The authors evaluated 23 distal anterior circulation occlusions during a 2-year period. Ten M1-segment occlusions and 10 cases without a vessel occlusion were also included. There was significant improvement in the sensitivity of detection of distal anterior circulation vessel occlusions, overall confidence, and time taken to interpret with multiphase CTA compared with single-phase CTA. The delayed vessel sign is a reliable indicator of anterior circulation vessel occlusion, particularly in cases involving distal branches.
Abstract
BACKGROUND AND PURPOSE:
Multiphase CTA, a technique to dynamically assess the vasculature in acute ischemic stroke, was primarily developed to evaluate collateral filling. We have observed that it is also useful in identifying distal anterior circulation occlusions due to delayed anterior circulation opacification on multiphase CTA, an observation we term the “delayed vessel sign.” We aimed to determine the usefulness of this sign by comparing multiphase CTA with single-phase CTA.
MATERIALS AND METHODS:
All 23 distal anterior circulation occlusions during a 2-year period were included. Ten M1-segment occlusions and 10 cases without a vessel occlusion were also included. All patients had follow-up imaging confirming the diagnosis. Initially, the noncontrast CT and first phase of the multiphase CTA study for each patient were blindly evaluated (2 neuroradiologists, 2 radiology trainees) for an anterior circulation occlusion. Readers' confidence, speed, and sensitivity of detection were recorded. Readers were then educated on the “delayed vessel sign,” and each multiphase CTA study was re-examined for a vessel occlusion after at least 14 days.
RESULTS:
There was significant improvement in the sensitivity of detection of distal anterior circulation vessel occlusions (P < .001), overall confidence (P < .001), and time taken to interpret (P < .001) with multiphase CTA compared with single-phase CTA. Readers preferred MIP images compared with source images in >90% of cases.
CONCLUSIONS:
The delayed vessel sign is a reliable indicator of anterior circulation vessel occlusion, particularly in cases involving distal branches. Assessment of the later phases of multiphase CTA for the delayed vessel sign leads to a significant improvement in the speed and confidence of interpretation, compared with single-phase CTA.
The major recent development in acute stroke care, clot retrieval by thrombectomy, reduces disability and improves the quality of life in patients with proximal large-vessel occlusions.1 Prompt brain imaging and precise localization of an intracranial vessel occlusion are important to aid in the selection of appropriate patients for treatment with intravenous thrombolysis and/or thrombectomy.2–4
CT angiography is performed immediately after noncontrast CT to assess vessel occlusion or stenoses and to evaluate the pial collateral status. Single-phase CTA (SPCTA, a single arterial phase study of the head and neck) is the most common CTA technique used to assess the intra- and extracranial vasculature.5 Multiphase CTA (MPCTA) is a new supplementary technique, distinct from brain perfusion CT, that acquires intracranial images at 3 time points (phases) rather than just a single phase.5 To date, MPCTA has been primarily used to obtain extra information on the extent of pial collateral filling.2,6,7
We have observed a simple imaging sign on MPCTA that assists in confirming the diagnosis of acute ischemic stroke. It appears especially useful when an occluded vessel is small (eg, M2 or M3 segments of the middle cerebral artery), a finding that may be easily overlooked with SPCTA alone. The “delayed vessel sign” refers to the presence of an artery distal to the point of occlusion/stenosis that is absent or poorly opacified on the initial angiographic phase but becomes more opacified on the delayed phases, appearing denser than the equivalent vessel on the opposite side (Figs 1 and 2). This sign can rapidly indicate the presence of an ipsilateral vessel occlusion.
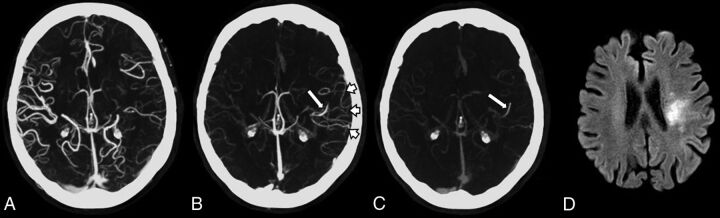
Fig 1.
Multiphase CTA and follow-up MR imaging of an 83-year-old woman presenting with acute right upper limb weakness and dysphasia. A, Axial MIP of the first phase demonstrates subtle paucity of vessels in the distribution of the left MCA compared with the right side. B, Axial MIP of the second phase demonstrates the delayed vessel sign (long arrow). There is delayed enhancement of the distal left MCA via pial collateral vessels (short arrows). This vessel is not seen on the first phase due to the presence of an M2 vessel occlusion. C, Axial MIP of the third phase also demonstrates the “delayed” left MCA vessel (long arrow). D, DWI b=1000 image 2 weeks postpresentation demonstrates a recent infarct (arrow) in the same left MCA territory.
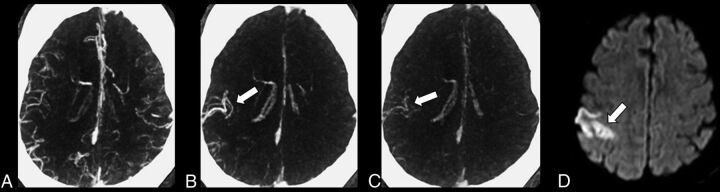
Fig 2.
Multiphase CTA in a 65-year-old man with acute left-sided weakness. A, Axial MIP of the first phase without obvious asymmetry. B and C, Axial MIPs of the second and third phases demonstrate the delayed vessel sign (arrow) with opacification of a distal right MCA branch, which was not opacified on the first angiographic phase due to a proximal M2 vessel occlusion. D, DWI b=1000 image 24 hours postpresentation demonstrates an acute infarct (arrow) in the same right MCA territory.
The aim of our study was to determine whether educating radiologists of all levels of experience (resident to expert) on the delayed vessel sign would improve sensitivity, confidence, and speed in the detection of distal anterior circulation vessel occlusions compared with SPCTA alone.
Materials and Methods
Patients and Distribution of Intracranial Vessel Occlusions
Approval for this study was obtained from the local ethics committee at Mater Misericordiae University Hospital. A senior radiology trainee (D.B.), not involved in the formal evaluation of the studies, selected all distal anterior (ie, M2/A2 segments or smaller) circulation occlusions in patients who presented to our institution after MPCTA became standard practice and who fulfilled the inclusion criteria (suspected acute ischemic stroke, having undergone NCCT and MPCTA, and follow-up cross-sectional imaging). This process identified 23 appropriate distal anterior circulation occlusions during the study period (January 2014 to September 2016). Ten consecutive M1 occlusions in the study period were also selected as a means of comparing the usefulness of the sign for the detection of larger vessel occlusions. In addition, the last 10 consecutive studies with normal findings with follow-up MR imaging available, obtained in the study period, were included to assess false-positives.
Of the 43 patients, 21 were women (mean age, 69 ± 16.5 years; range, 33–94 years). Before evaluation, vessel occlusions were deemed present or absent by a consensus of 2 neuroradiologists not involved in image evaluation who had access to follow-up imaging. The cases were randomly sorted to be evaluated by readers (neuroradiologists: P.J.M. with 10 years' experience and E.C.K. with 15 years' experience; radiology residents: G.S. with 2 years' experience, and E.S. with 3 years' experience).
Technical Parameters
Images were obtained with a 128-section multidetector CT scanner (Somatom Definition AS+; Siemens, Erlangen, Germany) at 120 kV, 90 mAs (effective) with a collimation of 128 × 0.6 mm. NCCT was performed from the skull base to the vertex. The first phase of the MPCTA was performed from the aortic arch to the skull vertex with the second and third phases performed from the skull base to the vertex. The first phase is timed to occur during the peak arterial phase with bolus monitoring of the descending thoracic aorta and is commenced after a 6-second delay. Eighty milliliters of intravenous contrast (iopamidol, Niopam; Bracco Imaging, Milan, Italy; 370 mg iodine per milliliter) is injected at a rate of 5 mL/s followed by 40 mL of saline at a similar rate. The second and third phases are acquired after 11 and 22 seconds, respectively.
Thin sections (1 mm) are reconstructed for each phase, and maximum intensity projections with 5-mm section thickness are reconstructed at the scanner workstation in 3 planes (axial, sagittal, and coronal) for each phase.
Image Interpretation
Stage 1.
The NCCT and SPCTA (ie, the first phase of an MPCTA study) were independently evaluated, and readers' confidence, speed, and sensitivity of detection were recorded. Readers were provided with a brief clinical history, NCCT, and source images of the SPCTA (ie, MIPs were initially not provided). On the NCCT, absence of hemorrhage, the presence or absence of a hyperdense vessel sign, and parenchymal infarction were recorded. Using SPCTA source images, readers were asked to record the presence and location of an intracranial vessel occlusion. A detailed analysis of all images generated was not expected. Intracranial vessel occlusions were classified as follows: right or left, anterior cerebral artery (A1, A2, or A3), and middle cerebral artery (M1, M2, M3). The time taken to complete the initial reading was recorded. Readers' degrees of confidence in their interpretation of each study were recorded with a 5-point Likert scale (1 = not at all confident, 2 = not very confident, 3 = neutral, 4 = confident, and 5 = very confident). Readers were then provided with the axial, coronal, and sagittal reconstructed MIP images and asked whether they preferred the source or reconstructed MIP images of the SPCTA.
Stage 2.
After an interval of at least 2 weeks, all readers were educated on the delayed vessel. Readers were advised to immediately assess the second and third phases of the MPCTA rather than the first phase to aid in the detection of delayed enhancement. A positive delayed vessel sign is only present if a suspect vessel on the delayed phases is poorly opacified on the initial phase. If the delayed vessel sign was identified, the readers were advised to follow the vessel proximally and correlate with the linked initial angiographic phase to aid in the detection of the point of vessel occlusion.
Following this education, all readers re-evaluated the 43 cases but with access to the source images from the entire MPCTA study rather than just the initial phase. MIP reconstructions were initially withheld. Readers were asked to record the same findings as described in stage 1, but to also comment on the presence or absence of the delayed vessel sign and on which phase it was most pronounced. Readers were also asked to record the presence of concomitant pial vessel hyperenhancement (which refers to the presence of relatively asymmetric enhancing pial vessels on delayed phases of a MPCTA ipsilateral to an intracranial vessel occlusion) and to record on which of the 3 phases this was most obvious. Readers were then provided with reconstructed MIP images and asked whether they preferred the source or reconstructed MIP images of the MPCTA.
Statistical Analysis
The significance level was set at P < .05. The McNemar test was used to compare SPCTA with MPCTA results. To evaluate interrater agreement between readers, we used unweighted κ statistics and 95% confidence intervals. Agreement was quantified as fair (κ = 0.21–0.40), moderate (κ = 0.41–0.60), substantial (κ = 0.61–0.80), or almost perfect (κ = 0.81–0.99).
Results
Distal Vessel Occlusions
There was a significant improvement in the sensitivity of the detection of distal (A2, A3, M2, M3) anterior circulation vessel occlusions on MPCTA compared with SPCTA (P < .001), from 75% to 100%. Among the 2 radiology trainees, 33 of 46 (total) distal occlusions were detected on SPCTA and all distal occlusions were detected with MPCTA. Among the 2 neuroradiologists, 36 of 46 (total) distal occlusions were detected on SPCTA and all distal occlusions were detected with MPCTA (Tables 1 and 2).
Table 1:
Results for radiology traineesa
| Distal Occlusions |
Proximal M1 Occlusions |
Normal Study Findings |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| SPCTA | MPCTA | P Value | SPCTA | MPCTA | P Value | SPCTA | MPCTA | P Value | |
| Sensitivity (%) | 71.7 (57.4–82.7) | 100 (92.2–100) | <.001 | 100 (83.8–100) | 100 (83.8–100) | NS | – | – | – |
| Confidence (1–5) | 3.8 (3.6–4.0) | 4.8 (4.6–4.9) | <.001 | 4.6 (4.3–4.8) | 5.0 | .08 | 3.6 (3.3–3.8) | 5.0 | <.001 |
| Time (sec) | 99 (90–107) | 44 (40–49) | <.001 | 72 (65–79) | 34 (30–37) | <.001 | 102 (93–112) | 56 (51–61) | <.001 |
Note:—NS indicates not significant.
Confidence intervals provided in parentheses.
Table 2:
Results for neuroradiologistsa
| Distal Occlusions |
Proximal M1 Occlusions |
Normal Study Findings |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| SPCTA | MPCTA | P Value | SPCTA | MPCTA | P Value | SPCTA | MPCTA | P Value | |
| Sensitivity (%) | 78.3 (64.4–87.7) | 100 (92.2–100) | .004 | 100 (83.8–100) | 100 (83.8–100) | NS | – | – | – |
| Confidence (1–5) | 3.8 (3.7–4.0) | 4.9 (4.8–5.0) | <.001 | 4.7 (4.5–4.9) | 5.0 | .08 | 3.7 (3.5–3.9) | 4.9 (4.7–5.0) | <.001 |
| Time (sec) | 84 (78–90) | 37 (34–41) | <.001 | 67 (60–74) | 29 (26–32) | <.001 | 81 (78–85) | 43 (39–47) | <.001 |
Confidence intervals provided in parentheses.
Among radiology trainees (Table 1), for distal anterior circulation occlusions, mean confidence improved from 3.8 for SPCTA to 4.8 for MPCTA (P < .001). The average time taken to interpret each case on SPCTA was 99 seconds and decreased to 44 seconds with MPCTA (P < .001). Among neuroradiologists (Table 2), for distal anterior circulation occlusions, the mean confidence was 3.8 for SPCTA, improving to 4.9 for MPCTA (P < .001). The average time taken to interpret each case on SPCTA was 84 seconds, decreasing to 37 seconds on MPCTA (P < .001).
M1 Occlusions
While all proximal (M1) occlusions were detected on both SPCTA and MPCTA by all readers, there was significant improvement in the speed of detection of M1 occlusions with MPCTA. There was also a trend toward improvement in the confidence of detection of M1 occlusions; however, this did not reach statistical significance (P = .08). Among radiology trainees, for proximal M1 occlusions, confidence improved from 4.6 with SPCTA to 5.0 with MPCTA (P = .08). The average time taken to interpret each case on SPCTA was 72 seconds, decreasing to 34 seconds on MPCTA (P < .001). Among neuroradiologists, for proximal M1 occlusions, the mean confidence was 4.7 for SPCTA, improving to 5.0 for MPCTA (P = .08). The average time taken to interpret each case on SPCTA was 67 seconds, decreasing to 29 seconds on MPCTA (P < .001).
Normal Study Findings
The average time taken to evaluate studies with normal findings without an intracranial vessel occlusion significantly decreased with MPCTA (49 seconds) compared with SPCTA (92 seconds) (P < .001), and readers' confidence significantly improved for studies with normal findings with MPCTA (4.9) compared with SPCTA (3.6) (P < .001).
General Findings
Both radiology trainees and neuroradiologists stated that in patients with an intracranial vessel occlusion, that they preferred and were more confident with their findings after MIP images were provided compared with source images (97.7% of MPCTA cases and 90.9% of SPCTA cases). Readers stated that they were more confident with MIP compared with source images in 100% of cases with normal findings without intracranial vessel occlusion.
Overall, the sensitivity of the delayed vessel sign for the presence of vessel occlusion was 96.2% (95% CI, 91.4%–98.3%) and specificity was 100% (95% CI, 91.2%–100%). Overall, the positive predictive value of the delayed vessel sign was 100% (95% CI, 97.1%–100%), and the negative predictive value was 88.9% (95% CI, 76.5%–95.1%).
With the Pearson correlation coefficient, there was a high correlation between the presence of the delayed vessel sign and pial vessel enhancement (r = 0.6465, P < .001).
Interrater agreement was almost perfect (κ = 0.83) between readers for distal anterior circulation intracranial occlusion detection with SPCTA and improved with MPCTA (κ = 0.95). Agreement was almost perfect for the presence of the delayed vessel sign in cases of distal anterior circulation intracranial vessel occlusion (κ = 0.91) and was substantial (κ = 0.75) for the presence of asymmetric pial vessel enhancement.
Discussion
In our institution, MPCTA is the standard angiographic technique in the investigation of suspected acute ischemic stroke. Acute cerebral ischemia is a dynamic process requiring a dynamic diagnostic approach.8 In this context, the evaluation of patients suspected of having acute ischemic stroke with single-phase CTA is inappropriate. While perfusion CT is an established stroke imaging technique (from which MPCTA-type images can also be generated),9,10 it is not universally available at all times of day, especially in non-stroke centers. MPCTA is an alternative technique for achieving dynamic vascular imaging and may be more widely applicable. Additional software-based postprocessing is not required, and in our experience, accurate interpretation by the nonexpert (especially if comfortable with SPCTA) is readily possible after brief training.
MPCTA was primarily developed to improve the assessment of pial collaterals; however, we have found, consistent with other reports, that it also aids in the diagnosis of distal anterior circulation occlusions.11,12 Our study provides an easily understood means by which MPCTA can improve the detection of more distal anterior circulation vessel occlusions. The previous studies,11,12 also demonstrating the superiority of MPCTA over SPCTA, did not provide a clear mechanism by which this benefit is derived. We have found that the delayed vessel sign on CTA can be communicated and understood quickly and easily by all medical professionals involved in acute stroke care. We have shown that the education of radiologists of different levels of expertise (but particularly trainee radiologists) on the delayed vessel sign leads to an increase in the sensitivity and speed of detection of distal anterior circulation vessel occlusions. We have also found that confidence in interpretation increases significantly.
It is likely that the delayed vessel (most commonly the more distal branches of the MCA) enhances slowly due to retrograde opacification via pial collaterals. A process of retrograde opacification is supported by the strong correlation between the presence of the delayed vessel sign and pial enhancement, an association most evident on the same delayed phase (either second or third). Our sign is distinct from the “clot outline sign,”13 which is seen on procedural arteriograms obtained before intra-arterial thrombolysis. This sign implies that the vessel in question is almost completely occluded and has minute blood flow past the clot (ie, antegrade flow). The delayed vessel sign, which we describe, is conceptually different because the vessel in question typically has total occlusion, which fills retrograde via collateral vessels. Unlike the clot outline sign, our study pertains to CTA, not procedural arteriography, and is used to assist in the diagnosis of distal anterior circulation occlusions rather than to predict treatment responders.
In this new era of endovascular treatment of proximal large-vessel occlusions, the detection and treatment of less severe stroke due to more distal vessel occlusion remain essential.14 Recent data suggest that patients with proximal M2 occlusions may benefit from endovascular treatment; therefore, early accurate detection of these occlusions will likely become more important.15,16 The role of intravenous thrombolysis with tenectaplase in mild stroke with distal vessel occlusion will be tested in the A Randomized Controlled Trial of TNK-tPA Versus Standard of Care for Minor Ischemic Stroke With Proved Occlusion trial (NCT02398656).
Patients are often initially triaged as having possible acute ischemic stroke by paramedics or other non-stroke specialists17 and are referred for NCCT after early rapid clinical assessment.17–20 The differential diagnosis of acute stroke from stroke mimics can be a challenge, especially in milder cases.21 Many patients present to the hospital off-hours22,23 when there may be less immediate access to senior radiology personnel and hence greater reliance on trainee radiologists for the initial interpretation of imaging.24,25 We think that this sign will aid in the rapid differentiation of stroke from stroke mimics.20,26 MPCTA comprises a large number of images that can seem daunting to interpret, especially in the urgent clinical scenario of suspected ischemic stroke. The key to a quick, confident, and accurate MPCTA interpretation, especially in patients with an uncertain clinical diagnosis, is to immediately assess the later phases rather than the initial phase for the presence of the delayed vessel sign; this assessment can then accelerate the identification of a vessel occlusion.
The purpose of MIPs is to give an overview of the target vessel by generating a DSA-like image.27 MIPs are known to increase the conspicuity of intracranial vessel occlusion and stenosis.28 In 97.7% of MPCTA cases and in 90.9% of SPCTA cases in this study, both radiology trainees and neuroradiologists stated that they preferred and were more confident with their interpretation when MIP images were provided compared with source images alone. The delayed vessel sign was often more apparent on MIP compared with source images (Fig 3). Readers also reported that symmetric vessel enhancement in cases without an intracranial vessel occlusion was easier to appreciate on MIP compared with source images and reassured readers that no intracranial vessel occlusion was present.
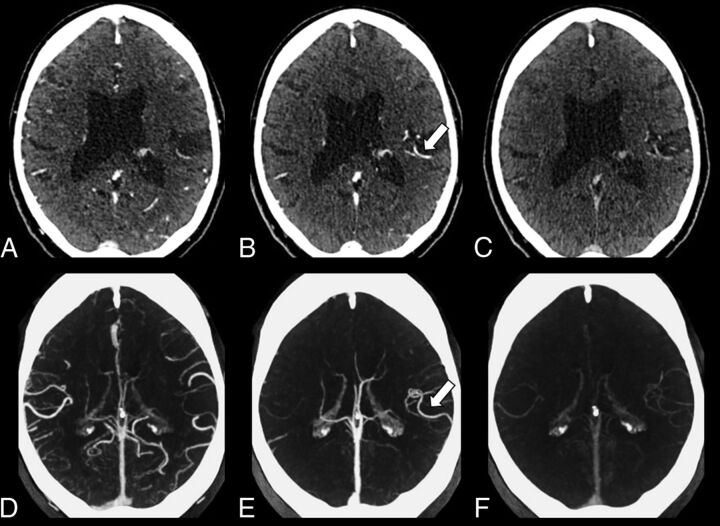
Fig 3.
Multiphase CTA in a 78-year-old man with expressive dysphasia and right-sided weakness. A, Axial source image of the first phase of MPCTA without obvious asymmetry of the intracranial vasculature. B, The delayed vessel sign is identified on the second phase (arrow), indicative of a proximal occlusion. C, Axial source image of the third phase of the MPCTA. D–F, Axial MIPs of the first, second, and third phases of the same patient. The delayed vessel sign is more apparent on MIP image (arrow) of the second phase (E) compared with source images.
This study was affected by several limitations. First, while all appropriate cases of distal anterior circulation occlusion were retrospectively selected, an element of bias in case selection cannot be excluded. For example, patients misdiagnosed as having a stroke mimic at presentation may not have received follow-up brain imaging and thus would not be included. Another limitation is that we did not use DSA as a criterion standard in the identification of an occlusion but rather used the consensus opinion of 2 neuroradiologists not involved in the study. Although the delayed vessel sign demonstrated a high negative predictive value in cases of distal occlusion, we hypothesize that the sign would be absent, or harder to appreciate, in patients with poor collateral supply in the ischemic territory. For example, in cases of basal ganglia and internal capsule infarction, territories supplied by lenticulostriate branches,29 the delayed vessel sign will likely be absent because these vessels are perforator arteries and lack collateral blood supply.30 In addition, the delayed vessel sign will be absent in cases of small cortical infarcts, which are often caused by occlusion of small pial vessels by microemboli.31 The sign may falsely indicate an acute intracranial occlusion in several scenarios not encountered in this study population, for example in the setting of chronic intracranial occlusions/stenoses and acute or chronic extracranial occlusions. Readers were not timed on their interpretation of the entire angiographic dataset but only on the time to establish the presence or absence of a vessel occlusion. The times recorded in this study are not a true reflection of the interpretation time for the entire study. Our inclusion criteria limited the study to anterior circulation stroke because this is the most common type of intracranial vessel occlusion; however, the sign may also be applicable to the posterior circulation.
Conclusions
The delayed vessel sign is a reliable indicator of anterior circulation vessel occlusion and is particularly useful in cases involving distal branches that are not easily identified on a single-phase angiographic examination. Immediate assessment of the MIP reconstructions of the later phases of an MPCTA examination for the delayed vessel sign leads to a significant improvement in the speed and confidence of detecting vessel occlusions.
ABBREVIATIONS:
- MPCTA
multiphase CTA
- SPCTA
single-phase CTA
Footnotes
Disclosures: Sean Murphy—UNRELATED: Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: travel grants to attend the International Stroke Conference, Houston, Texas, February 2017; sponsorship to attend the European Stroke Conference, Prague, May 2017; sponsorship to attend the UK Stroke Forum, November 2016.
References
- 1. Ganesalingam J, Pizzo E, Morris S, et al. Cost-utility analysis of mechanical thrombectomy using stent retrievers in acute ischemic stroke. Stroke 2015;46:2591–98 10.1161/STROKEAHA.115.009396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Menon BK, Campbell BC, Levi C, et al. Role of imaging in current acute ischemic stroke workflow for endovascular therapy. Stroke 2015;46:1453–61 10.1161/STROKEAHA.115.009160 [DOI] [PubMed] [Google Scholar]
- 3. Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 4. Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–30 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 5. Menon BK, d'Esterre CD, Qazi EM, et al. Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology 2015;275:510–20 10.1148/radiol.15142256 [DOI] [PubMed] [Google Scholar]
- 6. Kim EY, Shin DH, Noh Y, et al. Comparison of imaging selection criteria for intra-arterial thrombectomy in acute ischemic stroke with advanced CT. Eur Radiol 2016;26:2974–81 10.1007/s00330-015-4141-1 [DOI] [PubMed] [Google Scholar]
- 7. Bang OY, Goyal M, Liebeskind DS. Collateral circulation in ischemic stroke: assessment tools and therapeutic strategies. Stroke 2015;46:3302–09 10.1161/STROKEAHA.115.010508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liebeskind DS, Alexandrov AV. Advanced multimodal CT/MRI approaches to hyperacute stroke diagnosis, treatment, and monitoring. Ann N Y Acad Sci 2012;1268:1–7 10.1111/j.1749-6632.2012.06719.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Orrison WW Jr, Snyder KV, Hopkins LN, et al. Whole-brain dynamic CT angiography and perfusion imaging. Clin Radiol 2011;66:566–74 10.1016/j.crad.2010.12.014 [DOI] [PubMed] [Google Scholar]
- 10. Kortman HG, Smit EJ, Oei MT, et al. 4D-CTA in neurovascular disease: a review. AJNR Am J Neuroradiol 2015;36:1026–33 10.3174/ajnr.A4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Volny O, Cimflova P, Kadlecova P, et al. Single-phase versus multiphase CT angiography in middle cerebral artery clot detection: benefits for less experienced radiologists and neurologists. J Stroke Cerebrovasc Dis 2017;26:19–24 10.1016/j.jstrokecerebrovasdis.2016.08.023 [DOI] [PubMed] [Google Scholar]
- 12. Yu AY, Zerna C, Assis Z, et al. Multiphase CT angiography increases detection of anterior circulation intracranial occlusion. Neurology 2016;87:609–16 10.1212/WNL.0000000000002951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Christoforidis GA, Mohammad Y, Avutu B, et al. Arteriographic demonstration of slow antegrade opacification distal to a cerebrovascular thromboembolic occlusion site as a favorable indicator for intra-arterial thrombolysis. AJNR Am J Neuroradiol 2006;27:1528–31 [PMC free article] [PubMed] [Google Scholar]
- 14. Coutts SB, Modi J, Patel SK, et al. What causes disability after transient ischemic attack and minor stroke? Results from the CT and MRI in the Triage of TIA and minor Cerebrovascular Events to Identify High Risk Patients (CATCH) Study. Stroke 2012;43:3018–22 10.1161/STROKEAHA.112.665141 [DOI] [PubMed] [Google Scholar]
- 15. Sheth SA, Yoo B, Saver JL, et al. ; UCLA Comprehensive Stroke Center. M2 occlusions as targets for endovascular therapy: comprehensive analysis of diffusion/perfusion MRI, angiography, and clinical outcomes. J Neurointerv Surg 2015;7:478–83 10.1136/neurintsurg-2014-011232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Powers WJ, Derdeyn CP, Biller J, et al. ; American Heart Association Stroke Council. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015;46:3020–35 10.1161/STR.0000000000000074 [DOI] [PubMed] [Google Scholar]
- 17. Harbison J, Massey A, Barnett L, et al. Rapid ambulance protocol for acute stroke. Lancet 1999;353:1935 10.1016/S0140-6736(99)00966-6 [DOI] [PubMed] [Google Scholar]
- 18. Audebert HJ, Saver JL, Starkman S, et al. Prehospital stroke care: new prospects for treatment and clinical research. Neurology 2013;81:501–08 10.1212/WNL.0b013e31829e0fdd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goyal M, Menon BK, Hill MD, et al. Consistently achieving computed tomography to endovascular recanalization <90 minutes: solutions and innovations. Stroke 2014;45:e252–56 10.1161/STROKEAHA.114.007366 [DOI] [PubMed] [Google Scholar]
- 20. Zerna C, Assis Z, d'Esterre CD, et al. Imaging, intervention, and workflow in acute ischemic stroke: the Calgary approach. AJNR Am J Neuroradiol 2016;37:978–84 10.3174/ajnr.A4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamal N, Hill MD, Blacquiere DP, et al. Rapid assessment and treatment of transient ischemic attacks and minor stroke in Canadian emergency departments: time for a paradigm shift. Stroke 2015;46:2987–90 10.1161/STROKEAHA.115.010454 [DOI] [PubMed] [Google Scholar]
- 22. Yang CY, Chen YF, Lee CW, et al. Multiphase CT angiography versus single-phase CT angiography: comparison of image quality and radiation dose. AJNR Am J Neuroradiol 2008;29:1288–95 10.3174/ajnr.A1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy: ASPECTS Study Group—Alberta Stroke Programme Early CT Score. Lancet 2000;355:1670–74 10.1016/S0140-6736(00)02237-6 [DOI] [PubMed] [Google Scholar]
- 24. Streifler JY, Benderly M, Molshatzki N, et al. Off-hours admission for acute stroke is not associated with worse outcome–a nationwide Israeli stroke project. Eur J Neurol 2012;19:643–47 10.1111/j.1468-1331.2011.03603.x [DOI] [PubMed] [Google Scholar]
- 25. Sorita A, Ahmed A, Starr SR, et al. Off-hour presentation and outcomes in patients with acute ischemic stroke: a systematic review and meta-analysis. Eur J Intern Med 2014;25:394–400 10.1016/j.ejim.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 26. Coutts SB, Dubuc V, Mandzia J, et al. ; TEMPO-1 Investigators. Tenecteplase-tissue-type plasminogen activator evaluation for minor ischemic stroke with proven occlusion. Stroke 2015;46:769–74 10.1161/STROKEAHA.114.008504 [DOI] [PubMed] [Google Scholar]
- 27. Ota H, Takase K, Rikimaru H, et al. Quantitative vascular measurements in arterial occlusive disease. Radiographics 2005;25:1141–58 10.1148/rg.255055014 [DOI] [PubMed] [Google Scholar]
- 28. Lev MH, Farkas J, Rodriguez VR, et al. CT angiography in the rapid triage of patients with hyperacute stroke to intraarterial thrombolysis: accuracy in the detection of large vessel thrombus. J Comput Assist Tomogr 2001;25:520–28 10.1097/00004728-200107000-00003 [DOI] [PubMed] [Google Scholar]
- 29. Djulejić V, Djulejić S, Georgievski B, et al. Clinical significance of blood supply to the internal capsule and basal ganglia. J Clin Neurosci 2016;25:19–26 10.1016/j.jocn.2015.04.034 [DOI] [PubMed] [Google Scholar]
- 30. Kleine JF, Beller E, Zimmer C, et al. Lenticulostriate infarctions after successful mechanical thrombectomy in middle cerebral artery occlusion. J Neurointerv Surg 2017;9:234–39 10.1136/neurintsurg-2015-012243 [DOI] [PubMed] [Google Scholar]
- 31. Touaoussa A, El Youssi H, El Hassani I, et al. Disseminated intravascular coagulation: clinical and biological diagnosis [in French]. Ann Biol Clin (Paris) 2015;73:657–63 10.1684/abc.2015.1100 [DOI] [PubMed] [Google Scholar]