Abstract
BACKGROUND AND PURPOSE:
Whether ADC value predicts the therapy response and outcomes of primary central system lymphoma remains controversial. This study assessed the minimum ADC correlated with treatment response in patients with primary central nervous system lymphoma undergoing methotrexate-based chemotherapy.
MATERIALS AND METHODS:
Thirty-five patients with primary central nervous system lymphoma underwent conventional MR imaging and DWI before chemotherapy and after 1 and 5 cycles of chemotherapy. Treatment response was determined according to the International PCNSL Collaborative Group criteria and was classified as a complete response, partial response, or progressive disease. Pretreatment minimum ADC, minimum ADC after 1 cycle, minimum ADC after 5 cycles, and change in minimum ADC were compared among the different response groups. The Pearson correlation test was calculated between these ADC parameters and tumor response.
RESULTS:
The pretreatment minimum ADC of the progressive disease group was lower than that of the complete response and partial response groups, but there was no significant difference among them. The minimum ADC after 1 cycle and minimum ADC after 5 cycles were statistically significantly higher than the pretreatment minimum ADC. A comparison among groups showed that minimum ADC after 1 cycle, minimum ADC after 5 cycles, minimum ADC change, and the percentage of minimum ADC change were all significantly different among the 3 groups. A significant positive correlation was observed between the percentage of minimum ADC after 1 cycle of chemotherapy and the size reduction percentage after 5 cycles of chemotherapy. The minimum ADC change and the percentage of minimum ADC change performed better in the differentiation of the final treatment response, specifically in complete response and partial response from progressive disease.
CONCLUSIONS:
The minimum ADC after 1 cycle and minimum ADC changes were better correlated with the treatment response than the pretreatment minimum ADC. Minimum ADC after early therapy may potentially to be used to predict and monitor the response of primary central nervous system lymphoma to chemotherapy.
Primary central nervous system lymphoma (PCNSL) is a rare subtype of non-Hodgkin lymphoma, which is confined to the brain, eyes, and/or leptomeninges. The overall incidence of PCNSL in the immunocompetent population has been increasing during the past several years, and it is one of the few malignant primary brain tumors that is sensitive to both chemotherapy and radiation therapy.1,2 Methotrexate-based chemotherapy is the cornerstone of therapy in PCNSL, while whole-brain irradiation is recommended for patients with recidivation. Patients who are at low risk of tumor recidivation may benefit from methotrexate-based chemotherapy alone. On the contrary, high-risk patients may require further chemotherapy and/or whole-brain irradiation for consolidation of the response.3,4
Until recently, no proved imaging biomarkers could indicate tumor refractoriness to methotrexate-based chemotherapy and predict therapeutic outcomes in PCNSL treatment. Such biomarkers would provide clinicians with strong evidence for making clinical decisions, which might generate the early start of second-line salvage therapy in high-risk patients with PCNSL and likely contribute to personalized therapeutic strategies to detect tumor recurrence without delay and improve prognosis.5
Noninvasive MR diffusion-weighted imaging of the brain is based on the differential diffusion rates or the Brownian motion of water. It is an essential technique used to diagnose acute infarct in the brain, due to its ability to detect cytotoxic edema derived by altered water diffusion due to cellular damage. DWI has also been widely used in neuro-oncology to assess tumor pathology characteristics.6 Specifically, the apparent diffusion coefficient values derived from DWI have been shown to correlate with tumor cellularity, glioma grade, and treatment response.6–9 Some previous research has also suggested that pretherapeutic ADC values in tumor may be biomarkers noninvasively predictive of treatment response in patients with PCNSL.5 However, a number of other studies have not shown tumor ADC metrics to be predictive of response.10
To our knowledge, no research has reported whether dynamic changes of ADC values are related to treatment responses in patients with PCNSL. Thus, we assessed pre- and posttherapeutic ADC values in responsive or prognostic subgroups of patients with PCNSL in an effort to identify which tumors may experience recurrence and disease progression at an early stage.
Materials and Methods
Patient Population
Thirty-five patients with biopsy-proved PCNSL (26 men, 9 women; mean age, 58 ± 15 years; all immunocompetent) treated at the Huashan Hospital of Fudan University between October 2007 and February 2010 were selected for this retrospective study.
Inclusion criteria were patients being 18 years of age or older; histologic confirmation of PCNSL by stereotactic needle biopsy; serology negative for human immunodeficiency virus; no evidence of systemic non-Hodgkin lymphoma as demonstrated by PET-CT or CT of the chest, abdomen, and pelvis and bone marrow aspirate and biopsy; and baseline laboratory values being leukocytes ≥ 4000/L, platelets ≥ 100,000/L, and creatinine ≤ 1.5 mg/dL or creatinine clearance ≥ 50 cm3/min/1.73 m2 without any corticosteroids before treatment. All patients had a pathologic diagnosis of diffuse large B-cell PCNSL as defined by the World Health Organization and received methotrexate-based induction chemotherapy. Of the 53 patients who met the inclusion criteria for this investigation, 48 were studied on the basis of having undergone pretherapeutic contrast-enhanced MR imaging of the brain with DWI. Furthermore, 35 of the 48 patients had pretherapeutic, interval, and posttreatment follow-up contrast-enhanced MR imaging of the brain with DWI. Exclusion criteria consisted of any other active primary malignancy, preexisting immunodeficiency, and prior treatment for PCNSL. Pretreatment evaluations included baseline ophthalmologic examination (including dilated fundus examination, slit lamp examination, and color photography of the posterior pole) to assess ocular involvement and lumbar puncture to assess leptomeningeal involvement.
Treatment and Response Evaluation
Each methotrexate treatment cycle was administered in the hospital setting. Every patient received methotrexate-based chemotherapy, including methotrexate, 3–8 g/m2/day 1, + dexamethasone, 15 mg/days 1–3, + idarubicin, 15 mg/day 2 and every 21 days for 5 cycles. The response was assessed by using the International PCNSL Collaborative Group criteria, based on imaging, corticosteroid use, CSF cytology, and slit lamp examination in cases of CSF or ocular involvement (Table 1).11 Patients who achieved a complete response (CR) were defined as having resolution of contrast-enhancing lesions on follow-up MR imaging and, if indicated, by CSF cytologic analysis (if the CSF cytology was positive for malignant cells at the time of diagnostic staging). Patients who achieved a partial response (PR) were defined as an interval decrease in contrast-enhancing lesion volume. Progressive disease (PD) were defined as an interval increase in contrast-enhancing lesion volume or the development of new enhancing lesions on follow-up contrast-enhanced MR imaging or involve the eye or CSF.5 All follow-up imaging was performed no longer than 2 weeks after completion of all planned therapy to assess overall treatment response.12
Table 1:
Response criteria for primary central nervous system lymphoma
| Response | Brain Imaging | Corticosteroid Dose | Eye Examination Findings | CSF Cytology Findings |
|---|---|---|---|---|
| CR | No contrast enhancement | None | Normal | Negative |
| CRu | No contrast enhancement | Any | Normal | Negative |
| Minimal abnormality | Any | Minor RPE abnormality | Negative | |
| PR | 50% Decrease in enhancing tumor | Irrelevant | Minor RPE abnormality or normal | Negative |
| No contrast enhancement | Irrelevant | Decrease in vitreous cells or retinal infiltrate | Persistent or suspicious | |
| PD | 25% Increase in lesion | Irrelevant | Recurrent or new ocular disease | Recurrent or positive |
| Any new site of disease: CNS or systemic |
Note:—CRu indicates unconfirmed complete response; RPE, retinal pigment epithelium.
MR Imaging Protocol
Patients underwent MR imaging before receiving their first course of therapy (pretreatment); at an earlier stage of <48 hours after the end of the patient's first chemotherapy cycle, as indicated by the last chemotherapeutic injection (early treatment); and at the completion of all the standard treatments associated with the regular review of clinical time (posttreatment). None of the patients had begun corticosteroid treatment, radiation therapy, or chemotherapy or had a previous brain biopsy at the first MR imaging.
All patients were imaged by using a 3T clinical MR imaging scanner (Tim Trio; Siemens, Erlangen, Germany). MR imaging examinations included conventional contrast-enhanced T1-weighted imaging and DWI sequences obtained according to a standardized protocol: axial T1-weighted spin-echo (TR/TE, 2000/17 ms), axial T2-weighted fast spin-echo (TR/TE, 3000/98 ms), axial fluid-attenuated inversion recovery (TR/TE/TI, 8000/102/2200 ms), axial diffusion-weighted echo-planar imaging (TR/TE, 5000/82 ms; section thickness/intersection gap, 5/0 mm; matrix size, 130 × 130; FOV, 20 × 23 cm; 3 directions; b-value, 0 and 1000 s/mm2) acquired in the transverse plane throughout the infratentorial and supratentorial brain, and contrast-enhanced T1-weighted imaging. Gd-DTPA (Magnevist; Bayer HealthCare Pharmaceuticals, Wayne, New Jersey) was the intravenous contrast agent for the MR imaging study at 0.1 mmol/kg of body weight.
MR Image Processing
All MR imaging and DWI was analyzed by 2 experienced radiologists (W-Y.H. with 8 years of experience in clinical MR imaging and J.-B.W. with 5 years of experience in clinical MR imaging).
The ADC map was calculated from DWI, by using software provided by the manufacturer (syngo; Siemens). ROIs were manually placed within the solid components of the tumor. The ROIs were as large as possible and were placed to avoid areas of cystic, necrotic, or hemorrhagic components in the tumor. Each neuroradiologist drew 3 ROIs to obtain the minimum ADC (ADCmin). The minimum ADC value among these values was chosen as the result. An average of the results of 2 neuroradiologists was used as the patient's ADCmin. When multifocal lesions were observed, the ADCmin was measured for each lesion and the mean ADCmin was calculated for multifocal lesions (<5 lesions).
The volumetric response of tumors to treatment was evaluated according to the Response Evaluation Criteria In Solid Tumors criteria.13 The longest tumor diameter before and after treatment was measured on axial contrast-enhanced T1-weighted images, and the change of tumor diameter was calculated according to the following equations: Change in Tumor Diameter = Diameterpre − Diameterpost; Percentage of Change in Tumor Diameter = (Diameterpre − Diameterpost)/Diameterpre with “pre” and “post” indicating before and after.
The ADCmin change in tumor was calculated on the basis of the following equations: Change in ADCmin = ADCmin after 1 cycle (ADCminearly) − pretreatment ADCmin (ADCminpre); Percentage of Change in ADCmin = (ADCminearly − ADCminpre)/ADCminpre.
If the enhancing lesion was not detected after chemotherapy, then the ROIs were defined in the area where the tumor was initially present.
Statistical Analysis
Statistical analyses were performed by using statistical software (SPSS, Version 13.0; IBM, Armonk, New York). The intraclass correlation coefficient of ADCmin measure between the 2 radiologist was calculated. One-way analysis of variance was used to compare pretreatment ADCmin values, changes in ADCmin values, tumor diameter, and changes in tumor diameter among the CR, PR, and PD groups, and the comparison among groups was performed by using the least significant difference method for the post hoc evaluation. Linear regression models were used to examine size change and ADC measures. The Pearson correlation was performed to determine whether the pretreatment mean ADCmin value and percentage ADCmin change of the tumor after 1 month of chemotherapy were significantly related to the percentage size reduction of the tumor after chemotherapy. The differences between pretreatment and posttreatment ADC values as well as pretreatment and posttreatment tumor diameters were calculated by using a paired-samples t test. A P value < .05 was a statistically significant difference.
Results
Thirty-five patients with PCNSL were enrolled in this study. The mean pretreatment diameter of the tumors was 5.07 ± 2.02 cm (range, 1.75–11.02 cm). Standard International PCNSL Collaborative Group criteria follow-up of the tumor response classified 12 patients as having CR, 15 patients as having PR, and 8 patients as having PD. The intraclass correlation coefficient of ADC measures between the 2 radiologists was 0.73.
Pretreatment Prediction of Therapeutic Response
The pretreatment tumor diameter of the PR (n = 15) group was larger than that of the CR (n = 12) and PD (n = 8) groups, but there was no significant difference among them (F = 1.17, P = .33). The pretreatment ADCmin of the PD group was lower than that in the CR and PR groups, but there was no significant difference among them (F = 2.87, P = .07) (Table 2).
Table 2:
Comparison of ADCmin values among CR, PR, and PD groupsa
| No. | ADCminpre | ADCminearly | ADCminpost | ADCmin Changeb | Percentage ADCmin Changec | |
|---|---|---|---|---|---|---|
| CR | 12 | 566.56 ± 120.84 | 849.09 ± 182.45 | 858.06 ± 185.89 | 282.54 ± 110.40 | 51.17 ± 21.44 |
| PR | 15 | 487.54 ± 78.00 | 669.73 ± 130.28 | 677.14 ± 131.47 | 182.19 ± 88.28 | 37.52 ± 20.02 |
| PD | 8 | 476.13 ± 93.36 | 456.65 ± 93.36 | 432.55 ± 88.85 | −19.49 ± 13.46 | −4.17 ± 2.60 |
| Total | 35 | 512.03 ± 103.15 | 682.52 ± 203.52 | 683.26 ± 213.34 | 170.50 ± 142.03 | 32.68 ± 27.67 |
| P value | .072 | .000 | .000 | .000 | .000 | |
| CR-PR | .045 | .003 | .003 | .006 | .63 | |
| CR-PD | .051 | .000 | .000 | .000 | .000 | |
| PR-PD | .792 | .002 | .001 | .000 | .000 |
Note:—ADCminpost indicates ADCmin after 5 cycles.
All ADC values are reported as 100 × 10−6 mm2/s. Data are means.
ADCmin Change = ADCminearly − ADCminpre.
Percentage ADCmin Change = (ADCminearly − ADCminpre)/ADCminpre.
Monitoring and Early Assessment of Therapeutic Response
The mean ADCmin value for the CR and PR groups increased to different extents during chemotherapy (Table 2). For the CR group, the mean ADCmin value after 1 cycle of chemotherapy increased by 49.9%, and it was statistically significantly higher than the pretreatment ADC value (P < .001). The ADCmin value after 1 cycle and after 5 cycles of chemotherapy was statistically significantly higher than the pretreatment values (P < .001, P < .001), but there was no statistically significant difference between the ADCmin values after 1 and 5 cycles of chemotherapy (P = .17) (Fig 1).
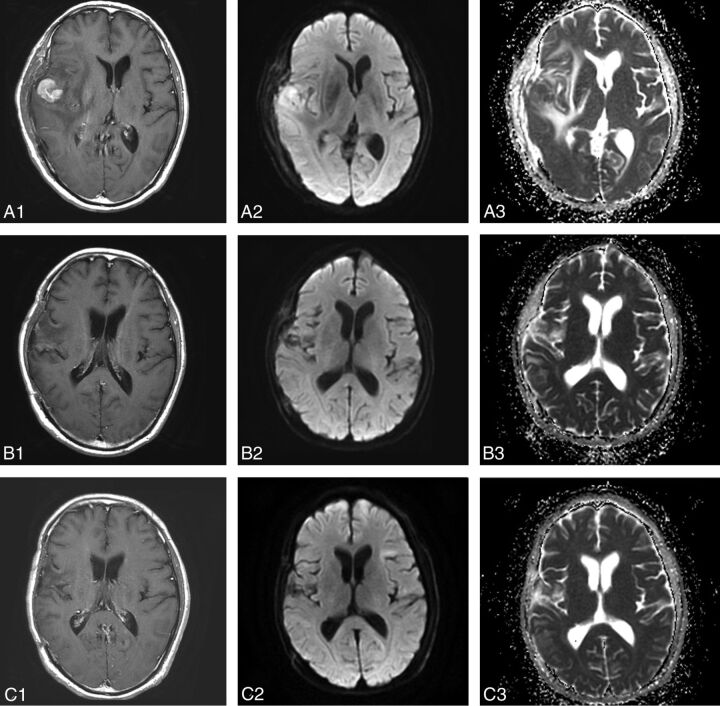
Fig 1.
MR images in a 54-year-old man with diffuse large B-cell PCNSL, belonging to the CR group (A1, A2, A3, before therapy; B1, B2, B3, after 1 cycle of chemotherapy; C1, C2, C3, after 5 cycles of chemotherapy). Contrast-enhanced T1-weighted image shows an apparent enhanced tumor on the right temporal lobe (A1). The tumor shows hyperintense on the DWI (A2, B2). The pretherapeutic ADCmin of the tumor was 668 × 10−6 mm2/s (A3). After 1 cycle of chemotherapy, the size of tumor has decreased significantly (B1, B2) and the ADCmin of the tumor has increased to 1014 × 10−6 mm2/s (B3). After 5 cycles of chemotherapy, the tumor has almost disappeared (C1), and the ADCmin has increased to 1026 × 10−6 mm2/s (C2, C3).
For the PR group, the ADCmin value after therapy increased gradually, though the increments were relatively small. The ADCmin values after 1 cycle and after 5 cycles of chemotherapy were statistically significantly higher than the pretreatment values (P < .001, P < .001), but there was no statistically significant difference between ADCmin values after 1 and 5 cycles of chemotherapy (P = .60) (Fig 2).
Fig 2.
MR images in a 53-year-old man with diffuse large B-cell PCNSL belonging to the PR group (A1, A2, A3, before therapy; B1, B2, B3, after 1 cycle of chemotherapy; C1, C2, C3, after 5 cycles of chemotherapy). An apparent enhanced tumor on the bilateral frontal lobe and corpus callosum (A1). On the DWI, the tumor is apparently hyperintense in relation to adjacent structures (A2, B2, C2). The baseline ADCmin of the tumor was 420 × 10−6 mm2/s (A3). After 1 cycle of chemotherapy, the size of the tumor has decreased slightly (B1, B2) and the ADCmin has increased to 764 × 10−6 mm2/s (B3). After 5 cycles of chemotherapy, the size of the tumor has continued to decrease (C1), while the ADCmin has decreased to 617 × 10−6 mm2/s (C2, C3).
The mean ADCmin value for the PD group decreased slightly or showed no obvious change during chemotherapy. The posttreatment ADCmin values for the patients with PD appeared to increase slightly compared with the pretreatment ADCmin values. The ADCmin values after 1 and 5 cycles of chemotherapy were statistically significantly higher than pretreatment values (P = .01, P = .04), but there was no statistically significant difference between the ADCmin values after 1 and 5 cycles of chemotherapy (P = .28) (Fig 3).
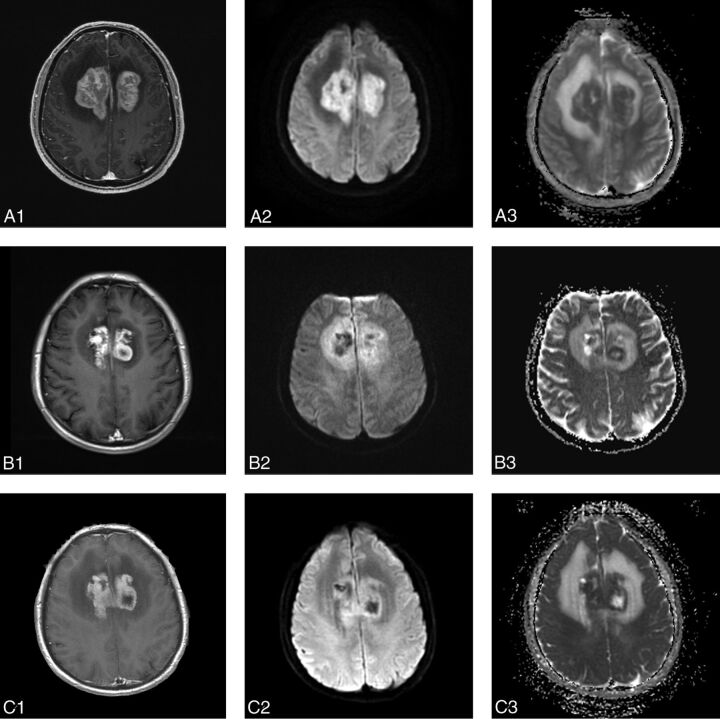
Fig 3.
MR images in a 68-year-old man with diffuse large B-cell PCNSL, belonging to the PD group (A1, A2, A3, before therapy; B1, B2, B3, after 1 cycle of chemotherapy; C1, C2, C3, after 5 cycles of chemotherapy). An apparent enhanced tumor on the left basal ganglia region (A1). On the DWI, the tumor is apparent hyperintense (A2, B2, C2). The baseline ADCmin of the tumor was 554 × 10−6 mm2/s (A3). After 1 cycle of chemotherapy, the size of tumor has decreased slightly (B1, B2). However, the ADCmin of the tumor has not increased but decreased to 536 × 10−6 mm2/s (B3). After 5 cycles of chemotherapy, the size of tumor (C1) has increased compared with A1 and B1 and the ADCmin of the tumor continued to decrease to 478 × 10−6 mm2/s (C2, C3).
The comparison among groups showed that ADCminearly, ADCmin after 5 cycles, ADCmin change, and percentage of ADCmin change were all significantly different among the 3 groups (CR, PR, and PD). Specifically, the mean percentage of ADCmin changes of the tumors after 1 cycle of chemotherapy was 55.68% for CR, 37.52% for PR, and −3.78% for PD, and there was a significant difference among these values (F = 22.10, P < .001) (Table 2). Comparison of pretreatment ADCmin, ADCmin early change, and percentage ADCmin change indicated that the ADCmin change and percentage ADCmin change performed better in differentiating the final treatment response, specifically differentiating the CR and PR groups from the PD group (Fig 4A, -B).
Fig 4.
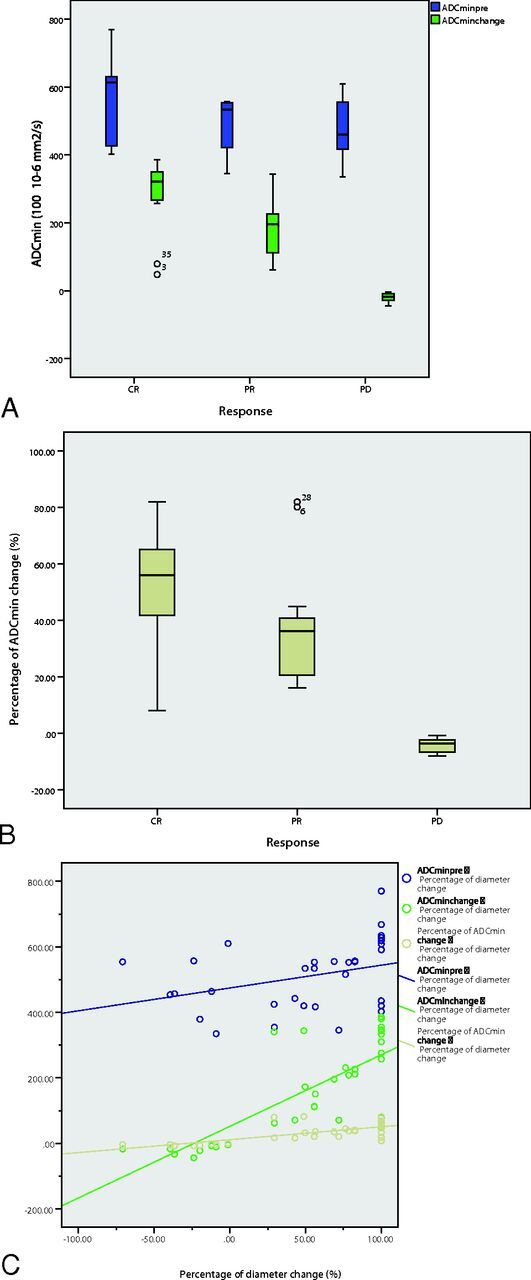
A boxplot of ADCminpre and ADCmin early change in the CR, PR, and PD groups (A). A boxplot of percentage ADCmin change in the CR, PR, and PD groups (B). ADCmin early change and percentage ADCmin change values can differentiate the 3 groups. The percentage ADCmin change performed better in differentiating the final treatment response, specifically differentiating the CR and PR groups from the PD group (A and B). C, Correlation between ADCminpre and percentage of the diameter (R2 = 0.046, blue), correlation between ADCmin early change and percentage of the diameter (R2 = 0.576, green), and the correlation between the percentage of ADCmin early change and percentage of the diameter (R2 = 0.717, yellow).
The mean percentage diameter of early changes of the tumors after 5 cycles of chemotherapy was 46.43% for CR, 20.83% for PR, and −1.46% for PD, and there was a significant difference among these values (F = 22.10, P < .001) (Table 3).
Table 3:
Comparison of tumor diameter among CR, PR, and PD groups
| No. | Diameterpre (cm) | Diameterearly (cm) | Diameterpost (cm) | Diameter Change (cm)a | Percentage Diameter Changeb | |
|---|---|---|---|---|---|---|
| CR | 12 | 4.41 ± 1.46 | 2.45 ± 1.08 | 0.00 ± 0.00 | 4.41 ± 1.46 | 100 |
| PR | 15 | 5.60 ± 2.35 | 4.71 ± 2.39 | 2.03 ± 1.19 | 3.57 ± 2.07 | 60.75 ± 18.53 |
| PD | 8 | 5.06 ± 2.02 | 5.23 ± 2.42 | 6.21 ± 2.50 | −1.16 ± 0.87 | 26.62 ± 22.16 |
| Total | 35 | 5.07 ± 2.02 | 4.03 ± 2.31 | 2.29 ± 2.71 | 2.78 ± 2.73 | 54.24 ± 50.38 |
| P value | .325 | .007 | .000 | .000 | .000 | |
| CR-PR | .137 | .008 | .001 | .201 | .000 | |
| CR-PD | .486 | .005 | .000 | .000 | .000 | |
| PR-PD | .543 | .570 | .000 | .000 | .000 |
Diameter Change = Diameterpre − Diameterpost.
Percentage Diameter Change = (Diameterpre − Diameterpost)/Diameterpre.
A positive correlation was observed between ADCminpre and the size reduction percentage after 5 cycles of chemotherapy (Pearson coefficient, 0.34; P = .05). In addition, a significant positive correlation was observed between the early ADCmin changes, early percentage of ADCmin changes after 1 cycle of chemotherapy, and size reduction percentage after 5 cycles of chemotherapy (Pearson coefficient, 0.58, P < .001; 0.72, P < .001, respectively) (Fig 4C).
Discussion
Traditionally, an assessment of solid cancer therapy effectiveness relies on comparison of changes in tumor size by images obtained before and after the therapeutic intervention. However, tumor size measurement by using images (CT or MR imaging) is insensitive to early treatment changes and cannot monitor changes in tissue structure at the cellular level.14 DWI can detect relatively small changes in tissue structure at the cellular level because it is specifically sensitive to cellular status, density, and microstructural organization.15 The combination of DWI with conventional MR imaging enables morphologic and physiologic changes to be assessed during the same examination. The extremely high DWI signal intensity is characteristic of MR imaging findings in PCNSL due to the high cell density, and cell density has a great differential value for tumors of the brain.16,17 Previous studies have shown a significant inverse correlation between cellularity and ADC values in PCNSL, suggesting ADC as a surrogate marker of tumor proliferation.17 ADC has shown promise in the prognostication of response to therapy for non-Hodgkin lymphoma, even if not technically in the central nervous system.18 DWI-derived ADC values provide an opportunity to quantitatively and serially follow treatment-induced changes in PCNSL, and specifically, ADCmin values were more powerful than ADC mean or ADC 25% values.5 Here, we aimed to confirm the validity of ADCmin measurement as an imaging biomarker of therapy response in immunocompetent patients with PCNSL.
The validity of pretherapeutic ADC as a predictor of clinical outcomes in patients with PCNSL remains controversial. Several studies have proposed that the lower the pretherapy baseline tumor ADC values, the shorter the progression-free survival and overall survival in patients with lymphoma.6,5,19 However, a new study showed no association between a higher baseline ADC and CR, and no differences were observed in progression-free survival or overall survival according to the baseline ADC among the CR, PR, and PD groups.10
In our study, the pretherapeutic ADCmin of the PD (476.14 ± 93.36 × 10−6 mm2/s) group was lower than that in the CR (566.56 ± 120.84 × 10−6 mm2/s) and PR (487.54 ± 78.00 × 10−6 mm2/s) groups; however, there was no significant difference among these values. The mean percentage of ADCmin change of the tumor after 1 cycle of chemotherapy was 55.68% for CR, 37.52% for PR, and −3.78% for PD, and there was a significant difference observed among them. Our results indicate that the ADCmin changes and percentage changes after early therapy could more precisely predict treatment response than the pretreatment ADCmin value. Although tumors with higher pretherapeutic ADC values and lower signal intensity at DWI responded better to treatment, neither the pretherapeutic diameter nor pretherapeutic ADCmin could completely predict the PCNSL outcome from methotrexate-based chemotherapy. A possible explanation is that pretherapeutic cellularity cannot precisely reflect the treatment response. However, cellularity reduction caused by chemotherapy drugs increases ADCmin. Thus, posttherapeutic ADCmin growth might indicate the later tumor regression or decelerated growth and enable early detection of tumor response. Change is a better assessment strategy based on pretheraputic data. Another explanation is the lack of sufficient statistical power due to the relatively small sample size.
Previous studies have confirmed that effective anticancer treatment resulted in an increase in water diffusion. Studies with animal models found that chemotherapy increased the tumor ADC value within days through reducing cellularity as a result of apoptosis and/or cell death.20,21 The ADC value also rose with greater histologic changes (such as a progressive increase in tumor extracellular space and an increase in pleomorphism, giant cells, and cells with the characteristic morphologic features of apoptosis) because water mobility was increased by the greater extracellular space and membrane permeability.22
In this study, the mean ADCmin values for patients with CR and PR all increased after treatment.13,23,24 The posttreatment ADCmin values of the CR group increased rapidly compared with the pretreatment values. In the PR group, the ADCmin value changed moderately, while in PD, it changed slightly and even decreased slightly after therapy. The percentage ADCmin change of the tumor after 1 cycle of chemotherapy correlated positively with the percentage size reduction of the tumor after 5 cycles of chemotherapy; this finding suggests that significant changes in tumor ADCmin values that occurred after treatment might indicate a better response to therapy. However, a minute change in tumor ADCmin values might indicate a less satisfactory outcome or even a therapeutically unresponsive tumor.
One hypothesis is that successful treatment of a tumor will result in significant damage to tumor cells in the form of a loss of cell membrane integrity with a subsequent reduction in tumor cell density. We observed that necrosis appeared in the center of the lesion in some cases after therapy, suggesting increases in the movement of water molecule diffusion, resulting in changes in tumor ADC values. Moreover, intratumoral edema, necrosis, and/or cysts may appear or increase due to damage to tumor cells or dynamic reorganization of the heterogeneous tumor structure after treatment. In addition, through dynamic changes of the ADCmin and diameter pre- and postchemotherapy, it was found that the change in mean ADCmin value occurred in advance of changes in tumor diameter, indicating that DWI is not dependent on relatively slow changes in tumor volume. Thus, DWI may be capable of providing earlier indications of therapeutic outcome due to molecular and cellular changes that typically precede observable macroscopic changes in gross tumor size. These findings offer a window of opportunity to modify the initial treatment regimen to improve the clinical outcome and minimize the morbidity associated with prolonged and ineffective treatment.
These results demonstrate the feasibility of using the ADCmin of DWI for the prediction of treatment outcomes in patients with PCNSL undergoing chemotherapy. Early identification of patients likely to have a poor response facilitates a chemoradiation regimen that may enable an early change in the treatment plan, such as addition of radiation therapy.
This study has a number of limitations. First, the follow-up time was short; thus, the correlation among the parameters obtained from DWI, the progression-free survival, and overall survival in patients with PCNSL could not be analyzed. Thus, further studies are needed to confirm the predictive value of ADCmin for the final prognosis. Second, the study included only cases of diffuse large B-cells of the PCNSL and did not contain other pathologic types, such as Burkitt or T-cell types. Third, the study size was relatively small for the comparison of the pretherapeutic ADCmin among groups.
Conclusions
Correlation between the percentage of ADCmin changes of a tumor after 1 cycle of chemotherapy and treatment outcomes provides a potential basis that may ultimately lead to the use of DWI for predicting and monitoring treatment response.
ABBREVIATIONS:
- ADCmin
minimum ADC
- ADCminearly
ADCmin after 1 cycle
- ADCminpre
pretreatment ADCmin
- CR
complete response
- PCNSL
primary central nervous system lymphoma
- PD
progressive disease
- PR
partial response
Footnotes
This research was supported by funding from the National Key Discipline Project, the Hainan Provincial Key Foundation Project (ZDXM2015066), and the National Natural Science Foundation of China under grant No. 81471627.
References
- 1. Nakajima S, Okada T, Yamamoto A, et al. Differentiation between primary central nervous system lymphoma and glioblastoma: a comparative study of parameters derived from dynamic susceptibility contrast-enhanced perfusion-weighted MRI. Clin Radiol 2015;70:1393–99 10.1016/j.crad.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 2. Bhagavathi S, Wilson JD. Primary central nervous system lymphoma. Arch Pathol Lab Med 2008;132:1830–34 [DOI] [PubMed] [Google Scholar]
- 3. Kansara R, Shenkier TN, Connors JM, et al. Rituximab with high dose methotrexate in primary central nervous system lymphoma. Am J Hematol 2015;90:1149–54 10.1002/ajh.24204 [DOI] [PubMed] [Google Scholar]
- 4. PDQ Adult Treatment Editorial Board. Primary CNS Lymphoma Treatment. Bethesda, Maryland: National Cancer Institute. Updated September 21, 2015. http://www.cancer.gov/types/lymphoma/patient/primary-cns-lymphoma-treatment-pdq. Accessed June 9, 2016. [Google Scholar]
- 5. Barajas RF Jr, Rubenstein JL, Chang JS, et al. Diffusion-weighted MR imaging derived apparent diffusion coefficient is predictive of clinical outcome in primary central nervous system lymphoma. AJNR Am J Neuroradiol 2010;31:60–66 10.3174/ajnr.A1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wieduwilt MJ, Valles F, Issa S, et al. Immunochemotherapy with intensive consolidation for primary CNS lymphoma: a pilot study and prognostic assessment by diffusion-weighted MRI. Clin Cancer Res 2012;18:1146–55 10.1158/1078-0432.CCR-11-0625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee EJ, Lee SK, Agid R, et al. Preoperative grading of presumptive low-grade astrocytomas on MR imaging: diagnostic value of minimum apparent diffusion coefficient. AJNR Am J Neuroradiol 2008;29:1872–77 10.3174/ajnr.A1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo AC, Cummings TJ, Dash RC, et al. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology 2002;224:177–83 10.1148/radiol.2241010637 [DOI] [PubMed] [Google Scholar]
- 9. Lee KC, Moffat BA, Schott AF, et al. Prospective early response imaging biomarker for neoadjuvant breast cancer chemotherapy. Clin Cancer Res 2007;13:443–50 10.1158/1078-0432.CCR-06-1888 [DOI] [PubMed] [Google Scholar]
- 10. Morris PG, Correa DD, Yahalom J, et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol 2013;31:3971–79 10.1200/JCO.2013.50.4910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abrey LE, Batchelor TT, Ferreri AJ, et al. ; International Primary CNS Lymphoma Collaborative Group. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 2005;23:5034–43 10.1200/JCO.2005.13.524 [DOI] [PubMed] [Google Scholar]
- 12. Iwadate Y, Suganami A, Ikegami S, et al. Non-deep-seated primary CNS lymphoma: therapeutic responses and a molecular signature. J Neurooncol 2014;117:261–68 10.1007/s11060-014-1379-4 [DOI] [PubMed] [Google Scholar]
- 13. Prasad SR, Jhaveri KS, Saini S, et al. CT tumor measurement for therapeutic response assessment: comparison of unidimensional, bidimensional, and volumetric techniques initial observations. Radiology 2002;225:416–19 10.1148/radiol.2252011604 [DOI] [PubMed] [Google Scholar]
- 14. Liua Y, Baia R, Suna H, et al. Diffusion-weighted imaging in predicting and monitoring the response of uterine cervical cancer to combined chemoradiation. Clin Radiol 2009;64:1067–74 10.1016/j.crad.2009.07.010 [DOI] [PubMed] [Google Scholar]
- 15. Server A, Kulle B, Maehlen J, et al. Quantitative apparent diffusion coefficients in the characterization of brain tumors and associated peritumoral edema. Acta Radiol 2009;6:683–89 10.1080/02841850902933123 [DOI] [PubMed] [Google Scholar]
- 16. Zacharia TT, Law M, Naidich TP, et al. Central nervous system lymphoma characterization by diffusion-weighted imaging and MR spectroscopy. J Neuroimaging 2008;18:411–17 10.1111/j.1552-6569.2007.00231.x [DOI] [PubMed] [Google Scholar]
- 17. Africa E, Pauciulo A, Vadalà R, et al. Primary central nervous system lymphoma: role of DWI in the differential diagnosis. Rays 2005;30:221–26 [PubMed] [Google Scholar]
- 18. Wu X, Kellokumpu-Lehtinen PL, Pertovaara H, et al. Diffusion-weighted MRI in early chemotherapy response evaluation of patients with diffuse large B-cell lymphoma: a pilot study—comparison with 2-deoxy-2-fluoro-D-glucose-positron emission tomography/computed tomography. NMR Biomed 2011;24:1181–90 10.1002/nbm.1689 [DOI] [PubMed] [Google Scholar]
- 19. Valles FE, Perez-Valles CL, Regalado S, et al. Combined diffusion and perfusion MR imaging as biomarkers of prognosis in immunocompetent patients with primary central nervous system lymphoma. AJNR Am J Neuroradiol 2013;34:35–40 10.3174/ajnr.A3165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fujimoto H, Kazama T, Nagashima T, et al. Diffusion-weighted imaging reflects pathological therapeutic response and relapse in breast cancer. Breast Cancer 2014;21:724–31 10.1007/s12282-013-0449-3 [DOI] [PubMed] [Google Scholar]
- 21. Chenevert TL, Stegman LD, Taylor JM, et al. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst 2000;92:2029–36 10.1093/jnci/92.24.2029 [DOI] [PubMed] [Google Scholar]
- 22. Chenevert TL, McKeever PE, Ross BD. Monitoring early response of experimental brain tumors to therapy using diffusion magnetic resonance imaging. Clin Cancer Res 1997;3:1457–66 [PubMed] [Google Scholar]
- 23. Moffat BA, Chenevert TL, Lawrence TS, et al. Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci U S A 2005;102:5524–29 10.1073/pnas.0501532102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moffat BA, Hall DE, Stojanovska J, et al. Diffusion imaging for evaluation of tumor therapies in preclinical animal models. MAGMA 2004;17:249–59 10.1007/s10334-004-0079-z [DOI] [PubMed] [Google Scholar]