The authors investigated the role of emergent endovascular stenting of long-segment carotid dissections in the acute ischemic stroke setting in 15 patients. They specifically evaluated long-segment carotid dissections requiring stent reconstruction with multiple tandem stents (≥ 3 stents) and presenting with acute (<12 hours) ischemic stroke symptoms (NIHSS score, ≥ 4). Carotid stent reconstruction was successful in all patients with no residual stenosis or flow limitation. Nine patients (60%) harbored intracranial occlusions, and 6 patients (40%) required intra-arterial thrombolysis/thrombectomy, achieving 100% TICI 2b–3 reperfusion. They conclude that emergent stent reconstruction of long-segment and flow-limiting carotid dissections in acute ischemic stroke intervention is safe and effective, with favorable clinical outcomes.
Abstract
BACKGROUND AND PURPOSE:
Although most cervical dissections are managed medically, emergent endovascular treatment may become necessary in the presence of intracranial large-vessel occlusions, flow-limiting and long-segment dissections with impending occlusion, and/or hypoperfusion-related ischemia at risk of infarction. We investigated the role of emergent endovascular stenting of long-segment carotid dissections in the acute ischemic stroke setting.
MATERIALS AND METHODS:
We retrospectively studied long-segment carotid dissections requiring stent reconstruction with multiple tandem stents (≥3 stents) and presenting with acute (<12 hours) ischemic stroke symptoms (NIHSS score, ≥4). We analyzed patient demographics, vascular risk factors, clinical presentations, imaging/angiographic findings, technical procedures/complications, and clinical outcomes.
RESULTS:
Fifteen patients (mean age, 51.5 years) with acute ischemic stroke (mean NIHSS score, 15) underwent endovascular stent reconstruction for vessel and/or ischemic tissue salvage. All carotid dissections presented with >70% flow limiting stenosis and involved the distal cervical ICA with a minimum length of 3.5 cm. Carotid stent reconstruction was successful in all patients with no residual stenosis or flow limitation. Nine patients (60%) harbored intracranial occlusions, and 6 patients (40%) required intra-arterial thrombolysis/thrombectomy, achieving 100% TICI 2b–3 reperfusion. Two procedural complications were limited to thromboembolic infarcts from in-stent thrombus and asymptomatic hemorrhagic infarct transformation (7% morbidity, 0% mortality). Angiographic and ultrasound follow-up confirmed normal carotid caliber and stent patency, with 2 cases of <20% in-stent stenosis. Early clinical improvement resulted in a mean discharge NIHSS score of 6, and 9/15 (60%) patients achieved a 90-day mRS of ≤2.
CONCLUSIONS:
Emergent stent reconstruction of long-segment and flow-limiting carotid dissections in acute ischemic stroke intervention is safe and effective, with favorable clinical outcomes, allowing successful thrombectomy, vessel salvage, restoration of cerebral perfusion, and/or prevention of recurrent thromboembolic stroke.
Cervical carotid or vertebral artery dissections are a common cause of acute ischemic stroke (AIS) in middle-aged and young adults.1–3 The prognosis of cervical dissections is favorable, with the standard of care being medical management as the majority of patients respond to anticoagulation/antiplatelet therapy.4,5 Delayed endovascular stenting of cervical dissections is reserved for patients presenting with recurrent ischemic symptoms and/or thromboembolic strokes refractory to medical management, progression of dissection-related stenosis, or symptomatic/enlarging dissecting pseudoaneurysms. Emergent endovascular treatment may also be required for cervical dissections presenting with concomitant intracranial thromboemboli/emergent large-vessel occlusion (ELVO), flow-limiting and long-segment lesions with impending occlusion, and/or hypoperfusion-related ischemia at risk of cerebral infarction.
Multiple randomized controlled trials have proved endovascular thrombectomy the standard of care in the treatment of ELVO.6–8 Since superimposed extracranial carotid or intracranial atherosclerotic disease and dissections are often an etiology of ELVO, recent studies have evaluated endovascular angioplasty/stenting techniques combined with intracranial thrombectomy. Adjunctive angioplasty/stenting techniques may be valuable in tandem carotid-intracranial occlusions secondary to acutely ruptured carotid atherosclerotic plaques, underlying intracranial atherosclerotic disease at risk for rethrombosis, or severe flow-limiting cervical/intracranial dissections. Furthermore, extracranial carotid stent placement may be necessary in emergent settings to provide distal access for intracranial thrombectomy, vessel salvage, or revascularization in hypoperfusion ischemic syndromes without sufficient intracranial collaterals. Several investigators have demonstrated the feasibility of emergency ICA stenting combined with intracranial thrombectomy for tandem ICA–MCA occlusions with acceptable rates of successful recanalization, complications, and clinical outcomes.9–14 In two of the recent multicenter trials that demonstrated a benefit of endovascular thrombectomy for AIS, carotid artery stent placement was necessary in 8.6%–12.9% of patients.6,7
Few studies have focused on the endovascular management of spontaneous cervical dissections with or without tandem intracranial ELVOs in the AIS setting, often limited to small sample sizes because most dissections can be managed medically postthrombectomy.15–17 We report on a unique cohort presenting for AIS intervention secondary to long-segment and flow-limiting carotid dissections requiring multiple tandem stents for endovascular reconstruction, irrespective of intracranial ELVO or successful thrombolysis/thrombectomy.
Materials and Methods
Patients
Patients presenting with spontaneous carotid artery dissections that underwent stent reconstruction between January 2011 and January 2015 were identified at the University of Massachusetts Medical Center or Northwestern University affiliated hospitals by using their neurointerventional databases. Institutional review board (IRB) approvals were acquired for a collaborative retrospective study. Emergent off-label use of Humanitarian Device Exemption (HDE) intracranial stents for the treatment of carotid dissections was reported to the respective IRBs and manufacturers as required. Medical record and PACS imaging data sharing was conducted under Health Insurance Portability and Accountability Act (HIPAA) guidelines. We included patients who were treated emergently in the AIS intervention window (<12 hours from symptom onset) with symptomatic (NIHSS ≥ 4) and long-segment carotid dissections requiring multiple tandem stents (≥3 stents) for endovascular reconstruction.
All patients had baseline CT/CTA and/or MRI/MRA available as components of AIS imaging protocols to exclude patients with intracranial hemorrhage and large ischemic infarcts of more than one-third of the MCA distribution and to identify suspected intracranial thromboembolic occlusions. CT/MR perfusion and MR diffusion-weighted imaging were not uniformly used, but were performed in 6/15 patients to estimate salvageable ischemic tissue and infarction volumes before intervention. If eligible, patients received IV tPA prior to attempted intra-arterial (IA) thrombolysis/mechanical thrombectomy of ELVOs and/or stent reconstruction of carotid dissections.
Procedures
All endovascular procedures were performed with the patient under monitored anesthesia care (n = 4) or general anesthesia (n = 11) with hemodynamic monitoring. Transfemoral access and retrograde advancement of 6F guide sheaths into the common carotid arteries, proximal to the dissections, provided guide sheath support. Microcatheter/microwire access was obtained across the dissection flaps with contrast injections confirming true lumen catheterization and opacification of the distal intracranial vasculature. Intravenous heparin anticoagulation was used in 14/15 cases with activated clotting time (ACT) monitoring (200–250 seconds). ELVOs were targeted with intra-arterial thrombolysis by using tPA and/or mechanical thrombectomy via direct aspiration or stent-retriever techniques. Due to persisting neurologic symptoms and/or severe flow limitations into the anterior circulation, carotid dissections were repaired using tandem stent reconstruction with partial overlapping techniques to prevent uncovered gaps across the stent constructs. Self-expanding or balloon-expanding stents were advanced and deployed over 0.014-inch exchange microwires, maintaining distal access across the length of the carotid dissections. Specific types of intracranial/carotid self-expanding stents (Wingspan/Neuroform intracranial stents; Stryker Neurovascular, Kalamazoo, Michigan; Precise carotid stent; Cordis, Fremont, California; Xact carotid stent; Abbott Laboratories, Abbott Park, Illinois) or peripheral/coronary balloon-expanding stents (Xience everolimus-eluting coronary stent; Abbott Laboratories; Express SD; Boston Scientific, Natick, Massachusetts; Resolute Integrity zotarolimus-eluting coronary stent; Medtronic, Minneapolis, Minnesota) were used at the discretion of the treating neurointerventionalist. Carotid stent reconstruction was performed serially to secure the distal intimal flap and proximal dissection inflow zone.
During carotid stent-placement procedures, intraoperative antiplatelet loading doses were administered with either orogastric/rectal aspirin 325–650 mg (n = 5), dual aspirin 325–650 mg, and clopidogrel 300–600 mg (n = 5), or intravenous glycoprotein IIb/IIIa inhibitors (eptifibatide 0.18 mg/kg, n = 3). The remaining two patients previously on dual aspirin/clopidogrel or warfarin/aspirin therapy were bridged to aspirin/clopidogrel. Patients who received intraoperative IIb/IIIa inhibitors or aspirin-only loading doses were subsequently loaded with clopidogrel 300–600 mg, within 12 hours postprocedure. All patients remained on dual aspirin 81–325 mg/clopidogrel 75 mg antiplatelet therapy for >6 months.
Clinical and Imaging Data Analysis
We studied patient demographics, vascular risk factors, presentations, NIHSS scores on admission, initial and follow-up imaging/angiographic findings, technical efficacy and safety, procedural complications, and clinical outcomes at discharge (NIHSS and mRS scores) and at 90 days (mRS). All carotid dissections were classified according to the Modified Carotid Artery Injury Grading Scale on DSA and were measured for the length of vessel involvement on initial CTA/MRA studies.18 Final post-procedural DSA studies after carotid stent reconstruction and adjunctive IA thrombolysis/thrombectomy techniques were evaluated for residual cervical segmental stenosis (significance >50% by NASCET criteria),19 flow limitation, and cerebral reperfusion according to the modified TICI scale.
Follow-up imaging with noncontrast CT head studies postprocedure and at 24–72 hours was assessed for any intracranial hemorrhagic (reperfusion or infarct transformation) complications according to the European Cooperative Acute Stroke Study (ECASS) criteria20 and evolving infarction. Symptomatic intracranial hemorrhage was defined as an association with any clinical deterioration or increase in the NIHSS score of >4. Delayed carotid Doppler ultrasound and CTA and/or conventional angiographic follow-up studies at 3–6 months post-procedure were evaluated to assess midterm carotid and stent patency.
All retrospective clinical and imaging data analysis was agreed upon by two senior neurointerventionalists at each site. Statistical analysis was limited to mean and standard deviation calculations for patient age, NIHSS score, and mean dissection lengths, and median calculations for the number of stent constructs and mRS scores.
Results
We retrospectively identified 15 patients (11 men: 4 women; mean age 51.5 years) who underwent endovascular stent reconstruction with ≥3 stents for long-segment and symptomatic (mean NIHSS score 15) carotid dissections in the AIS setting (<12 hours from symptom onset). Baseline demographics data, presenting signs and symptoms, and precipitating risk factors are presented in the Table. Procedural data regarding pretreatment IV tPA thrombolysis, IA recanalization of concurrent intracranial occlusion, anatomic extent of dissections, length and degree of flow limitation, number/type of stents for carotid reconstruction, complications, and clinical outcomes are noted in the On-line Table.
Patient demographics and presentations
| Demographics/Presentations | |
|---|---|
| Age (mean) (yr) | 51.5 (range 37–79 years) |
| Sex (F/M) | 4/11 |
| Presenting symptoms (No.) (%) | |
| Hemiplegia/hemiparesis | 12 (80) |
| Aphasia | 6 (40) |
| Headache | 4 (26.7) |
| Facial droop | 4 (26.7) |
| Sensory deficit | 3 (20) |
| Hemineglect | 2 (13.3) |
| Visual deficit | 2 (13.3) |
| Risk factors (No.) (%) | |
| Hypertension | 7 (46.7) |
| Dyslipidemia | 5 (33.3) |
| Diabetes mellitus | 1 (6.7) |
| Fibromuscular dysplasia | 1 (6.7) |
| No significant risk factor | 6 (40) |
All carotid dissections were classified as at least grade 2b lesions (>70% stenosis with flow limitation) per the Modified Carotid Artery Injury Grading Scale18 with a minimum lesion length of 3.5 cm (mean 6.7 cm; range 3.5–9 cm) and involved the distal cervical segment; 10/15 dissections extended past the skull base into the petrous-cavernous segments of the ICA without intracranial extension. Additionally, 5 (33%) patients presented with associated dissecting pseudoaneurysms (grade 3b), and 4 (27%) patients had progressed to an acute cervical carotid occlusion (grade 4), requiring extracranial thromboaspiration in a single case for revascularization across the occluded vessel. Multiple stents were utilized, with a median of 5 stent constructs per patient, including self-expanding peripheral/carotid stents (13/15 patients), coronary balloon-expanding stents (4/15 patients), and self-expanding intracranial stents (12/15 patients) for distal cervical and skull base pathology. Long-segment carotid stent reconstruction was technically successful in all patients with no significant (>50%) residual stenosis/occlusion or flow limitation, immediate reduction in subintimal inflow, and contrast stasis visualized in associated pseudoaneurysms on post-procedure DSA analysis.
Nine patients (60%) also presented with intracranial thromboemboli, either proximal large-vessel (n = 5) or distal small-vessel (n = 4) occlusions. Additional endovascular intracranial interventions were performed in 6/15 (40%) patients, consisting of IA tPA thrombolysis (n = 3) and/or thrombectomy with thromboaspiration (n = 1) or stent-retriever (n = 3) techniques. TICI 2b/3 reperfusion was achieved in all patients (100%) postthrombolysis/thrombectomy and carotid stent reconstruction.
Interventions were relatively safe with procedural complications limited to a single patient (patient 6) developing multifocal thromboembolic infarcts in the left middle cerebral artery distribution secondary to in-stent thrombus. Subsequent clinical deterioration and a poor clinical outcome at discharge resulted in overall procedural morbidity of 1/15 (7%). Although no symptomatic intracranial hemorrhages occurred to suggest reperfusion complications, a left temporal lobe intraparenchymal hemorrhage without neurologic sequelae was consistent with hemorrhagic infarct conversion (HI-2 grade by ECASS criteria) on follow-up CT head studies. There was no procedure-related mortality at discharge or at 90-day follow-up.
Rapid improvement in post-procedural clinical outcomes was observed from a mean NIHSS score of 15 ± 8 on admission to NIHSS 6 ± 5 on discharge. On clinical follow-up, most patients obtained further functional independence from a median mRS of 2 at discharge to an mRS 1 at 90-day follow-up, with 9/15 patients (60%) achieving an mRS of ≤2 at discharge and 90-day follow-up as noted in the On-line Table. There were no interval recurrent ischemic symptoms, TIAs, or strokes during the course of clinical follow-up.
Follow-up carotid Doppler ultrasound and CTA/DSA studies at 3–6 months were available in 14/15 patients, with 12 patients demonstrating stent patency, complete restoration of carotid artery caliber, and no evidence of in-stent thrombosis or significant stenosis. Two patients exhibited either mild persisting vessel irregularity/tapering or intimal hyperplasia causing <20% segmental in-stent stenosis. In addition, no new or persisting carotid pseudoaneurysms were identified, with interval thrombosis/healing of dissecting aneurysms in all 5 patients, suggesting successful stent-associated flow diversion and intimal flap reconstruction.
Illustrative Case
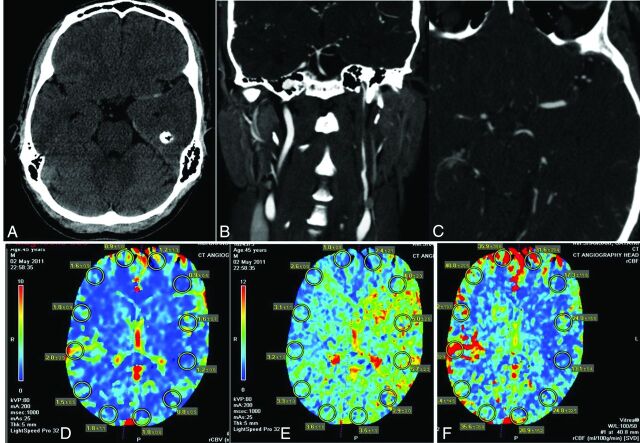
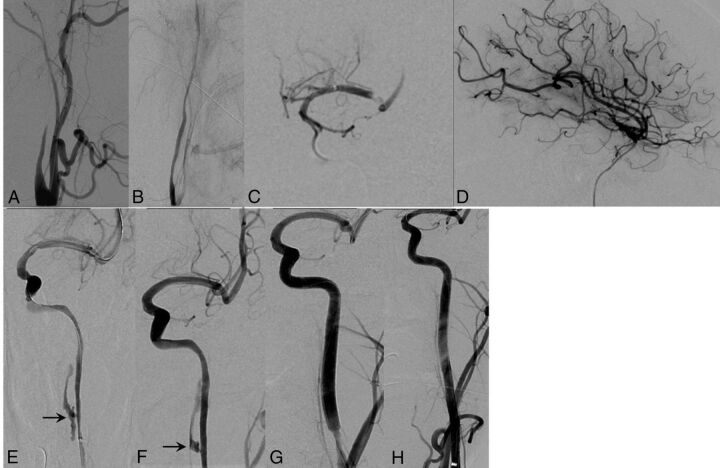
A 45-year-old man (patient 13) presented to the emergency department with acute onset of aphasia, right hemineglect, hemiparesis, and left-sided gaze deviation with an NIHSS score of 20. CT/CTA studies identified a 9-cm-long-segment left ICA dissection from the carotid bifurcation to the petrous segment and a distal thromboembolic occlusion of the left M1 segment (Fig 1A–C). CT brain perfusion studies confirmed a large left hemispheric perfusion abnormality, suggesting an ischemic penumbra and salvageable tissue (Fig 1D, -F). Following IV tPA infusion with no neurologic improvement, the patient was placed under general anesthesia in the neurointerventional suite with hemodynamic vasopressor support to augment pial collaterals. Following 6F guide sheath placement in the left common carotid artery, DSA studies confirmed a severe >80% flow-limiting cervical left ICA dissection and distal M1 segment thromboembolus/ELVO (Fig 2A, -B). A Penumbra aspiration coaxial catheter system (Penumbra, Alameda, California) was advanced over a Transend EX 0.014-inch microwire (Stryker), which enabled access across the true lumen of the ICA dissection into the M1 segment of the left MCA, confirmed on intermittent microcatheter angiograms (Fig 2C). Intra-arterial tPA (5 mg) infusion and mechanical thrombectomy with vacuum aspiration techniques resulted in complete recanalization of the left MCA distribution consistent with TICI 2b reperfusion. Since the long-segment left ICA dissection remained at risk for re-thrombosis/occlusion due to persisting flow limitation despite intracranial thrombectomy (Fig 2D), we initiated tandem and partially overlapping stent reconstruction of the left ICA from the petrocavernous junction to the proximal cervical segment (Fig 2E−G).
Fig 1.
Illustrative case (patient 13). A, NCCT axial image demonstrates no large regional infarction or intracranial hemorrhage, but a hyperdense left MCA sign suggestive of a large vessel occlusion. Coronal (B) and axial (C) CTA head/neck images demonstrate a long-segment left ICA dissection involving the proximal cervical-to-horizontal petrous segment with an associated distal M1 segment left MCA occlusion. D, CT brain perfusion study with preserved relative cerebral blood volume, markedly elevated relative mean transit time (E), and mildly decreased relative cerebral blood flow (F), consistent with severe hemodynamic impairment and hypoperfusion-related ischemia in the left cerebral hemisphere.
Fig 2.
Illustrative case (patient 13). A and B, Lateral DSA images demonstrate a tapered and severely narrowed left ICA dissection with flow limitation that extends across the cervical segment into the skull base. C, Anteroposterior DSA image of an aspiration thrombectomy catheter navigated across the left ICA cervical dissection and placed just proximal to the M1 segment thromboembolus. D, Lateral DSA image demonstrates successful thrombolysis/thrombectomy, resulting in complete recanalization and eventual TICI 3 reperfusion of the left MCA distribution. E–H, Serial anteroposterior DSA images demonstrate stent reconstruction of the long-segment left ICA dissection after MCA thrombectomy, distal to the petrocavernous junction into the proximal cervical left ICA, resulting in near-normal vessel caliber, with no residual stenosis or flow limitation. Note spontaneous thrombosis of the carotid pseudoaneurysm (arrow) and no residual subintimal contrast in the midcervical segment after stent-induced apposition of the intimal flap.
The patient received eptifibatide 10.8 mg intravenously during carotid vessel wall reconstruction. With deployment of multiple tandem intracranial and carotid stents, the left ICA normalized in caliber with no evidence of a residual intimal flap, pseudoaneurysm, in-stent thrombosis/stenosis, or flow limitation into the intracranial circulation, consistent with TICI 3 reperfusion. After a post-procedure CT head study excluded reperfusion hemorrhage or hemorrhagic infarct conversion, the patient was loaded with dual-antiplatelet therapy (aspirin 325 mg and clopidogrel 600 mg). The patient made an excellent neurologic recovery with a discharge NIHSS score of 0 and functionally independent clinical outcome with a discharge and 90-day mRS 0.
Discussion
We present a rare series of long-segment and flow-limiting carotid artery dissections with or without associated ELVO, presenting as a primary AIS etiology and requiring endovascular stent reconstruction with multiple stents due to hemodynamic insufficiency. We performed adjunctive IA thrombolysis/thrombectomy in 40% of patients with tandem intracranial ELVO, and all patients achieved >TICI 2b reperfusion on the final angiographic assessment. Low complication, symptomatic intracranial hemorrhage, and morbidity (7%) rates translated into favorable clinical outcomes (60%, mRS ≤ 2). Although not always necessary as noted in prior studies,15,16 stent reconstruction can salvage acutely occluded carotid dissections and confer an ability to navigate larger guide or intermediate catheters for the management of associated extra- and intracranial ELVOs. Moreover, re-establishing robust cervical antegrade flow can be inherently thrombolytic for small-vessel intracranial occlusions, prevent recurrent thromboemboli or reocclusion after successful cervical recanalization/intracranial thrombectomy, and provide hemodynamic augmentation to pial collaterals supplying any residual ischemic tissue. Even in the absence of ELVO or distal small intracranial thromboemboli (40% of patients in our study), severe flow-limiting cervical dissections without adequate circle of Willis or pial collateral supply can present with profound hypoperfusion-related ischemia, requiring emergent endovascular stent reconstruction and cerebral reperfusion to salvage the ischemic penumbra.
Multiple published studies have described internal carotid artery stent placement for the endovascular management of complicated dissections for various indications and using multiple stents to treat long-segment cervical dissections,21–23 but the maximum number of stents or the length of the dissection pathology was not reported. Biondi et al24 and Coric et al25 also had individual case reports about treating complicated long-segment carotid dissections with multiple stents, presenting with refractory ischemic symptoms after the failure of medical management. In our series, we did not use a precise definition of “long-segment” but included dissections that required the use of multiple (≥3) tandem stents to cover the entire dissection flap, which nearly always involved the distal cervical segment of the ICA. Therefore, we selected severely symptomatic, flow-limiting dissections (>2b Modified Carotid Artery Injury Grading Scale) that required emergent intervention. Additionally, these lesions presented with characteristic long-segment (>3.5 cm) and distal cervical/skull base involvement of the vessel wall, perhaps due to unrestrained subintimal extension or spiral dissection. Recent developments in intravascular ultrasound optical coherence tomography, and high-resolution, vessel wall MR imaging techniques may improve the cross-sectional assessment of intramural pathology for more accurate diagnosis and treatment planning.26,27
Furthermore, we limited our study to flow-limiting carotid dissections that presented with severe, acute ischemic symptoms (mean NIHSS score of 15) within <12 hours from symptom onset, requiring emergent multiple tandem stent reconstructions with or without adjunctive IA thrombolysis/thrombectomy. Other than a few case reports,28,29 limited series have been published to suggest the technical and clinical efficacy of stent placement in cervical dissections in the AIS setting. In the larger traumatic carotid dissection series by Cohen et al,23 14/23 patients presented with AIS and underwent carotid stent placement with 16/23 patients having severe or flow-limiting dissections (subocclusive grade IV/occlusive grade V). Although they reported excellent clinical outcomes (92.3%, mRS 0–3 at 90 days), this series focused on traumatic carotid dissections, a separate pathology with often delayed presentations, unclear intervention times from symptom onset (<17 hours) in the AIS subgroup, and only 2 patients with ELVO that required tandem IA thrombolysis/thrombectomy.23 Lavallée et al17 compared 6 patients with tandem ICA/MCA occlusions who underwent endovascular stent placement of underlying carotid dissections and intracranial mechanical thrombectomy versus 4 patients who received IV tPA alone. Endovascular treatment was associated with markedly improved ICA/MCA recanalization rates and correlated directly with independent functional outcomes, mRS 0–2 (100% versus 25%). Fields et al16 reported a small cohort of 8 ICA and 1 vertebral artery dissections and tandem intracranial occlusions from the mechanical thrombectomy Merci registry, but carotid/vertebral stent placement was needed in only 5/9 patients. This relatively young cohort (mean age, 48 years) obtained 60% TICI >2b reperfusion rates, without significant complications or symptomatic intracerebral hemorrhage, and excellent clinical outcomes (80%, mRS ≤ 2). More recently, Marnat et al15 reported their experience with 20/258 patients (7.6%) from the prospective RECOST (Prognostic Factors Related to Clinical Outcome Following Thrombectomy in Ischemic Stroke) Study, who presented with tandem carotid artery dissections and anterior circulation ELVOs with severe AIS (mean NIHSS score 17) within 6 hours of symptom onset. In comparison to patients with isolated ELVO, they identified a statistically younger population; prolonged interventional procedure times; no difference in TICI 2b/3 recanalization, complications, or symptomatic intracerebral hemorrhage rates; and equivalent-to-better clinical outcomes (70%, mRS ≤ 2) in this cohort. However, 15/20 (75%) carotid dissections were managed medically in this series with no need for endovascular stent reconstruction after angiographic confirmation of an adequate circle of Willis (anterior/posterior communicating arteries) and <2-second venous phase delay in the affected cerebral hemisphere.15
Multiple case reports and case series have demonstrated the successful technical use of self-expanding carotid or peripheral stents and covered stent grafts in the endovascular reconstruction of cervical dissections and dissecting aneurysms. In fact, after stent realignment of the intimal flap, acute dissecting aneurysms will often undergo spontaneous thrombosis due to flow diversion and reduced inflow into the false aneurysm lumen, as observed in all 5 associated pseudoaneurysms in our series. Long-segment carotid dissections may extend into the distal cervical, petrocavernous, and even intracranial segments, requiring off-label applications of lower profile balloon-expanding coronary stents or flexible/navigable self-expanding intracranial stents, as seen in nearly all our patients.30 Although intracranial stents are highly trackable and accommodating to the tortuous distal cervical vasculature with adequate radial force for the treatment of cervical dissections, this application is considered an off-label use of an HDE device, requiring institutional review board and manufacturer notification. Alternatively, balloon-expanding coronary stents can be advantageous in severe dissection-related stenoses with focal intramural hemorrhage, spiral dissections, or in the constrained osseous compartment of the petrous carotid canal, provide greater angioplasty-dependent radial force and precise placement for vessel reconstruction. Mechanical thrombectomy of tandem ICA−MCA occlusions has been described by several authors, including a proximal-to-distal approach with carotid stent placement to re-establish antegrade flow and access to the intracranial circulation for thrombectomy.9–14 Despite this being necessary in acutely occluded or severe atherosclerotic carotid stenoses, acute cervical dissections can usually be traversed with distal-access catheter technology to target distal ELVOs, first with either direct aspiration and/or stent-retriever thrombectomy for rapid cerebral reperfusion. In our experience, the use of proximal balloon-guide catheters was deferred in preference to lower profile and distal-access aspiration catheters with or without stent retrievers to prevent further injury to the cervical carotid wall during thrombectomy. Subsequently, tandem stent reconstruction of cervical dissections was performed as described from a distal-to-proximal approach, maintaining access across the true lumen and securing the distal extension of the flap to the proximal inflow zone.
Although stent reconstruction of long-segment cervical lesions avoids anticoagulation use in the AIS setting, dual antiplatelet therapy is mandatory with preoperative or intraoperative loading preferred to prevent intraprocedural in-stent thromboembolic/occlusion complications. Deployment of multiple tandem stents with extensive vessel wall exposure to foreign metallic material increases the risk of platelet aggregation and thromboemboli. However, even antiplatelets may be contraindicated or used with trepidation in the AIS setting after IV tPA thrombolysis and/or mechanical thrombectomy, especially if there is a high risk of reperfusion hemorrhage or hemorrhagic infarct transformation. We used several different antiplatelet strategies in this precarious setting, but in a few patients, we provided an intravenous glycoprotein IIb/IIIA inhibitor (eptifibatide, 0.18 mg/kg) during carotid stent reconstruction. Several advantages of IIb/IIIa inhibitors include immediate antiplatelet protection without the need for oral aspirin/clopidogrel loading and a reduced time to reach peak platelet inhibition (minutes versus hours depending on dose and class of oral thienopyridines). As a reversible competitive inhibitor with a short half-life, eptifibatide rapidly decays with normalizing platelet function over hours, providing time to initiate aspirin/clopidogrel loading or terminate antiplatelet therapy if hemorrhagic complications are suspected. If CT head findings are equivocal or concerning, clopidogrel loading can be delayed until a repeat follow-up CT head study definitively excludes evolving intracranial hemorrhage. New dual-energy CT applications may assist with the earlier diagnosis of contrast staining versus hemorrhagic infarct transformation after endovascular stroke interventions; hence, allowing confident initiation of dual-antiplatelet therapy and protection from stent-related thromboembolic complications in the AIS setting.31
Our study had several limitations as a retrospective and nonrandomized study of a small cohort that lacked a control population for comparison. Variable carotid stent placement protocols, equipment preferences, and antiplatelet management could not be standardized in our study design. However, severe flow-limiting and long-segment carotid dissections are rare pathologies in the setting of AIS, and it would be difficult to conduct a large cohort study without multicenter involvement. Even the Merci registry of 980 patients yielded only 5 patients with cervical dissections requiring stent reconstruction with intracranial thrombectomy.16 Furthermore, severe acute presentations in this population warrant intervention, at least for associated intracranial ELVO and hypoperfusion-related ischemia at risk for infarction in the absence of sufficient intracranial collaterals. However, if sufficient flow across an acute cervical dissection is re-established after ELVO thrombectomy or adequate intracranial collaterals are present with a neurologic response and symptomatic improvement, this population could potentially be studied, comparing emergent stent reconstruction versus conservative medical management using an early transition to anticoagulation therapy.
Conclusions
In this series, we demonstrate the technical feasibility, safety, and clinical efficacy of multiple tandem stents to reconstruct long-segment and flow-limiting carotid dissections presenting in the AIS setting. As an independent or adjunctive methodology to IA thrombolysis/thrombectomy, it is a valuable technique in AIS intervention. Carotid stent reconstruction of severe flow-limiting dissections may prevent further dissection propagation, carotid occlusion, recurrent thromboembolic complications, and/or perfusion-dependent ischemia/infarction. Although midterm technical and clinical efficacy is promising, diligent antiplatelet management and further validation with larger multicenter studies and long-term outcome assessment are required.
Supplementary Material
ABBREVIATIONS:
- AIS
acute ischemic stroke
- ELVO
emergent large-vessel occlusion
- IA
intra-arterial
Footnotes
Disclosures: Muhib Khan—UNRELATED: Other: Women's Health Initiative, Comments: stroke-outcome adjudication. Michael C. Hurley—UNRELATED: Payment for Lectures including Service on Speakers Bureaus: Penumbra. Matthew J. Gounis—UNRELATED: Consultancy: Codman Neurovascular, Stryker Neurovascular; Grants/Grants Pending: National Institutes of Health, Asahi Intecc, Blockade Medical, CereVasc LLC, Codman Neurovascular, Cook, Gentuity LLC, Fraunhofer Institute, InNeuroCo Inc, Lazarus Effect, Medtronic Neurovascular, MicroVention/Terumo, Neuravi, Philips Healthcare, Stryker Neurovascular, Wyss Institute for Biologically Inspired Engineering*; Stock/Stock Options: InNeuroCo. Ajay K. Wakhloo—UNRELATED: Consultancy: Stryker Neurovascular, Codman Neurovascular, Philips Healthcare; Expert Testimony: McCoy LLC, Coral Gables, Florida, Comments: expert testimony for defendant physician; Grants/Grants Pending: Philips Healthcare, Comments: equipment grant*; Payment for Lectures including Service on Speakers Bureaus: Harvard Postgraduate Course, Miami Vascular Institute; Stock/Stock Options: InNeuroCo, Penumbra; Other: Pulsar Vascular Comments: bridge loan. Ajit S. Puri—UNRELATED: Consultancy: Stryker Neurovascular, Medtronic, Codman, Comments: proctor for Stryker and Medtronic; Grants/Grants Pending: Stryker Neuromuscular and Medtronic*; Stock/Stock Options: InNeuroCo. *Money paid to the institution.
References
- 1. Bogousslavsky J, Regli F. Ischemic stroke in adults younger than 30 years of age. Cause and prognosis. Arch Neurol 1987;44:479–82 10.1001/archneur.1987.00520170009012 [DOI] [PubMed] [Google Scholar]
- 2. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med 2001;344:898–906 10.1056/NEJM200103223441206 [DOI] [PubMed] [Google Scholar]
- 3. Lucas C, Moulin T, Deplanque D, et al. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke 1998;29:2646–48 10.1161/01.STR.29.12.2646 [DOI] [PubMed] [Google Scholar]
- 4. Engelter ST, Lyrer PA, Kirsch EC, et al. Long-term follow-up after extracranial internal carotid artery dissection. Eur Neurol 2000;44:199–204 10.1159/000008236 [DOI] [PubMed] [Google Scholar]
- 5. Schievink WI. The treatment of spontaneous carotid and vertebral artery dissections. Curr Opin Cardiol 2000;15:316–21 10.1097/00001573-200009000-00002 [DOI] [PubMed] [Google Scholar]
- 6. Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 7. Jovin TG, Chamorro A, Cobo E, et al. ; REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–306 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 8. Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–30 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 9. Stampfl S, Ringleb PA, Möhlenbruch M, et al. Emergency cervical internal carotid artery stenting in combination with intracranial thrombectomy in acute stroke. AJNR Am J Neuroradiol 2014;35:741–46 10.3174/ajnr.A3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cohen JE, Gomori JM, Rajz G, et al. Extracranial carotid artery stenting followed by intracranial stent-based thrombectomy for acute tandem occlusive disease. J Neurointerv Surg 2015;7:412–17 10.1136/neurintsurg-2014-011175 [DOI] [PubMed] [Google Scholar]
- 11. Matsubara N, Miyachi S, Tsukamoto N, et al. Endovascular intervention for acute cervical carotid artery occlusion. Acta Neurochir (Wien) 2013;155:1115–23 10.1007/s00701-013-1697-x [DOI] [PubMed] [Google Scholar]
- 12. Papanagiotou P, Roth C, Walter S, et al. Carotid artery stenting in acute stroke. J Am Coll Cardiol 2011;58:2363–69 10.1016/j.jacc.2011.08.044 [DOI] [PubMed] [Google Scholar]
- 13. Malik AM, Vora NA, Lin R, et al. Endovascular treatment of tandem extracranial/intracranial anterior circulation occlusions: preliminary single-center experience. Stroke 2011;42:1653–57 10.1161/STROKEAHA.110.595520 [DOI] [PubMed] [Google Scholar]
- 14. Spiotta AM, Lena J, Vargas J, et al. Proximal to distal approach in the treatment of tandem occlusions causing an acute stroke. J Neurointerv Surg 2015;7:164–69 10.1136/neurintsurg-2013-011040 [DOI] [PubMed] [Google Scholar]
- 15. Marnat G, Mourand I, Eker O, et al. Endovascular management of tandem occlusion stroke related to internal carotid artery dissection using a distal to proximal approach: insight from the RECOST Study. AJNR Am J Neuroradiol 2016;37:1281–88 10.3174/ajnr.A4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fields JD, Lutsep HL, Rymer MR, et al. ; Merci Registry Investigators. Endovascular mechanical thrombectomy for the treatment of acute ischemic stroke due to arterial dissection. Interv Neuroradiol 2012;18:74–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lavallée PC, Mazighi M, Saint-Maurice JP, et al. Stent-assisted endovascular thrombolysis versus intravenous thrombolysis in internal carotid artery dissection with tandem internal carotid and middle cerebral artery occlusion. Stroke 2007;38:2270–74 10.1161/STROKEAHA.106.481093 [DOI] [PubMed] [Google Scholar]
- 18. Seth R, Obuchowski AM, Zoarski GH. Endovascular repair of traumatic cervical internal carotid artery injuries: a safe and effective treatment option. AJNR Am J Neuroradiol 2013;34:1219–26 10.3174/ajnr.A3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–53 10.1056/NEJM199108153250701 [DOI] [PubMed] [Google Scholar]
- 20. Larrue V, von Kummer RR, Müller A, et al. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke 2001;32:438–41 [DOI] [PubMed] [Google Scholar]
- 21. Bejjani GK, Monsein LH, Laird JR, et al. Treatment of symptomatic cervical carotid dissections with endovascular stents. Neurosurgery 1999;44:755–60; discussion 760–61 10.1097/00006123-199904000-00037 [DOI] [PubMed] [Google Scholar]
- 22. Malek AM, Higashida RT, Phatouros CC, et al. Endovascular management of extracranial carotid artery dissection achieved using stent angioplasty. AJNR Am J Neuroradiol 2000;21:1280–92 [PMC free article] [PubMed] [Google Scholar]
- 23. Cohen JE, Gomori JM, Itshayek E, et al. Single-center experience on endovascular reconstruction of traumatic internal carotid artery dissections. J Trauma Acute Care Surg 2012;72:216–21 10.1097/TA.0b013e31823f630a [DOI] [PubMed] [Google Scholar]
- 24. Biondi A, Katz JM, Vallabh J, et al. Progressive symptomatic carotid dissection treated with multiple stents. Stroke 2005;36:e80–82 10.1161/01.STR.0000177883.50262.cf [DOI] [PubMed] [Google Scholar]
- 25. Coric D, Wilson JA, Regan JD, et al. Primary stenting of the extracranial internal carotid artery in a patient with multiple cervical dissections: technical case report. Neurosurgery 1998;43:956–59 10.1097/00006123-199810000-00139 [DOI] [PubMed] [Google Scholar]
- 26. Swartz RH, Bhuta SS, Farb RI, et al. Intracranial arterial wall imaging using high-resolution 3-Tesla contrast-enhanced MRI. Neurology 2009;72:627–34 10.1212/01.wnl.0000342470.69739.b3 [DOI] [PubMed] [Google Scholar]
- 27. Xu P, Lv L, Li S, et al. Use of high-resolution 3.0-T magnetic resonance imaging to characterize atherosclerotic plaques in patients with cerebral infarction. Exp Ther Med 2015;10:2424–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen M. Mechanical recanalization of acute carotid terminus occlusion from traumatic arterial dissection. Front Neurol 2010;1:123 10.3389/fneur.2010.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu W, Binder D, Foster-Barber A, et al. Endovascular embolectomy of acute basilar artery occlusion. Neurology 2003;61:1421–23 10.1212/WNL.61.10.1421 [DOI] [PubMed] [Google Scholar]
- 30. Ansari SA, Thompson BG, Gemmete JJ, et al. Endovascular treatment of distal cervical and intracranial dissections with the Neuroform stent. Neurosurgery 2008;62:636–46; discussion 636–46 10.1227/01.NEU.0000311350.25281.6B [DOI] [PubMed] [Google Scholar]
- 31. Phan CM, Yoo AJ, Hirsch JA, et al. Differentiation of hemorrhage from iodinated contrast in different intracranial compartments using dual-energy head CT. AJNR Am J Neuroradiol 2012;33:1088–94 10.3174/ajnr.A2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.