Abstract
BACKGROUND AND PURPOSE:
Large-bore catheters allow mechanical thrombectomy in ischemic stroke by engaging and retrieving clots without additional devices (direct aspiration first-pass technique [ADAPT]). The purpose of this study was to establish a model for minimal catheter diameters needed for ADAPT.
MATERIALS AND METHODS:
We established a theoretic model for the calculation of minimal catheter diameters needed for ADAPT. We then verified its validity in 28 ADAPT maneuvers in a porcine in vivo model. To account for different mechanical thrombectomy techniques, we factored in ADAPT with/without a hypothetic 0.021-inch microcatheter or 0.014-inch microwire inside the lumen of the aspiration catheter and aspiration with a 60-mL syringe versus an aspiration pump.
RESULTS:
According to our calculations, catheters with an inner diameter of >0.040 inch and >0.064 inch, respectively, are needed to be effective in the middle cerebral artery (2.5-mm diameter) or in the internal carotid artery (4 mm) in an average patient. There was a significant correlation between predicted and actual thrombectomy results (P = .010). Our theoretic model had a positive and negative predictive value of 78% and 79%, respectively. Sensitivity and specificity were 88% and 64%, respectively.
CONCLUSIONS:
Our theoretic model allows estimating the minimal catheter diameters needed for successful mechanical thrombectomy with ADAPT, as demonstrated by the good agreement with our animal experiments. Our model will be helpful to interventionalists in avoiding selecting catheters that are likely too small to be effective.
Endovascular mechanical thrombectomy (MT) with stent retrievers is the most effective treatment option for acute ischemic stroke caused by large-vessel occlusion.1 Newly developed large-bore catheters, which can be placed in close proximity to the intracranial occlusion site, allow engagement and retrieval of a clot without additional devices (so-called direct aspiration first-pass technique [ADAPT]).2 The concept of ADAPT is to engage a clot, clog the catheter tip, and retrieve the catheter and clot together. This simple technique is promising for establishing MT in a wider range of hospitals and may reduce the risk of procedure-related subarachnoid hemorrhage.3,4 However, the effectiveness of ADAPT is an issue that needs to be resolved; failure rates for ADAPT and the need to change the MT strategy have been reported in 22%–44% of cases.4–7
The size and composition of the clot on the one hand and suction force at the catheter tip on the other are supposedly the most crucial factors for successful MT with ADAPT.8 While the size and composition of a clot cannot be influenced, the force at the catheter tip is the product of applied pressure and cross-sectional area of the catheter. Consequently, the catheter with the largest tip diameter will apply the greatest force. Instinctively, one would think it would be best to follow the principle of “the bigger the better” and use the largest available catheter, but smaller catheters leave more spare lumen in the access catheter for proximal aspiration and have the advantage of better maneuverability and therefore allow easier and less traumatic access to the occlusion site. Hence, the ideal catheter is as small as possible and as large as necessary. However, the force needed to engage a given clot is unknown. The purpose of this study was to develop a theoretic model for calculating the minimal catheter diameters necessary for MT with ADAPT and to validate this model in an in vivo porcine experiment.
Materials and Methods
Theoretic Model
For clot retrieval, the force at the catheter tip must exceed the force that keeps the clot in position. In a minimal model, the total force effectively acting on a clot can be expressed as FTotal = FAspiration + FAdhesion (Fig 1). To estimate these forces (see below), we used a simplified model, in which the clot is a fully occlusive impervious rigid body, the vessel is a rigid tube with a constant diameter, and there is no flow in the aspiration catheter and consequent head loss. Furthermore, our model neglects possible contributing factors such as the thickness of the catheter wall and pulsatile blood flow around the clot.
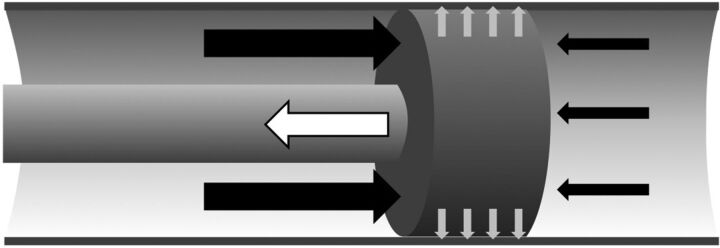
Fig 1.
Schematic depicting forces affecting the clot. The suction force at the tip of the catheter (white arrow) must exceed the force of the blood pressure (black arrows) and the adhesion force that hold the clot in its position (gray arrows). Arrows represent the direction and idealized amount of force.
FAspiration is the suction force at the tip of the aspiration catheter and is defined as FAspiration = ACatheterΔPCatheter, with ACatheter being the area at the tip and ΔPCatheter being the pressure in the tubing system (white arrow in Fig 1). The respective surface is subtracted from the area of the catheter tip to account for variations of the ADAPT technique with a microcatheter or a microwire introduced into the catheter.
FPressure is determined by the pressure difference before and behind the clot and the surface of the clot exposed to this pressure. Hence FPressure = AclotΔPVessel, with AClot being the area of the clot minus the area of the catheter tip and ΔPVessel being the intravascular pressure difference before and behind the clot (black arrows in Fig 1). The effective pressure difference depends on the presence of collaterals that maintain blood pressure behind the clot.9 In our model, we use a gradient of 60 mm Hg, which Sorimachi et al10 have assessed on average in occlusions of the internal carotid artery and the M1 segment of the MCA in 36 patients with stroke.
The adhesion force (FAdhesion) between the clot and the vessel wall is unknown and may be small when it is determined by mechanical friction alone or very large if the clot is wedged or if there is protein binding between the clot and the vessel wall (gray arrows in Fig 1).11 Because a clot does not constantly migrate in a vessel, the minimal adhesion force that keeps the clot in position can be estimated as FAdhesion ≥ FPressure, which equals FAdhesion = C FPressure, with C being a constant that is ≥1. Romero et al11 estimated the adhesion forces between clots and the vessel wall in the MCA with a bond graph model for the aspiration device and found that typical adhesion forces ranged between 0.01 and 0.1 N. Chueh et al12 experimentally determined adhesion forces in a rabbit model and indicated adhesion forces of 0.7 N after 5 hours of clot/vessel interaction. These values lie in the range of our model, which provides for C = 1 adhesion forces between 0.014 and 0.16 N for clotted vessels with diameters between 1.5 and 5 mm, respectively.
Hence, FTotal = π(RCatheter2 − RInnerCatheter2)ΔPCatheter + π(RVessel2 − RCatheter2)ΔPVessel + πRVessel2ΔPVessel, with R representing the respective radii. The minimal catheter radius needed to move the clot, FTotal ≤ 0, can be calculated as follows:
We calculated the catheter diameters needed for ADAPT in vessels with diameters from 1.5 to 5 mm, which are typical for cerebral arteries. To take into account different MT techniques, we compared ADAPT techniques with/without a hypothetic 0.021-inch microcatheter (outer diameter, 0.8 mm) or a 0.014-inch microwire (outer diameter, 0.46 mm) inside the lumen of the aspiration catheter and factored in vacuum pressure generated with a 60-mL syringe (experimentally determined maximal vacuum pressure of −0.89 bar) or generated with an aspiration pump ([Pump MAX; Penumbra, Alameda, California], vacuum pressure of −0.86 bar, which corresponds to the manufacturer's recommended pressure).
Animal Model
To verify our theoretic model, we performed ADAPT maneuvers in an in vivo porcine animal model and compared the predictions with the actual results. All experiments were performed in 2 female Landrace swine (average weight, 58 kg) with peri- and intrainterventional management as reported previously.13 The experiments were performed in accordance with the German legislation governing animal studies following the Guide for the Care and Use of Laboratory Animals (National Research Council, 8th ed, 2011) and the “Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes” (Official Journal of the European Union, 2010). Official permission was granted from the governmental animal care and use office (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen, Recklinghausen, Germany).
One week before the procedures, we produced whole-blood clots in a Chandler loop to have radiopaque and solid clots that can be engaged with the aspiration catheter without being aspirated into it.14,15 We then injected the clots into various branches of the subclavian artery with diameters ranging from 1.5 to 6 mm. Before the ADAPT maneuvers, we measured the diameters of the occluded vessels as well as the pressure before and behind the clot with a Trevo Pro 18 microcatheter (Stryker, Kalamazoo, Michigan) using a PowerLab 16/35 workstation (AdInstruments, Dunedin, New Zealand) and LabChart 8 Software (AdInstruments). Before the ADAPT maneuvers, there was a pause of at least 10 minutes to allow reocclusion of the channel produced by the microcatheter. ADAPT maneuvers were conducted approximately 20–60 minutes after injection of the clots. We performed ADAPT maneuvers with Sofia 5F and 6F aspiration catheters (MicroVention, Tustin, California), which were introduced through an 8F long sheath (Flexor Shuttle Guiding Sheath; Cook, Bloomington, Indiana). Aspiration was applied with a an aspiration pump (Penumbra Pump MAX), which was connected to the aspiration catheter via a standard 3-way valve (Discofix C; Braun, Melsungen, Germany) and a hemostatic Gateway Adapter (Boston Scientific, Fremont, California) with the standard tubing, on the recommended setting of 25.5 inHg (≈0.86 bar). Because our aim was to verify our ADAPT model, ADAPT was only considered successful if the clot could be engaged (indicated by clogging of the aspiration catheter) and removed with 1 pass (Fig 2), whereas complete ingestion of the clot was not regarded as successful. To prevent ingestion of a clot, the thrombectomy maneuver was performed 5–10 seconds after presumed contact between the catheter and the clot.
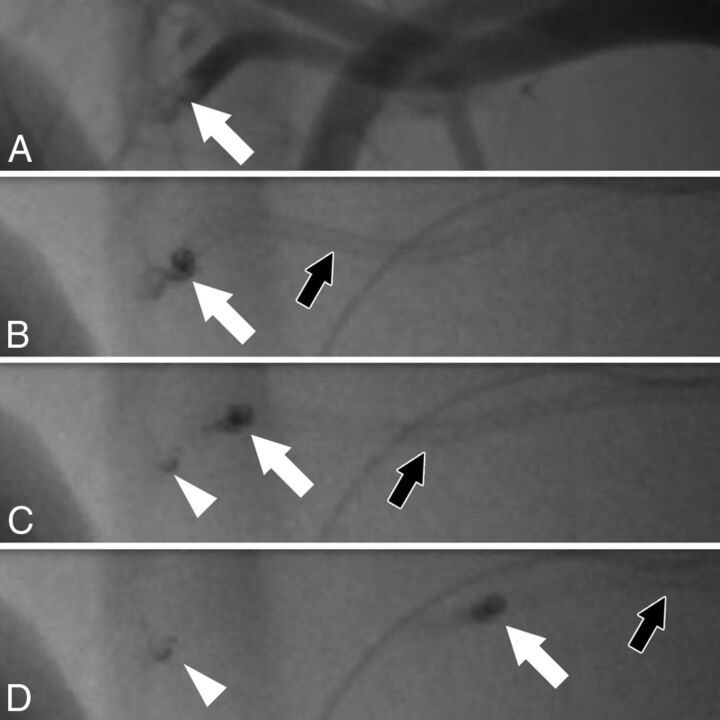
Fig 2.
Fluoroscopic angiography of an ADAPT maneuver. Angiography shows a clot (A, white arrow) in a branch of the axillary artery with a diameter of 1.9 mm. The pressure gradient before and behind the clot is 38 mm Hg, necessitating an aspiration catheter with an inner diameter of at least 1.9F for clot removal, according to our calculations. The clot (A–D: white arrow), which is partially radiopaque, is engaged with a Sofia 5F catheter (B, black arrow). When the catheter is pulled back (C and D, black arrow), the larger portion of the clot can be removed (C and D, white arrow). However, there is fragmentation of the clot, with a small portion of the clot remaining in the vessel (C and D, arrowhead).
Statistical Analysis
Continuous parametric variables are presented as means ± SD; ordinal and nonparametric variables, as medians; and categoric variables, as frequencies. Fisher exact, χ2, Student t, and Mann-Whitney U tests were used whenever applicable after testing our data for normal distribution with a Shapiro-Wilk test. P values of an α level ≤ .05 were significant. All statistical analyses were performed with SPSS 23 software (IBM, Armonk, New York).
Results
Theoretic Model
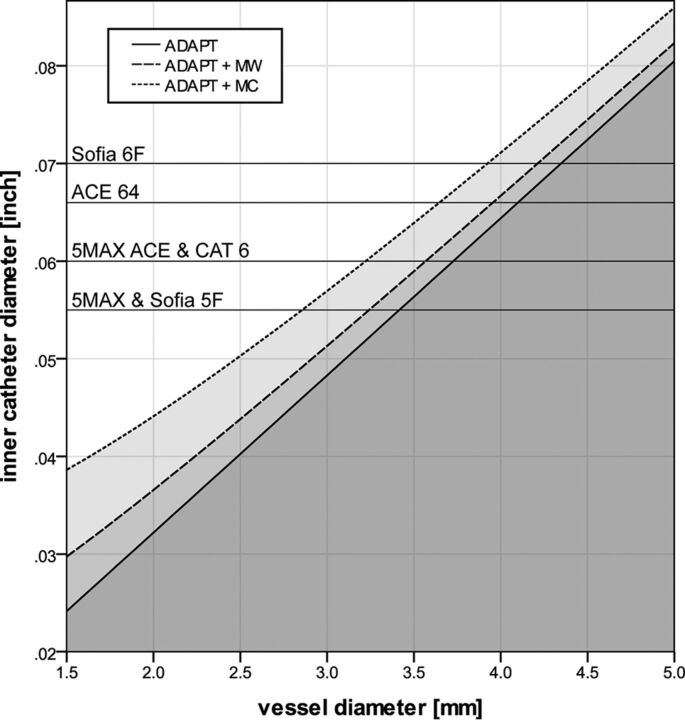
Figure 3 depicts the minimum inner diameters needed to overcome the force that keeps a clot in its position in a vessel with a given diameter using ADAPT with manual aspiration in an average patient with stroke. Using an additional microcatheter necessitates a significantly larger aspiration catheter (P ≤ .024), but introducing a microwire does not (P ≥ .368). When a pump instead of a syringe is used for aspiration, catheters need to be approximately 1% larger than indicated in Fig 3. However, the use of a pump instead of a syringe has no significant impact on the required catheter diameters (P = .839).
Fig 3.
Correlations between minimal catheter size and vessel diameter in an average patient. Graphs represent the minimal inner catheter diameter (y-axis) needed to overcome the force that keeps a clot in its position in a vessel with a given diameter (x-axis), using ADAPT with manual aspiration in an average patient (ie, a pressure gradient of 60 mm Hg before and behind the clot). The continuous line represents the ADAPT technique without microcatheters or microwires in the aspiration catheter. Dotted lines represent the ADAPT technique with an additional microcatheter (MC) or microwire (MW) in the aspiration catheter. Gray areas under the curves correspond to catheter diameters that are not large enough for ADAPT. Given the lower pressure provided by a pump, catheters need to be approximately 1% larger than indicated in the figure when a pump instead of a syringe is used for aspiration. Black horizontal lines represent the inner diameters of various commercially available catheters: Sofia (MicroVention); AXS Catalyst 6 (CAT6; Stryker); and 5MAX, 5MAX ACE, and ACE64 (Penumbra).
Animal Model
We performed 28 ADAPT maneuvers, 19 of which were with the Sofia 5F catheter and 9 with the Sofia 6F catheter. The mean vessel diameter was 3.8 ± 1.1 mm, ranging from 1.9 to 5.9 mm. The median pressure gradient before and behind the clot was 39.5 mm Hg (interquartile range, 34 mm Hg), ranging from 2 to 116 mm Hg. According to our model predictions, our catheters were oversized by 0.11 ± 0.020 inch on average, ranging from largely undersized cases (−0.030 inch) to largely oversized cases (+0.043 inch).
Nine maneuvers were performed with catheters that were supposedly too small for successful thrombectomy according to our calculations (Sofia 5F, n = 6, and Sofia 6F, n = 3). In fact, 7 of these 9 maneuvers failed, and 2 were successful (1 case each with a Sofia 5F and 6F). Conversely, 4 of 19 maneuvers that were predicted to be successful failed (2 cases each with a Sofia 5F and 6F). There was a significant correlation between predicted and actual thrombectomy results (P = .010). Our theoretic model had a positive and negative predictive value of 78% and 79%, respectively. Sensitivity and specificity were 88% and 64%, respectively.
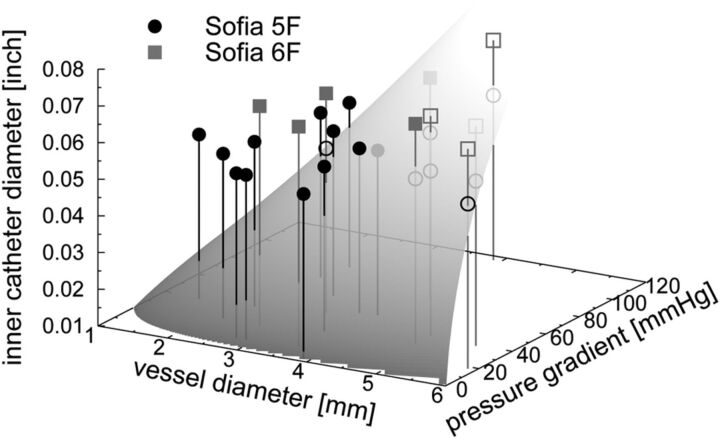
Figure 4 depicts the correlation among vessel diameter, pressure gradient, catheter diameter, and predicted-versus-actual outcome of all 28 ADAPT maneuvers. Successful ADAPT maneuvers were performed in significantly smaller vessels (3.1 ± 0.8 mm versus 4.7 ± 0.9 mm; P < .001) and with significantly oversized catheters (+0.21 ± 0.016 inch versus −0.006 ± 0.013 inch; P < .001). Pressure gradients were comparable in successful and unsuccessful cases (median, 38.0 mm Hg, versus 41.0 mm Hg; P = .378). The 4 cases in which the ADAPT failed unexpectedly were performed in significantly larger vessels compared with cases with correct predictions (4.9 ± 1.3 mm versus 3.5 ± 1.1 mm; P = .025). Catheters in both groups had a comparable marginal oversize (+0.008 ± 0.006 inch versus +0.012 ± 0.022 inch; P = .437), and pressure gradients did not differ significantly (median, 29.5 mm Hg, versus 39.0 mm Hg; P = .607).
Fig 4.
Plot of all 28 ADAPT experiments. The shaded plane indicates the minimal inner catheter diameter (z-axis) needed to engage a clot in a vessel with a given diameter (x-axis) when a specific pressure gradient (y-axis) is applied. Filled and open symbols indicate experiments, in which the removal of the clot was successful or failed, respectively. In case of perfect agreement between theory and experiment, all filled symbols lie above the dividing plane, whereas all open symbols lie below it. Note that most deviating points (open symbols above the shaded plane and filled symbols below the shaded plane) are located so close to the dividing surface that small variations in the measurement of the vessel diameter and/or pressure gradient could lead to a substantially better agreement between theory and experiments.
Discussion
The rationale of the ADAPT technique is to engage a clot with a large-bore catheter and establish constant adherence between the clot and the catheter with suction force. This force can be calculated easily, given that it is the product of the cross-sectional area at the catheter tip and the pressure. Because the cross-sectional area increases by the square of the radius, small changes of diameter result in a large change of force. For instance, a 27% increase of the inner diameter of the Sofia 6F catheter compared with the Sofia 5F catheter (0.07 versus 0.055 inch) results in a 62% increase of force. Recently, Nikoubashman et al16 and Hu and Stiefel8 have characterized various commercially available catheters and calculated the flow through them and the force at the tip of these catheters. However, knowledge of this force is of little use if the force needed to engage a clot is unknown. We approached this problem with a simplified theoretic model and calculated the minimal catheter diameter necessary for clot retrieval with ADAPT. A significant correlation between our experimental and theoretic models validates the latter and provides a justification for the approximations used (Fig 4).
We estimated a minimal catheter diameter below which MT is unlikely to be successful in an average patient with stroke (Fig 3). The negative predictive value of 78% and the positive predictive value of 79% derived from our in vivo experiment support the hypothesis that there is a minimal catheter size required for ADAPT but that larger catheters do not necessarily result in successful recanalization. We calculated that in an average patient, catheters with an inner diameter of >0.040 and >0.064 inch, respectively, are needed to be effective in an MCA with a diameter of 2.5 mm or in the terminal segment of the internal carotid artery with a diameter of 4 mm (Fig 3). If collaterals are better than in the average patient and there is a pressure gradient of 40 mm Hg instead of 60 mm Hg, catheters with an inner diameter of >0.033 and >0.053 inch, respectively, would be sufficient to be effective in the MCA and terminal segment of the internal carotid artery. Hence, our calculations imply that in most cases, the available aspiration catheters should be sufficient for ADAPT in the MCA and that the aspiration catheters of the newest generation are sufficient to extract most occlusions in the average patient (Fig 3). This finding is in accordance with results by Turk et al,5 who reported that recanalization (TICI 2b/3a) was achieved often with the 5MAX catheter (Penumbra) (75%) but significantly more often with the larger 5MAX ACE catheter (Penumbra) (82%). Conversely, this result is also in line with our clinical experience that ADAPT with smaller catheters (ie, 5F) is more frequently (but not always) successful in the MCA than in the internal carotid artery. However, conclusive data to support this hypothesis are lacking because no study has specifically addressed the correlation between target vessel and recanalization, to our knowledge.
Turk et al5 published a series of 100 ADAPT cases with 15% ICA occlusions, which were predominantly treated with 5MAX and 5MAX ACE catheters and reported successful recanalization (TICI 2b/3) in 78% of all cases. Supporting the hypothesis that ADAPT recanalization in the ICA is less likely to be successful, Kowoll et al7 had a higher proportion of ICA occlusions (26%) and a lower recanalization rate of 56% in their series of 54 patients while exclusively using the larger 5MAX ACE catheter. However, Delgado Almandoz et al,4 who treated 45 patients with an even higher proportion of ICA occlusions (42%), reported a comparably high recanalization rate of 71%, using the 5MAX ACE in most cases (89%). Also on the contrary, Möhlenbruch et al,6 who had a comparably low proportion of ICA occlusions (17%) in their series of 85 patients, used the largest available catheter (Sofia 6F) and reported a relatively low recanalization rate of 65%. This lower rate may be partly due to varying study designs, because Möhlenbruch et al performed 1.5 passes on average before changing the MT strategy, whereas Delgado Almandoz et al performed 2.5 passes. Also, various nonmanipulable factors such as collateral situation (ie, pressure gradient along the clot) and clot composition may have had an impact on recanalization results.
In summary, our theoretic model is a helpful tool to select the most efficient catheters, also for techniques in which a combination of stent-retriever thrombectomy and ADAPT is used.17,18 The strength of our ADAPT model is that it considers all relevant forces in the system that play a role when the clot is engaged with the catheter. Flows, however, which are altered by resistors such as 3-way valves, hemostatic valves, and additional tubing for the aspiration system, play no considerable role in ADAPT when contact between the catheter and the clot is established and the force at its tip becomes constant. Hence, our model requires only knowledge of the applied vacuum pressure and the diameter of the vessel to estimate the necessary catheter diameter. Thus, our model lends itself to clinical situations in which time is of the essence.
However, even though we made great effort to validate our model, it has several limitations that might affect its accuracy: First, the nature of our experiments did not allow a systematic analysis of all possible settings because we were restricted by the porcine anatomy. Also, our theoretic model does not consider actual collateral status and adhesion force and clot burden; this feature is likely the reason why our model did not correctly predict the failure of clot removal in very large vessels. In addition, our model does not account for wedging of clots, which may occur in bifurcations and make thrombectomy more difficult. Our model also neglects clot composition, which possibly has an impact on clot fragmentation (Fig 2) and thrombectomy by clot aspiration. This is a potentially important mechanism in the context of soft clots and large-bore catheters and may have occurred unnoticed in some of our experiments. Last, vessel access (ie, balloon catheter versus large sheath), which may have an impact on thrombectomy results, was an unstudied factor in our model. Despite these limitations, the good agreement between our theoretic model and our experimental results implies that our theoretic model captures the essential physics of the problem, and our study provides a useful step toward better understanding and controlling clot removal with ADAPT.
Conclusions
Our theoretic model allows estimating the minimal catheter diameters needed for successful MT with ADAPT, as demonstrated by the good agreement with our animal experiments. Our model may be helpful to interventionalists in avoiding selecting catheters that are too small to be effective.
Acknowledgments
The authors thank Johanna Sandmann, Franziska Müschenich, and Thorsten Sichtermann for their helpful contributions during data acquisition. Arash Nikoubashman acknowledges support from the German Research Foundation (DFG) under project number NI 1487/2-1.
ABBREVIATIONS:
- ADAPT
direct aspiration first-pass technique
- MT
mechanical thrombectomy
Footnotes
Disclosures: Martin Wiesmann—UNRELATED: Consultancy: Stryker Neurovascular; Payment for Lectures Including Service on Speakers Bureaus: Bracco, Siemens, Stryker Neurovascular; Payment for Development of Educational Presentations: Abbott, abmedica, Acandis, Bayer HealthCare, Bracco, B. Braun Melsungen, Codman Neurovascular, Covidien, Dahlhausen, MicroVention, Penumbra, phenox, Philips Healthcare, Siemens, Silk Road Medical, St. Jude, Stryker Neurovascular.
References
- 1. Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 2. Turk AS, Spiotta A, Frei D, et al. Initial clinical experience with the ADAPT technique: a direct aspiration first pass technique for stroke thrombectomy. J Neurointerv Surg 2014;6:231–37 10.1136/neurintsurg-2013-010713 [DOI] [PubMed] [Google Scholar]
- 3. Nikoubashman O, Reich A, Pjontek R, et al. Postinterventional subarachnoid haemorrhage after endovascular stroke treatment with stent retrievers. Neuroradiology 2014;56:1087–96 10.1007/s00234-014-1424-1 [DOI] [PubMed] [Google Scholar]
- 4. Delgado Almandoz JE, Kayan Y, Young ML, et al. Comparison of clinical outcomes in patients with acute ischemic strokes treated with mechanical thrombectomy using either Solumbra or ADAPT techniques. J Neurointerv Surg 2016;8:1123–28 10.1136/neurintsurg-2015-012122 [DOI] [PubMed] [Google Scholar]
- 5. Turk AS, Frei D, Fiorella D, et al. ADAPT FAST study: a direct aspiration first pass technique for acute stroke thrombectomy. J Neurointerv Surg 2014;6:260–64 10.1136/neurintsurg-2014-011125 [DOI] [PubMed] [Google Scholar]
- 6. Möhlenbruch MA, Kabbasch C, Kowoll A, et al. Multicenter experience with the new SOFIA Plus catheter as a primary local aspiration catheter for acute stroke thrombectomy. J Neurointerv Surg 2016. December 20. [Epub ahead of print] 10.1136/neurintsurg-2016-012812 [DOI] [PubMed] [Google Scholar]
- 7. Kowoll A, Weber A, Mpotsaris A, et al. Direct aspiration first pass technique for the treatment of acute ischemic stroke: initial experience at a European stroke center. J Neurointerv Surg 2016;8:230–34 10.1136/neurintsurg-2014-011520 [DOI] [PubMed] [Google Scholar]
- 8. Hu YC, Stiefel MF. Force and aspiration analysis of the ADAPT technique in acute ischemic stroke treatment. J Neurointerv Surg 2016;8:244–46 10.1136/neurintsurg-2014-011563 [DOI] [PubMed] [Google Scholar]
- 9. Sorimachi T, Fujii Y, Tsuchiya N, et al. Blood pressure in the artery distal to an intraarterial embolus during thrombolytic therapy for occlusion of a major artery: a predictor of cerebral infarction following good recanalization. J Neurosurg 2005;102:870–78 10.3171/jns.2005.102.5.0870 [DOI] [PubMed] [Google Scholar]
- 10. Sorimachi T, Morita K, Ito Y, et al. Blood pressure measurement in the artery proximal and distal to an intra-arterial embolus during thrombolytic therapy. J Neurointerv Surg 2011;3:43–46 10.1136/jnis.2010.003061 [DOI] [PubMed] [Google Scholar]
- 11. Romero G, Higuera I, Martinez ML, et al. Analysis and simulation of the adhesion forces between clot and the artery wall for a novel thrombectomy device applied to the middle cerebral artery. In: Proceedings of the 2010 12th International Conference on Computer Modelling and Simulation (UKSim 2010), Cambridge UK. March 24–26, 2010;195–200 [Google Scholar]
- 12. Chueh J, Kuhn AL, Mehra M, et al. Embolus adhesion to activated endothelium after embolization: a parameter to predict outcomes of mechanical thrombectomy in acute ischemic stroke. Stroke 2012;43(suppl 1):A3750 [Google Scholar]
- 13. Nikoubashman O, Pjontek R, Brockmann MA, et al. Retrieval of migrated coils with stent retrievers: an animal study. AJNR Am J Neuroradiol 2015;36:1162–66 10.3174/ajnr.A4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Robbie LA, Young SP, Bennett B, et al. Thrombi formed in a Chandler loop mimic human arterial thrombi in structure and RAI-1 content and distribution. Thromb Haemost 1997;77:510–15 [PubMed] [Google Scholar]
- 15. Gralla J, Schroth G, Remonda L, et al. A dedicated animal model for mechanical thrombectomy in acute stroke. AJNR Am J Neuroradiol 2006;27:1357–61 [PMC free article] [PubMed] [Google Scholar]
- 16. Nikoubashman O, Alt JP, Nikoubashman A, et al. Optimizing endovascular stroke treatment: removing the microcatheter before clot retrieval with stent-retrievers increases aspiration flow. J Neurointerv Surg 2017;9:459–62 10.1136/neurintsurg-2016-012319 [DOI] [PubMed] [Google Scholar]
- 17. Massari F, Henninger N, Lozano JD, et al. ARTS (Aspiration-Retriever Technique for Stroke): initial clinical experience. Interv Neuroradiol 2016;22:325–32 10.1177/1591019916632369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maus V, Behme D, Kabbasch C, et al. Maximizing first-pass complete reperfusion with SAVE. Clin Neuroradiol 2017. February 13. [Epub ahead of print] 10.1007/s00062-017-0566-z [DOI] [PubMed] [Google Scholar]