Abstract
BACKGROUND AND PURPOSE:
The introduction of liquid embolic agents has revolutionized endovascular approach to cranial vascular malformations. The aim of the study was to retrospectively assess the efficacy and safety of Precipitating Hydrophobic Injectable Liquid (PHIL), a new nonadhesive liquid embolic agent, in the treatment of patients with cranial dural arteriovenous fistulas. The primary end point was the rate of complete occlusion of dural arteriovenous fistulas. Secondary end points included the incidence of adverse events and clinical status at 3-month follow-up.
MATERIALS AND METHODS:
This was a retrospective multicenter study. Twenty-six consecutive patients with dural arteriovenous fistulas (de novo or previously treated) treated by injection of PHIL only or with PHIL in combination with other embolization products (such as Onyx or detachable coils) were included in the study. Recruitment started in August 2014 and ended in September 2015.
RESULTS:
Twenty-two (85%) patients were treated with PHIL only, with 3 patients treated with both PHIL and Onyx, and 1, with both PHIL and coils. Immediate complete angiographic occlusion was achieved in 20 (77%) patients. Of the 6 patients with residual fistulas, 3 were retreated with PHIL and 1 achieved angiographic cure. An adverse event was seen in 1 patient who developed worsening of preexisting ataxia due to acute thrombosis of the draining vein.
CONCLUSIONS:
PHIL appears to be safe and effective for endovascular treatment of cranial dural arteriovenous fistulas. Short-term angiographic and clinical results are comparable with those of Onyx, with the added advantage of easier preparation and improved homogeneous cast visualization. The use of iodine as a radio-opacifier also produces considerably less artifacts on CT compared with tantalum-based embolic materials.
Dural arteriovenous fistulas (DAVFs) are a rare type of acquired intracranial vascular malformation consisting of a pathologic shunt located within the dura mater of the brain.1–3 These lesions have been categorized by Awad et al,4 Borden et al,5 and Cognard et al6 according to their locations and patterns of venous drainage. Acute presentation with intracranial hemorrhage occurs in up to 65% of patients,6 and patients with a previous intracranial hemorrhage may have up to a 35% risk of another neurologic event within 2 weeks.7
Endovascular embolization has become the primary treatment approach for DAVFs.1,8,9 The goal of endovascular therapy is to achieve complete obliteration of the fistulous point between the feeding arteries and the draining veins. This can be safely accomplished by occluding the draining veins, which often results in complete closure of the lesion, unlike in cerebral arteriovenous malformations.
The introduction of liquid embolic agents has a significant impact on the endovascular approach to DAVFs. Precipitating Hydrophobic Injectable Liquid (PHIL; MicroVention, Tustin, California) is a new nonadhesive liquid embolic agent comprising a copolymer dissolved in dimethyl-sulfoxide (DMSO). It is delivered by slow and controlled injection through a DMSO-compatible microcatheter under fluoroscopic control. An iodine component is chemically bonded to the copolymer to provide homogeneous radio-opacity during fluoroscopic visualization. When it comes in contact with human blood, the DMSO solvent dissipates, causing the copolymer to precipitate in situ into a coherent embolus. The PHIL liquid embolic system is available in 3 formulations: 25%, 30%, and 35%. PHIL 25% will travel more distally and penetrate deeper into the fistula due to its lower viscosity compared with PHIL 30% or 35%. The latter 2 are more appropriate for use in high-flow arteriovenous shunts with ≥1 direct fistula. Higher strength formulations are also preferred when increased fluoroscopic visibility is desirable.
The aim of our study was to assess the efficacy and safety of PHIL in the treatment of patients with cranial DAVFs.
Materials and Methods
Study Design and Setting
This was a retrospective multicenter study. Five European institutions with experience with the PHIL embolic agent for DAVF treatment participated in the study: Queen Elizabeth Hospital Birmingham (United Kingdom), Copenhagen Righospitalet (Denmark), Alfried Krupp Krankenhaus in Essen (Germany), Hospital Rosario in Madrid (Spain), and Ospedale Niguarda Ca' Granda in Milan (Italy).
Participants
Twenty-six consecutive patients with DAVFs (de novo or previously treated) treated by embolization with PHIL only or PHIL used in combination with other embolization products such as Onyx (Covidien, Irvine, California) or detachable coils were included in the study. Recruitment started in August 2014 and ended in September 2015.
Study End Points
The primary end point was the rate of complete angiographic occlusion of the DAVF. Secondary end points included the incidence of adverse events and clinical status at 3-month follow-up.
Data Collection
Patient information including sociodemographic data, medical history, description of the DAVF, procedure details, procedure-related adverse events, and follow-up angiographic and clinical results were collected by the participating investigators, by using an electronic case report form developed specifically for the evaluation. The principal investigator reviewed all reported data for inconsistencies/missing information and sought clarification with individual investigators when needed.
Data Analysis
Only descriptive analyses were performed.
Results
Patient Details
From August 2014 to September 2015, 26 consecutive patients (13 men and 13 women) with cranial DAVFs were treated with the PHIL embolic agent. Among the 26 patients, 30 total procedures were performed, with an average of 1.15 procedures per patient. The mean age of these patients was 60 ± 13 years (median, 57 years; range, 43–90 years).
Seven patients presented acutely, with intracranial hemorrhage (2 patients), hydrocephalus, seizure, venous sinus thrombosis, lower limb monoparesis, and visual loss. The remaining 19 patients (73%) were asymptomatic. Most (17/26, 65%) of the DAVFs were de novo lesions; while 8/26 (30%) DAVFs had been previously embolized with Onyx. Information regarding previous treatment was not available for 1 patient.
The locations of DAVFs were the transverse sinus (8/26), vein of Galen (7/26), superior sagittal sinus (4/26), and others (7/26). Most patients (20/26, 77%) presented with aggressive DAVFs (type IIb or higher), with >50% of cases being types III and IV. Five of 26 (19%) patients had type I or IIa DAVFs. The DAVF grade was not reported in 1 patient (Tables 1 and 2).
Table 1:
Baseline characteristics of patients
| Patients (No.) | |
|---|---|
| Clinical status | |
| mRS | |
| Scale 0 | 9 (34%) |
| Scale 1 | 14 (54%) |
| Scale 2 | 1 (4%) |
| Scale 4 | 1 (4%) |
| Unknown | 1 (4%) |
| DAVF type | |
| Cognard classification | |
| Type I | 2 (7%) |
| Type IIa | 3 (12%) |
| Type IIa + IIb | 2 (7%) |
| Type IIb | 3 (12%) |
| Type III | 9 (33%) |
| Type IV | 5 (19%) |
| Type V | 1 (4%) |
| Unknown | 1 (4%) |
Table 2:
Immediate posttreatment angiographic results by DAVF type
| DAVF Type (No.) |
||||
|---|---|---|---|---|
| ≤Type IIa | >Type IIa | Unknown Type | All Types | |
| Complete occlusion | 5 (100%) | 14 (70%) | 1 (100%) | 20 (77%) |
| Partial occlusion | 0 | 6 (30%) | 0 | 6 (23%) |
Procedure Description
Twenty-two (85%) patients were treated with PHIL only, 3 patients were treated with PHIL and Onyx, and 1, with PHIL and detachable coils. Commonly used microcatheters for injection were Headway Duo (10/26; MicroVention), Apollo (7/26; Covidien), and Marathon (4/26; Covidien). Treatment was performed through the injection of the middle meningeal (19/26), occipital (1/26), and vertebral (1/26) arteries and others (5/26). Remodeling balloon catheters were used for venous sinus protection in 7 patients. PHIL 25% was used in most patients (29/30, 90%). The mean duration of the injection was 32 minutes, and the mean volume injected was 1.25 mL. The extent of reflux along the catheter tip was <1 cm in 10/30 procedures, between 1 and 2 cm in 7/30 procedures, and >2 cm in 11/30 procedures. In 2 procedures, the severity of reflux was not reported.
Postprocedure Results
Posttreatment control runs demonstrated complete occlusion of the DAVF in 20/26 patients (77%), including all 5 patients with DAVF types ≤IIa and 14/20 (70%) patients with DAVF types >IIa (Table 2). The results by location showed a higher rate of complete occlusion in those with vein of Galen (86%) and superior sagittal sinus (100%) lesions (Table 3). Three of 6 patients with partial occlusion underwent additional embolization with PHIL: One of them achieved an angiographic cure, but the other 2 still have a residual shunt.
Table 3:
Immediate posttreatment angiographic results by location of the DAVF
| DAVF Location (No.) |
||||
|---|---|---|---|---|
| Transverse Sinus | Vein of Galen | Superior Sagittal Sinus | Others | |
| Complete occlusion | 5 (63%) | 6 (86%) | 4 (100%) | 5 (71%) |
| Partial occlusion | 3 (37%) | 1 (14%) | 0 | 2 (29%) |
We had 1 adverse event in our series. This involved a patient with a complex type III vein of Galen DAVF who developed worsening of ataxia after initial embolization due to acute thrombosis of the draining vein.
Follow-Up Results
A follow-up assessment of angiographic occlusion and clinical outcome (mRS) was conducted for 11/26 (42%) patients at 3 months. The mean duration between the final embolization procedure and the follow-up visit was 89 days. The mRS score was evaluated in 10 patients, and angiographic assessment was performed in all 11 patients (9 DSAs and 2 MRAs). Seven of 10 patients had not experienced a change in the mRS score since the postprocedure visit, while 3/10 patients reported an improvement in the mRS score from grade 1 to 0.
Angiographic controls showed 9/11 patients with complete DAVF occlusion at 3 months. Two patients with residual shunts received additional treatment with the PHIL, and 1 of them was successfully occluded. Of the 9 patients with complete angiographic occlusion at 3 months, 2 underwent follow-up angiography at 1 year, which confirmed persistent DAVF occlusion.
Discussion
The objective of endovascular treatment of DAVFs is to completely obliterate the fistulous point between the feeding arteries and draining veins. In our study of 26 consecutive patients treated with PHIL, 20/26 (77%) achieved complete angiographic occlusion at the end of the initial embolization. We identified 1 procedure-related adverse event in a patient who developed worsening of preexisting ataxia due to acute thrombosis of the draining vein.
Our study is, however, limited by the relatively low number of short-term angiographic and clinical follow-ups. Persistent occlusion was observed in 9/11 (82%) patients who had a 3-month follow-up visit, 3 patients showed improved clinical status, and the remaining patients reported stable neurologic functions at 3-month follow-up.
Similar results in terms of complete occlusion and the rate of adverse events were observed in studies conducted in patients treated with Onyx. The largest retrospective study reviewed 53 DAVFs embolized with n-BCA or Onyx from November 2003 to November 2008 in the Massachusetts General Hospital (Boston).10 In this study, 83% (29/35) of DAVFs treated with Onyx showed complete occlusion at the 3-month postprocedure visit, while only 33% (7/21) of patients treated with n-BCA had complete occlusion. Three patients treated with Onyx experienced major neurologic adverse events, including 2 facial nerve palsies and an asymptomatic clot formation in the external carotid artery.
Cognard et al11 included 30 patients with cranial DAVFs treated with Onyx in a prospective study between July 2003 and November 2006. Twenty-four of 30 (80%) patients who underwent treatment were found to have complete closure of the fistula on the immediate posttreatment angiographic control. They reported 2 adverse events: One patient woke up with third and fourth cranial nerve palsies as well as fifth cranial nerve territory pain; the other patient developed acute cerebellar syndrome.
Nogueira et al12 performed a retrospective analysis of 12 consecutive patients with intracranial DAVFs who were treated with Onyx as the only embolic agent between March 2006 and February 2007. Seventeen procedures were performed in 12 patients. Complete angiographic cure on immediate posttreatment angiography was achieved in 10 patients (80%). There was 1 technical complication that resulted in asymptomatic extracranial vertebral artery dissection.
Lv et al13 reported 31 cases of patients with DAVFs treated by embolization with Onyx between February 2005 and February 2007 at Beijing Tiantan Hospital. There was angiographic evidence of complete shunt elimination and symptomatic resolution in 19 patients (61.3%). Adverse events occurred in 5/31 patients (16%), including hemifacial palsy, jaw pain, and posterior fossa infarction.
The new-generation nonadhesive liquid embolic agents such as Onyx and PHIL have been described as having advantages over n-BCA. These agents are cohesive and less thrombogenic, allowing deeper and more controlled penetration of the fistulas.
The use of PHIL requires no prior preparation. PHIL 25%, used in most of the cases, is less radiopaque compared with Onyx 18, allowing better visualization through the copolymer cast (Figs 1 and 2) but has the drawback of reduced visibility in case of small-vessel penetration. Operators with experience in both liquid embolic agents often notice the difference in fluoroscopic visibility. In our experience, the risk of inadvertent embolization of potentially dangerous collaterals is not increased compared with other liquid embolic agents.
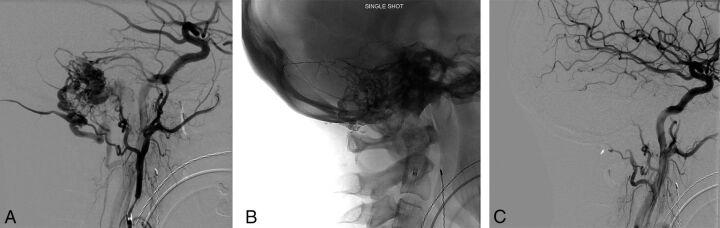
Fig 1.
A, Right sigmoid sinus DAVF receives arterial supply from branches of the right occipital, posterior auricular, and middle meningeal arteries as demonstrated on the pre-embolization DSA. B, Posttreatment unsubtracted single-shot image demonstrates the PHIL cast. C, Control run confirms complete angiographic occlusion of the DAVF.
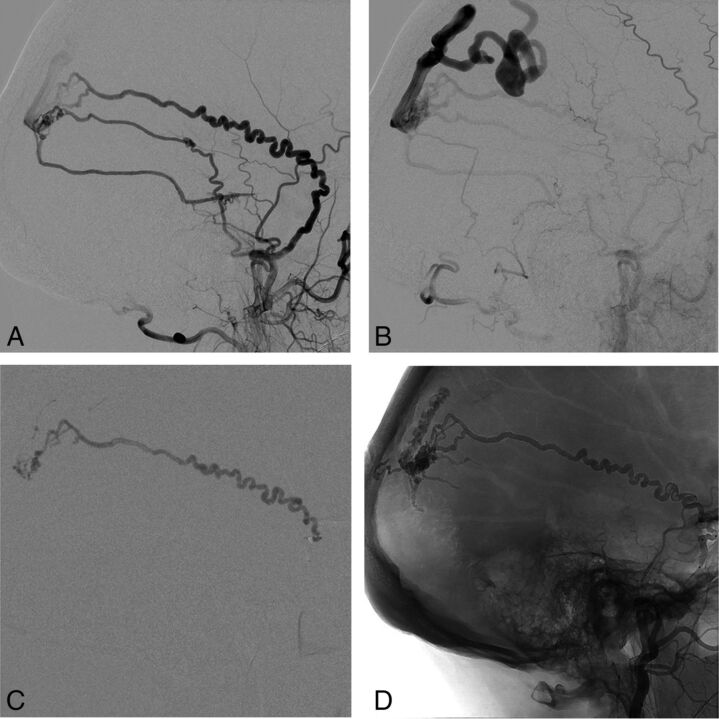
Fig 2.
A and B, Initial DSA showing a high-grade right parietal DAVF supplied by a markedly tortuous petrosquamal branch of the right middle meningeal artery. C, PHIL injection through the Scepter XC balloon (MicroVention) in the proximal middle meningeal artery demonstrates good penetration of the DAVF. D, Unsubtracted image showing the PHIL cast in the draining vein, middle meningeal artery, and other arterial feeders filled in a retrograde manner.
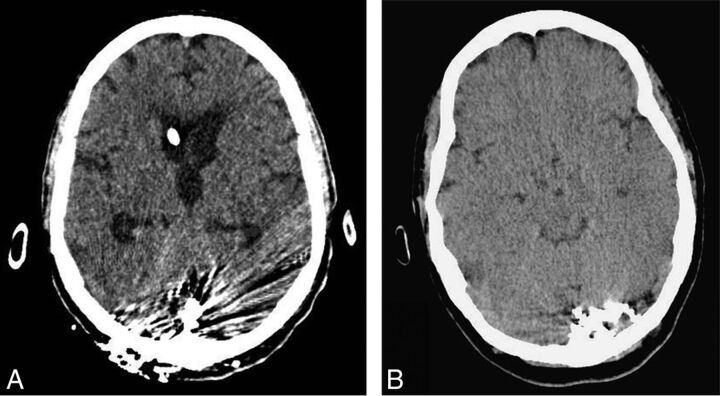
Due to its iodine-based composition, which is chemically bonded to the copolymer, PHIL is also believed to produce a more homogeneous fluoroscopic appearance, eliminating the risk of tantalum precipitation seen during prolonged Onyx injections. In addition, the use of iodine as a radio-opacifier produces significantly less beam-hardening artifacts on CT compared with tantalum-based embolic agents. As a consequence, more information can be obtained from the postoperative CT scan (Fig 3).
Fig 3.
Plain CT images comparing CT artifacts associated with a tantalum-based embolic agent (A) and PHIL (B) casts.
There is no significant technical difference between PHIL and Onyx in terms of reflux control and prevention of catheter retention. In our experience, PHIL appears less adhesive and therefore offers slightly better forward penetration, which probably explains the shorter mean injection time in our study compared with other published results conducted with Onyx injections.11
In high-flow fistulas, PHIL casts have the tendency to fragment and move to the venous side, as opposed to streaming as one would expect with Onyx. Both embolic agents otherwise behave similarly in the treatment of all DAVF types.
Conclusions
PHIL appears to be a safe and effective option for endovascular treatment of cranial DAVFs. Postembolization short-term angiographic and clinical results are comparable with those of Onyx, with the added advantage of easier preparation and improved homogeneous cast visualization. In our experience, the use of iodine as a radio-opacifier also produces significantly less artifacts on CT compared with tantalum-based embolic materials.
ABBREVIATIONS:
- DAVF
dural arteriovenous fistula
- DMSO
dimethyl-sulfoxide
- PHIL
Precipitating Hydrophobic Injectable Liquid
Footnotes
Disclosures: Saleh Lamin—RELATED: Provision of Writing Assistance, Medicines, Equipment, or Administrative Support: assistance from MicroVention (assisted in coordinating between the various centers and finalizing the manuscript); UNRELATED: Payment for Lectures including Service on Speakers Bureaus: Microvention; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: MicroVention (support to attend World Federation of Interventional and Therapeutic Neuroradiology, Australia); Other: proctoring fees, Microvention. Swarupsinh Chavda—RELATED: Provision of Writing Assistance, Medicines, Equipment, or Administrative Support: Microvention facilitated communication among institutions involved in the study; UNRELATED: Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Microvention provided sponsorship to a meeting of the American Society of Neuroradiology, Montreal. Allan Thomas—RELATED: Support for Travel to Meetings for the Study or Other Purposes: MicroVention (assistance to attend the annual meeting of the Society of NeuroInterventinal Surgery, 2015); UNRELATED: Consultancy: Sequent Medical (physician proctor for use of the Woven EndoBridge); Payment for Lectures including Service on Speakers Bureaus: MicroVention (payment for speaking at the Society of NeuroInterventinal Surgery industry evening on the use of the Woven EndoBridge). Mats E. Cronqvist—RELATED: Other: MicroVention, Comment: I was 1 author/physician writing the “Instruction for Use” of the PHIL liquid embolic agent. However, there has been no payment or any financial or other compensation for this work per my request. I am employed as a MD, PhD, at the Section of Neuroradiology, Department of Radiology, Rigshopsitalet University Hospital Copenhagen, Denmark. Markus Holtmannspolter—UNRELATED: Consultancy: MicroVention, Covidien, Sequent Medical (irregular activities as proctor and consultant); Payment for Lectures including Service on Speakers Bureaus: MicroVention, Covidien, Sequent Medical (irregular activities as proctor and consultant); Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: MicroVention, Covidien, Sequent Medical (irregular activities as a proctor and consultant). Alfredo Casasco—RELATED: Support for Travel to Meetings for the Study or Other Purposes: Microvention. Rene Chapot—UNRELATED: Consultancy: MicroVention*; Payment for Lectures including Service on Speakers Bureaus: Microvention.* *Money paid to the institution.
References
- 1. Gandhi D, Chen J, Pearl M, et al. Intracranial dural arteriovenous fistulas: classification, imaging findings, and treatment. AJNR Am J Neuroradiol 2012;33:1007–13 10.3174/ajnr.A2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim MS, Han DH, Kwon OK, et al. Clinical characteristics of dural arteriovenous fistula. J Clin Neurosci 2002;9:147–55 10.1054/jocn.2001.1029 [DOI] [PubMed] [Google Scholar]
- 3. Sarma D, ter Brugge K. Management of intracranial dural arteriovenous shunts in adults. Eur J Radiol 2003;46:206–20 [DOI] [PubMed] [Google Scholar]
- 4. Awad IA, Little JR, Akarawi WP, et al. Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg 1990;72:839–50 10.3171/jns.1990.72.6.0839 [DOI] [PubMed] [Google Scholar]
- 5. Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 1995;82:166–79 [DOI] [PubMed] [Google Scholar]
- 6. Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 1995;194:671–80 10.1148/radiology.194.3.7862961 [DOI] [PubMed] [Google Scholar]
- 7. Duffau H, Lopes M, Janosevic V, et al. Early rebleeding from intracranial dural arteriovenous fistulas: report of 20 cases and review of the literature. J Neurosurg 1999;90:78–84 10.3171/jns.1999.90.1.0078 [DOI] [PubMed] [Google Scholar]
- 8. Chung SJ, Kim JS, Kim JC, et al. Intracranial dural arteriovenous fistulas: analysis of 60 patients. Cerebrovasc Dis 2002;13:79–88 10.1159/000047755 [DOI] [PubMed] [Google Scholar]
- 9. Rammos S, Bortolotti C, Lanzino G. Endovascular management of intracranial dural arteriovenous fistulae. Neurosurg Clin N Am 2014;25:539–49 10.1016/j.nec.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 10. Rabinov J, Yoo AJ, Ogilvy CS, et al. ONYX versus n-BCA for embolization of cranial dural arteriovenous fistulas. J Neurointerv Surg 2013;5:306–10 10.1136/neurintsurg-2011-010237 [DOI] [PubMed] [Google Scholar]
- 11. Cognard C, Januel AC, Silva NA Jr, et al. Endovascular treatment of intracranial dural arteriovenous fistulas with cortical venous drainage: new management using Onyx. AJNR Am J Neuroradiol 2008;29:235–41 10.3174/ajnr.A0817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nogueira RG, Dabus G, Rabinov JD, et al. Preliminary experience with Onyx embolization for the treatment of intracranial dural arteriovenous fistulas. AJNR Am J Neuroradiol 2008;29:91–97 10.3174/ajnr.A0768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lv X, Jiang C, Li Y, et al. Results and complications of transarterial embolization of intracranial dural arteriovenous fistulas using Onyx-18. J Neurosurg 2008;109:1083–90 10.3171/JNS.2008.109.12.1083 [DOI] [PubMed] [Google Scholar]