Abstract
BACKGROUND AND PURPOSE:
Smoking is a major risk factor for patients with intracranial aneurysms, yet the effects of smoking on outcomes of aneurysm with flow-diverter treatment remain unknown. We studied the impact of smoking on long-term angiographic and clinical outcomes after flow-diverter treatment of intracranial aneurysms.
MATERIALS AND METHODS:
We retrospectively reviewed data from patients treated with the Pipeline Embolization Device and included in the International Retrospective Study of the Pipeline Embolization Device, the Pipeline for Uncoilable or Failed Aneurysms Study, and the Aneurysm Study of Pipeline in an Observational Registry. Patients were stratified according to smoking status into 3 groups: 1) never smoker, 2) current smoker, and 3) former smoker. We studied angiographic and clinical outcomes. Outcomes were compared by using χ2 and Student t tests. A multivariate analysis was performed to determine whether smoking was independently associated with poor outcomes.
RESULTS:
Six hundred sixteen patients with 694 aneurysms were included. Current smokers had a smaller mean aneurysm size compared with the other 2 groups (P = .005) and lower rates of multiple Pipeline Embolization Device use (P = .015). On multivariate analysis, former smokers (OR, 1.08; 95% CI, 0.43–2.71; P = .57) and current smokers (OR, 0.70; 95% CI, 0.27–1.77; P = .38) had similar odds of long-term angiographic incomplete occlusion compared with never smokers. Former smokers (OR, 1.27; 95% CI, 0.64–2.52; P = .25) and current smokers (OR, 0.74; 95% CI, 0.37–1.46; P = .22) had similar odds of major morbidity and neurologic mortality compared with never smokers.
CONCLUSIONS:
These results suggest that smoking is not associated with angiographic and clinical outcomes among patients treated with the Pipeline Embolization Device. Nonetheless, patients with intracranial aneurysms should continue to be counseled about the risks of tobacco smoking.
Tobacco smoking is one of the most important risk factors for intracranial aneurysm formation and subarachnoid hemorrhage.1–7 Previous studies have shown that cigarette smoking is associated with a 6-fold increased risk of SAH.1,6 Controversy exists regarding smoking as a risk factor for aneurysm recurrence after endovascular coiling of intracranial aneurysms.8,9 It is possible that smoking could affect aneurysm occlusion rates following flow-diverter therapy because a number of preclinical studies have demonstrated that cigarette smoking reduces the number of circulating endothelial progenitor cells,10 cells essential to aneurysm healing following flow-diverter therapy.11 In addition, smoking has been associated with poor postoperative clinical outcomes for a number of surgical and endovascular procedures.12,13
Given the widespread acceptance and use of flow-diverter therapy in the treatment of intracranial aneurysms, it is important to know what affect, if any, modifiable risk factors such as smoking have on clinical and angiographic outcomes. To gain a better understanding of the impact of smoking on long-term outcomes after flow diversion for intracranial aneurysms, we studied angiographic and clinical outcomes of patients included in 3 large clinical studies of the Pipeline Embolization Device (PED; Covidien, Irvine, California): the International Retrospective Study of the Pipeline Embolization Device (IntrePED), the Pipeline for Uncoilable or Failed Aneurysms study (PUFS), and the Aneurysm Study of Pipeline in an Observational Registry (ASPIRE), stratifying patients into 3 groups: 1) never smoker, 2) current smoker, and 3) former smoker. The goal of this study was to determine whether smoking is an independent risk factor for long-term aneurysm incomplete occlusion and major neurologic morbidity-mortality after PED treatment. We hypothesized that patients who smoked or had a history of smoking would have a lower rate of aneurysm occlusion and higher morbidity-mortality rates.
Materials and Methods
Patient Population
Patients were selected from the PUFS,14 IntrePED,15 and the ASPIRE (https://www.clinicaltrials.gov/ct2/show/NCT01557036) studies. PUFS was a prospective single-arm clinical trial of 108 patients with 108 aneurysms, including only patients with wide-neck (≥4 mm) and large (10–24.9 mm) or giant (≥25 mm) aneurysms of the internal carotid artery from the petrous to the superior hypophyseal segments with a follow-up of 5 years. IntrePED was a retrospective postmarket registry of 793 patients with 906 aneurysms with no size or location criteria with a follow-up of 3 years. ASPIRE was a prospective postmarket registry with 191 patients with 207 aneurysms, in which size and location inclusion criteria followed the country-specific PED instruction for use with a follow-up of 2 years. The patients included in this study have already been included in previous studies that did not focus on the impact of smoking.
We pooled data from these 3 studies including patients with unruptured and ruptured aneurysms in which information on smoking status was available. Patients were divided into 3 groups based on smoking status: 1) current smoker, 2) previous smoker and 3) never smoker. For previous smokers, no data were available regarding the last time the patient smoked cigarettes. We collected and analyzed the following baseline characteristics: age, sex, number of aneurysms, aneurysm size, aneurysm type (saccular, fusiform, dissecting, and other), aneurysm location, rupture status, and use of multiple PEDs.
Outcomes
The primary outcomes of this study were complete aneurysm occlusion at last follow-up and major neurologic morbidity and neurologic mortality. Secondary outcomes included major ipsilateral ischemic stroke, ipsilateral intracranial hemorrhage, all-cause mortality, and in-stent stenosis at last follow-up. “Major” adverse events were defined as ongoing clinical deficits at 7 days following the event. All major adverse events are included in the neurologic morbidity and mortality rates. All adverse events were adjudicated by the Adverse Events Review Committee of each study. An independent core lab adjudicated all angiographic outcomes. ASPIRE, IntrePED, and PUFS all reported clinical outcomes while only ASPIRE and PUFS reported angiographic outcomes.
Statistical Analysis
Statistical analyses were performed by using SAS, Version 9.1 or higher (SAS Institute, Cary, North Carolina). Summary statistics are presented for all data available by using means and SDs for continuous variables and frequency tabulations for categoric variables. Comparisons among groups for continuous variables were evaluated by using t tests or ANOVAs and the Fisher exact or Pearson χ2 test for binary categoric variables. Most statistical analyses were performed across patient groups—that is, on a per-patient basis. Because some patients had >1 aneurysm, however, each patient's first aneurysm treated was used to classify patients into the 4 anatomic/size subgroups and the largest aneurysm was used to classify patients into the 3 aneurysm-size categories. The first aneurysm treated was defined a priori. A multivariate logistic regression analysis was performed to determine whether smoking status was independently associated with the above outcomes. Adjusted variables in this model were baseline variables that were significantly different among groups. For the multivariate analysis, the never-smoker group was the reference group. Given the wide variability in the length of follow-up, we performed a survival analysis on aneurysm occlusion by smoking status.
Results
Baseline Patient and Aneurysm Characteristics
Six hundred sixteen patients with 694 treated aneurysms were included. Long-term clinical follow-up was available for 616 patients. Angiographic follow-up >6 months was available for 210 patients. Baseline demographics and aneurysms characteristics according to the smoking status are presented in the On-line Table.
The mean age of all patients was 57.4 ± 14.2 years. The mean length of follow-up was 22.2 ± 18.5 months for the clinical evaluation and 28.9 ± 23.7 months for the angiographic follow-up. One hundred seventy-nine patients with 214 aneurysms (30.8%) were current smokers, 111 patients with 120 aneurysms (17.3%) were former smokers, and 326 patients with 360 aneurysms (51.9%) had never smoked. In general, baseline characteristics were similar among groups except that current smokers had a smaller mean aneurysm size (11.2 ± 7.1 mm) compared with the previous smoker (13.4 ± 7.6 mm) and never smoker (13.2 ± 8.1 mm) groups (P = .005). Fewer patients in the current smoker group were treated with multiple PEDs (31.0%, 66/213) than in the previous smoker (44.5%, 53/119) and never smoker (41.8%, 150/359) groups (P = .015).
Univariate Analysis
Univariate analysis is presented in Table 1. Major neurologic morbidity and mortality rates were similar among groups (7.3% for current smokers, 14.4% for previous smokers, and 10.5% for never smokers, P = .15). Complete occlusion rates at last follow-up were similar among groups as well (86.2% for current smokers, 79.6% for previous smokers, and 82.5% for never smokers, P = .64). Previous smokers did have higher rates of major ipsilateral ischemic stroke (9.9%, 11/111) compared with current smoker (3.4%, 6/179) and never smoker groups (4.3%, 14/325) (P = .04). Previous smokers also had higher rates of major neurologic morbidity (13.5%, 15/111) compared with current smokers (4.5%, 8/179) and those who never smoked (8.0%, 26/325) (P = .02). There were no differences in rates of major ipsilateral intracranial hemorrhage (P = .22), neurologic mortality (P = .93), all-cause mortality (P = .56), and in-stent stenosis (P = .80).
Table 1:
Univariate analysis of patient groups
| Outcome | Current Smoker | Previous Smoker | Never Smoker | Total | P Value |
|---|---|---|---|---|---|
| Major ipsilateral ischemic stroke | 3.4% (6/179) | 9.9% (11/111) | 4.3% (14/325) | 5.0% (31/615) | .043a |
| Major ipsilateral intracranial hemorrhage | 1.1% (2/179) | 3.6% (4/111) | 3.7% (12/325) | 2.9% (18/615) | .216 |
| Major morbidity | 4.5% (8/179) | 13.5% (15/111) | 8.0% (26/325) | 8.0% (49/615) | .024a |
| Neurologic mortality | 3.9% (7/179) | 4.5% (5/111) | 4.6% (15/325) | 4.4% (27/615) | .931 |
| Major morbidity and neurologic mortality | 7.3% (13/179) | 14.4% (16/111) | 10.5% (34/325) | 10.2% (63/615) | .146 |
| All-cause mortality | 4.5% (8/179) | 7.2% (8/111) | 5.2% (17/325) | 5.4% (33/615) | .564 |
| Complete aneurysm occlusion | |||||
| 180 days (−20/+42 days) | 75.0% (30/40) | 73.7% (28/38) | 76.1% (51/67) | 75.2% (109/145) | .969 |
| 1 yr (±42 days) | 96.9% (31/32) | 80.6% (25/31) | 80.9% (38/47) | 85.5% (94/110) | .068 |
| 3 yr | 95.2% (20/21) | 92.0% (23/25) | 93.3% (28/30) | 93.4% (71/76) | 1.000 |
| 5 yr | 94.1% (16/17) | 100.0% (19/19) | 92.3% (24/26) | 95.2% (59/62) | .615 |
| Last follow-up visit | 86.2% (50/58) | 79.6% (39/49) | 82.5% (80/97) | 82.8% (169/204) | .638 |
| In stent stenosis at last angiographic follow-up | |||||
| >50%–75% | 1.9% (1/52) | 2.2% (1/45) | 0.0% (0/81) | 1.1% (2/178) | .296 |
| >75% | 1.9% (1/52) | 0.0% (0/45) | 2.5% (2/81) | 1.7% (3/178) | .795 |
Significant.
Multivariate Analysis
The multivariate logistic regression analysis is presented in Table 2. Previous smokers had similar odds of major neurologic morbidity and mortality compared with the never smoker group (OR, 1.27; 95% CI, 0.64–2.52; P = .25). The same was true for current smokers (OR, 0.74; 95% CI, 0.37–1.46; P = .22). Previous smokers also had similar odds of incomplete angiographic occlusion at last follow-up compared with the never smoker group (OR, 1.08; 95% CI, 0.43–2.71; P = .57). The same was true for current smokers (OR, 0.70; 95% CI, 0.27–1.77; P = .38). The odds of all other complications were similar between never smokers and current/previous smokers as well.
Table 2:
Multivariate logistic regression analysis
| Outcome/Smoke Status | Odds Ratio (CI 95%) | P Value |
|---|---|---|
| Major ipsilateral ischemic stroke | ||
| Current vs never | 0.94 (0.36–2.42) | .40 |
| Previous vs never | 1.91 (0.79–4.60) | .12 |
| Major ipsilateral intracranial hemorrhage | ||
| Current vs never | 0.41 (0.11–1.56) | .18 |
| Previous vs never | 1.04 (0.35–3.08) | .41 |
| Major morbidity | ||
| Current vs never | 0.65 (0.29–1.44) | .11 |
| Previous vs never | 1.49 (0.73–3.06) | .09 |
| Neurologic mortality | ||
| Current vs never | 0.85 (0.33–2.16) | .76 |
| Previous vs never | 0.96 (0.33–2.78) | .94 |
| Major morbidity and neurologic mortality | ||
| Current vs never | 0.74 (0.37–1.46) | .22 |
| Previous vs never | 1.27 (0.64–2.52) | .25 |
| All-cause mortality | ||
| Current vs never | 0.88 (0.36–2.14) | .46 |
| Previous vs never | 1.50 (0.61–3.67) | .29 |
| Without complete aneurysm occlusion at last follow-up | ||
| Current vs never | 0.70 (0.27–1.77) | .38 |
| Previous vs never | 1.08 (0.43–2.71) | .57 |
| Stenosis >50% at last follow-up | ||
| Current vs never | 2.30 (0.37–14.21) | .45 |
| Previous vs never | 1.46 (0.17–12.43) | .97 |
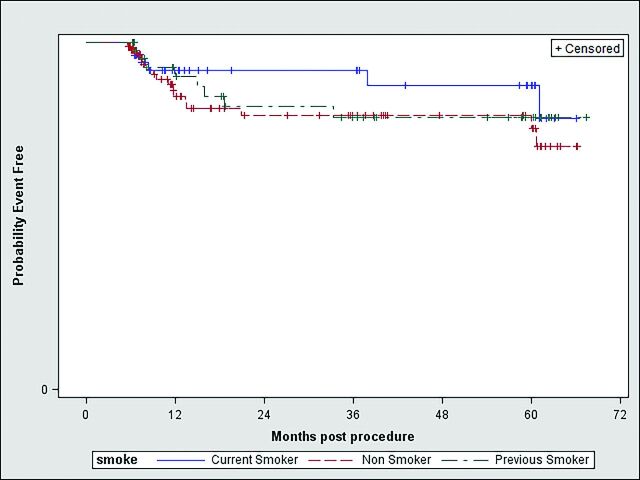
According to the survival analysis on aneurysm occlusion by smoking status, there was no significant difference in freedom from occlusion across the smoking-status groups (log-rank test, P value = .52); survival curves are presented in the Figure.
Figure.
Survival analysis on aneurysm occlusion by smoking status. Log-rank test, P value = .52.
Discussion
Our study of >600 patients with nearly 700 treated aneurysms demonstrates that tobacco smoking is not independently associated with aneurysm occlusion rates or higher rates of poor clinical outcome following PED embolization of intracranial aneurysms. In both uni- and multivariate analyses of the entire cohort and in a subgroup analysis, we failed to detect an association between smoking and long-term angiographically confirmed occlusion rates or with combined neurologic morbidity and mortality. However, the multivariate analysis showed a tendency for lower rates of complete occlusion for current smokers. The nonsignificance of these results may be due to potential lack of statistical power of the analyses even if the population of the study was quite large. Rates of stroke, hemorrhage, and in-stent stenosis were similar between groups on multivariate analysis. These findings suggest that smoking status should not be a factor for excluding patients from PED embolization of intracranial aneurysms.
This study is the first, to our knowledge, to specifically analyze the impact of smoking on angiographic and clinical outcomes after PED treatment of intracranial aneurysms. Understanding the effect of smoking on outcomes related to the PED is important because previous studies have shown that cigarette smoking is a risk factor for both intracranial aneurysm formation and recurrence after endovascular coiling.9 In their study of 100 patients, Ortiz et al9 found that cigarette smokers had higher odds of recanalization following endovascular coiling than never smokers. However, larger follow-up studies by Brinjikji et al8 and Chen et al16 demonstrated no association between aneurysm occlusion and smoking status.
Our study found no association between smoking status and clinical outcomes following flow-diverter treatment of intracranial aneurysms with the PED. While no prior studies have examined the association between clinical outcomes and flow-diverter treatment, other studies have reported clinical outcomes following stent placement of intracranial arteries. In a study of 125 patients undergoing stent-assisted coiling with the Enterprise self-expanding stent (Codman & Shurtleff, Raynham, Massachusetts), Song et al17 found that active smoking was associated with higher rates of delayed thromboembolic events. In a study of 45 aneurysms in 41 patients receiving covered stents for treatment of distal internal carotid and vertebral artery aneurysms, Zhu et al18 found that smoking was an independent predictor of late in-stent stenosis. In the setting of carotid stenosis, a subgroup analysis of the Carotid Revascularization Endarterectomy versus Stenting Trial found that smoking predicted an increased rate of restenosis after carotid endarterectomy but not after carotid stent placement.19
Prior studies have demonstrated that smoking is associated with worse clinical outcomes following stent placement in other locations as well. In a study of >9000 patients undergoing percutaneous coronary intervention with drug-eluting stents, Matteau et al20 found that smoking was an independent risk factor for postoperative ischemic events and bleeding. Similar to patients with PEDs, patients with percutaneous coronary intervention are required to receive dual antiplatelet therapy following their intervention. Yeo et al21 found that active smoking was independently associated with higher rates of in-stent thrombosis following percutaneous coronary intervention. In a subgroup analysis of the Synergy Between Percutaneous Coronary Intervention With TAXUS and Cardiac Surgery (See more at http://www.acc.org/Latest-in-Cardiology/Clinical-Trials/2014/08/19/16/32/SYNTAX#sthash.u3T0dfPw.dpuf), Zhang et al22 found that smoking was associated with poor clinical outcomes after revascularization in patients with complex coronary artery disease with higher rates of in-stent thrombosis, death, myocardial infarction, and stroke. Smoking has also been associated with decreased odds of primary stent patency following endovascular treatment of subclavian artery disease.12
Limitations
Our study has several limitations. Some patients from the PUFS, IntrePED, and ASPIRE studies were not included in the present pooled analysis because of missing data regarding their smoking status. This noninclusion of some patients could be a potential selection bias, but we doubt that this has skewed the results because the availability of their smoking status is not likely to be related to their actual smoking habits. The patients in our study were stratified into those who never, currently, or formerly smoked, but we did not further stratify smokers by pack-year because such data were not available. Previous studies have shown that increased pack-years are associated with an increased risk of SAH.23,24 Also, we performed the analysis on the basis of the smoking status of the patients at the time of the treatment and did not have information regarding their smoking habits after the treatment, which might have changed during the course of follow-up. We acknowledge that this feature might introduce a bias if many patients stopped smoking after the treatment or restarted smoking during the follow-up period. Because our study was retrospective, we did not perform a power calculation before data collection. This omission might introduce a potential bias due to low statistical power, and multiple comparisons may raise the false-positivity issue or not reach it as well. However, our study is the largest study examining the association between smoking and outcomes of intracranial aneurysm treatment to date. Last, we have no data or information as to whether smokers were managed differently than never smokers. It is possible that smokers were more likely to undergo more careful intraprocedural and periprocedural monitoring of antiplatelet and anticoagulation status or closer angiographic follow-up.
Conclusions
The results of our study show that smoking is not an independent risk factor for worse clinical outcomes, aneurysm occlusion rates, or in-stent stenosis after PED treatment of intracranial aneurysms. Nonetheless, patients with intracranial aneurysms should continue to be counseled about the risks of cigarette smoking.
Supplementary Material
ABBREVIATION:
- PED
Pipeline Embolization Device
Footnotes
Disclosures: Giuseppe Lanzino—UNRELATED: Consultancy: Covidien/Medtronic.* Tibor Becske—UNRELATED: Consultancy: Covidien/Medtronic; Other: proctoring fees from Covidien/Medtronic. David F. Kallmes—RELATED: Grant: Medtronic (Principal Investigator of clinical trial)*; Consulting Fee or Honorarium: Medtronic (Steering Committee participation)*; UNRELATED: Board Membership: GE Healthcare (Cost-Effectiveness Board)*; Consultancy: Medtronic,* Comments: planning and implementing clinical trials; Grants/Grants Pending: MicroVention,* Sequent Medical,* SurModics,* Codman Neurovascular,* ev3/Covidien/Medtronic,* NeuroSigma*; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Medtronic,* Comments: presentation at FDA panel meeting. *Money paid to the institution.
References
- 1. Bonita R. Cigarette smoking, hypertension and the risk of subarachnoid hemorrhage: a population-based case-control study. Stroke 1986;17:831–35 10.1161/01.STR.17.5.831 [DOI] [PubMed] [Google Scholar]
- 2. Juvela S, Hillbom M, Numminen H, et al. Cigarette smoking and alcohol consumption as risk factors for aneurysmal subarachnoid hemorrhage. Stroke 1993;24:639–46 10.1161/01.STR.24.5.639 [DOI] [PubMed] [Google Scholar]
- 3. Juvela S, Poussa K, Porras M. Factors affecting formation and growth of intracranial aneurysms: a long-term follow-up study. Stroke 2001;32:485–91 10.1161/01.STR.32.2.485 [DOI] [PubMed] [Google Scholar]
- 4. Knekt P, Reunanen A, Aho K, et al. Risk factors for subarachnoid hemorrhage in a longitudinal population study. J Clin Epidemiol 1991;44:933–39 10.1016/0895-4356(91)90056-F [DOI] [PubMed] [Google Scholar]
- 5. Kang HS, Han MH, Kwon BJ, et al. Repeat endovascular treatment in post-embolization recurrent intracranial aneurysms. Neurosurgery 2006;58:60–70; discussion 60–70 10.1227/01.NEU.0000194188.51731.13 [DOI] [PubMed] [Google Scholar]
- 6. Petitti DB, Wingerd J. Use of oral contraceptives, cigarette smoking, and risk of subarachnoid haemorrhage. Lancet 1978;2:234–35 [DOI] [PubMed] [Google Scholar]
- 7. Sacco RL, Wolf PA, Bharucha NE, et al. Subarachnoid and intracerebral hemorrhage: natural history, prognosis, and precursive factors in the Framingham Study. Neurology 1984;34:847–54 10.1212/WNL.34.7.847 [DOI] [PubMed] [Google Scholar]
- 8. Brinjikji W, Lingineni RK, Gu CN, et al. Smoking is not associated with recurrence and retreatment of intracranial aneurysms after endovascular coiling. J Neurosurg 2015;122:95–100 10.3171/2014.10.JNS141035 [DOI] [PubMed] [Google Scholar]
- 9. Ortiz R, Stefanski M, Rosenwasser R, et al. Cigarette smoking as a risk factor for recurrence of aneurysms treated by endosaccular occlusion. J Neurosurg 2008;108:672–75 10.3171/JNS/2008/108/4/0672 [DOI] [PubMed] [Google Scholar]
- 10. Wei HJ, Wang D, Chen JL, et al. Mobilization of circulating endothelial progenitor cells after endovascular therapy for ruptured cerebral aneurysms. Neurosci Lett 2011;498:114–18 10.1016/j.neulet.2011.04.061 [DOI] [PubMed] [Google Scholar]
- 11. Kadirvel R, Ding YH, Dai D, et al. Cellular mechanisms of aneurysm occlusion after treatment with a flow diverter. Radiology 2014;270:394–99 10.1148/radiol.13130796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soga Y, Tomoi Y, Fujihara M, et al. Perioperative and long-term outcomes of endovascular treatment for subclavian artery disease from a large multicenter registry. J Endovasc Ther 2015;22:626–33 10.1177/1526602815590579 [DOI] [PubMed] [Google Scholar]
- 13. Musallam KM, Rosendaal FR, Zaatari G, et al. Smoking and the risk of mortality and vascular and respiratory events in patients undergoing major surgery. JAMA Surg 2013;148:755–62 10.1001/jamasurg.2013.2360 [DOI] [PubMed] [Google Scholar]
- 14. Becske T, Kallmes DF, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology 2013;267:858–68 10.1148/radiol.13120099 [DOI] [PubMed] [Google Scholar]
- 15. Kallmes DF, Hanel R, Lopes D, et al. International retrospective study of the Pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol 2015;36:108–15 10.3174/ajnr.A4111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen JX, Lai LF, Zheng K, et al. Influencing factors of immediate angiographic results in intracranial aneurysms patients after endovascular treatment. J Neurol 2015;262:2115–23 10.1007/s00415-015-7824-2 [DOI] [PubMed] [Google Scholar]
- 17. Song J, Yeon JY, Kim JS, et al. Delayed thromboembolic events more than 30 days after self-expandable intracranial stent-assisted embolization of unruptured intracranial aneurysms. Clin Neurol Neurosurg 2015;135:73–78 10.1016/j.clineuro.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 18. Zhu YQ, Li MH, Lin F, et al. Frequency and predictors of endoleaks and long-term patency after covered stent placement for the treatment of intracranial aneurysms: a prospective, non-randomised multicentre experience. Eur Radiol 2013;23:287–97 10.1007/s00330-012-2581-4 [DOI] [PubMed] [Google Scholar]
- 19. Lal BK, Beach KW, Roubin GS, et al. ; CREST Investigators. Restenosis after carotid artery stenting and endarterectomy: a secondary analysis of CREST, a randomised controlled trial. Lancet Neurol 2012;11:755–63 10.1016/S1474-4422(12)70159-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matteau A, Yeh RW, Camenzind E, et al. Balancing long-term risks of ischemic and bleeding complications after percutaneous coronary intervention with drug-eluting stents. Am J Cardiol 2015;116:686–93 10.1016/j.amjcard.2015.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yeo KK, Armstrong EJ, Soni K, et al. Long-term outcomes of angiographically confirmed coronary stent thrombosis: results from a multicentre California registry. EuroIntervention 2015;11:188–95 10.4244/EIJV11I2A33 [DOI] [PubMed] [Google Scholar]
- 22. Zhang YJ, Iqbal J, van Klaveren D, et al. Smoking is associated with adverse clinical outcomes in patients undergoing revascularization with PCI or CABG: the SYNTAX trial at 5-year follow-up. J Am Coll Cardiol 2015;65:1107–15 10.1016/j.jacc.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 23. Anderson CS, Feigin V, Bennett D, et al. ; Australasian Cooperative Research on Subarachnoid Hemorrhage Study (ACROSS) Group. Active and passive smoking and the risk of subarachnoid hemorrhage: an international population-based case-control study. Stroke 2004;35:633–37 10.1161/01.STR.0000115751.45473.48 [DOI] [PubMed] [Google Scholar]
- 24. Kim CK, Kim BJ, Ryu WS, et al. Impact of smoking cessation on the risk of subarachnoid haemorrhage: a nationwide multicentre case control study. J Neurol Neurosurg Psychiatry 2012;83:1100–03 10.1136/jnnp-2012-302538 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.