|---|---|---|
| Age, years, median (IQR) | 53 (20) | 53 (20) | 18,658/18,658 | 1.000 |
| Gender, male, n (%) | 8207 (44.0) | 8207 (44.0) | 18,658/18,658 | 1.000 |
| Follow-up center, n (%) Public hospitals University hospitals Private centers |
13,797 (73.9) 1030 (5.5) 3831 (20.5) |
14,431 (77.3) 720 (3.9) 3507 (18.8) |
18,658/18,658 |
<0.001 |
| Education (≥9 years - n,%) | 604 (28.6) | 587 (26.9) | 2112/2182 | 0.21 |
| BMI, kg/m2, median (IQR) | 30.0 (7.1) | 27.1 (6.4) | 2050/1193 | <0.001 |
| Clinical severity | ||||
| Hospitalization, n (%) | 8172 (43.8) | 5485 (29.4) | 18,658/18,658 | <0.001 |
| Hospital stay > 7 days, n (%) | 4080 (57.4) | 2689 (55.8) | 7109/4819 | 0.09 |
| ICU admission, n (% of those hospitalized) | 1706 (20.9) | 1078 (19.7) | 8151/5473 | 0.08 |
| ICU stay > 6 days, n (%) | 913 (53.7) | 584 (54.3) | 1700/1076 | 0.77 |
| Death, n (%) | 1162 (6.2) | 733 (3.9) | 18,657/18,657 | <0.001 |
| Chest CT on admission consistent with COVID-19, n (%) | 5648 (31.8) | 4076 (23.1) | 17,771/17,672 | <0.001 |
| Laboratory values | ||||
| Glucose, mg/dL, median (IQR) | 126 (76) | 107 (28) | 1708/628 | <0.001 |
| HbA1c, %, [mmol/mol] median (IQR) | 6.9 (2.3) [52 (2)] | ------- | 8193/------ | |
| HbA1c > 7% [53 mmol/mol], n (%) | 3798 (46.4) | ------- | 8177/------- | |
| Total chol, mg/dL, median (IQR) | 197 (64) | 201 (57) | 1155/247 | 0.214 |
| Triglycerides, mg/dL, median (IQR) | 136 (100) | 121 (79) | 1711/370 | <0.001 |
| HDL-chol, mg/dL, median (IQR) | 47 (18) | 50 (17) | 1281/265 | 0.005 |
| LDL-chol, mg/dL, median (IQR) | 120 (53.9) | 127.8 (51.5) | 1364/270 | 0.048 |
| AST, >ULN, n (%) | 481 (22.6) | 191 (23.6) | 2127/810 | 0.58 |
| ALT, >ULN, n (%) | 493 (23.1) | 138 (16.6) | 2132/833 | <0.001 |
| D-dimer > ULN, n (%) | 594 (58.0) | 254 (68.6) | 1024/370 | <0.001 |
| CRP, >ULN, n (%) | 3023 (69.8) | 1018 (77.4) | 4332/1316 | <0.001 |
| Procalcitonin, >ULN, n (%) | 108 (15.7) | 44 (19.7) | 689/223 | 0.16 |
| Lactate dehydrogenase, >ULN, n (%) | 1139 (44.8) | 472 (53.1) | 2545/889 | <0.001 |
| Ferritin, >100 ng/mL, n (%) | 1462 (62.4) | 509 (70.9) | 2344/718 | <0.001 |
| Fibrinogen, >ULN, n(%) | 234 (79.9) | 96 (78.0) | 293/123 | 0.68 |
| Lymphopenia, Lym # <1000/µL, n (%) | 2009 (19.7) | 1713 (20.5) | 10,212/8346 | 0.15 |
| Comorbid conditions | ||||
| Smoking (current smoker - n, %) | 1960 (15.5) | 2225 (17.1) | 12,681/13,039) | <0.001 |
| Hypertension, n (%) | 12,455 (66.8) | 7870 (42.2) | 18,658/18,658 | <0.001 |
| Dyslipidemia, n (%) | 8333 (44.7) | 2456 (13.2) | 18,658/18,658 | <0.001 |
| COPD/Asthma, n (%) | 5845 (31.3) | 3929 (21.1) | 18,658/18,658 | <0.001 |
| Obesity, n (%) | 1024 (50.0) | 344 (28.8) | 2049/1193 | <0.001 |
| Heart failure, n (%) | 1392 (7.5) | 631 (3.4) | 18,658/18,658 | <0.001 |
| Coronary artery disease, n (%) | 5399 (28.9) | 2839 (15.2) | 18,658/18,658 | <0.001 |
| Peripheral artery disease, n (%) | 1318 (7.1) | 646 (3.5) | 18,658/18,658 | <0.001 |
| Stroke, n (%) | 577 (3.1) | 301 (1.6) | 18,658/18,658 | <0.001 |
| Diabetic retinopathy, n (%) | 433 (2.3) | ----- | 18,658/----- | |
| Diabetic neuropathy, n (%) | 2112 (11.3) | ----- | 18,658/----- | |
| Chronic kidney disease, n (%) | 916 (16.9) | 410 (18.5) | 5417/2219 | 0.10 |
| Cancer, n (%) | 1209 (6.5) | 790 (4.2) | 18,658/18,658 | <0.001 |
| Treatments | ||||
| Insulin-based regimen, n (%) | 3340 (17.9) | ------ | 18,658/------ | |
| RAS blocker, n (%) | 8371 (44.9) | 4491 (24.1) | 18,658/18,658 | <0.001 |
| Statin, n (%) | 4727 (25.3) | 1106 (5.9) | 18,658/18,658 | <0.001 |
| Acetylsalicylic acid, n (%) | 5148 (27.6) | 2550 (6.8) | 18,658/18,658 | <0.001 |
3.2.2. Endpoint analysis
In the T2DM group, the rate of hospitalization was 1.5-fold (43.8% vs. 29.4%), and 30-day mortality 1.6-fold (6.2% vs. 3.9%), and pulmonary involvement 1.4-fold (31.8% vs. 23.1%) higher when compared to the Non-DM group. The ratio of longer hospital stay (>7 days), ICU admission, and longer ICU stay (>6 days) were numerically higher, and lung involvement on CT was markedly more common in the T2DM group.
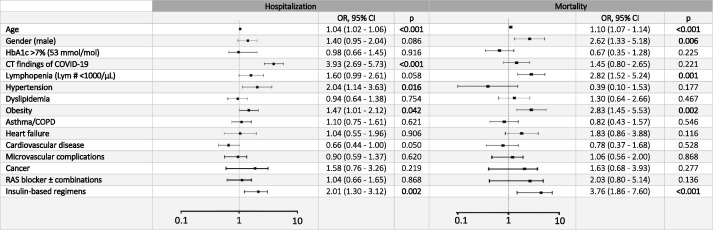
In the T2DM group, multivariate regression analysis showed independent associations of age (OR: 1.04, 95% CI: 1.02–1.06), pulmonary involvement (3.93, 2.69–5.73), hypertension (2.04, 1.14–3.63), obesity (1.47, 1.01–2.12), and any insulin therapy (2.02, 1.30–3.12) with hospitalization; whereas age (1.10, 1.07–1.14), male gender (2.62, 1.33–5.18), lymphopenia (2.82, 1.45–5.53), obesity (2.83, 1.45–5.53), and insulin treatment (3.76, 1.86–7.60) with mortality (Fig. 2 and Supplementary Table 1).
Fig. 2.
Forest plot graph of multivariable logistic regression analysis of patients with type 2 diabetes mellitus in Istanbul (dependent variables: hospitalization, and mortality).
In the Non-DM group, while pulmonary involvement and obesity were associated with a higher risk of hospitalization; age, male gender, lymphopenia, pulmonary involvement, heart failure, and CKD were positively associated with mortality. However, the ORs of common associates were smaller than the T2DM group, and obesity appeared inversely associated with hospitalization (Supplementary Table 2).
4. Discussion
The purpose of this population-based observational study was to describe the differences in clinical characteristics and outcomes (hospitalization and mortality) of COVID-19 patients with and without T2DM. As expected, compared to Non-DM patients, T2DM patients were older and heavier, had more comorbidities, and more severe clinical disease (including inflammatory response, pulmonary involvement, and admission to ICU). Therefore, they were more likely to be hospitalized and had a higher mortality rate.
Diabetes, in particular, T2DM is one of the most frequently evaluated comorbidities in COVID-19. Earlier small-scale case series [6], [16] and the subsequent larger single-center studies [17], [18] from China have stated that the prevalence of diabetes ranged from 10.1% to 23.3%. Single-center reports from other territories demonstrated a sharp rise from 33.8% to 68.3% [7], [11] as the pandemic expanded. In fact, the frequency of diabetes among patients with COVID-19 may depend on the diabetes prevalence of the general population. For instance, the prevalence was reported 68.3% in Saudi Arabia [4], 33.8% in New York [11], and 20.9% in Greece [19]. In a meta-analysis, the prevalence of diabetes was 10% in China [20], and 26.5% in studies outside of China [21]. Likewise, the prevalence of diabetes ranged from 13.0% to 40.2% in multicenter studies reported in similar time frames to our study [10], [11], [22], [23], [24]. Similarly, the pooled prevalence of T2DM in systematic reviews and meta-analysis on COVID-19 was in a wide range, between 10.1% and 22% [8], [21], [25], [26], [27].
Diabetes prevalence is better understood with the few published population-based studies. Accordingly, diabetes prevalence in hospitalized COVID-19 patients was 12% in Italy [9], 18.9% in Spain [4], and 9% in South Korea (including ambulatory and hospitalized cases) [12]. Although, diabetes prevalence in COVID-19 studies from several metropolitan cities was in a wide range from 3.8% in Tehran [28] to 68.3% in Riyadh [7]. Studies from other cities (i.e., New York, Wuhan, Atlanta, Milan, Daegu, Madrid, London, and Detroit) were generally based on limited case series [11], [18], [29], [30], [31], [32], [33], [34], and the community-based studies were scarce [29]. The present population-based study from Istanbul metropolitan city included both outpatient and inpatient COVID-19 cases with a T2DM prevalence of 22.6%.
Generally, in studies published on COVID-19, the average patient age has been reported above 60 [10], [19], [22], [23], [24], [35], [36], [37], the majority was male [22], [23], [24], [27], [35], [36], [37], and patients with T2DM tended to be older [17], [18], [22]. Unlike most of the studies, the median age of our cohort was younger and the proportion of men was lower in the T2DM group due to the smaller percentage of the elderly population in our society as compared to European countries, e.g. Italy (8.6% vs. 22.7%) [38], as well as prompt, strict quarantine restrictions limited to the older people which may keep them uninfected [3], [38]. Moreover, as outpatients might be relatively younger, the inclusion of both ambulatory and hospitalized patients in our study may also play a role. The female predominance of COVID-19 patients with T2DM is shown in similar reports from outpatient and inpatient settings, particularly in younger patient samples [9], [12].
Hypertension and obesity were the most prevalent comorbidities in hospitalized COVID-19 patients. In several population reports, hypertension ranged from 32% to 66% [7], [11], [27], [39], and obesity between 29% and 42% [7], [11], [27]. Besides, comorbidities are much more common in COVID-19 patients with T2DM. In the present study, the prevalence of hypertension was higher in the T2DM group, compared to the Non-DM group (66.4% vs. 22.8%), and it did not change after age- and sex-matching. The median BMI of our T2DM cohort was in the obesity cut-off, though, obesity prevalence was higher (50.2% vs. 28.8%).
In general, comorbidities predict poor outcomes in COVID-19 patients. In a meta-analysis from the US, Europe, and China, the prevalence of heart disease was 37% and CKD 27% [39]. In a multicenter study from Turkey among patients with CKD, 21.9% admitted to ICU, and 14.2% died [40]. The interactions between CKD and diabetes in COVID‐19 may render these patients highly vulnerable to death. In a registry study enrolled COVID-19 patients from seven countries, those with underlying heart disease (24%) had worse outcomes with a 39.7% mortality rate [41].
Compared with the Non-DM group, the higher prevalence of comorbidities such as dyslipidemia, COPD/asthma, heart failure, CVD, and cancer in our T2DM group was consistent with published studies [19], [32], [39], [41]. In contrast, CKD did not differ in patients with or without T2DM probably due to missing data.
Diabetes frequency differs with the clinical severity of SARS-CoV-2 infection. In meta-analyses from China, diabetes prevalence was higher in critical/mortal patients as compared to non-critical patients [7], [8], [12], [21]. The correlation between diabetes and severe COVID-19 is not fully understood. Suggested mechanisms include higher affinity cellular binding and efficient virus entry, decreased viral clearance, delayed Th1/Th17 activation, blunted interferon response, increased susceptibility to hyperinflammation, and cytokine storm syndrome [42]. Furthermore, the low-grade chronic inflammation in diabetes may favor the development of an exaggerated inflammatory response [43]. SARS-CoV-2 may also directly disrupt pancreatic beta-cells through interaction with angiotensin-converting enzyme-2 [42], [43].
COVID-19 mortality is high among hospitalized patients. It was reported 24.5% in New York [11], 27.8% in Northern Italy [35]. In-hospital mortality is significantly higher in COVID-19 patients with diabetes. Pre-existing diabetes, compared to those without has accounted for 24% vs. 10.2% in a report from China [7]; 28.8% vs. 6.2% and 7.8% vs. 2.7% in multicenter studies from US [22], and China [44]; 24% vs. 20% in a meta-analysis [39], and 10.6% vs. 2.1% in a South Korean population-based study [12]. While in Sicily, although diabetes was not a risk factor for COVID-19, it was associated with a higher fatality [45].
The proportion of severe to critical cases and mortality rate are known to increase in older COVID-19 patients, and men with T2DM were more likely to die. In line with other studies [32], [35], [42], in the T2DM group of the present study, older age and hypertension were associated with increased risk of both hospitalization and mortality. However, male-gender was strongly linked with the risk of mortality but not with hospitalization; whereas, hypertension appeared to increase the likelihood of hospitalization but not mortality.
Elevated CRP, decreased lymphocytes, and increased LDH are the most common laboratory abnormalities in COVID-19. In our whole T2DM cohort, more patients had higher than ULN levels of D-dimer, CRP, LDH, and ferritin as compared with the Non-DM cohort (Table 1). However, after matching for age and sex, fewer patients in the T2DM group showed ULN levels of D-dimer, CRP, LDH, and ferritin (Table 2). We confirmed this by comparing the actual levels between groups. In the T2DM group, the median (IQR) levels of D-dimer, CRP, LDH, and ferritin were significantly higher than the Non-DM group (Supplementary Table 3). During the first wave of the COVID-19 in Turkey, there was positive discrimination for the hospitalization of patients with chronic diseases including T2DM. This might have been created a bias. As a result, T2DM patients with less severe disease may be hospitalized while patients without T2DM may not be hospitalized unless they have a more severe disease of COVID-19. In addition, laboratory tests may be more likely to be ordered in hospitalized patients. Considering these, a higher frequency of patients with high D-dimer, CRP, LDH, and ferritin levels in the Non-DM group can be expected. Pulmonary involvement on CT has been the leading indication for hospitalization in COVID-19. In a report from Greece, among hospitalized patients with COVID-19, 81% developed pneumonia, and one-fifth of them was died [19]. In our T2DM group, pulmonary involvement appeared as one of the strongest associates of hospitalization but not with mortality, and lymphopenia was linked with the risk of mortality but not with hospitalization.
While we and others could not demonstrate an association between HbA1c and worse outcomes in T2DM patients with COVID-19, some studies stated that HbA1c was associated with adverse outcomes [22], [46], and another study from New York verified no such associations [47]. The association between higher HbA1c and SARS-CoV-2 complications may be mediated by pre-existing complications such as CKD and CVD.
Insulin is a preferred treatment modality in emergencies, especially in respiratory distress, including SARS-CoV-2 infection, and sepsis [48]. However, in some studies, insulin use has been implicated with poor prognosis in patients with COVID-19 through enhanced systemic inflammation, and severe vital organ damage [17], [47]. Our study has confirmed that any pre-existing insulin therapy was strongly associated with the risk of hospitalization and 30-day mortality. Further analysis of the outcomes of our T2DM patient groups with and without insulin-based therapy revealed that patients on insulin-based therapy had higher hospitalization (61.4% vs. 40%, p < 0.001), admission to ICU (30.6% vs. 17.7%, p < 0.001), and mortality (14.6% vs. 4.4%, p < 0.001) rates than those on non-insulin-based regimens (Supplementary Table 4). Insulin-treated patients are likely to have a longer duration of diabetes with established diabetes complications or may have a decreased beta-cell function. Nevertheless, we could not confirm this because we did not have data regarding the duration of diabetes, diabetes complications, or recent glucose profile and hypoglycemia during the hospitalization of our patients. Although this issue needs to be studied further, we think that insulin therapy is indispensable when necessary in T2DM, especially in cases with clinically severe COVID-19 infection.
This population-based study of COVID-19 patients in Istanbul substantiated that hospitalization and 30-day mortality rates were higher in patients with T2DM as compared with those without diabetes, and even after age- and sex-matching both outcomes remained 1.5-fold, and 1.6-fold higher. Although our hospitalization rate was higher than those observed by others [9], [29], [37], the mortality rates in groups with or without T2DM were significantly lower than studies reported across the world, and this is one of the most striking results of our study. Compared with middle-income countries, the mortality of COVID-19 in the US and the wealthiest European countries (i.e., Spain, Germany, Italy, France, UK, Denmark, Norway, Sweden, and The Netherlands) remains quite high [4], [24], [35], [36], [46], [49]. This may suggest that public health interventions may be less accessible in these countries, among many other interplaying factors [50]. Countries with the highest life expectancy have demographically transitioned to greater proportions of older and frailer populations, which are susceptible to infections and non-communicable diseases. Such comorbidities were associated with a higher risk of COVID-19-related death. On the other hand, chronic diseases, especially diabetes contribute significantly to the cost of COVID-19 care, therefore, some patients may not be admitted to the hospitals despite their need. In the case of Turkey, almost all residents are under the state-wide health insurance system. Before the pandemic, the MoH established a Scientific Advisory Board for COVID-19 to work on pandemic preparedness (public health measures, test criteria, and standard treatment protocols). During the first hit of COVID-19 in Istanbul, all symptomatic ambulatory and hospitalized patients received antiviral therapy (in the order of hydroxychloroquine, lopinavir/ritonavir, and favipiravir) following standard protocols. Besides, more advanced therapies (i.e., low molecular weight heparin-LMWH, glucocorticoids, high-flow nasal oxygen, mechanical ventilation, and immunomodulators (e.g. tocilizumab, anakinra) were procured by the MoH whenever needed [3]. Since the health system was not overwhelmed in the first period of the epidemic, and all hospital services including ICU admission and ventilators were provided free of charge to all patients in need. Patients with underlying diseases such as diabetes were positively discriminated against to provide hospitalization. The healthcare facilities, which are offered equally to everyone including those with T2DM, may at least partly explain the low mortality rate during the first wave of the pandemic in Istanbul. Nonetheless, other factors such as the age structure of our population, and the inclusion of both ambulatory and hospitalized patients might also be promoted the low mortality rate. Supporting, other population-based studies including the out-and in-patient settings from South Korea [12] and Italy [37] demonstrated relatively high mortality in hospitalized cohorts with respect to the outpatient cohorts but the mortality was still higher than our rates.
There are several limitations to our study. First, the study groups could not be strictly matched and some clinical data, especially of those on ambulatory care were missing, probably, physicians may not have time to order tests. The applied multivariate modeling might be limited by residual confounding and bias. Second, this study was a retrospective observation, which could not establish a causal effect relationship between T2DM and severity and mortality of COVID-19. Third, clinical information, such as diabetes duration, urinary albumin levels, and daily glucose variations in the T2DM group were not available. Finally, an earlier started treatment may have a favorable role in reducing mortality. Supporting, the death rate of severe COVID-19 in T2DM has started to decrease dramatically, in parallel to the accumulation of treatment experience.
The most important strength of our study comes from its feature that reflects population-based national data with adequate follow-up (30 days). To our knowledge, this is one of the few studies representing such a large consecutive confirmed COVID-19 patients followed with a standard protocol. Mostly, the studies available in the literature cover a limited number of case series, which were seen in the first weeks or months of the epidemic. Subsequently published nation-wide and meta-analysis studies had a sample size, just over a couple of thousands of patients. It is important to remind that, our clinical data were obtained from the national database for COVID-19 covering outpatient and inpatient settings. Additionally, all patients were treated at the pandemic hospitals or outpatient clinics designated to COVID-19. The population-based nature of this study can exclude the possible bias due to the selection of hospitals caused by incomplete follow-up or assessment of in-hospital mortality only.
In conclusion; our findings demonstrated that T2DM patients infected with SARS-CoV-2 as compared to those without diabetes were exhibited more severe clinical disease with a higher hospitalization, admission to ICU, and mortality rates. Based on these findings it is emphasized that clinicians should pay more attention to the monitoring and treatment of COVID-19 patients with diabetes. Besides the fact that our study covered in- and outpatient settings, and our relatively young population, the low mortality observed in patients with or without T2DM may be related to the healthcare coverage of the society, and the treatment approaches implemented throughout the state.
Acknowledgments
Acknowledgments
We would like to thank all of our healthcare professionals for working with great devotion during the COVID-19 outbreak, treating and monitoring patients, and ensuring these data were collected. We also would like to express our respect and sincere gratitude in front of the memories of all healthcare professionals who lost their lives in the epidemic.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
IS, AS, IT, and SS were involved in the conception and design of the study. Staff from the MoH (NA, OC, and MC) were responsible for the data download and verification. ID and CH cleaned the data. ID, IT, CH, and KYA analyzed the data. ID, IS, and AS prepared the figures and tables. IS, AS, and KYA drafted the manuscript. All authors were involved in the interpretation, critically reviewed the first draft, and approved the final version.
All authors declare no competing interests.
Data availability
The national surveillance system database was established by the MoH, Turkey for the management of hospitalization and follow-up of patients COVID-19. The data is available to researchers who meet the criteria for access (requests are evaluated by the General Directorate of Health Information Systems). The authors are not permitted to share these data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diabres.2021.108753.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard. Available at ‘https://covid19.who.int/’. Accessed on Feb 17, 2021.
- 2.Worldometer COVID-19 Coronavirus Pandemic. Available at ‘https://www.worldometers.info/coronavirus/’. Accessed on Feb 17, 2021.
- 3.Republic of Turkey Ministry of Health. COVID-19 Information Page. Available at ‘https://covid19.saglik.gov.tr/TR-66935/genel-koronavirus-tablosu.html’. Accessed on Feb 17, 2021.
- 4.Carrasco-Sánchez F.J., López-Carmona M.D., Martínez-Marcos F.J., Pérez-Belmonte L.M., Hidalgo-Jiménez A., Buonaiuto V., Suárez Fernández C., Freire Castro S.J., Luordo D., Pesqueira Fontan P.M., Blázquez Encinar J.C., Magallanes Gamboa J.O., de la Peña Fernández A., Torres Peña J.D., Fernández Solà J., Napal Lecumberri J.J., Amorós Martínez F., Guisado Espartero M.E., Jorge Ripper C., Gómez Méndez R., Vicente López N., Román Bernal B., Rojano Rivero M.G., Ramos Rincón J.M., Gómez Huelgas R. Admission hyperglycaemia as a predictor of mortality in patients hospitalized with COVID-19 regardless of diabetes status: data from the Spanish SEMI-COVID-19 Registry. Ann Med. 2021;53(1):103–116. doi: 10.1080/07853890.2020.1836566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Y., Guan X., Jia L., Xing N., Cheng L., Liu B.o., et al. Independent and combined effects of hypertension and diabetes on clinical outcomes in patients with COVID‐19: a retrospective cohort study of Huoshen Mountain Hospital and Guanggu Fangcang Shelter Hospital. J Clin Hypertens. 2021;23(2):218–231. doi: 10.1111/jch.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D., Hu B.o., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alguwaihes A.M., Al-Sofiani M.E., Megdad M., Albader S.S., Alsari M.H., Alelayan A., Alzahrani S.H., Sabico S., Al-Daghri N.M., Jammah A.A. Diabetes and Covid-19 among hospitalized patients in Saudi Arabia: a single-centre retrospective study. Cardiovasc Diabetol. 2020;19(1) doi: 10.1186/s12933-020-01184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu L., Wang B., Yuan T., Chen X., Ao Y., Fitzpatrick T., Li P., Zhou Y., Lin Y.-F., Duan Q., Luo G., Fan S., Lu Y., Feng A., Zhan Y., Liang B., Cai W., Zhang L., Du X., Li L., Shu Y., Zou H. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J Infect. 2020;80(6):656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giorgi Rossi P., Marino M., Formisano D., Venturelli F., Vicentini M., Grilli R. Reggio Emilia COVID-19 Working Group. Characteristics and outcomes of a cohort of COVID-19 patients in the Province of Reggio Emilia, Italy. PLoS One. 2020;15:e0238281. doi: 10.1371/journal.pone.0238281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodacre S., Thomas B., Lee E., Sutton L., Loban A., Waterhouse S., et al. Characterisation of 22445 patients attending UK emergency departments with suspected COVID-19 infection: Observational cohort study. PLoS One. 2020;15:e0240206. doi: 10.1371/journal.pone.0240206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323(20):2052. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee S.G., Park G.U., Moon Y.R., Sung K. Clinical characteristics and risk factors for fatality and severity in patients with coronavirus disease in Korea: a nationwide population-based retrospective study using the Korean health insurance review and assessment service (HIRA) database. Int J Environ Res Public Health. 2020;17:8559. doi: 10.3390/ijerph17228559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.TurkStat Statistic Data Portal. Address-Based Population Registration System 2019. Available at ‘https://biruni.tuik.gov.tr/medas/’. Accessed on December 14, 2020).
- 14.Satman I., Omer B., Tutuncu Y., Kalaca S., Gedik S., Dinccag N., Karsidag K., Genc S., Telci A., Canbaz B., Turker F., Yilmaz T., Cakir B., Tuomilehto J. Twelve-year trends in the prevalence and risk factors of diabetes and prediabetes in Turkish adults. Eur J Epidemiol. 2013;28(2):169–180. doi: 10.1007/s10654-013-9771-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., 3rd, Feldman H.I., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan Y., Yang Y., Wang F., Ren H., Zhang S., Shi X., Yu X., Dong K. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diab Res Care. 2020;8(1):e001343. doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y., Yang D., Cheng B., Chen J., Peng A., Yang C., et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Dia Care. 2020;43(7):1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 18.Yu B.o., Li C., Sun Y., Wang D.W. Insulin treatment is associated with increased mortality in patients with COVID-19 and type 2 diabetes. Cell Metab. 2021;33(1):65–77.e2. doi: 10.1016/j.cmet.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrakis V., Panagopoulos P., Papazoglou D., Papanas N. Diabetes mellitus and hypertension as major risk factors of mortality from Covid-19 pneumonia. Exp Clin Endocrinol Diabetes. 2020 Dec 9 doi: 10.1055/a-1325-0381. [DOI] [PubMed] [Google Scholar]
- 20.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – A systematic review, meta-analysis, and meta-regression. Diabet Metabol Syndrome: Clin Res Rev. 2020;14(4):395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shang L., Shao M., Guo Q., Shi J., Zhao Y., Xiaokereti J., et al. Diabetes mellitus is associated with severe infection and mortality in patients with COVID-19: a systematic review and meta-analysis. Arch Med Res. 2020;51(7):700–709. doi: 10.1016/j.arcmed.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bode B., Garrett V., Messler J., McFarland R., Crowe J., Booth R., et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14(4):813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodman KE, Magder LS, Baghdadi JD, Pineles L, Levine A.R. , Perencevich E.N. , et al. Impact of sex and metabolic comorbidities on COVID-19 mortality risk across age groups: 66,646 inpatients across 613 U.S. hospitals . Clin Infect Dis 2020: ciaa1787. 10.1093/cid/ciaa1787. [DOI] [PMC free article] [PubMed]
- 24.Karagiannidis C., Mostert C., Hentschker C., Voshaar T., Malzahn J., Schillinger G., et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respirat Med. 2020;8:853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mantovani A., Byrne C.D., Zheng M.-H., Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: A meta-analysis of observational studies. Nutr, Metabol Cardiovasc Diseases. 2020;30(8):1236–1248. doi: 10.1016/j.numecd.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo L.i., Shi Z., Zhang Y.a., Wang C., Do Vale Moreira N.C., Zuo H., Hussain A. Comorbid diabetes and the risk of disease severity or death among 8807 COVID-19 patients in China: A meta-analysis. Diabetes Res Clin Pract. 2020;166:108346. doi: 10.1016/j.diabres.2020.108346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moazzami B., Chaichian S., Kasaeian A., Djalalinia S., Akhlaghdoust M., Eslami M., et al. Metabolic risk factors and risk of Covid-19: a systematic review and meta-analysis. PLoS One. 2020;15:e0243600. doi: 10.1371/journal.pone.0243600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikpouraghdam M., Jalali Farahani A., Alishiri GholamHossein, Heydari S., Ebrahimnia M., Samadinia H., Sepandi M., Jafari N.J., Izadi M., Qazvini A., Dorostkar R., Tat M., Shahriary A., Farnoosh G., Hosseini Zijoud S.R., Taghdir M., Alimohamadi Y., Abbaszadeh S., Gouvarchin Ghaleh H.E., Bagheri M. Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in IRAN: A single center study. J Clin Virol. 2020;127:104378. doi: 10.1016/j.jcv.2020.104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chishinga N, Gandhi NR, Onwubiko UN, Telford C, Prieto J, Smith S, et al. Characteristics and risk factors for hospitalization and mortality among persons with COVID-19 in Atlanta Metropolitan Area. medRxiv: The Preprint Server for Health. Sciences 2020: 2020.12.15.20248214. 10.1101/2020.12.15.20248214. [DOI]
- 30.Ciceri F., Castagna A., Rovere-Querini P., De Cobelli F., Ruggeri A., Galli L., Conte C., De Lorenzo R., Poli A., Ambrosio A., Signorelli C., Bossi E., Fazio M., Tresoldi C., Colombo S., Monti G., Fominskiy E., Franchini S., Spessot M., Martinenghi C., Carlucci M., Beretta L., Scandroglio A.M., Clementi M., Locatelli M., Tresoldi M., Scarpellini P., Martino G., Bosi E., Dagna L., Lazzarin A., Landoni G., Zangrillo A. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol. 2020;217:108509. doi: 10.1016/j.clim.2020.108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S.W., Kim S.M., Kim Y.K., Kim J.Y., Lee Y.M., Kim B.O., et al. Clinical Characteristics and outcomes of COVID-19 cohort patients in Daegu Metropolitan City outbreak in 2020. J Korean Med Sci. 2021;36:e12. doi: 10.3346/jkms.2021.36.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Gonzalez C.G., Chamorro-de-Vega E., Valerio M., Amor-Garcia M.A., Tejerina F., Sancho-Gonzalez M., Narrillos-Moraza A., Gimenez-Manzorro A., Manrique-Rodriguez S., Machado M., Olmedo M., Escudero-Vilaplana V., Villanueva-Bueno C., Torroba-Sanz B., Melgarejo-Ortuño A., Vicente-Valor J., Herranz A., Bouza E., Muñoz P., Sanjurjo M. COVID-19 in hospitalised patients in Spain: a cohort study in Madrid. Int J Antimicrob Agents. 2021;57(2):106249. doi: 10.1016/j.ijantimicag.2020.106249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Izzi-Engbeaya C., Distaso W., Amin A., Yang W., Idowu O., Kenkre J.S., Shah R.J., Woin E., Shi C., Alavi N., Bedri H., Brady N., Blackburn S., Leczycka M., Patel S., Sokol E., Toke-Bjolgerud E., Qayum A., Abdel-Malek M., Hope D.C.D., Oliver N.S., Bravis V., Misra S., Tan T.M., Hill N.E., Salem V. Adverse outcomes in COVID-19 and diabetes: a retrospective cohort study from three London teaching hospitals. BMJ Open Diab Res Care. 2021;9(1):e001858. doi: 10.1136/bmjdrc-2020-001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suleyman G., Fadel R.A., Malette K.M., Hammond C., Abdulla H., Entz A., et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in Metropolitan Detroit. JAMA Netw Open. 2020;3(6):e2012270. doi: 10.1001/jamanetworkopen.2020.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferroni E., Giorgi Rossi P., Spila Alegiani S., Trifirò G., Pitter G., Leoni O., et al. Survival of hospitalized COVID-19 patients in Northern Italy: a population-based cohort study by the ITA-COVID-19 Network. Clin Epidemiol. 2020;12:1337–1346. doi: 10.2147/CLEP.S271763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piroth L., Cottenet J., Mariet A-S., Bonniaud P., Blot M., Tubert-Bitter P., et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respiratory Med. 2021;9(3):251–259. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Potalivo A., Montomoli J., Facondini F., Sanson G., Lazzari Agli L.A., Perin T., et al. Sixty-day mortality among 520 Italian hospitalized COVID-19 patients according to the adopted ventilatory strategy in the context of an integrated multidisciplinary clinical organization: a population-based cohort study. Clin Epidemiol. 2020;12:1421. doi: 10.2147/CLEP.S278709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naharci M.I., Katipoglu B., Tasci I. Coronavirus 2019 (COVID-19) outbreak and geropsychiatric care for older adults: a view from Turkey. Int. Psychogeriatr. 2020;32(10):1193–1197. doi: 10.1017/S1041610220001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dorjee K., Kim H., Bonomo E., Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: a comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS One. 2020;15:e0243191. doi: 10.1371/journal.pone.0243191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozturk S., Turgutalp K., Arici M., Odabas A.R., Altiparmak M.R., Aydin Z., et al. Mortality analysis of COVID-19 infection in chronic kidney disease, haemodialysis and renal transplant patients compared with patients without kidney disease: a nationwide analysis from Turkey. Nephrol Dial Transplant. 2020;35:2083–2095. doi: 10.1093/ndt/gfaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Núñez-Gil I.J.J., Fernández-Ortiz A., Maroud Eid C., Huang J., Romero R., Becerra-Muñoz V.M., et al. Underlying heart diseases and acute COVID-19 outcomes. Cardiol J. 2020 Dec;21 doi: 10.5603/CJ.a2020.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muniyappa R., Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lima-Martínez MM, Carrera Boada C, Madera-Silva MD, Marín W, Contreras M. COVID-19 and diabetes: a bidirectional relationship. Clin Investig Arterioscler 2020;S0214-9168(20)30105-4. English, Spanish. 10.1016/j.arteri.2020.10.001. [DOI] [PMC free article] [PubMed]
- 44.Zhu L., She Z.-G., Cheng X.u., Qin J.-J., Zhang X.-J., Cai J., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silverii G.A., Monami M., Cernigliaro A., Vigneri E., Guarnotta V., Scondotto S., et al. Are diabetes and its medications risk factors for the development of COVID-19? Data from a population-based study in Sicily. Nutr Metab Cardiovasc Dis. 2021;31(2):396–398. doi: 10.1016/j.numecd.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holman N., Knighton P., Kar P., O'Keefe J., Curley M., Weaver A., Barron E., Bakhai C., Khunti K., Wareham N.J., Sattar N., Young B., Valabhji J. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agarwal S., Schechter C., Southern W., Crandall J.P., Tomer Y. Preadmission diabetes-specific risk factors for mortality in hospitalized patients with diabetes and coronavirus disease 2019. Diabetes Care. 2020;43(10):2339–2344. doi: 10.2337/dc20-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bornstein S.R., Rubino F., Khunti K., Mingrone G., Hopkins D., Birkenfeld A.L., Boehm B., Amiel S., Holt R.IG., Skyler J.S., DeVries J.H., Renard E., Eckel R.H., Zimmet P., Alberti K.G., Vidal J., Geloneze B., Chan J.C., Ji L., Ludwig B. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8(6):546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahrenfeldt L.J., Otavova M., Christensen K., Lindahl-Jacobsen R. Sex and age differences in COVID-19 mortality in Europe. Wien Klin Wochenschr. 2020:1–6. doi: 10.1007/s00508-020-01793-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bilinski A., Emanuel E.J. COVID-19 and excess all-cause mortality in the US and 18 comparison countries. JAMA. 2020;324:2100–2102. doi: 10.1001/ja. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The national surveillance system database was established by the MoH, Turkey for the management of hospitalization and follow-up of patients COVID-19. The data is available to researchers who meet the criteria for access (requests are evaluated by the General Directorate of Health Information Systems). The authors are not permitted to share these data.