Abstract
BACKGROUND AND PURPOSE:
Spontaneous or progressive occlusion of the posterior fossa dural sinuses is often observed in patients with vein of Galen malformation, which can affect the clinical course. The aim of this study was to examine the patency of the posterior fossa dural sinuses in patients with vein of Galen malformation and to analyze the clinical and angiographic course of this condition.
MATERIALS AND METHODS:
We retrospectively reviewed 61 consecutive children with vein of Galen malformations. Clinical presentation, management, outcome, and angiographic change were analyzed for the patients with attention paid to all dural sinus occlusions.
RESULTS:
Twenty patients (32.8%) demonstrated spontaneous sinus occlusion, mostly in the sigmoid sinus. This condition was not observed in neonates and was first discovered during infancy or childhood. Progression of sinus occlusion was seen in 10 patients, and the conditions of 6 of them deteriorated in accordance with the progression of sinus occlusion. After total or subtotal obliteration of the malformation by transarterial glue embolization, 13 patients recovered to healthy, 3 patients had only mild developmental delay, and 4 patients remained neurologically disabled.
CONCLUSIONS:
Spontaneous sinus occlusion is not a rare condition and can result in neurologic deterioration in the natural history of untreated vein of Galen malformation. If signs of progressive sinus occlusion are noticed, early arteriovenous shunt reduction or elimination by transarterial glue embolization is expected to prevent permanent brain damage.
Vein of Galen malformations (VGMs) are direct arteriovenous shunts in the subarachnoid space of the velum interpositum cistern and quadrigeminal cistern, supplied by the choroidal and quadrigeminal arteries and drained by the dilated median prosencephalic vein of Markowski, the embryonic precursor of the vein of Galen.1,2 This malformation is considered to form between 6 and 11 weeks of gestational age when this transient embryonic vein exists.
The clinical presentation of VGM varies depending on the age of the patient. Neonates typically present with high-output congestive heart failure due to high-flow shunt producing cardiac overload, whereas infants and children usually present with hydrovenous disorders, such as macrocrania, prominent facial and scalp veins, and hydrocephalus.2,3 Hydrovenous disorders result from diminished absorption of CSF due to cerebral venous hypertension.4 Development of outflow restriction in the posterior fossa dural sinuses will improve the cardiac overload but will lead to further intracranial venous hypertension, which will not only interfere with absorption of CSF but will also create congestion within cerebral veins. Venous hypertension consequently results in brain tissue loss and hence developmental delay.2,3
Thus, posterior fossa dural sinus occlusion is thought to aggravate the clinical course of VGM. However, its incidence, pathogenic mechanism, and the effect of endovascular embolization are unknown. The aim of this study was to analyze the clinical and angiographic course of patients with VGM with sinus occlusion and to determine whether embolization can prevent clinical deterioration in these patients.
Materials and Methods
Retrospective review of the institutional data base during January 2005 to December 2012 revealed 61 patients with VGM. We collected information from the clinical case records, MR images at presentation and during follow-up, and DSA at the time of each endovascular embolization and follow-up.
All patients were examined for the patency of the posterior fossa dural sinuses, including transverse, sigmoid, and occipital sinuses and jugular bulbs, on the basis of bilateral common or internal carotid and at least unilateral vertebral arteriography.
All patients except 1 who demonstrated spontaneous thrombosis of the VGM on the initial angiogram were treated with single or multiple sessions of endovascular treatment at the Hyman Newman Institute for Neurology and Neurosurgery. All patients were treated primarily by transarterial embolization by using n-BCA. Transarterial embolization with ethylene vinyl alcohol copolymer (Onyx; Covidien, Irvine, California) mostly via dural feeders or a transvenous approach with detachable coils was added, if necessary, to obtain complete obliteration of the VGM.
Fifteen patients who were referred to us after partial embolization at an outside institution were also included in this study. Although available data were limited, the initial status of the posterior fossa dural sinuses could be evaluated with DSA or MR venography in all of these cases. At outside institutions, 4 of 15 patients had been treated with n-BCA in the same manner as ours, 7 patients had undergone proximal feeder occlusion with coils, and the remaining 4 patients had been treated exclusively with Onyx.
To clarify the impact of the age of the patient on occlusive changes in the posterior fossa dural sinuses, we categorized the patients into 3 age groups: neonates (≤30 days after birth), infants (1–24 months of age), and children (2–15 years of age), according to the age at the initial treatment either in our center or outside.
Angiographic changes in the posterior fossa dural sinuses were categorized into progression, no change, and regression. New development of sinus occlusion or an increase in the extent of the occluded segment before the first treatment by us or during the course of treatment was defined as “progression.” Reopening of the occluded sinus or a decrease in the extent of the occluded segment was defined as “regression.”
Clinical factors were compared between patients with and without sinus occlusion by using Fisher exact tests, and we considered a P value <.05 statistically significant.
Results
Demographics and Clinical Characteristics
We identified 61 patients with VGM referred to our center between January 2005 and December 2012 (Table 1). Nineteen patients were first treated within 30 days after birth (neonate group); 34 patients, at 1 to 24 months of age (infant group); and 8 patients at 2–15 years of age (children's group).
Table 1:
Demographics and clinical characteristics of 61 patients with VGM comparing patients with and without sinus occlusion
| Sinus Occlusion Group (n = 20) | Nonocclusion Group (n = 41) | P Valuea | |||
|---|---|---|---|---|---|
| Male/female | 9:11 | 24:17 | NS | ||
| Age at the first treatment | |||||
| Neonates (0–30 days) | 2 | 10.0% | 17 | 41.5% | <.05 |
| Infants (1–24 mo) | 13 | 65.0% | 21 | 51.2% | NS |
| Children (2–15 yr) | 5 | 25.0% | 3 | 7.3% | NS |
| Presentation | |||||
| Congestive heart failure | 3 | 15.0% | 20 | 48.8% | <.05 |
| Hydrovenous disorder | 15 | 75.0% | 12 | 29.3% | <.001 |
| Developmental delay | 5 | 25.0% | 2 | 4.9% | <.05 |
| Seizure | 3 | 15.0% | 2 | 4.9% | NS |
| Headache | 0 | 0.0% | 1 | 2.4% | NS |
| Asymptomatic | 0 | 0.0% | 9 | 22.0% | <.05 |
| Embryonic sinus | |||||
| Falcine sinus | 13 | 65.0% | 24 | 58.5% | NS |
| Occipital sinus | 14 | 70.0% | 25 | 61.0% | NS |
Note:—NS indicates not significant.
P value was calculated using the Fisher exact test.
Twenty patients (32.8%) demonstrated occlusion of the posterior fossa dural sinuses. In the neonate group, sinus occlusion was considerably less likely to occur compared with the other groups. All 19 neonates demonstrated well-developed sigmoid sinuses and internal jugular veins on the initial angiograms. Occipital sinuses were also observed in all neonates. Only 2 (cases 16 and 19) of them, who had been referred late to our center owing to previous complications after early treatment, developed sinus occlusion during their infancy.
With respect to the clinical presentation, hydrovenous disorders, such as macrocrania, prominent facial and scalp veins, and hydrocephalus, were most closely correlated with sinus occlusion (75% in sinus occlusion group and 29.3% in nonocclusion group; P < .001). Developmental delay was also more common in the patients with sinus occlusion (25% in the sinus occlusion group and 4.9% in the nonocclusion group, P < .05). All asymptomatic patients were not associated with sinus occlusion. Although the persistence of the occipital sinus could function as an alternative venous outlet when the sigmoid sinus was occluded, the prevalence of embryonic sinuses, such as the falcine and occipital sinuses, was not correlated statistically with the development of posterior fossa dural sinus occlusion.
Representative Cases
Case 8.
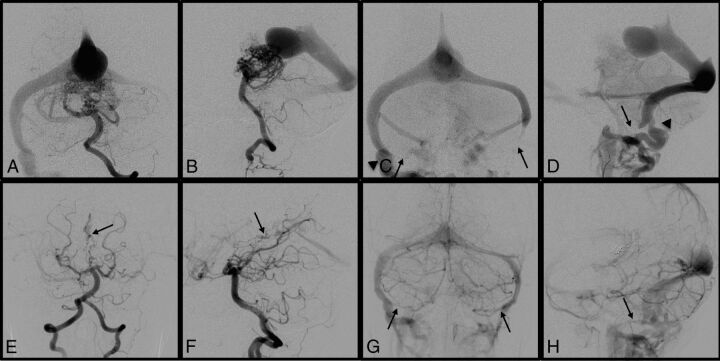
A 13-month-old boy, who had been diagnosed with VGM due to macrocrania and prominent scalp and facial veins at 6 months of age, was referred to our center for endovascular treatment (Fig 1). The initial angiogram demonstrated VGM and complete occlusion of the left sigmoid sinus and near-occlusion of the right sigmoid sinus. There was an alternative venous pathway via the emissary vein to the jugular bulb on the right side. After subtotal obliteration of the VGM achieved by staged transarterial embolization, the bilateral sigmoid sinuses reopened and the patient has been developing normally.
Fig 1.
Case 8. A 13-month-old boy was diagnosed with VGM due to macrocrania and prominent scalp and facial veins. Left vertebral artery angiograms before the first embolization demonstrated a VGM in the arterial phase (A and B) and complete occlusion of the left sigmoid sinus and near-occlusion of the right sigmoid sinus in the venous phase (C and D, arrows). There was an alternative venous pathway via the emissary vein to the jugular bulb on the right side (C and D, arrowheads). Left vertebral artery angiograms in the midarterial phase (E and F) and the late venous phase (G and H) after subtotal obliteration of VGM achieved by staged transarterial embolization (E and F, arrows) demonstrate the reopened bilateral sigmoid sinuses (G and H, arrows). The patient is developing normally.
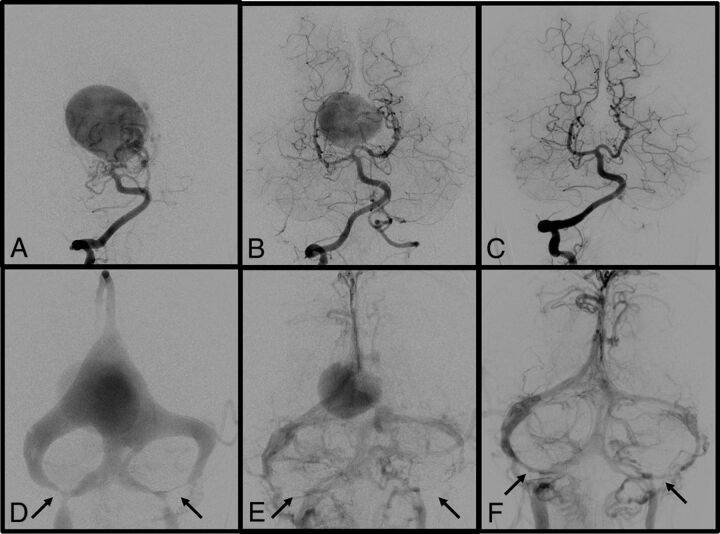
Case 14.
A 3-month-old girl with a prenatal diagnosis of VGM presented with hydrocephalus (Fig 2). She underwent the first embolization at 3 months of age. Angiography showed stenosis of the left sigmoid sinuses and jugular bulbs. Intraventricular hemorrhage occurred after the first embolization procedure, and resulted in progression of hydrocephalus. However, the patient recovered well after ventriculoperitoneal shunt placement. She underwent the second embolization at 7 months of age. Angiography showed a decrease in size of the VGM and progression to bilateral sigmoid sinus occlusion. The third embolization at 13 months of age led to subtotal obliteration of the VGM (data not shown). At 2 years of age, angiography showed total exclusion of the VGM and regression of the bilateral sigmoid sinus occlusion. Her neurologic status has improved except for a slight developmental delay at the last follow-up.
Fig 2.
Case 14. A 3-month-old girl with a prenatal diagnosis of VGM who presented with hydrocephalus. Right vertebral artery angiograms before the first embolization at 3 months of age (A and D) show a VGM with multiple feeders and stenosis of bilateral sigmoid sinuses and jugular bulbs (D, arrows). The first embolization was complicated by intraventricular hemorrhage resulting in hydrocephalus. However, the patient recovered well after ventriculoperitoneal shunt placement. Right vertebral artery angiograms before the second embolization at 7 months of age (B and E) show a decrease in the size of the VGM and progression to bilateral sigmoid sinus occlusion (E, arrows). Note the partial regression of the occipital sinuses. The third embolization at 13 months of age led to subtotal obliteration of the VGM (data not shown). Right vertebral artery angiograms at 2 years of age (C and F) show total exclusion of VGM and regression of bilateral sigmoid sinuses (F, arrows). Her neurologic status had improved except for a slight developmental delay at the last follow-up.
Clinical and Angiographic Courses
With respect to the location of occluded sinuses, 9 patients had unilateral and 11 patients had bilateral occlusion, most commonly at the sigmoid sinus (Table 2 and Fig 3). Sinus occlusion was not observed in neonates and was first discovered during infancy in 13 patients and during childhood in 7 patients. In these patients, we found spontaneous occlusion at the first angiogram but before the first treatment in 13 patients and newly developed occlusions during the course of treatment in 7 patients. In all of these 7 patients, angiograms after any session of embolization showed no embolic material in the area where sinus occlusion later occurred.
Table 2:
Summary of patients with posterior fossa dural sinus occlusion
| Case No. | Sex | Presentation |
Location of Sinus Occlusion |
Procedural Complications | Neurologic Deficit | ||
|---|---|---|---|---|---|---|---|
| Initial | Later | Right | Left | ||||
| Neonates | |||||||
| 16 | F | CHF | HC, DD, SZ | SJ | Ischemic stroke | Severe DD, hemiparesis | |
| 19 | M | CHF | HC, DD | TSJ | SJ | SDH and ischemic stroke | Severe DD |
| Infants | |||||||
| 6 | M | MC | HC | S | S | None | None |
| 11 | F | HC | S | None | None | ||
| 14 | F | HC | DD | S | S | IVH | Mild DD |
| 12 | F | MC, HC | S | S | None | None | |
| 7 | F | MC, HC | TSJ | T | None | None | |
| 13 | M | MC | HA, DD | TSJ | TSJ | None | Mild DD |
| 15 | M | HC, DD, SZ | TSJ | Ischemic stroke | Moderate DD, hemiparesis | ||
| 4 | M | MC | S | TSJ | None | None | |
| 1 | M | HC | S | None | None | ||
| 2 | M | MC, HC | S | None | None | ||
| 10 | F | MC | S | SJ | None | None | |
| 8 | M | MC | S | SJ | None | None | |
| 5 | F | SZ | TSJ | None | None | ||
| Children | |||||||
| 3 | F | MC | SJ | None | None | ||
| 20 | M | MC, DD | S | S | Ischemic stroke | Moderate DD, hemiparesis | |
| 18 | M | MC, DD | SJ | None | Mild DD | ||
| 9 | F | DD | SJ | SJ | None | None | |
| 17 | M | MC | SJ | None | None | ||
Note:—CHF indicates congestive heart failure; MC, macrocrania; HC, hydrocephalus; DD, developmental delay; SZ, seizure; HA, headache; T, transverse sinus; S, sigmoid sinus; J, jugular bulb; SDH, subdural hematoma; IVH, intraventricular hemorrhage.
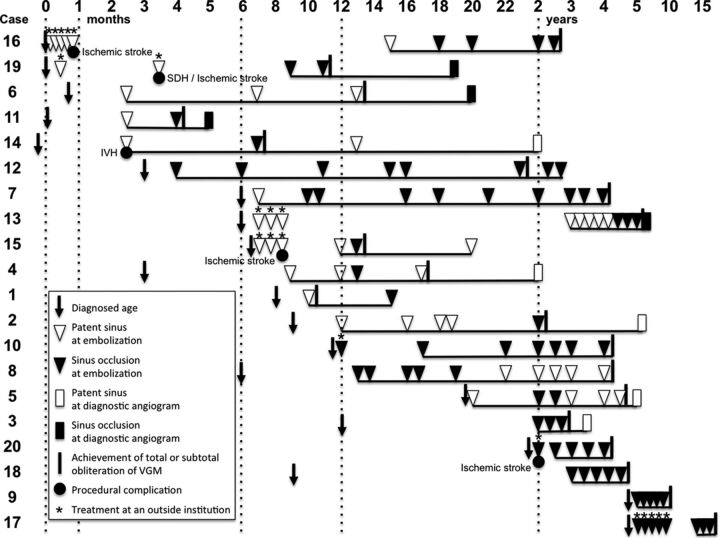
Fig 3.
Angiographic patency of the posterior fossa dural sinuses in each patient is chronologically shown. Time points of the initial diagnosis and total or subtotal obliteration of the VGM and procedural complications are also shown. Underlines indicate the follow-up periods in our center. SDH indicates subdural hematoma; IVH, intraventricular hemorrhage.
Progression of sinus occlusion was identified in 10 patients, and conditions of 6 of them deteriorated in accordance with the progression of sinus occlusion. Regression of sinus occlusion was also identified in 7 patients (3 patients first showed progression and showed regression afterward during the course of treatment).
In 8 of 10 patients who showed progression, sinus occlusion was identified while a significant amount of shunt flow of the VGM still existed, largely from 2 months to 3 years of age. The remaining 2 patients (cases 1 and 6) demonstrated sinus occlusion with well-developed collateral venous pathways when the VGM was totally obliterated. Regression of sinus occlusion was found when VGM was totally or subtotally excluded in all 7 patients (6 infants and a 2-year-old child). There was no change in 6 patients (2 infants and 4 children).
Seven (70%) of 10 patients with hydrocephalus, including 1 patient (case 11) who developed progressive hydrocephalus during treatment, showed a decrease in the ventricular size after embolization and did not have any symptom or neurologic deficit on the last follow-up. Of the patients with sinus occlusion, only case 14 underwent a shunt operation for hydrocephalus. Four (50%) (cases 9, 13, 14, and 18) of 8 patients who presented with developmental delay demonstrated significant improvement on the last follow-up.
Five patients had procedural complications. One patient who underwent glue embolization at our center had a small thalamic hemorrhage with ventricular extension after embolization and had mild developmental delay (case 14). Four patients who underwent early treatment at outside institutions had ischemic stroke (cases 15, 16, 19, and 20), resulting in hemiparesis in 3 and moderate-to-severe developmental delay in 4. On the last follow-up, 13 patients were neurologically intact, 3 patients had only mild developmental delay without focal signs, and all 4 patients who underwent early treatment at outside institutions had significant neurologic deficits.
Discussion
Posterior fossa dural sinus occlusion is a common finding in patients with VGM.2,5 However, few descriptions have focused on this condition in the previous literature, and the pathogenic mechanism remains unknown. Raybaud et al,1 in their series of 23 patients with VGM, identified complete angiographic absence of both transverse and sigmoid sinuses in 5 patients, and they found that the venous drainage channels are patent more often in neonates than in older children. Geibprasert et al6 reported that significant jugular bulb stenosis was present in 7 of 25 patients (28%) and was associated with hydrocephalus in 6 patients. Chow et al,7 in their recent review of 41 patients with VGM, also identified 16 patients (39.0%) with sinus stenosis and 4 patients (4.9%) with venous sinus thrombus or occlusion on the angiograms. The overall frequency (32.8%) of this condition and rare incidence in neonates in our series are consistent with those in these previous studies.
As the pathogenic mechanism of sinus occlusion, dysmaturation of the jugular bulb due to the arteriovenous shunt or persistence of the occipital sinus,2 embryologic segmental sinus atresia,1 progressive jugular bulb stenosis resulting from expansion of the cranial vault,8 and the induced intimal hyperplasia from increased shear stress due to the high-flow shunt have been speculated.8
In the normal development of the cerebral venous system, adult arrangement of the transverse and sigmoid sinuses and the jugular bulbs can be seen at 3 months of gestational age.9 After 4 months of gestational age, the transverse sinus begins to expand with marked increase in venous flow from the rapidly growing cerebral hemispheres. In contrast to the ballooning of the transverse sinus, the sigmoid sinus and jugular bulb are poorly developed at this stage because the jugular bulb, which is surrounded by cartilaginous and osseous structures, has difficulty expanding during fetal life. After birth, the hemodynamic change from a fetal to a postnatal type of circulation eventually promotes the jugular bulb maturation.10 Meanwhile, considerable high-flow shunts from VGM could presumably trigger the development of the sigmoid sinus and the jugular bulb by overcoming the surrounding cartilaginous and osseous structures during fetal life or immediately after birth. Consequently, neonates with VGM who present with high-output congestive heart failure usually have well-developed sigmoid sinuses and jugular bulbs as observed in our series. This finding suggests that posterior fossa dural sinus occlusion in VGM is not developmental but is acquired after birth, as we have also seen in other high-flow intracranial AVFs presenting in neonates and infants (A.B., unpublished data, date unknown).
Recently, we reported that the outcome of patients with VGM who presented with heart failure in the neonatal period has significantly improved by timely endovascular treatment.11 Those patients who undergo appropriate treatment from the neonatal period will not develop sinus occlusion and can achieve angiographic obliteration of VGMs with normal neurologic development.
When shunt flow through the VGM is relatively low, the patient is asymptomatic during the neonatal period and is often diagnosed after developing hydrovenous disorders during infancy. In such patients, enlargement of the cranial vault and specific growth patterns of the posterior fossa may interfere with the development of the jugular foramen.2 In our series, most sinus occlusion occurred during the infantile period when the cranial vault was expanding. Progression of sinus occlusion might be associated with the growth pattern of the posterior fossa during infancy.
Posterior fossa dural sinus occlusion will be a cause of worsening of the hydrovenous disorder; at the same time, it may be a secondary change due to intracranial hypertension. Sainte-Rose et al12 reported that the increased superior sagittal sinus pressure in infantile hydrocephalus is due to a reversible collapse of the sinus caused by the intracranial hypertension. It is very likely that the collapse takes place in the posterior fossa at the level of the sigmoid sinuses. Also in patients with VGM, persistent intracranial high pressure may mechanically compress the sigmoid sinus causing reversible stenosis/occlusion. In our series, 7 patients (6 infants and a 2-year-old child) demonstrated regression of sinus occlusion when intracranial pressure decreased by total or subtotal obliteration of the VGM. However, long-term compression of the sinus may cause irreversible occlusion. The patients exposed to prolonged intracranial hypertension due to treatment delay demonstrated persistent occlusion of the posterior fossa dural sinuses despite successful shunt reduction by endovascular treatment.
Hydrovenous Disorder due to Sinus Occlusion
In a patient with a VGM, high-flow arteriovenous shunts draining into the torcular Herophili increase the superior sagittal sinus pressure.13 Raised pressure in the superior sagittal sinus causes cortical cerebral vein stagnation, resulting in increased pial venous pressure, resulting in progressive impairment of CSF absorption, which is further aggravated by the occlusion, and consequently progressive diffuse brain damage. Therefore, the prognosis of an untreated VGM depends on pial venous pressure, which is affected by the timing of the development of sinus occlusion and alternative venous pathways to drain the VGM and the brain. If sinus occlusion develops slowly, collateral pathways may develop and adapt to the increased venous pressure, allowing the patients to grow normally at the beginning.
However, the long-term pial venous congestion may eventually result in developmental delay due to chronic ischemia in untreated VGM.2 Therefore, patients with increased pial venous pressure have an urgent need to reduce shunt flow of the VGM to avoid permanent brain damage. Reduction in shunt flow of the VGM by embolization might stop progression of sinus occlusion and consequently prevent progression of symptoms.
Effectiveness of Endovascular Embolization
If endovascular embolization is started in the neonatal period and substantial reduction of the shunt flow of the VGM is achieved in time, patency of the posterior fossa dural sinuses seems to be preserved, as seen in 16 patients of our 19 neonates, excluding 3 patients who died within 30 days, achieved total obliteration of the VGM before 2 years of age. Among them, the posterior fossa dural sinuses remained patent during the course of treatment in 14 patients treated early in our series. In contrast, 2 patients who were referred to us late after early initial treatment developed sinus occlusion.
We believe that transarterial glue embolization is the best way to achieve on-target obliteration at the fistula site of the VGM, leading to effective shunt reduction. Although sinus occlusion may initially progress despite effective glue embolization in the early phases of the staged embolization, continuous shunt reduction by further glue embolization will eventually stop progression and sometimes reopen occluded sinuses; this treatment will prevent or even reverse development of symptoms of the hydrovenous disorder.
Proximal feeder occlusion should be avoided because it not only leaves the fistula site patent but also eliminates future transarterial access to the fistula. In the patient with sinus occlusion, the transvenous access route is often impeded. Transvenous sinus stent placement in patients with progressive jugular bulb stenosis has been reported14 but should be performed only when the transarterial approach is not possible because of limited experience with and unknown long-term effects of this placement.
Two patients who underwent proximal coil occlusion without sufficient shunt reduction developed hydrocephalus and sinus occlusion and consequently had significant developmental delay despite late total exclusion of the VGM. Because proximal coil occlusion did not close the fistula itself, there was persistent prolonged intracranial venous hypertension before the fistulas were sufficiently occluded with n-BCA.
Therefore, it is important to shorten the duration of patient exposure to high-flow shunts leading to intracranial venous hypertension. Careful observation of head circumference and developmental milestones will be helpful in detecting a patient who is predisposed to progressive posterior fossa dural sinus occlusion. When a patient with VGM shows signs of progressive hydrovenous disorder, early arteriovenous shunt reduction by transarterial glue embolization is the best option to prevent permanent brain damage.
Conclusions
Patients with VGM are frequently associated with posterior fossa dural sinus occlusion, typically during their infancy. Sinus occlusion will aggravate a hydrovenous disorder and vice versa. Early arteriovenous shunt flow reduction and total obliteration by transarterial glue embolization may have the potential to prevent progressive sinus occlusion and its symptoms and may even reverse some of the symptoms. Therefore, recognition of the frequent occurrence of this condition and careful observation of early signs of hydrovenous disorder are important for the management of patients with VGM.
ABBREVIATION:
- VGM
vein of Galen malformation
References
- 1. Raybaud CA, Strother CM, Hald JK. Aneurysms of the vein of Galen: embryonic considerations and anatomical features relating to the pathogenesis of the malformation. Neuroradiology 1989;31:109–28 10.1007/BF00698838 [DOI] [PubMed] [Google Scholar]
- 2. Lausjaunias P, ter Brugge KG, Berenstein A. Surgical Neuroangiography: Vol. 3: Clinical and Interventional Aspects in Children. Berlin: Springer-Verlag; 2006 [Google Scholar]
- 3. Lasjaunias PL, Chng SM, Sachet M, et al. The management of vein of Galen aneurysmal malformations. Neurosurgery 2006;59:S184–94; discussion S3–S13 [DOI] [PubMed] [Google Scholar]
- 4. Zerah M, Garcia-Monaco R, Rodesch G, et al. Hydrodynamics in vein of Galen malformations. Childs Nerv Syst 1992;8:111–17; discussion 117 10.1007/BF00298261 [DOI] [PubMed] [Google Scholar]
- 5. Lasjaunias P, Ter Brugge K, Lopez Ibor L, et al. The role of dural anomalies in vein of Galen aneurysms: report of six cases and review of the literature. AJNR Am J Neuroradiol 1987;8:185–92 [PMC free article] [PubMed] [Google Scholar]
- 6. Geibprasert S, Krings T, Armstrong D, et al. Predicting factors for the follow-up outcome and management decisions in vein of Galen aneurysmal malformations. Childs Nerv Syst 2010;26:35–46 10.1007/s00381-009-0959-7 [DOI] [PubMed] [Google Scholar]
- 7. Chow ML, Cooke DL, Fullerton HJ, et al. Radiological and clinical features of vein of Galen malformations. J Neurointerv Surg 2015;7:443–48 10.1136/neurintsurg-2013-011005 [DOI] [PubMed] [Google Scholar]
- 8. Raybaud C. Normal and abnormal embryology and development of the intracranial vascular system. Neurosurg Clin N Am 2010;21:399–426 10.1016/j.nec.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 9. Padget DH. The development of the cranial venous system in man, from the viewpoint of comparative anatomy. Contrib Embryol 1957;36:79–140 [Google Scholar]
- 10. Okudera T, Huang YP, Ohta T, et al. Development of posterior fossa dural sinuses, emissary veins, and jugular bulb: morphological and radiologic study. AJNR Am J Neuroradiol 1994;15:1871–83 [PMC free article] [PubMed] [Google Scholar]
- 11. Berenstein A, Fifi JT, Niimi Y, et al. Vein of Galen malformation in neonates: new management paradigms for improving outcomes. Neurosurgery 2012;70:1207–14 10.1227/NEU.0b013e3182417be3 [DOI] [PubMed] [Google Scholar]
- 12. Sainte-Rose C, LaCombe J, Pierre-Kahn A, et al. Intracranial venous sinus hypertension: cause or consequence of hydrocephalus in infants? J Neurosurg 1984;60:727–36 10.3171/jns.1984.60.4.0727 [DOI] [PubMed] [Google Scholar]
- 13. Quisling RG, Mickle JP. Venous pressure measurements in vein of Galen aneurysms. AJNR Am J Neuroradiol 1989;10:411–17 [PMC free article] [PubMed] [Google Scholar]
- 14. Brew S, Taylor W, Reddington A. Stenting of a venous stenosis in vein of Galen aneurysmal malformation: a case report. Interv Neuroradiol 2001;7:237–40 [DOI] [PMC free article] [PubMed] [Google Scholar]