We read with great interest the review article entitled “Aneurysms Associated with Brain Arteriovenous Malformations” by Rammos et al.1 This article summarizes the different subtypes of intracranial aneurysms that may be associated with brain AVMs (bAVMs). We congratulate the authors for their effort to clarify the nomenclature of such aneurysms. However, we would like to raise our disagreement with the following statement: “Given that pathologic specimens of resected AVM nidi consist of a conglomerate of venous tangles and loops, implicating that venous drainage begins at the level of the nidus, intranidal aneurysms are de facto venous.” Histopathologically, the nidus has been reported to comprise both arteries and veins with disorganized walls, as well as vessels of ambiguous nature.2–5 Even though we agree that some intranidal aneurysms may have a venous nature and may be responsible for some bAVM bleeding events (Fig 1), in our experience, most intranidal aneurysms in bAVMs with hemorrhagic presentation are rather false aneurysms arising from the dysplastic vessels belonging to the nidus and represent the bleeding site.6 Indeed, as reported by numerous series,7,8 in patients with intracranial bAVM-related bleeding events, intranidal aneurysms are frequently observed. A higher rebleeding rate has also been reported in patients with bAVMs harboring intranidal aneurysms.9 Nidal aneurysms have been defined by a joint experts group as those with any portion contained in the bAVM nidus.10 As underlined by Redekop et al,8 these aneurysms are often seen on early phase of the DSA, before substantial venous filling, and may present a contrast media stagnation on late phase (Figs 2 and 3). Depiction of intranidal aneurysms is both difficult and subject to a high rate of interobserver disagreement. The review of Rammos et al1 is lacking a discussion on advanced imaging tools that may help to demonstrate these aneurysms and further our understanding of their origin. Indeed, high-rate (6 frames per second or more) DSA acquisitions, 3D rotational angiography (RA), 4D-RA technology,11 and ultraselective angiography, or even more recent algorithms used on 3D-RA such as segmentation algorithms12 or anamorphosis algorithm,13 may help to depict these aneurysms more accurately. Wall enhancement of the intranidal aneurysm on MR imaging has also been proposed as a radiologic feature that may help to confirm the bleeding site.14
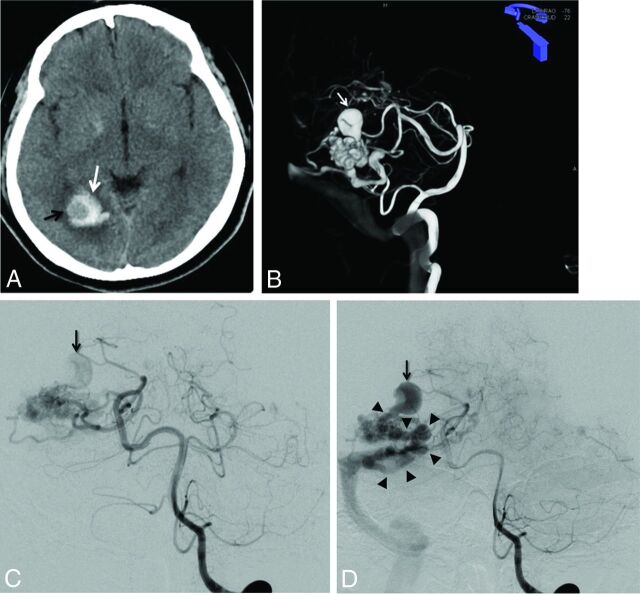
Fig 1.
A, Unenhanced brain CT scan (axial section) in a 40-year-old woman with headache. Right occipital hematoma is seen (white arrow). Note a round hypoattenuated shape surrounded by the hematoma, corresponding to the intranidal aneurysm (black arrow). B, Volume rendering reconstruction from the 3D-RA acquisition through the left vertebral artery, showing a large intranidal aneurysm (white arrow). C and D, Left vertebral DSA in anteroposterior projection at early phase (C) and late phase (D). At early phase, an intranidal aneurysm is seen (C, arrow). On late phase, this nidal aneurysm appears clearly connected to the main draining vein (D, arrowheads), confirming the venous nature of this intranidal aneurysm.
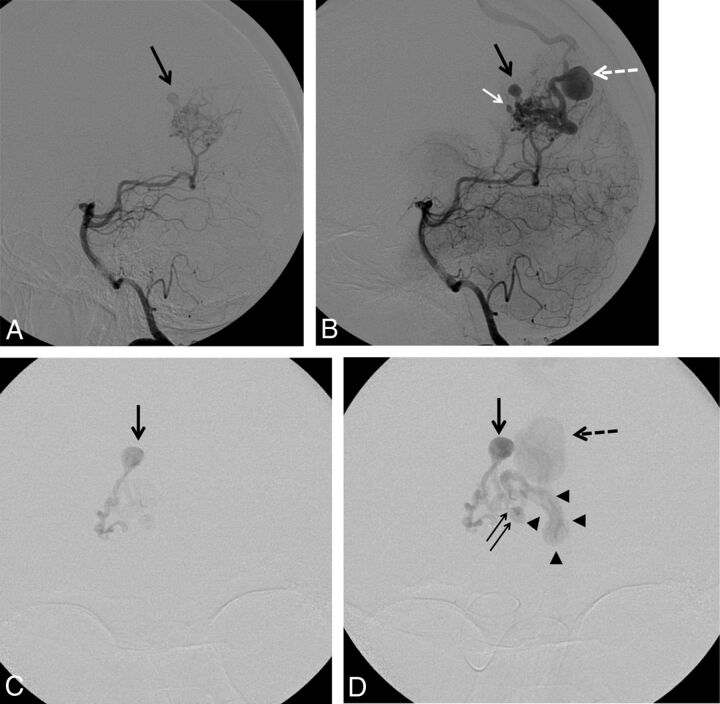
Fig 2.
A and B, Left vertebral artery DSA in lateral projection in a 41-year-old woman with a ruptured right parieto-occipital bAVM. A, Early phase showing a nidal aneurysm (black arrow) before filling of the draining vein. B, Intermediate phase showing a second intranidal aneurysm (white arrow) in addition to the first one (black arrow). Note a focal venous ectasia on the main draining vein (white dotted arrow). C and D, Selective DSA in anteroposterior projection from the parieto-occipital branch of the right posterior cerebral artery through a flow-dependent microcatheter. C, Early phase showing the filling of both the nidus and an intranidal aneurysm before any venous filling. D, Intermediate phase showing, in addition to the previously described intranidal aneurysm (black arrow), a second smaller nidal aneurysm (double arrow) close to the origin of the main draining vein (arrowheads). Note the presence of a focal venous ectasia on the main draining vein (black dotted arrow).
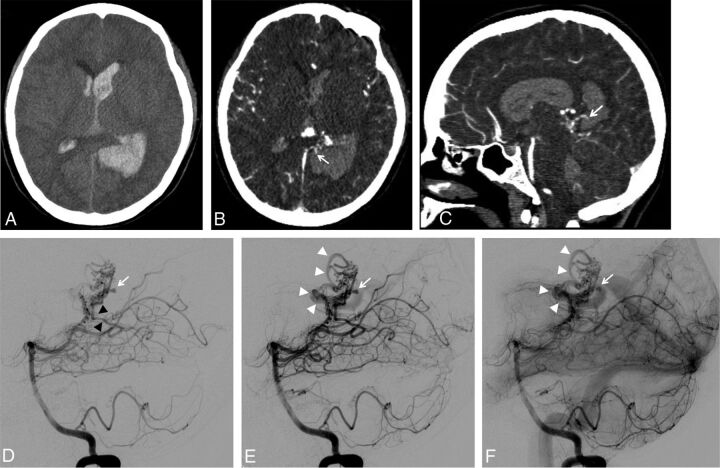
Fig 3.
A, Unenhanced brain CT scan (axial section) showing a left parieto-occipital hematoma associated with intraventricular hemorrhage in a 19-year-old woman. B and C, Brain CT angiography: axial section (B) and sagittal reconstruction (C). Small false aneurysm arising from the nidus and close to the hematoma is seen on both axial and sagittal images (B and C, white arrows). The close relationship between the intranidal aneurysm and the hematoma suggests the aneurysm as being the cause of the bleeding. D–F, Left vertebral artery DSA in lateral projection at very early phase (D), early phase (E), and intermediate phase (F). At very early phase, opacification of the nidus is seen, supplied mainly by the left posterolateral choroidal artery (D, black arrowheads). Note the opacification of an intranidal aneurysm located at the posterior aspect of the nidus (D, white arrow) before any substantial filling of the venous drainage. At later phase, the origin of the draining vein is filling (E, white arrowheads) while the nidal aneurysm is still visible (E, white arrow). On intermediate phase, stagnation of the nidal aneurysm is seen (F, white arrow) while the venous drainage is more clearly seen (F, white arrowheads).
Finally, we agree with Rammos et al1 on the fact that the depiction of these intranidal aneurysms is of tremendous importance because they may prompt the interventional neuroradiologists or neurosurgeons to perform in an emergency a target treatment or whole resection of the bAVM to prevent early rebleeding.9
Footnotes
Disclosures: Frédéric Clarençon—UNRELATED: Payment for lectures including service on speakers bureaus: Medtronic. Nader Sourour—UNRELATED: Consultancy: Medtronic, Microvention; Stock/stock options: Medina/Medtronic.
References
- 1. Rammos SK, Gardenghi B, Bortolotti C, et al. Aneurysms associated with brain arteriovenous malformations. AJNR Am J Neuroradiol 2016. June 23. [Epub ahead of print] 10.3174/ajnr.A4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCormick WF. The pathology of vascular (“arteriovenous”) malformations. J Neurosurg 1966;24:807–16 10.3171/jns.1966.24.4.0807 [DOI] [PubMed] [Google Scholar]
- 3. Mandybur TI, Nazek M. Cerebral arteriovenous malformations. A detailed morphological and immunohistochemical study using actin. Arch Pathol Lab Med 1990;114:970–73 [PubMed] [Google Scholar]
- 4. Jellinger K. Vascular malformations of the central nervous system: a morphological overview. Neurosurg Rev 1986;9:177–216 [DOI] [PubMed] [Google Scholar]
- 5. Deshpande DH, Vidyasagar C. Histology of the persistent embryonic veins in arteriovenous malformations of brain. Acta Neurochir (Wien) 1980;53:227–36 [DOI] [PubMed] [Google Scholar]
- 6. Mjoli N, Le Feuvre D, Taylor A. Bleeding source identification and treatment in brain arteriovenous malformations. Interv Neuroradiol 2011;17:323–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turjman F, Massoud TF, Viñuela F, et al. Correlation of the angioarchitectural features of cerebral arteriovenous malformations with clinical presentation of hemorrhage. Neurosurgery 1995;37:856–60; discussion 860–62 [DOI] [PubMed] [Google Scholar]
- 8. Redekop G, TerBrugge K, Montanera W, et al. Arterial aneurysms associated with cerebral arteriovenous malformations: classification, incidence, and risk of hemorrhage. J Neurosurg 1998;89:539–46 10.3171/jns.1998.89.4.0539 [DOI] [PubMed] [Google Scholar]
- 9. Meisel HJ, Mansmann U, Alvarez H, et al. Cerebral arteriovenous malformations and associated aneurysms: analysis of 305 cases from a series of 662 patients. Neurosurgery 2000;46:793–800; discussion 800–02 [DOI] [PubMed] [Google Scholar]
- 10. Atkinson RP, Awad IA, Batjer HH, et al. Reporting terminology for brain arteriovenous malformation clinical and radiographic features for use in clinical trials. Stroke 2001;32:1430–42 [DOI] [PubMed] [Google Scholar]
- 11. Lescher S, Gehrisch S, Klein S, et al. Time-resolved 3D rotational angiography: display of detailed neurovascular anatomy in patients with intracranial vascular malformations. J Neurointerv Surg 2016. August 4. [Epub ahead of print] 10.1136/neurintsurg-2016-012462 [DOI] [PubMed] [Google Scholar]
- 12. Clarençon F, Maizeroi-Eugène F, Bresson D, et al. Elaboration of a semi-automated algorithm for brain arteriovenous malformation segmentation: initial results. Eur Radiol 2015;25:436–43 10.1007/s00330-014-3421-5 [DOI] [PubMed] [Google Scholar]
- 13. Clarençon F, Maizeroi-Eugène F, Maingreaud F, et al. Interest of convex spherical anamorphosis in better understanding of brain AVMs' angioarchitecture. J Neurointerv Surg 2016;8:959–64 10.1136/neurintsurg-2015-011888 [DOI] [PubMed] [Google Scholar]
- 14. Omodaka S, Endo H, Fujimura M, et al. High-grade cerebral arteriovenous malformation treated with targeted embolization of a ruptured site: wall enhancement of an intranidal aneurysm as a sign of ruptured site. Neurol Med Chir (Tokyo) 2015;55:813–17 10.2176/nmc.cr.2015-0052 [DOI] [PMC free article] [PubMed] [Google Scholar]