Abstract
BACKGROUND AND PURPOSE:
There is an uncertainty about the association between intracranial aneurysms and aortic dissection. We aimed to determine the prevalence of intracranial aneurysms in patients with aortic dissection and evaluate the independent risk factors for the presence of intracranial aneurysms in these patients.
MATERIALS AND METHODS:
Seventy-one patients with a confirmed aortic dissection who underwent additional brain imaging were enrolled as the aortic dissection group, and 2118 healthy individuals with brain imaging, as controls. Demographic data were obtained from their medical records, including age, sex, comorbidities, and arch vessel involvement of aortic dissection. Two readers reviewed all brain images independently regarding the presence, morphology, size, and location of intracranial aneurysms. Baseline characteristics were compared between the aortic dissection group and controls by propensity score matching, and logistic regression analysis was performed for independent risk factors for the presence of intracranial aneurysms.
RESULTS:
The prevalence of intracranial aneurysms was 12.96% in the aortic dissection group and 1.85% in controls (P = .022). The mean diameter of intracranial aneurysms was significantly larger in the aortic dissection group (5.79 ± 3.26 mm in aortic dissection versus 3.04 ± 1.57 mm in controls; P = .008), and intracranial aneurysms of >7 mm were also more common in the aortic dissection group (28.6% in aortic dissection versus 5.3% in controls, P = .003). On multivariate analysis, arch vessel involvement of aortic dissection was an independent risk factor for the presence of intracranial aneurysms (odds ratio, 6.246; 95% confidence interval, 1.472–26.50; P = .013).
CONCLUSIONS:
Patients with aortic dissection have a high prevalence of intracranial aneurysms, and selective screening for brain vessels could be considered in these patients with arch vessel involvement. A further prospective study is needed to demonstrate a substantial prevalence of intracranial aneurysms.
Intracranial aneurysms (IAs) are found in approximately 3% of the general population,1 and IA rupture with subarachnoid hemorrhage is a life-threatening event with substantial morbidity and mortality.2,3 With the advancement of the imaging modalities, early diagnosis of IA is relevant, especially in at-risk patients with selected conditions associated with an increased occurrence of IAs.4
IAs and aortic diseases are different disease entities but have a similar pathophysiologic mechanism, which may be caused by excessive hemodynamic stress to the vessel wall or genetic factors for vascular fragility. The guidelines for unruptured IAs4 have suggested that there is an increased the risk of aneurysm formation in some aortic pathologies such as bicuspid aortic valve and coarctation of the aorta. Recently, some authors5–7 have published the link between IA and aortic aneurysm, which showed an IA incidence of 9%–11% in patients with aortic aneurysms. However, there are a limited number of genetic or experimental studies8–10 and case reports11–13 for the association between IA and aortic dissection (AD).
Therefore, we aimed to demonstrate the prevalence of IA in patients with AD and investigate independent risk factors for the presence of IA in these patients.
Materials and Methods
Patients
After approval of the institutional review board (Gangnam Severance Hospital) for this retrospective study, informed consent was waived. Patients with AD were recruited from the medical data base of this tertiary hospital between January 2009 and June 2016. Inclusion criteria for the AD group were as follows: 1) patients with a confirmed AD as the indication for the CT angiography of the aorta; and 2) patients with AD who had undergone additional brain imaging such as brain CTA or MR angiography for the evaluation of any neurologic symptoms and follow-up of known IAs. Exclusion criteria were patients with traumatic AD and known connective tissue diseases such as Marfan, Ehlers-Danlos, and Loeys-Dietz syndromes.
Among healthy individuals at the Health Promotion Center of our tertiary hospital between January 2011 and December 2012, those who paid for their brain imaging at their own expense were consecutively enrolled as a control group. This Health Promotion Center was not advertised, and each examinee voluntarily decided to visit and choose brain imaging for his or her health examination.
We obtained demographic data of all patients from medical records: age, sex, and comorbidities (hypertension, diabetes, hyperlipidemia, and smoking); history of cerebrovascular disease, including intracerebral hemorrhage or cerebral infarction; and arch vessel involvement. Arch vessel involvement was defined as extension of the AD into any cervicobrachial vessels, such as the right brachiocephalic artery, left common carotid artery, or left subclavian artery.
Image Acquisition
Brain CTA was performed with a 128-channel multidetector CT scanner (Somatom Definition AS+; Siemens, Erlangen, Germany) with iopromide (Ultravist 370 mg iodine/mL; Schering Korea, Seoul, Korea). The imaging parameters were as follows: an exposure setting of 120 kV and 140 mA with automatic tube current modulation (CARE Dose4D; Siemens), a collimation of 64 × 0.6 mm, a section acquisition of 128 × 0.6 mm, and a pitch of 0.45. The imaging volume ranged from the vertex of the skull to the posterior arch of the C1 vertebra.
Brain MRA was performed with a 3T scanner (Discovery MR750; GE Healthcare, Milwaukee, Wisconsin). 3D time-of-flight MRA protocol parameters were as follows: TR/TE, 23/2.5 ms; flip angle, 20°; FOV, 210 × 185 mm; 4 slabs (176 sections); section thickness, 1.4 mm; matrix, 416 × 224; and acquisition time, 5 minutes 9 seconds.
All axial source data of CTA and MRA were reconstructed for the maximum intensity projections (axial, sagittal, and coronal) and volume-rendered images of the cerebral arteries.
Image Analysis
Two experienced neuroradiologists (S.H.S., S.J.A.) reviewed the axial brain images independently on the PACS, together with all reconstructed images of the brain vessels. The presence of IAs was determined in a consensus reading, and any clinical information or knowledge about this study was not provided to the readers.
IA was defined as an abnormal focal outpouching of the cerebral artery on brain CTA or MRA.14 Aneurysm size was measured by the largest diagonal diameter. Aneurysm locations were classified as the internal carotid artery, anterior cerebral artery including the anterior communicating artery, middle cerebral artery, and vertebrobasilar artery; and the morphology of the aneurysm was categorized as saccular or fusiform.
Statistical Analysis
Differences between the AD group and controls were evaluated with a χ2 or Fisher exact test for all categoric variables and an independent 2-sample t test for all continuous variables. Simple logistic regression analysis was performed to determine independent risk factors for the presence of IA in the AD group. Multiple logistic regression analysis was performed on variables with an unadjusted effect and P < .05 on simple logistic regression analysis. P < .05 was considered statistically significant. All statistical analyses were performed with SAS, Version 9.2 (SAS Institute, Cary, North Carolina).
Propensity score matching was performed to minimize the intergroup difference in baseline characteristics. The propensity score was developed with multiple logistic regression with respect to age, sex, hypertension, diabetes mellitus, hyperlipidemia, smoking, and a history of cerebrovascular disease. Using the propensity score of each patient, we matched a selected case with controls by a 1:2 matching. The nearest-neighbor-matching algorithm with a “greedy method” was used to match patients. After patient matching, we performed the paired t and McNemar tests. Balance between both groups for each variable was evaluated by propensity score distributions, and absolute standardized differences before and after matching were calculated. After patient matching, absolute standardized differences of <0.10 implied good balance between both groups.
Results
Baseline Characteristics
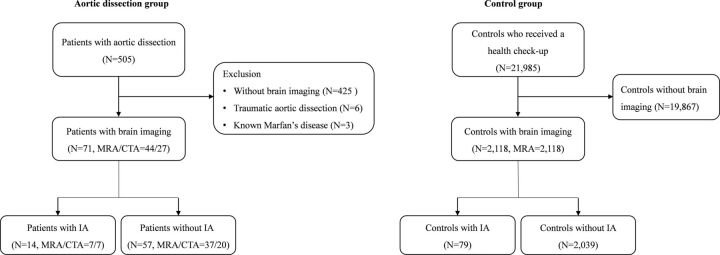
A total of 505 patients with confirmed AD were collected between January 2009 and June 2016 (Fig 1). Patients without brain imaging (n = 425), patients with traumatic aortic dissection (n = 6), and those with Marfan disease (n = 3) were excluded from this study. Of these patients, 71 with brain imaging were enrolled as the AD group. Brain CTA was performed in 27 patients (38%), and brain MRA, in 44 (62%). Among 71 patients, 45 (63.38%) underwent brain imaging for the evaluation of nonspecific neurologic symptoms; 23 (32.39%), for the evaluation of headache; and 3 (4.23%), for the follow-up imaging of surgically clipped IA.
Fig 1.
Flowchart of the study population.
Of potential controls with a health examination, 2118 with brain MRA were enrolled as the controls.
As shown in Table 1, the mean age of the AD group was significantly higher than that of controls (58.6 ± 13.6 years versus 53.9 ± 9.6 years, respectively; P = .004). Hypertension (60.6% in AD versus 29.4% in controls, P < .0001) and cerebrovascular disease history (21.1% in AD versus 0.94% in controls, P < .0001) were significantly associated with the AD group. After a 1:2 matching, there was no significant difference between both groups regarding age, sex, comorbidities, or cerebrovascular disease history.
Table 1:
Comparison of baseline characteristics between the overall and matched study population
| Characteristics | Overalla |
Matchedb |
||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 2189) | AD Group (n = 71) | Controls (n = 2118) | P Value | Total (n = 162) | AD Group (n = 54) | Controls (n = 108) | P Value | |
| Presence of IA (No.) (%) | 93 (4.25) | 14 (19.72) | 79 (3.73) | <.0001 | 9 (5.55) | 7 (12.96) | 2 (1.85) | .022 |
| Age (mean) | 54.03 ± 9.78 | 58.66 ± 13.6 | 53.87 ± 9.59 | .004 | 59.20 ± 11.73 | 58.98 ± 13.09 | 59.31 ± 11.05 | .808 |
| Female sex (No.) (%) | 963 (43.99) | 33 (46.48) | 930 (43.91) | .668 | 67 (41.36) | 24 (44.44) | 43 (39.81) | .189 |
| Hypertension (No.) (%) | 665 (30.38) | 43 (60.56) | 622 (29.37) | <.0001 | 67 (41.36) | 24 (44.44) | 43 (39.81) | .43 |
| DM (No.) (%) | 246 (11.24) | 5 (7.04) | 241 (11.38) | .255 | 97 (59.88) | 31 (57.41) | 66 (61.11) | .436 |
| Hyperlipidemia (No.) (%) | 191 (8.73) | 2 (2.82) | 189 (8.92) | .073 | 15 (9.26) | 4 (7.41) | 11 (10.19) | .313 |
| CVD (No.) (%) | 35 (1.6) | 15 (21.13) | 20 (0.94) | <.0001 | 5 (3.09) | 1 (1.85) | 4 (3.7) | >.9999 |
| Smoking (No.) (%) | 725 (33.12) | 15 (21.13) | 710 (33.52) | .087 | 127 (78.39) | 43 (79.63) | 84 (77.78) | 0.732 |
Note:—DM indicates diabetes mellitus; CVD, cerebrovascular disease.
P values before matching were calculated with a χ2 or independent 2-sample t test.
P values after matching were calculated with the McNemar or paired t test.
Among 71 patients with AD, 46 were included in the Stanford type A group (64.8%) and 25 were type B (35.2%). Among 14 patients with AD and IA, 9 were included in the Stanford type A group (64.2%) and 5 were type B (35.8%). In the AD group, arch vessel involvement was found in 14: 6 of 14 patients with IA (42.8%) and 8 of 57 patients without IA (14%).
Prevalence and Characteristics of IA
IAs were found in 14 of 71 patients with AD (19.72%) versus 79 of 2118 controls (3.73%), which was significantly different (P < .0001). After propensity score matching, there were IAs in 7 of 54 patients with AD (12.96%) versus 2 of 108 controls (1.85%), which also showed a significant difference (P = .022).
In Table 2, multiple IAs were noted in 11 of 79 controls and not in the AD group. Of these 11 controls, 2 IAs were found in eight; 3 IAs, in 1; and 4 IAs, in 2. All IAs were saccular except 1 fusiform IA in a control. The mean diameter of IAs was significantly larger in the AD group (5.79 ± 3.26 mm in AD versus 3.04 ± 1.57 mm in controls, P = .008), and IAs of >7 mm were also more common in the AD group (28.6% in AD versus 5.3% in controls, P = .003). In the AD group, IAs were frequently situated in the MCA (35.7% in AD versus 8.4% in controls, P = .012) or vertebrobasilar artery (21.4% in AD versus 2.1% in controls, P = .015), while ICA aneurysms were more common in the controls (77.9% in controls versus 28.5% in AD, P < .001).
Table 2:
Characteristics of intracranial aneurysms between the AD group and controls
| Characteristics | AD Group (n = 14) | Controlsa (n = 95) | P Value |
|---|---|---|---|
| Location | |||
| MCA | 5 (35.7) | 8 (8.4) | .012 |
| ACA | 2 (14.3) | 11 (11.6) | .673 |
| ICA | 4 (28.5) | 74 (77.9) | <.0001 |
| VBA | 3 (21.4) | 2 (2.1) | .015 |
| Aneurysm size | |||
| Mean diameter (mm) | 5.79 ± 3.26 | 3.04 ± 1.57 | .008 |
| ≥7 mm | 4 (28.6) | 5 (5.3) | .003 |
| Aneurysm morphology | |||
| Saccular type | 14 (100) | 94 (98.9) | |
| Arch vessel involvement | 6 (42.8) | 0 | |
| Diagnostic imaging modality | |||
| Brain CTA | 7 (50) | 0 | |
| Brain MRA | 7 (50) | 95 (100) |
Note:—ACA indicates anterior cerebral artery; VBA, vertebrobasilar artery.
Multiple aneurysms in 11 of 79 controls: 2 IAs in 8, three IAs in 1, and 4 IAs in 2.
Independent Risk Factors for the Presence of IA in the AD Group
Univariate analysis showed that arch vessel involvement (OR, 4.594; 95% CI, 1.257–16.78; P = .021) was significantly correlated with the presence of IAs. On multivariate analysis, arch vessel involvement (OR, 6.246; 95% CI, 1.472–26.50; P = .013) was also significantly associated with the presence of IAs (Table 3).
Table 3:
Independent predictors of intracranial aneurysms in the AD group
| Predictors | Univariate |
Multivariate |
||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Female sex | 0.833 (0.256–2.71) | .762 | ||
| Age | 1.028 (0.982–1.077) | .232 | ||
| Hypertension | 2.865 (0.721–11.38) | .135 | ||
| SBP | 1.007 (0.987–1.028) | .473 | ||
| DBP | 1.027 (0.994–1.063) | .113 | ||
| Diabetes mellitus | 0.576 (0–3.374) | .643 | ||
| Hyperlipidemia | 0.765 (0.018–33.015) | .889 | ||
| CVD | 1.673 (0.441–6.345) | .449 | ||
| Smoking | 0.341 (0.014–8.188) | .507 | ||
| Arch vessel involvement | 4.594 (1.257–16.78) | .021 | 6.246 (1.472–26.5) | .013 |
Note:—SBP indicates systolic blood pressure; DBP, diastolic blood pressure; CVD, cerebrovascular disease.
Relationship between Arch Vessel Involvement and IAs
Five of 6 patients and AD and arch vessel involvement showed right brachiocephalic artery involvement, and in 4 of these 5 patients, an IA was detected in the ipsilateral ICA, MCA, or VA (Table 4).
Table 4:
Summary of the association between arch vessel involvement and intracranial aneurysm location in 6 patients with AD
| Involved Arch Vessels | IA Location | IA Size (mm) |
|---|---|---|
| Right BCA, left SCA | Right VA | 11 |
| Right BCA, left CCA | Right MCA bifurcation | 4 |
| Right BCA | Right cavernous ICA | 9 |
| Right BCA | Right cavernous ICA | 9 |
| Left CCA, Left SCA | Right MCA bifurcation | 6 |
| Right BCA | Anterior communicating artery | 5 |
Note:—BCA indicates brachiocephalic artery; CCA, common carotid artery; VA, vertebral artery.
Discussion
This study showed a much higher prevalence of IAs in patients with AD than in the control group or general population.1 In the AD group, IAs of >7 mm in diameter were frequently found, and IAs were located predominantly within the MCA or vertebrobasilar artery. An independent risk factor for the presence of IAs in patients with AD was arch vessel involvement.
After propensity score matching, IA prevalence was estimated at 12.96%, which was 7 times higher than that in the controls and was comparable with that in patients with various aortic diseases.5,6,15,16 Compared with the general population,1 IA prevalence in this study was much higher by 4-fold. Curtis et al15 described a 10.3% prevalence in patients with coarctation of the aorta, and Schievink et al16 evaluated 9.8% in the bicuspid aortic valve. Some authors5,6 reported >11% of IA concurrence in patients with aortic aneurysm. To our knowledge, this is the first retrospective study to determine IA prevalence in patients with AD.
Although the reason for the high IA prevalence in patients with AD is unclear, recent studies proposed several potential explanations for the association between IAs and AD. Regalado et al10 suggested the possibility of a genetic link between IA and thoracic aortic aneurysm and dissection in a cohort of 514 families, in which 29 IAs were found. They found an autosomal dominant inheritance between IA and thoracic aortic aneurysm and dissection. In addition, hypertension was a major risk factor of AD and IA.4,17 Fukuda et al8 demonstrated that both IA and AD were caused by chronic inflammation of the arterial wall in hypertensive rats treated with the prostaglandin receptor antagonist.
In this study, IAs of >7 mm were frequently found in the AD group, and they were located predominantly in the MCA or vertebrobasilar artery. Several large-cohort studies14,18 demonstrated size (>7 mm) and location (posterior cerebral distribution) of the aneurysm as independent predictors of IA rupture. In a pooled analysis of 6 prospective cohort studies,19 predictors of aneurysm rupture were also aneurysm size (>7 mm), location including the vertebrobasilar artery, and ethnic difference. Although the treatment of unruptured IAs is still controversial, clinicians should consider the likelihood of IA rupture during an aortic operation for such patients because cerebral perfusion pressure may increase temporarily by clamping or balloon occlusion of the aorta.15
Our study showed that arch vessel involvement was an independent risk factor for the presence of IA in patients with AD. While its significance is ambiguous, experimental studies20 have shown that vascular fragility may result from abnormal development of neural crest–derived cells, which comprise the tunica media of the aortic arch and its branches; and the neural crest defect has been implicated previously in the association of aortocephalic arterial dissections and congenital bicuspid aortic valves. Inamasu et al11 recently reported a rare case of concurrent IA rupture and acute AD of Stanford type A, in which the right brachiocephalic trunk was involved. Moreover, our study showed right brachiocephalic artery involvement of AD in 5 patients, and IAs were detected along the ipsilateral ICA, MCA, or VA in 4 of 5, which may be induced by altered hemodynamics in the cranial vessels from the involvement of great vessel origins. Therefore, selective screening for brain vessels should be weighed carefully in these patients with arch vessel involvement.
There are several limitations in our study. First, only a small number of patients with brain imaging were retrospectively enrolled, which may be a selection bias; and the possibility of overestimation or underestimation of IA incidence cannot be excluded. Second, the diagnosis of IA was based on brain MRA or CTA, which may make it difficult to diagnose small IAs of <3 mm and to differentiate small aneurysms from junctional dilations.21,22 Third, ethnic differences for these 2 diseases should be considered for generalization. In addition, no pedigree or genetic study was available in our retrospective study. Finally, we could not exclude the possibility that the healthy controls might have an asymptomatic aortic pathology. For validation of the link between IA and AD, a further prospective, large-cohort study is needed.
Conclusions
In our study, patients with AD have a high prevalence of IAs, and selective screening for brain vessels could be considered prudent in case of arch vessel involvement. A further prospective, large-scale cohort study is needed to demonstrate a substantial prevalence of IAs.
Acknowledgments
All authors appreciate Hye Sun Lee and Sinae Kim for their assistance in the statistics of this study.
ABBREVIATIONS:
- AD
aortic dissection
- IA
intracranial aneurysm
Footnotes
This work was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (grant No. HC15C1056).
References
- 1. Vlak MH, Algra A, Brandenburg R, et al. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol 2011;10:626–36 10.1016/S1474-4422(11)70109-0 [DOI] [PubMed] [Google Scholar]
- 2. Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med 2006;355:928–39 10.1056/NEJMra052760 [DOI] [PubMed] [Google Scholar]
- 3. van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet 2007;369:306–18 10.1016/S0140-6736(07)60153-6 [DOI] [PubMed] [Google Scholar]
- 4. Thompson BG, Brown RD Jr, Amin-Hanjani S, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention, American Heart Association, American Stroke Association. Guidelines for the Management of Patients with Unruptured Intracranial Aneurysms: a Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015;46:2368–400 10.1161/STR.0000000000000070 [DOI] [PubMed] [Google Scholar]
- 5. Rouchaud A, Brandt MD, Rydberg AM, et al. Prevalence of intracranial aneurysms in patients with aortic aneurysms. AJNR Am J Neuroradiol 2016;37:1664–68 10.3174/ajnr.A4827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shin YW, Jung KH, Moon J, et al. Site-specific relationship between intracranial aneurysm and aortic aneurysm. Stroke 2015;46:1993–96 10.1161/STROKEAHA.115.009254 [DOI] [PubMed] [Google Scholar]
- 7. Kuzmik GA, Feldman M, Tranquilli M, et al. Concurrent intracranial and thoracic aortic aneurysms. Am J Cardiol 2010;105:417–20 10.1016/j.amjcard.2009.09.049 [DOI] [PubMed] [Google Scholar]
- 8. Fukuda M, Aoki T, Manabe T, et al. Exacerbation of intracranial aneurysm and aortic dissection in hypertensive rat treated with the prostaglandin F-receptor antagonist AS604872. J Pharmacol Sci 2014;126:230–42 10.1254/jphs.14148FP [DOI] [PubMed] [Google Scholar]
- 9. Regalado ES, Guo DC, Villamizar C, et al. Exome sequencing identifies SMAD3 mutations as a cause of familial thoracic aortic aneurysm and dissection with intracranial and other arterial aneurysms. Circ Res 2011;109:680–86 10.1161/CIRCRESAHA.111.248161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Regalado E, Medrek S, Tran-Fadulu V, et al. Autosomal dominant inheritance of a predisposition to thoracic aortic aneurysms and dissections and intracranial saccular aneurysms. Am J Med Genet A 2011;155A:2125–30 10.1002/ajmg.a.34050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inamasu J, Suzuki T, Wakako A, et al. Concurrence of aneurysmal subarachnoid hemorrhage and Stanford type A acute aortic dissection. J Stroke Cerebrovasc Dis 2016;25:e86–88 10.1016/j.jstrokecerebrovasdis.2016.03.034 [DOI] [PubMed] [Google Scholar]
- 12. Sakata N, Hamasaki M, Iwasaki H, et al. Dissecting aneurysms involving both anterior cerebral artery and aorta. Pathol Int 2007;57:224–28 10.1111/j.1440-1827.2007.02085.x [DOI] [PubMed] [Google Scholar]
- 13. Inaba S, Iwata S, Kayano T, et al. Perioperative management of a patient with subarachnoid hemorrhage complicated with descending aortic dissection [in Japanese]. Masui 2005;54:680–82 [PubMed] [Google Scholar]
- 14. Morita A, Kirino T, Hashi K, et al. ; UCAS Japan Investigators. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 2012;366:2474–82 10.1056/NEJMoa1113260 [DOI] [PubMed] [Google Scholar]
- 15. Curtis SL, Bradley M, Wilde P, et al. Results of screening for intracranial aneurysms in patients with coarctation of the aorta. AJNR Am J Neuroradiol 2012;33:1182–86 10.3174/ajnr.A2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schievink WI, Raissi SS, Maya MM, et al. Screening for intracranial aneurysms in patients with bicuspid aortic valve. Neurology 2010;74:1430–33 10.1212/WNL.0b013e3181dc1acf [DOI] [PubMed] [Google Scholar]
- 17. Erbel R, Aboyans V, Boileau C, et al. ; ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult—the Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873–926 10.1093/eurheartj/ehu281 [DOI] [PubMed] [Google Scholar]
- 18. International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: risk of rupture and risks of surgical intervention. N Engl J Med 1998;339:1725–33 10.1056/NEJM199812103392401 [DOI] [PubMed] [Google Scholar]
- 19. Greving JP, Wermer MJ, Brown RD Jr, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol 2014;13:59–66 10.1016/S1474-4422(13)70263-1 [DOI] [PubMed] [Google Scholar]
- 20. Schievink WI, Mokri B. Familial aorto-cervicocephalic arterial dissections and congenitally bicuspid aortic valve. Stroke 1995;26:1935–40 10.1161/01.STR.26.10.1935 [DOI] [PubMed] [Google Scholar]
- 21. Wang H, Li W, He H, et al. 320-detector row CT angiography for detection and evaluation of intracranial aneurysms: comparison with conventional digital subtraction angiography. Clin Radiol 2013;68:e15–20 10.1016/j.crad.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 22. Sailer AM, Wagemans BA, Nelemans PJ, et al. Diagnosing intracranial aneurysms with MR angiography: systematic review and meta-analysis. Stroke 2014;45:119–26 10.1161/STROKEAHA.113.003133 [DOI] [PubMed] [Google Scholar]