Abstract
We have reviewed a considerable amount of recent scientific papers relating inflammation caused by air pollution with chronic and severe medical conditions. Furthermore, there are evidences relating organ inflammation caused by not only outdoor long-term but also short-term inhaled radioisotopes contained in high polluted air or in household natural radioactive background aerosols, in addition to SARS-COV-2 attached to bioaerosols, which are related with a worst evolution of severe acute respiratory syndrome patients. Reactive oxygen species (ROS) production induced by the interaction with environmental ionizing radiation contained in pollution is pointed out as a critical mechanism that predispose mainly to elder population, but not excluding young subjects, presenting previous chronic conditions of lung inflammation or neuroinflammation, which can lead to the most serious consequences.
Keywords: Alzheimer’s disease, ARDS, COVID-19, NLRP3 inflammasome, Pollution, Radioactivity
Abbreviations: ACE-2, Angiotensin-converting enzyme-2; AD, Alzheimer’s disease; ARDS, Acute respiratory distress syndrome; AT1, angiotensin II receptor type 1; Ca2+, Calcium ion; CO, Carbon monoxide; CO2, Carbon dioxide; COVID-19, Coronavirus disease-2019; DAMP, Damage-associated molecular pattern; DNA, Deoxyribonucleic acid; GSDMD, Gasdermin D; HSPCs, Hematopoietic stem/progenitor cells; IFN-γ, Interferon-gamma; K+, Potassium ion; mBq, miliBecquerel; MERS, Middle east respiratory syndrome; mSv, miliSievert; Na+,K+-ATPase, Sodium-potassium adenosine triphosphatase; NLRP3, Nucleotide binding domain and repeated inflammation of leucine-rich protein 3; NF-κB, Nuclear factor-kappa B; NO2, Nitrogen dioxide; oAβ, Oligomeric amyloid beta; ORF, Open reading frame; PAMP, Pathogen-associated molecular pattern; PM10, Particulate matter with diameter <10 µm; PM2.5, Particulate matter with diameter < 2.5 µm; ROS, Reactive oxygen species; SARS-COV-2, Severe acute respiratory syndrome coronavirus-2; SO2, Sulfur dioxide; Sv, Sievert; TGF-β, Transforming growth factor beta; THP-1, Tamm-Horsfall Protein-1; TLR, Toll like receptor; TNF-α, Tumor necrosis factor-alpha; TSNA, Tobacco-specific nitrosamines; USEPA, United States Environmental Protection Agency; µSv, microSievert; WHO, World Health Organization
Background
Reduced life expectancy and increased risks of stroke, cardiovascular disease, and mortality is associated with air pollution, especially related with PM2.5 [1]. The World Health Organization (WHO) reports 3.7 million premature deaths in 2012 to outdoor polluted air, and 4.3 million to household pollution, on disease attributed to preventable environmental hazards. The breathing of particulate matter gives them a way deep into lungs and the bloodstream, giving rise to respiratory and cardiovascular disease, or premature death [1].
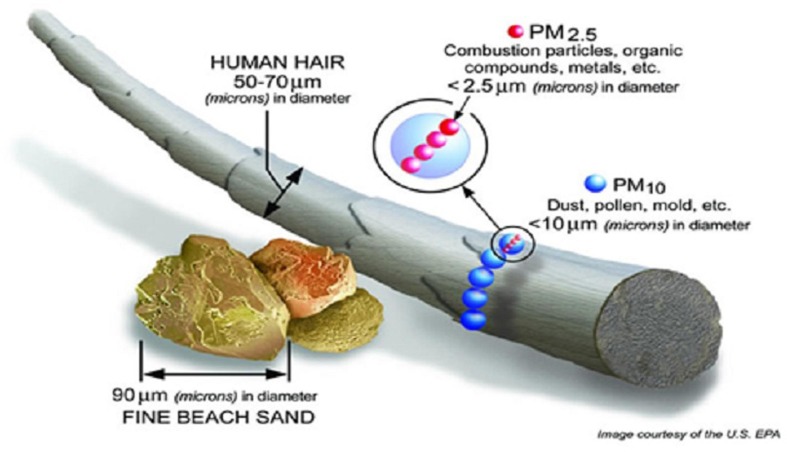
Ionizing radiation from particulate matter is a critical element in air quality. Outdoor air pollution releases low-level long-term radiation due to its content on radioactive particles, an attribute of the exposure that has been largely neglected by health effects studies. Radiation may cause damage to macromolecules and thereby increase the risk of aging-related diseases [2]. An increasing association of PM2.5, PM10 (see particle size illustration in Fig. 1 ), nitrogen dioxide (NO2) and carbon monoxide (CO) concentrations with the risk of adverse outcomes has been experience in the last decades. Inhaled particles could act as transport vehicles for radionuclides in their way inside the lungs, which may emit radiation after inhalation and deposition in the respiratory tract promoting an acute rise in blood pressure by changing the vascular structure or function via inflammation [2]. Carbon dioxide (CO2) is also another important atmospheric pollutant, as well as sulfur dioxide (SO2).
Fig. 1.
Particulate matter diameter comparison. Source: United States Environmental Protection Agency (USEPA).
PM2.5 induces the activation of the NLRP3 inflammasome.
The nucleotide binding domain and repeated inflammation of leucine-rich protein 3 (NLRP3) inflammasome plays a central role in pulmonary inflammation related diseases like chronic obstructive pulmonary disease, asthma, and acute respiratory distress syndrome (ARDS). It has been implicated in a broad range of diseases, like Alzheimer’s disease (AD), Prion diseases, type 2 diabetes, and some infectious diseases [3]. It detects a wide range of microbial motifs, endogenous danger signals and environmental irritants [4]. Its activation involves the recognition of a pathogen-associated molecular pattern (PAMP) or a damage-associated molecular pattern (DAMP), which runs the nuclear factor-kappa B (NF-κB) pathway releasing precursor forms of Interleukin-1β and Interleukin-18 [5]. The NLRP3 inflammasome is turned on by lysosome-mediated cathepsin B, K+ efflux, reactive oxygen species (ROS) production via dysfunctional mitochondria, the release of mitochondrial deoxyribonucleic acid (DNA) in oxidized form, and alterations in Ca2+ concentration, followed by the conversion of pro-Interleukin-1β and pro-Interleukin-18 into their respective mature forms, finally released from the cell via pores generated by gasdermin D (GSDMD) [6].
Although few studies have described which molecules included in PM2.5 would be responsible of the NLRP3 inflammasome activation, Zheng et al. [7] found PM2.5 sustained in polluted air can be internalized into Tamm-Horsfall Protein-1 (THP-1) cells through multiple approaches, such as phagocytosis, macropicnocytosis, clathrin-mediated endocytosis, or caveolin-mediated endocytosis, and have potentials to activate the inflammasome through three distinct mechanisms including cathepsin B release, reactive oxygen species (ROS) production and K+ efflux ending up with an increase of vascular permeability leading to shrinkage of cytosolic F-actin fibers and collagen deposition, forming lung fibrosis. They also found PM2.5 induce fibrosis as well as lung inflammation in balb/c mice, increasing tumor growth factor-β1 (TGF-β1) and Interleukin-1β levels, resulting in small bronchi collagen deposition after a few weeks of breathing exposure.
Yegambaram et al. [8] suggest that the effects of PM2.5 over Alzheimer’s disease is possibly linked to the nucleotide binding domain and repeated inflammation of leucine-rich protein 3 (NLRP3) inflammasome activation triggered by reactive oxygen species (ROS). Interleukin-1β production in oligomeric amyloid beta (oAβ) stimulated microglia induced by PM2.5 possibly depends on that activation. Wang et al. [9] pointed out that 30% of Alzheimer’s disease risk include human lifestyle patterns and environmental facts like inorganic and organic hazards, pesticides, exposure to toxic metals, chemicals from industry and particulate matter in polluted air. Prolonged exposures to pollutants together with bioaccumulation along an individual’s lifetime are speculated to induce neuropathology and neuroinflammation, stepping the way for developing AD. There is growing evidence that cerebrovascular reactivity to carbon dioxide is affected in Alzheimer's disease [10].
Thom et al. [11] think CO2 elevations, found in modern buildings due to the high consumption of electrical energy and heating, will stimulate leukocytes to produce microparticles and activate the NLRP3 inflammasome caused by mitochondrial oxidative stress.
NLRP3 inflammasome activation by environmental radioactivity sustained in PM2.5.
Radioactive particles can be found in polluted air from exhausted gasses in industrial human activity and transportation, or in nature, as natural background radiation from terrestrial environment, which varies tremendously worldwide within different countries.
Ionizing radiation induces reactive oxygen species (ROS) when interacts with tissues. Those atomic interactions might change the function and number of immune system cells increasing the levels of lymphocytes T and macrophages, given rise to the secretion of several inflammatory intermediators: Nuclear factor-kappa B (NF-κB), Interleukin-1, Interleukin-2, Interleukin-6, Interleukin-8, Interleukin-33, tumor necrosis factor-α (TNF-α), tumor growth factor- β (TGF-β), and interferon-γ (IFN-γ) [12]. Reactive oxygen species (ROS) production and damage have been shown to trigger the nucleotide binding domain and repeated inflammation of leucine-rich protein 3 (NLRP3) inflammasome [13] and could be the starting mechanism when interaction with radiation is produced.
Radioactivity suspended in PM2.5 could shed some light on the suspicion about the origin of that inflammatory activation as ionizing radiation may produce cell damage. Enzymatic activity of the Na+/K+ pump or sodium-potassium pump (Na+, K+-ATPase) shows up an exponential decrease as the applied radiation dose contained in PM2.5 is increased [14]. There is evidence that the inactivation is due to an interaction between free of water radiolysis and the ion pump [15].
Potassium, uranium, thorium, and their radioactive decay products (radium, radon, etc.), are the primary radioactive elements leading to human exposure on the Earth’s surface. For instance, Radon-222 is a radioactive gaseous element, part of the Uranium-238 disintegration chain that continues to decay and release ionizing radiation after inhalation and been deposited in the lungs. This is associated with biomarkers of inflammation and endothelial dysfunction [16]. Radon gas does not usually have high levels outdoors but tends to accumulate in homes and can result in high concentrations, especially in areas with highly permeable soils or with a high Radium-226 content.
Li et al. [17] studied the workers exposed to natural background radiation from uranium mines in China where high concentrations of indoor Radon-222 exists. They separate subjects in two different categories: a “control group” worked underground for <5 years and the “experiment group” working underground for ⩾5 years. Compared to the “control group”, long-term exposed subjects showed upregulated expression of proinflammatory cytokines, such as interferon-γ (IFN-γ), Interleukin-10, Interleukin-6, and tumor necrosis factor-α (TNF-α).
Polonium-210 suspended in PM2.5 can be generated as well in the Uranium-238 and Radium-226 disintegration chain. Hodgson [18] in 2018 showed in most of the countries the natural levels of radioactive Polonium-210 in urine were below 30 mBq.day-1 in 95% of the studied population but China and Italy, with values greater than 20% above that level, were exceptions.
Since the 1960’s tobacco manufacturers have been aware of the α-radioactivity contained in smoke [19] as several studies pointed out the principal source of Polonium-210 are tobacco plants fertilizers, which are rich in polyphosphates containing Radium-226, as well as its decay products Lead-210 and Polonium-210. According to Papastefanou [20], the annual effective dose due to inhalation for adult smokers is shown in Table 1 .
Table 1.
Annual effective dose due to inhalation for adults smokers [20].
| Radioisotope | Range μSv/year | Average μSv/year |
|---|---|---|
| Radium-226 | 42.5 to 178.6 | 79.7 |
| Radium-228 | 19.3 to 116.0 | 67.1 |
| Lead-210 | 47.0 to 134.9 | 104.7 |
| Radium-226 + Radium-228 + Lead-210 | 151.9 to 401.3 | 251.5 |
To give an idea of such figures, the annual effective dose from Cs-137 of Chernobyl origin was three orders of magnitude lower as it varied from 70.4 to 410.4 nSv/year (average 199.3 nSv/year).
Radford at al. [21] reported that for an individual smoking two packs of cigarettes a day, the radiation dose from Polonium-210 inhaled in cigarette smoke to bronchial epithelium probably is at least seven times that from background sources. Also, in some areas may be up to 10 Sv or more in 25 years. Winters at al. [22] later reported that in a person smoking one and a half packs of cigarettes a day, the radiation dose to the bronchial epithelium in areas of bifurcation is 80 mSv/year (skin dose equivalent to 300 chest X-ray films/year). There are indeed many other harmful chemical elements in cigarettes. Although nicotine itself is not carcinogenic, it can be likely converted into highly carcinogenic tobacco-specific nitrosamines (TSNA). Natural alkaloids combine with nitrate given rise to carcinogen nitrosamines during the tobacco curing and processing, formed from nicotine and related compounds by a nitrosation reaction, converting organic compounds into nitroso groups.
According to the Agency for Toxic Substances and Disease Registry’s Priority Substance List, Lead (Pb) abundance and toxicity makes it the second most dangerous environmental poison. Pb compounds are still being used in aviation fuels despite leaded petrol has been removed away from use in many countries [23]. Apart of the well-known Pb poisoning of its stable nuclei, there are also unstable radioactive forms of this element in PM2.5, like Lead-214 which is a radioactive beta emitter. It promotes inflammatory responses producing Interleukin-2, Interleukin-4, Interleukin-8, Interleukin-1β, Interleukin-6, tumor necrosis factor- α (TNF-α) and interferon-γ (IFN-γ), and influencing the immune system cells (T and B lymphocytes, Langerhans cells, macrophages) and the secretion of immunoglobulin A, Immunoglobulin E, Immunoglobulin G, endothelin, and histamine [23].
Lead (Pb) enhances expression of factors associated with inflammation [24]. RadNet stationary sampling stations provide airborne particulate samples on synthetic fiber filters [23]. After five to fifteen days, short-lived radon progenies, like Lead-214 and Bismuth-214, were attached on the filters and gross beta radioactivity were measured. Despite the decay of most of the short-lived radionuclides, there was still residual radioactivity, especially from the last one to two days of the sampled particles, which can be related to the relatively long-lived Radon-222 progenies (Lead-210 and Bi-210) [23].
Experimental studies have also shown nitrogen dioxide or its chemical products in PM2.5 remaining in the lungs when they are inhaled for prolonged periods [25]. The major source of nitrogen dioxide is burning of fossil fuels. Most of the NO2 in the cities comes from exhausted gasses in motor vehicles. Radioactivity associated with labelled nitrogen, originally within nitrogen dioxide, was detectable in extrapulmonary sites as well. There are certain processes in nature that produce many of these radioactive isotopes. For instance, some radioactive nitrogen is produced in the atmosphere when high energy cosmic radiation from the sun hits the earth. Nitric and nitrous acids or their salts have been observed in the blood and urine after exposure to nitrogen dioxide [25].
There are about seven known radioactive isotopes of nitrogen (N-13, N-12, N-16, N-18, N-19, N-20, N-21), and other two which are stable and non-radioactive (N-14, N-15). Nitrogen-14 is much more common (99.63% of natural nitrogen), and Nitrogen-15, slightly heavier, makes up the remaining 0.37%. The longest half-life of the radioactive nitrogen is less than ten minutes. Hence, the only way to keep nitrogen around is to be making it all the time as it occurs naturally in the atmosphere. Nitrogen-compounds continuously undergo chemical exchange of their nitrogen atoms with atmospheric nitrogen under standard conditions, becoming radioactive.
Radiocarbon (Carbon-14) is a naturally occurring radioactive isotope of carbon that is constantly produced in the upper atmosphere through the impact of cosmic radiation on nitrogen molecules (N2), splitting them apart, and which naturally decays away at a constant rate. Carbon-14 atoms react rapidly to form mostly CO (carbon monoxide), which subsequently oxidizes at a slower rate to form CO2 (radioactive carbon dioxide). This gas mixes quickly and spreads evenly distributed throughout the atmosphere in the order of weeks.
Sulfur dioxide (SO2) is other of the constituents of polluted air coming from industrial activity [26]. It is a source of sulfate (SO4) in the atmosphere that promote the production of acid rain and reduce atmospheric visibility. SO2 is common in areas surrounding coal-fired electric generating plants in industrialized countries [27]. Acid rain causes accelerated mobilization of many natural and anthropogenic radionuclides materials in soils, especially Radium-226 and Cesium-137. There is an estimation that a decrease in pH of 1 unit in the soil may increase the mobility of Radium and Cesium by a factor of 2 or more. This will lead to similar increases in plant uptake and radiological dose to man [28].
Lung and other organs severe complications.
Several studies have shown a possible link between air pollution and COVID-19 pulmonary severity leading to fatality. Early on April 2020, Wu et al. [29] reported a relation between an increment of 1 μg.m-3 in PM2.5 concentrations with an increase of 15% in the observed death rate by SARS-COV-2, representing a 20-fold death risk increase due to pollution compared to deaths by other issues (pulmonary complication or cardiac disease).
Correlation between high level in SARS-COV-2 lethality and the atmospheric pollution in Northern Italy has been studied by Conticini et al. [30]. Lombardy and Emilia Romagna were Italian regions with both the highest level of virus lethality in the world and one of Europe’s most polluted areas. These authors suggest that high levels of pollution in Northern Italy could be considered an additional co-factor of the high level of mortality recorded in that area. In fact, prolonged exposure to air pollution could lead to a chronic inflammatory stimulus, even in young and healthy subjects.
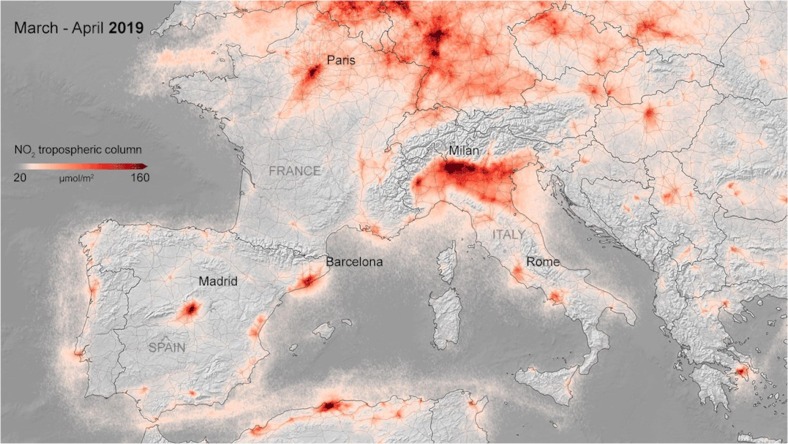
Ogen [31] reports high concentrations of the tropospheric NO2 over Europe in Northern Italy and Madrid metropolitan area, as detected by the Sentinel-5P satellite (see Fig. 2 ). NO2 buildup close to the surface is linked to downwards airflows and can cause high incidence of respiratory problems and inflammation. This chronic exposure to NO2 could be an important contributor to the high COVID-19 fatality rates observed in these regions.
Fig. 2.
Tropospheric nitrogen dioxide concentrations over Europe between March and April 2019. Levels are the same over the last few years. Copernicus Sentinel-5P (https://maps.s5p-pal.com/).
Frontera et al. [32] find, as well, regions of the world with high concentration of air pollutants, especially PM2.5 and NO2, have higher infection rates from SARS-COV-2 and with a higher mortality. Chronic exposures correlate with alveolar angiotensin-converting enzyme-2 (ACE-2) receptor overexpression leading to more severe COVID-19 infection. Frontera proposed a “double-hit hypothesis”:
-
(1)
Chronic exposure to PM2.5 causes alveolar ACE-2 receptor overexpression. This may increase viral load in patients exposed to pollutants in turn depleting ACE-2 receptors and impairing host defenses.
-
(2)
High atmospheric NO2 may provide a second hit causing a severe form of SARS-COV-2 in ACE-2 depleted lungs resulting in a worse outcome.
Epidemiological studies show evidence that PM2.5 long-term exposure is associated with mortality and morbidity [33]. This evidence is weaker for PM10. There are also strong relations between daily exposures and immediately mortality and morbidity during the next days. Repeated exposures in several days can result in health effects greater than single-day effects. The hazards of long-term exposure are much greater than those observed for short-term exposure suggesting that relation is not only with severity but may also be due to the progression of the underlying diseases [33]. There is significant evidence from toxicological and clinical studies on the effects of combustion-derived particles that short-lived maximum exposures (ranging from less than an hour to a few hours) lead to immediate physiological changes [33].
SARS-COV-2 infection induce an hyperinflammatory status.
Inflammasome activation by COVID-19 has been recently described by Conti et al. [34]. When upper and lower respiratory tract cells are infected, an acute respiratory syndrome bringing out pro-inflammatory cytokines, like Interleukin-1β and Interleukin-6 could occur in severe cases. Binding of the virus to the toll like receptor (TLR) causes release of pro-Interleukin-1β which is adhered closely by caspase-1, followed by inflammasome activation and production of active mature Interleukin-1β which a mediator of lung inflammation, fever, and fibrosis. The hyperinflammation status, showing increased blood levels of Interleukin-1, Interleukin-6 and tumor necrosis factor-α (TNF-α) is observed in poor prognosis COVID-19 patients, with and without known risk factors and elevated hyperinflammation markers (D-Dimers/Ferritin) [35].
McGonagle et al. [36] also refers to Interleukin-1β as the inducing agent to cytokine hyperproduction in COVID-19 induced pneumonia and macrophage activation syndrome-like disease, particularly Interleukin-6 and tumor necrosis factor-α (TNF-α). Conticini et al. [30] refers to the importance of the innate immune system hyperactivation in the overproduction of inflammatory cytokines and chemokines, such as tumor necrosis factor-α (TNF-α), Interleukin-1β, Interleukin-6, Interleukin-8, Interleukin-17 and Interleukin-18, and several growth factors. They found similar evidence to this condition in patients affected by severe viral pneumoniae such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS).
SARS-COV virus genome encodes a group of open reading frame (ORF) proteins, as described by Lara et al. [37]. Open reading frame 3a (ORF3a) protein amplifies the oligomerization of SARS ORF3a protein, driving to necrotic cell death. SARS ORF3a protein also runs dysfunction and lysosomal damage via the NLRP3 inflammasome activation. Consistent with pyroptotic cell death in macrophages, open reading frame 8b (ORF8b) protein robustly activates the NLRP3 inflammasome [37].
Dalan et al. [38] found out that SARS-COV-2 use the same pathway as SARS-CoV, infecting humans through the angiotensin-converting enzyme-2 (ACE-2) receptor [34]. Neuron and glial cells are also a potential target of COVID-19 due to the overexpression of ACE-2 receptors in dementia or Alzheimer’s Disease patients [39]. Ratajczak et al. [40] reports, as well, SARS-COV-2 infection and overactivation of the NLRP3 inflammasome is a trigger of the cytokine storm and a risk factor for damage of hematopoietic stem cells. SARS-COV-2 virus entry receptor ACE-2, and receptor for angiotensin II receptor type 1 (AT1), are importantly expressed on hematopoietic stem/progenitor cells (HSPCs) surface.
Viruses airborne transmission through bioaerosols.
Several studies revealed that bioaerosols might have higher significant effects on urban air quality during the haze days than during non-haze days. Meteorological variables correlations with bioaerosol levels of airborne viable bacteria and fungi and particulate concentrations showed that the daily average concentrations during the haze days were not only much higher than those during the non-haze days, but also exceeded the recommended permissible limit values [41]. Furthermore, more allergic, and infectious genera in bioaerosols were found during the haze days.
Although droplets of viruses larger than 100 µm fall rapidly to the floor in a time of very few seconds in a diameter of 2 m distance from the dispersing individual, there is a noticeably clear and scientifically proven evidence of transmission of SARS-COV-2 by bioaerosol inhalation spreading to other people at greater distances [42].
SARS-COV-2 transported by aerosols, defined as suspensions of microscopic particles less than 100 µm in diameter of solids or liquids in air or other gas, can fly much greater distances during a considerable number of seconds or even hours, and in a high concentration near the infected subjects. If this occurs in poorly ventilated enclosed places may result in the infection of many people [42].
Zoran et al. [43] looked for a correlation between the spreading diffusion and lethality of SARS-COV-2 and surface air pollution in Milan metropolitan area (Lombardy region). Air quality and climate parameters were analyzed during the first quarter of 2020 together with the average daily concentrations of inhalable PM2.5 and PM10 at ground level. They found out strong links of particulate matter ground levels significantly associated with average surface air temperature and air relative humidity on COVID-19 cases.
Discussion
Age is one of the most important prognostic factors associated to COVID-19 lethality. Recent studies suggest a role for the NLRP3 inflammasome activation in lung inflammation and fibrosis in SARS-COV and SARS-COV-2 infections in such population [37].
Air pollution is well known to have increased amounts of radioactivity due to a variety of reasons as part of a transported “chemical soup” in the atmosphere. Furthermore, in environments with high levels of polluting aerosols, the virus contained in bioaerosols may be easily attached to particulate matter to fly around. Both, long-term breathed PM2.5 and pollution gasses as well as inhaled SARS-COV-2 bioaerosols, initiate the production of anti-inflammatory mechanisms promoted by the activation of the NLRP3 inflammasome. A pathway initiator of this inflammasome is produced by ionizing radiation macrophage lysosomal damage after reactive oxygen species creation [6]. As a result, potassium, sodium, chloride, and calcium abnormalities are found in most severe COVID-19 patients [14]. Subjects with these extreme symptoms tend to display a higher proportion of hypokalemia at baseline compared to those with less severe forms of disease. Hypokalemia could be thought to be caused by long exposure to radiation and it is a link between radioactivity contained in pollution and the NLRP3 inflammasome activation.
Our analysis propose a “Quadrupole Effect Hypothesis” (QEH) related to pollution:
-
(1)
Eldest people, after long-term living in highly polluted areas, could express a basal predisposition to increase the hyperinflammation cascade of events leading to pulmonary fibrosis or other organ damage due to pollution interaction and further reactive oxygen species activation of the NLRP3 inflammasome.
-
(2)
Dementia or AD, developed mainly in eldest population, is overexpressing angiotensin-converting enzyme-2 (ACE-2) via reactive oxygen species production and the subsequent NLRP3 inflammasome activation.
-
(3)
SARS-COV-2 infection establish an inflammatory condition via ACE-2 receptors.
-
(4)
Pollution aerosols facilitate the spread of the virus.
This hypothesis is backed up by several facts:
-
(a)
High level pollution regions are suffering the fatality fate due to pulmonary complications related to COVID-19 disease.
-
(b)
China, Iran, Brazil, and India are between the countries with higher natural background radioactivity levels on Earth [44]. They are having many severe endings in comparison with other regions of the planet because of pulmonary complications related to COVID-19 disease (see Fig. 3 ).
-
(c)
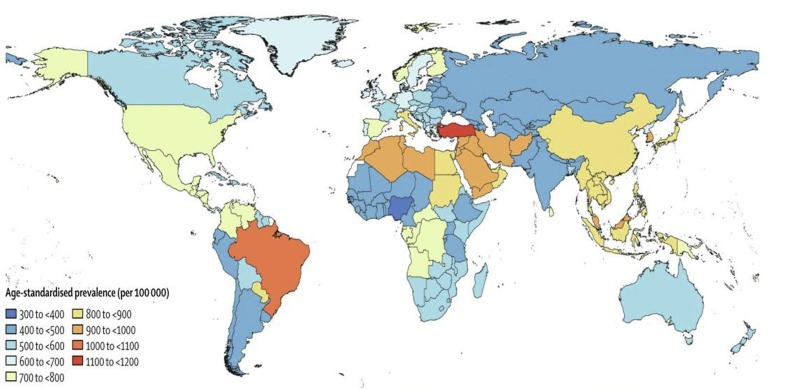
Between March and April 2020, most common condition of dying for people infected by SARS-COV-2 in England and Wales was dementia and Alzheimer’s disease, with 6,887 deaths (20.4% of total) [45]. It is shown in Fig. 4 the age-standardized prevalence for Alzheimer’s disease and other dementias per 100,000 population by location for both sexes over the world [46].
-
(d)
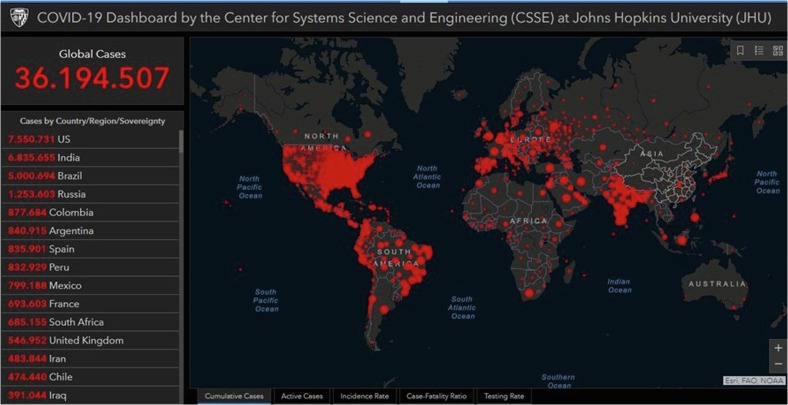
Several global reports last August 2020 informed that India overtakes Brazil as second most COVID-infected country. The greater the number of cases, the higher the number of deaths (Fig. 5 ).
-
(e)
There are strong correlations between all the world maps shown along the text and the regions over the planet with higher rate of COVID-19 complications. Those countries have high levels of air pollution and/or high levels of natural background radioactivity.
Fig. 3.
Natural background radiation worldwide (https://ppt-online.org/303224).
Fig. 4.
Age-standardized prevalence for Alzheimer’s disease worldwide.
Fig. 5.
Cumulative confirmed cases last updated 10/8/2020 10:23 a. m. Source: John Hopkins University of Medicine. (https://coronavirus.jhu.edu/map.html). India, Brazil, and Iran are in between the most affected countries in the world.
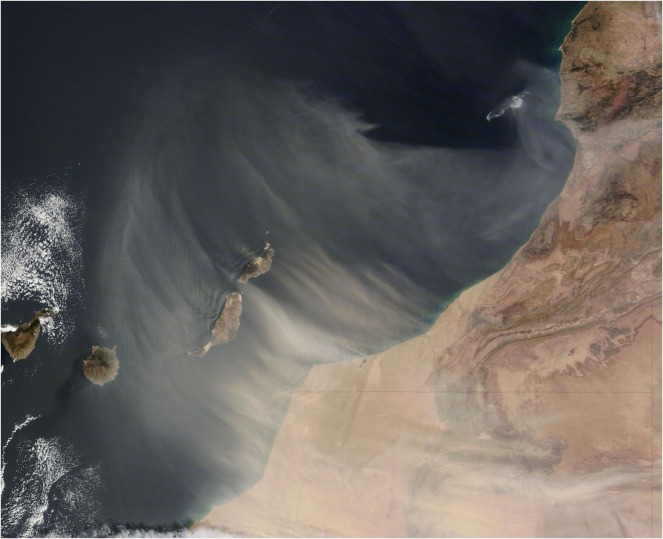
To discuss a little more in detail by setting an example, a local important and unconventional event occurred last 22nd to 24th February 2020, over the Canary Islands, in Spain. It was suffered an intense and strong episode of dust storm, shown in Figs. 6 and 7 , from the Northern coast of Africa affecting the Spanish archipelago. These islands are in northwestern Africa, near the coast of southern Morocco and northern Western Sahara, between coordinates 27° 37' and 29° 25' north latitude and 13° 20' and 18° 10' west longitude. It was Carnival main week at that time, very celebrated and popular locally, and hundreds of people did not remain at home as it was strongly advised by the Government Authorities, especially in Santa Cruz de Tenerife, where some of the public celebrations were not suspended despite the unhealthy conditions.
Fig. 6.
NASA resource (https://images.app.goo.gl/WM4B3LCz2GgZWeqo8).
Fig. 7.
Tenerife Auditorium near view through dense dust. Santa Cruz de Tenerife. February 2020 (https://images.app.goo.gl/XJfwnsRTgxAemws77).
Canary Islands were the most polluted regions on Earth for a while. Increments ranging from 849% to 1778% against the background average levels, reaching pollutant concentrations greater than 3000 μg.m-3 [47]. Dust storms are repeated along the year several times but not as huge as this one. Desert dust episodes have been linked with cardiovascular hospital admissions and mortality [33].
Santa Cruz de Tenerife is normally showing higher Air Quality Index (AQI) levels than the observed concentrations in Las Palmas de Gran Canaria. We have been checking the AQI numbers continuously along 2020 through the mobile application AirVisual (https://www.iqair.com/air-quality-app) and this issue is normally repeated almost as a rule throughout the year. The so called Trade winds prevent pollutants from concentrating, something that happens, especially in Las Palmas de Gran Canaria, located on an isthmus, and to a lesser extent in Santa Cruz de Tenerife, located in the Massif of Anaga, which blocks air flows to run away. Air Quality Index (AQI) translates unintuitive pollutant concentration measurements into one easy-to-understand scale to represent the health risk posed by ambient air pollution. It usually considers up to six main pollutants (PM2.5, PM10, carbon monoxide, sulfur dioxide, nitrogen dioxide and ground level ozone), and calculates the respective health risk (or AQI number) for each one at any given time. The index goes from 0 to 500, where high index values indicate higher levels of air pollution and higher potential for adverse health effects. Any value larger than 300 is considered hazardous, while values of 0-50 represents good air quality.
Along January and September 2020, the number of fatalities or severe cases in Santa Cruz de Tenerife has been astonishingly higher than in Las Palmas de Gran Canaria (metropolitan areas). It seems that people in the western islands had a more silent pre-inflammatory and chronic pulmonary condition due to regular and higher pollution levels which has been exacerbated by the infection of SARS-COV-2.
As a proof of this observation, during the second wave of infections (August 2020), despite a worse social initial oversight in Las Palmas de Gran Canaria, there were more cumulated and less recovered cases in Tenerife, as well as much more deceased patients (see Table 2 ).
Table 2.
COVID-19 evolution between 28th January and 30th September 2020. Resource: Gobierno de Canarias (https://www3.gobiernodecanarias.org/sanidad/scs/content/dcb400c5-6504-11ea-9a8e-719d4b52bf6c/InformeCasosCOVID-19.pdf).
| Area of residency | Active cases |
Closed cases |
Total | Deceased Difference (%) | |||
|---|---|---|---|---|---|---|---|
| At home | Admitted |
Deceased | Recovered | ||||
| In hospital ward | ICU | ||||||
| Tenerife | 1824 | 60 | 21 | 125 | 1829 | 3859 | 142.05 |
| Gran Canaria | 4192 | 131 | 37 | 88 | 2994 | 7442 | |
Although it seems a possible link based on our analysis, it is a very difficult task to relate background ionizing radiation to chronic organ inflammation as effects of radioactive PM10 transported in dust storms along the year, containing gross alpha, gross beta, Potassium-40, Cesium-137, Lead-210, and Berilium-7 activities, as measured for instance in atmospheric aerosols collected in Tenerife during that big storm last February, as shown by López-Pérez et al. [47]. They do not provide PM2.5 readings in this case. There are reasons to believe that the existence of radioactive particles near the Canary Islands could be causally related in part to the nuclear assays by France in Algeria during the sixties, in the past century [48].
Furthermore, the number of death cases associated with Alzheimer’s disease by island between Tenerife and Gran Canaria shows a much higher amount in the case of Tenerife over several past years (Table 3 ).
Table 3.
Alzheimer’s Disease death cases in the Canary Islands. Resource: Instituto Canario de Estadística (http://www.gobiernodecanarias.org/istac/).
| Year | Gran Canaria | Tenerife | Difference between islands % |
|---|---|---|---|
| 2017 | 147 | 243 | 165,3 |
| 2016 | 139 | 204 | 146,8 |
| 2015 | 157 | 218 | 138,9 |
Emissions of CO2 to the atmosphere by Volcano Teide in the island of Tenerife was about 390±58 tons a day in 2014 (Instituto Volcanológico de Canarias, INVOLCAN, http://www.involcan.org/). Due to the mentioned growing evidence of cerebrovascular reactivity to carbon dioxide in Alzheimer's disease patients [10], it is plausible to think that CO2 may be related with such a big difference.
Atmospheric measurements of CO2 are composed by fossil fuel combustion and carbon emissions from other sources, such as volcanic natural emissions. Using Carbon-14 as a marker, it is possible parsed out CO2 sources. Fossil fuels are made of carbon-based materials (ancient plants and animals) that is millions of years old. When the carbon gets to a fossil fuel state it is completely devoid of Carbon-14. The Carbon-14 in fossil fuels has long since stopped the process of radioactive decay. Non-radioactive volcanic CO2 aerosols could be acting as a transport in the case of SARS-COV-2 but also as an initiator of the NLRP3 when it comes to atmospheric CO2.
Another reason that might explain these health issues in Santa Cruz de Tenerife rather than in other islands is the petrol refinery that was working in the metropolitan area between 1930 and 2015, exhaling polluting gases for long years but never considered consistently and publicly as a serious hazardous. Refining units were stopped for economic and regulatory reasons.
Conclusions
Pollution radioisotopes and droplets of SAR-COV-2 suspended in aerosols trigger the hyperinflammation mechanisms of the NLRP3 inflammasome representing a serious factor of severity and mortality over the COVID-19 infected population. People breathing unhealthy air is predisposed to have severe complications because they receive the virus with a “preinstalled” chronic inflammation.
Particulate matter high concentrations, either due to pollution or natural background radioactivity, or both at the same time, have much to do over the previous health condition and predispose their innate immune system behavior under the influence of a SARS-COV-2 bioaerosol infection or other inflammatory processes, like in the Alzheimer’s disease affected subjects, as stated in our “Quadrupole Effect Hypothesis”.
The effects of radioactivity contained in pollution is not sufficiently studied outside the framework of cancer. Better knowledge of the damages in the population is needed. What seems to be clear is that reactive oxygen species (ROS) produced by ionizing radiation starts the NLRP3 inflammasome chronic activation helping other diseases in violent endings.
Since negative effects of pollution are also noticeable in the short-term, releasing temporarily vehicles from cities in times such as this we are living with the second wave of the someway uncontrolled pandemic could serve to shield the severity of the virus a little.
Patient or public involved
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Dissemination declaration
Dissemination results to study participants or patient organizations is not applicable.
Patient consent statement
There are no patients involved in this work so there is no need for a patient consent statement.
Authors contribution
David Macias-Verde, Pedro C Lara and Javier Burgos-Burgos conceived the idea, wrote the paper, and did the revision of the paper.
Funding
Grant 2019-0001 Hospitales Universitarios San Roque/Universidad Fernando Pessoa Canarias.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Figures copyright
All the figures have been taken from public reports stated in the bibliography or as free access on the internet.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.110396.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.https://www.who.int/mediacentre/news/releases/2014/air-pollution/en/.
- 2.Gao X., Koutrakis P., Blomberg A.J. Short-term ambient particle radioactivity level and renal function in older men: insight from the normative aging study. Environ Int. 2019;131:105018. doi: 10.1016/j.envint.2019.105018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y., Wang H., Kouadir M. Recent advances in the mechanisms of NLRP3 inflammasome activation and its Inhibitors. Cell Death Dis. 2019;131:105018. doi: 10.1038/s41419-019-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swanson K.V., Meng D. Ting JP-Y: the NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019 Aug;19:477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pirzada R.H., Javaid N., Choi S. The roles of the NLRP3 inflammasome in neurodegenerative and metabolic diseases and in relevant advanced therapeutic. interventions. Genes (Basel) 2020 Jan;27(131) doi: 10.3390/genes11020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelley N., Jeltema D., Duan Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng R., Tao L., Jian H. NLRP3 inflammasome activation and lung fibrosis caused by airborne fine particulate matter. Ecotoxicol Environ Safety. 2019;163:612–619. doi: 10.1016/j.ecoenv.2018.07.076. [DOI] [PubMed] [Google Scholar]
- 8.Yegambaram M., Manivannan B., Beach T.G. Role of environmental contaminants in the etiology of Alzheimer’s disease: a review. Curr Alzheimer Res. 2015;12:116–146. doi: 10.2174/1567205012666150204121719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang B., Shi J., Ge N. PM2.5 exposure aggravates oligomeric amyloid beta-induced neuronal injury and promotes NLRP3 inflammasome activation in an in vitro model of Alzheimer’s disease. J Neuroinflamm. 2018 May 2;15(1):132. doi: 10.1186/s12974-018-1178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glodzik L., Randall C., Rusinek H., de Leon M.J. Cerebrovascular reactivity to carbon dioxide in Alzheimer's disease. J Alzheimers Dis. 2013;35(3):427–440. doi: 10.3233/JAD-122011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thom S.R., Bhopale V.M., Hu J.P. Increased carbon dioxide levels stimulate neutrophils to produce microparticles and activate the nucleotide-binding domain-like receptor 3 inflammasome. Free Radical Biol Med. 2017;106:406–416. doi: 10.1016/j.freeradbiomed.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Yahyapour R, Amini P, Rezapour S, et al. Radiation-induced inflammation and autoimmune diseases. Military Med Res 1186:40779-018. 10.1186/s40779-018-0156-7. [DOI] [PMC free article] [PubMed]
- 13.Hendry J.H., Simon S.L., Wojcik A. Human exposure to high natural background radiation: what can it teach us about radiation risks? J Radiol Prot. 2009;29:29–42. doi: 10.1088/0952-4746/29/2A/S03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lippi G., South A.M., Henry B.M. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19) Ann Clin Biochem. 2020;57:262–265. doi: 10.1177/0004563220922255. Epub 2020 May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mense M., Stark G., Apell H.J. Effects of free radicals on partial reactions of the Na, K-ATPase. J Membrane Biol. 2020;156:63–71. doi: 10.1007/s002329900188. [DOI] [PubMed] [Google Scholar]
- 16.Nyhan M.M., Ricec M., Blomberg A. Associations between ambient particle radioactivity and lung function. Environ Int. 2019;130 doi: 10.1016/j.envint.2019.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li K., Chen Y., Li X. Alteration of cytokine profiles in uranium miners exposed to long-term low-dose ionizing radiation. Sci World J. 2014 doi: 10.1155/2014/216408. Epub 2014 Apr 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodgson A. Natural levels of Polonium-210 in urine. J Radiol Prot. 2018;38:923–933. doi: 10.1088/1361-6498/aac698. Epub 2018 May 22. [DOI] [PubMed] [Google Scholar]
- 19.Zagà V, Lygidakis C, Chaouachi K, et al., Polonium and lung cancer. J Oncol 2011. 10.1155/2011/860103. Epub 2011 Jun 23. [DOI] [PMC free article] [PubMed]
- 20.Papastefanou C. Radioactivity of tobacco leaves and radiation dose induced from smoking. Int J Environ Res Public Health. 2009;6:558–567. doi: 10.3390/ijerph6020558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radford EPJr, Hunt VR, Polonium- 210: a volatile radioelement in cigarette. Science 1964;143:247–249. 10.1126/science.143.3603.247. [DOI] [PubMed]
- 22.Winters T.H., Di Franza J.R. Radioactivity in cigarette smoking. N Engl J Med. 1982;306:364–365. doi: 10.1056/NEJM198202113060612. [DOI] [PubMed] [Google Scholar]
- 23.USEPA (United States Environmental Protection Agency). RadNet Monitoring Network. (2017). http://www.epa.gov/radnet.
- 24.Metryka E., Chibowska K., Gutowska I. Lead (Pb) exposure enhances expression of factors associated with inflammation. Int J Mol Sci. 2018 Jun 20;19(6):1813. doi: 10.3390/ijms19061813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO Regional Office for . Denmark; Copenhagen: 2000. Europe. [Google Scholar]
- 26.Shprentz D.S., Bryner G.C., Shprentz J.S., Hawkings D.G. National Resources Defense Council; Washington, DC: 1996. National Resources Defense Council, Breath taking: premature mortality due to air pollution in 239 American cities; p. 99. [Google Scholar]
- 27.Petruzzi S., Musim B., Bignami G. Acute and chronic sulfur dioxide (SO2) exposure: and overview of its effects on humans and laboratory animals. Review. Ann Ist Super Sanita. 1994;30:151–156. PMID: 7832407. [PubMed] [Google Scholar]
- 28.Sheppard S.C., Sheppard M.I. Modeling estimates of the effect of acid rain on background radiation dose. Environ Health Perspect. 1988 Jun;78:197–205. doi: 10.1289/ehp.8878197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu X., Nethery R.C., Sabath B.M. Exposure to air pollution and COVID-19 mortality in the United States: a nationwide cross-sectional study. medRxiv. 2020 doi: 10.1101/2020.04.05.20054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conticini E., Frediani B., Caro D. Can atmospheric pollution be considered a co-factor in extremely high level of SARS-COV-2 lethality in Northern Italy? Environ Pollut. 2020;261:114465. doi: 10.1016/j.envpol.2020.114465. Epub 2020 Apr 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogen Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID-19) fatality. Sci Total Environ. 2020;726:138605. doi: 10.1016/j.scitotenv.2020.138605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frontera A., Cianfanelli L., Vlachos K. Severe air pollution links to higher mortality in COVID-19 patients: the “double-hit” hypothesis. J Infect. 2020 doi: 10.1016/j.jinf.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Review of evidence on health aspects of air pollution – REVIHAAP Project Technical Report. World Health Organization; 2013. [PubMed]
- 34.Conti P., Ronconi G., Caraffa A. Induction of pro-inflammatory cytokines (Interleukin-1 and Interleukin-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV- 2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2) doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 35.Mehta P., McAuley D.F., Brown M. COVID- 19: consider cytokine storm syndromes and immunosuppression. Lancet Correspondence. 2020;28:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGonagle D., Sharifa K., O'Regand A. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020 Jun;19:102537. doi: 10.1016/j.autrev.2020.102537. Epub 2020 Apr 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lara PC, Macías-Verde D, Burgos-Burgos J. Age-induced NLRP3 inflammasome over-activation increases lethality of SARS-COV-2 pneumonia in elderly patients. Aging Dis 2020;11(4). http://dx.doi.org/10.14336/AD.2020.0601. [DOI] [PMC free article] [PubMed]
- 38.Dalan R, Bornstein SR, El-Armouche A, et al. The ACE-2 in COVID- 19: foe or friend? Horm Metab Res 2020;52:257–263. 10.1055/a-1155-0501. Epub 2020 Apr 27. PMID: 32340044. DOI: 10.1055/a-1155-0501. [DOI] [PMC free article] [PubMed]
- 39.Bostanciklioglu M. Severe acute respiratory syndrome Coronavirus 2 is penetrating to dementia research. Curr Neurovasc Res. 2020 doi: 10.2174/1567202617666200522220509. [DOI] [PubMed] [Google Scholar]
- 40.Ratajczak M.Z., Kucia M. SARS-COV-2 infection and overactivation of NLRP3 inflammasome as a trigger of cytokine “storm” and risk factor for damage of hematopoietic stem cells. Leukemia. 2020;34:1726–1729. doi: 10.1038/s41375-020-0887-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y., Fu H., Wang W., Liu J., Meng Q. Characteristics of bacterial and fungal aerosols during the autumn haze days in Xian, China. Atmos Environ. December 2015;122:439–447. doi: 10.1016/j.atmosenv.2015.09.070. [DOI] [Google Scholar]
- 42.Prather KA, LC Marr, Schooley RT et al. Airborne transmission of SARS-COV-2. Science 05 Oct 2020. DOI: 10.1126/science.abf0521. [DOI] [PubMed]
- 43.Zoran M.A., Savastru R.S., Savastru D.M. Assessing the relationship between surface levels of PM2.5 and PM10 particulate matter impact on COVID-19 in Milan, Italy. Sci Total Environ. 2020;738:139825. doi: 10.1016/j.scitotenv.2020.139825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hendry J.H., Simon S.L., Wojcik A. Human exposure to high natural background radiation: what can it teach us about radiation risks? J Radiol Prot. 2009;29:A29–A42. doi: 10.1088/0952-4746/29/2A/S03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alzheimer’s Society. UK. https://www.alzheimers.org.uk/news/2020-05-15/ons-figures-show-dementia-main-underlying-condition-covid-19-deaths-alzheimers.
- 46.GBD Dementia Collaborators: global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2016;2020(18):88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopez-Pérez M., Lorenzo-Salazar J.M., Expósito F.J. Impact of a massive dust storm on the gross alpha, gross beta, 40K, 137Cs, 210Pb, 7Be activities measured in atmospheric aerosols collected in Tenerife, Canary Islands. Atmos Environ. 2020;239:117806. doi: 10.1016/j.atmosenv.2020.117806. [DOI] [Google Scholar]
- 48.Danesia P.R., Moreno J., Makarewicz M. Residual radionuclide concentrations and estimated radiation doses at the former French nuclear weapons test sites in Algeria. Appl Radiat Isotopes Environ Radioact. 2008;66(1671–1674) doi: 10.1016/j.apradiso.2007.08.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.