Abstract
BACKGROUND AND PURPOSE:
Although core needle biopsy was introduced as a diagnostic alternative to fine-needle aspiration, the utility and safety of core needle biopsy for thyroid nodules in a large population has yet to be studied comprehensively. We evaluate core needle biopsy yields on a large-scale basis to investigate its potential in the preliminary diagnosis of thyroid nodules.
MATERIALS AND METHODS:
Between March 2005 and December 2013, 2448 initially detected thyroid nodules from 2120 consecutive patients who underwent core needle biopsy were retrospectively evaluated. Of these, 72 thyroid nodules from 63 patients were excluded due to prior fine-needle aspiration attempts. The inconclusive and conclusive result rates, diagnostic accuracy, sensitivity, specificity, positive predictive value, negative predictive value, and unnecessary surgery rate of core needle biopsy were evaluated.
RESULTS:
With core needle biopsy as the first-line method, the inconclusive result rate was 11.9% (283/2376) and the conclusive result rate was 88.1% (2093/2376). The diagnostic accuracy, sensitivity, specificity, positive predictive value, and negative predictive value of core needle biopsy for the diagnosis of malignancy were 96.7% (1160/1200), 89.7% (347/387), 100% (813/813), 100% (347/347), and 95.3% (813/853), respectively. There were no major complications and 12 minor complications.
CONCLUSIONS:
We have demonstrated that first-line use of core needle biopsy may well improve diagnostic accuracy in thyroid nodules, reducing inconclusive or false-negative results and unnecessary operations. Such benefits underscore the promising role of core needle biopsy in managing thyroid nodules and optimizing related surgical decision-making.
Although ultrasonography (US)-guided fine-needle aspiration (FNA) is a safe, accurate, and cost-effective method for diagnosing malignancy in thyroid nodules, there are limitations.1,2 A major drawback is the frequency of inconclusive results (ie, indeterminate or inadequate results), accounting for 25%–30% of FNA results.2,3 In such instances, even repeat FNA attempts may still be nondiagnostic (9.9%–47.8% incidence).4–6 Nodules with inconclusive FNA results are commonly referred for diagnostic surgery at reported rates of 22.2%–94.7%.7–9 Although several studies suggest that biomarkers (molecular or genetic) and clinical or sonography parameters may serve to support FNA outcomes,10–12 surgical confirmation is often still required.1,2,12
Core needle biopsy (CNB) was introduced as a diagnostic alternative to FNA or tissue diagnosis. It is well-tolerated and safe and associated with a low incidence of complications.3,4,6,13–16 However, its role has remained second-line, largely serving as a supplement in patients with inconclusive FNA results. However, a number of studies have reported that a diagnosis was established via CNB in up to 98% of nodules with indeterminate FNA results; and by performing CNB and repeat FNA in combination, 97% of nodules with prior inadequate FNA yields are eventually diagnosed.4–6,14–16 Most interesting, there have been few studies to date on the use of CNB as a first-line examination for the diagnosis of thyroid nodules.17–19 Consequently, the utility and safety of CNB for thyroid nodules in a large population have not yet been studied comprehensively.
This study was conducted on the premise that highly diagnostic yields are achievable via CNB, without undue or major complications. We therefore evaluated CNB yields on a large-scale basis to investigate its full potential in the preliminary diagnosis of thyroid nodules.
Materials and Methods
Study Population
This observational study was approved by the Institutional Review Board of Konyang University Hospital and Daejeon Sun Hospital, with written informed consent for data access waived. However, all patients undergoing CNB at our facility granted prior informed consent.
Between March 2005 and December 2013, 2448 thyroid nodules detected in 2120 consecutive patients at 2 institutions, Konyang University Hospital (n = 634) and Daejeon Sun Hospital (n = 1814), were subjected to ultrasound-guided CNB. Of these, 72 thyroid nodules in 63 patients were excluded on the basis of prior FNA attempts. Finally, 2376 initially detected thyroid nodules from 2057 consecutive patients (594 men and 1463 women; mean age, 50.8 ± 12.6 years, range, 11–91 years) were enrolled in this study. These enrolled thyroid nodules underwent CNB due to suspicious US findings (n = 1538), heavy calcifications (n = 296), high vascularity (n = 289), and requests of a small group of referring physicians (n = 253). The physicians of this cohort preferred the CNB rather than FNA in an attempt to avoid inconclusive FNA results.
Final diagnoses in malignant nodules were confirmed by postsurgical histopathology or other pathologic documentation (including biopsy-proved lymphoma or metastasis). Benign nodules were also confirmed by postsurgical histopathology, by sequential benign CNB or FNA outcomes (at least twice with intervals of >6 months), or by benign CNB findings with a nodule that was stable or decreased in size of after 1 year (at minimum).
Analysis of US Findings
The US images were reviewed independently by 2 radiologists (Y.J.K., and H.Y.H). The US finding of the nodules were evaluated for following features20,21: the size of thyroid nodules, composition (solid, predominantly solid, predominantly cystic, or cyst), shape (ovoid to round or irregular), orientation (parallel or nonparallel), margin (smooth, spiculated, or ill-defined), echogenicity (isoechoic, hypoechoic, markedly hypoechoic, or hyperechoic), and calcifications (none, macrocalcifications, or microcalcifications). The suspicious US findings were defined as nonparallel orientation, spiculated margin, marked hypoechogenicity, and the presence of micro- or macrocalcifications.20,21 A suspicious malignant nodule was defined if 1 of the above findings was present. If there were discrepancies in the US findings, the radiologists resolved them by consensus.
Sonography-Guided CNB Procedures
US examinations were performed by using 1 of 3 US systems: an iU22 or HDI-5000 U (Philips Healthcare, Best, the Netherlands) or a Logiq 9 ultrasound (GE Healthcare, Milwaukee, Wisconsin), each equipped with a high-frequency linear probe (7–12 MHz). All US examinations and US-guided CNBs were performed by 1 of 5 radiologists (Y.J.K., Y.S.P., D.H.O., H.Y.H., or J.M.Y.) with ≥5 years of clinical experience in performing and interpreting US images of the thyroid gland. If the nodule had a cystic portion of >50% or necrosis, the internal fluid of the nodule was aspirated at first and then US-guided CNB was performed on the remaining solid portion.
Disposable 1.1-cm excursion 18-ga double-action spring-activated needles (TSK Ace-cut; Create Medic, Yokohama, Japan) were used for CNB, following local anesthetic injection (lidocaine 1%). Before insertion, power Doppler US was used to carefully evaluate vessels along the biopsy course to avoid hemorrhage. With a freehand technique, the needle was advanced into a nodule or across its margin to obtain a tissue core, but the thyroid capsule was avoided to prevent vessel injury. Once the nodule was pierced, adjacent vessels were again evaluated to minimize injury and bleeding. We measured the distance of travel (1.1 cm) before sequential firing of the needle stylet and cutting cannula.
Tissue cores were placed in 10% buffered formalin immediately at the completion of the procedure for conventional processing. Each patient was then monitored for 10–20 minutes with firm local compression of the biopsy site.
Analysis of CNB Results
All CNB specimens were reviewed by board-certified attending staff pathologists with ≥5 years of clinical experience (S.Y.P., Y.M.K., B.K.K., and H.J.L.), though thyroid CNB diagnostic criteria were not yet standardized. For this study, the 6 categories of the Bethesda System were used to classify histopathologic CNB results.22
In the absence of any identifiable follicular elements or with scant normal follicular content, a CNB was considered nondiagnostic. Benign CNB readings were those demonstrating colloid or hyperplastic nodules and lymphocytic thyroiditis. CNB specimens containing nodules with some atypical cells not diagnostic of malignancy were interpreted as atypia (atypia of undetermined significance [AUS]) or follicular lesions of undetermined significance (FLUSs). These included cellular follicular nodules that were difficult to distinguish (follicular neoplasm versus hypercellular/hyperplastic nodule). Nodules with histologic features favoring follicular neoplasm were categorized as suggestive of follicular neoplasm or consistent with follicular neoplasm. “Suspicious for malignancy” included specimens that displayed atypia of a borderline nature. Unequivocal malignant features were needed for a diagnosis of malignancy.
Statistical Analysis
The statistical analysis relied on standard software (SPSS Version 18.0 for Windows: IBM, Armonk, New York). Rates of nondiagnostic results, malignancy, inconclusive and conclusive results, unnecessary surgery (considered malignant by CNB but confirmed as benign or viewed as a follicular neoplasm by CNB but proved to be adenomatous hyperplasia), and complications were determined. Major complications were defined as events that might result in admission to a hospital for therapy, an unplanned increase in the level of care, lengthened hospital stay, or events that might lead to substantial morbidity or disability. Other complications such as perithyroid hemorrhage or edema were considered minor complications.23
Diagnosis of malignancy included nodules with suspicious for malignancy or malignant CNB results. Inconclusive results included nondiagnostic and AUS/FLUS readings. With respect to thyroid cancer, CNB was analyzed for diagnostic accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
Subgroup analysis related to sample adequacy was performed according to the nodule size (<10 mm and ≥10 mm), location (upper/lower and mid), composition (cyst; cystic component ≥50%; and solid, cystic component <50%), and the presence and type of calcification. Subgroup analysis related to inconclusive results was also performed according to the nodule size, composition, location, and suspicious US findings. The parameters of the 2 groups were compared by using Student t tests and the χ2 or Fisher exact test. Statistical significance was set at P < .05.
Results
In all patients, CNB procedures were well-tolerated without immediate complications. The mean nodule size was 14.3 ± 9.6 mm (range, 4–93 mm), with nodules ≥10 mm accounting for 62.6% (1488/2376) of the sample. Among the 888 nodules of <10 mm, 634 nodules underwent CNB due to suspicious US findings. Two hundred fifty-four nodules with indeterminate US findings underwent CNB to decide the extent of the surgery for multiple thyroid nodules or to evaluate primary malignancy when cervical lymph nodes were diagnosed as metastatic. The mean follow-up was 27.5 ± 21.9 months. CNB results (n = 2376) and final diagnoses (n = 1200) are summarized in Table 1.
Table 1:
Core-needle biopsy results and final diagnosis for initially detected thyroid nodulesa
| Total CNB (n = 2376) | Final Diagnosis (n = 1200) |
CNB <10 mm (n = 888) | Final Diagnosis (n = 455) |
CNB ≥10 mm (n = 1488) | Final Diagnosis (n = 745) |
||||
|---|---|---|---|---|---|---|---|---|---|
| Benign (n = 813) | Malignant (n = 387) | Benign (n = 232) | Malignant (n = 223) | Benign (n = 581) | Malignant (n = 164) | ||||
| Nondiagnostic | 117 (4.9) | 19 (2.3) | 8 (2.1) | 45 (5.1) | 4 (1.7) | 7 (3.1) | 72 (4.8) | 15 (2.6) | 1 (0.6) |
| Benign | 1549 (65.2) | 734 (90.3) | 8 (2.1) | 483 (54.4) | 218 (94.0) | 6 (2.7) | 1066 (71.6) | 516 (88.8) | 2 (1.2) |
| AUS or FLUS | 166 (7.0) | 44 (5.4) | 16 (4.1) | 55 (6.2) | 6 (2.6) | 9 (4.0) | 111 (7.5) | 38 (6.5) | 7 (4.3) |
| FN or SFN | 70 (2.9) | 16 (2.0) | 8 (2.1) | 18 (2.0) | 4 (1.7) | 1 (0.5) | 52 (3.5) | 12 (2.1) | 7 (4.3) |
| Suspicious for malignancy | 25 (1.1) | 0 | 21 (5.4) | 14 (1.6) | 0 | 12 (5.4) | 11 (0.7) | 0 | 9 (5.5) |
| Malignancy | 449 (18.9) | 0 | 326 (84.2) | 273 (30.7) | 0 | 188 (84.3) | 176 (11.8) | 0 | 138 (84.1) |
Note:—FN indicates follicular neoplasm; SFN, suspicious for a follicular neoplasm.
Data are the number of nodules with percentages in parentheses. Percentages do not add up to 100% because of rounding.
Final Diagnosis
Final histopathologic diagnoses were ultimately acquired in 1200 of 2376 nodules (50.5%), all included in the outcome analyses. Of 2376 nodules, 1176 (49.5%) were neither followed adequately nor surgically removed to confirm prior CNB diagnostic assessments. Malignancies (n = 387) were diagnosed following surgical resections (n = 379) or biopsy-confirmed specific pathologic results, including metastasis (n = 7) or lymphoma (n = 1). Benign nodules (n = 813) were confirmed by an operation (193/813, 23.7%), sequential benign FNA or CNB readings (twice at least) (83/813, 10.2%), or a minimum 1-year follow-up of stable or shrinking nodules considered benign by CNB (537/813, 66.1%).
Diagnostic Utility of First-Line US-Guided CNB
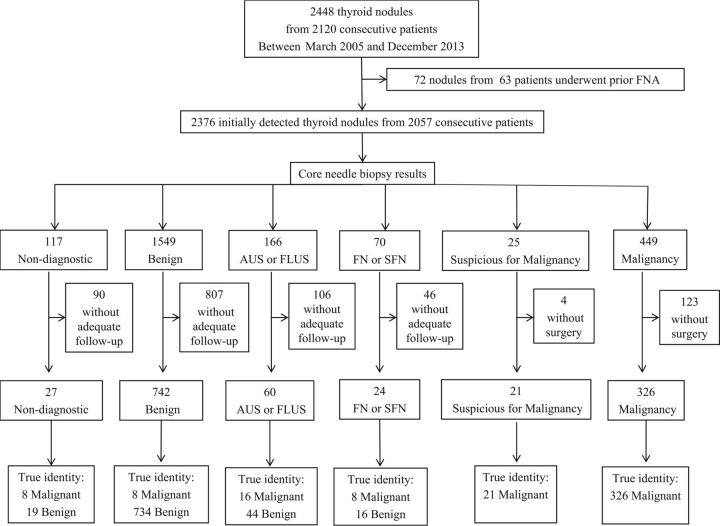
Study outcomes of CNB as a first-line procedure for a preliminary diagnosis of thyroid nodules are summarized in Fig 1 and Table 2. In terms of detecting malignancy, CNB displayed a diagnostic accuracy of 96.7%, a sensitivity of 89.7%, a specificity of 100%, a PPV of 100%, and an NPV of 95.3%. The false-negative rate was 1.1% (8/742), with no false-positive results in this study. The diagnostic accuracy and NPV were significantly higher for nodules of ≥10 mm than for nodules of <10 mm. The malignancy rate was significantly higher for nodules of <10 mm (32.3%) than for nodules of ≥10 mm (12.6%). Moreover, sensitivity, specificity, and PPV did not show significant differences according to nodule size. Diagnostic accuracy was not associated with the composition and location of thyroid nodules (On-line Table 1).
Fig 1.
Flow and study outcomes in study patients. Numbers are the number of thyroid nodules. FN indicates follicular neoplasm; SFN, suspicious for a follicular neoplasm.
Table 2:
Outcome of CNB for initially detected thyroid nodules
| Study Outcomes | Incidence (Total) | 95% CI | Incidence (<10 mm) | 95% CI | Incidence (≥10 mm) | 95% CI | P Value |
|---|---|---|---|---|---|---|---|
| Nondiagnostic | 4.9% (117/2376) | (4.1–5.8) | 5.1% (45/888) | (3.6–6.6) | 4.8% (72/1488) | (3.8–5.9) | .803 |
| Inconclusive | 11.9% (283/2376) | (10.6–13.2) | 11.3% (100/888) | (9.2–13.4) | 12.3% (183/1488) | (10.6–14.0) | .450 |
| Conclusive | 88.1% (2093/2376) | (86.8–89.4) | 88.7% (788/888) | (86.6–90.8) | 87.7% (1305/1488) | (86.0–89.4) | .450 |
| Malignancy | 19.9% (474/2376) | (18.3–21.6) | 32.3% (287/888) | (29.1–35.6) | 12.6% (187/1488) | (10.9–14.3) | <.001 |
| Diagnostic accuracy | 96.7% (1160/1200) | (95.7–97.7) | 94.9% (432/455) | (92.8–96.9) | 97.7% (728/745) | (96.6–98.8) | .012 |
| Sensitivity | 89.7% (347/387) | (86.6–92.7) | 89.7% (200/223) | (85.3–93.4) | 89.6% (147/164) | (84.9–94.1) | 1.000 |
| Specificity | 100% (813/813) | (100.0–100.0) | 100% (232/232) | (100.0–100.0) | 100% (581/581) | (100.0–100.0) | 1.000 |
| PPV | 100% (347/347) | (100.0–100.0) | 100% (200/200) | (100.0–100.0) | 100% (147/147) | (100.0–100.0) | 1.000 |
| NPV | 95.3% (813/853) | (93.9–96.7) | 91.0% (232/255) | (87.3–94.2) | 97.2% (581/598) | (95.8–98.3) | <.001 |
| Unnecessary surgery | 0.6% (2/363) | (0–1.4) | 0% (0/163) | 1.0% (2/200) | (0–2.5) | .504 | |
| Major complication | 0 | 0 | 0 | ||||
| Minor complication | 0.5% (12/2376) | (0.3–0.8) | 0.6 (5/888) | (0.1–1.1) | 0.5% (7/1488) | (0.1–0.9) | .758 |
Sample Adequacy and Conclusiveness
For CNB readings, the nondiagnostic rate was 4.9% (117/2376). Of 117 nodules, 38 contained a mix of fibromuscular tissue or normal thyroid tissue, owing to inaccurately targeted biopsies; 59 showed little or no cellular content due to cystic change or necrosis of a nodule; and 20 showed only hemorrhage. Nodule size (10 mm and ≥10 mm) and calcification did not affect the sample adequacy. The composition and location of the nodules were associated with the nondiagnostic results (Table 3).
Table 3:
Univariate analysis for factors associated with nondiagnostic result on CNB
| Study Outcomes | Nondiagnostic Results | Diagnostic Results | P Value |
|---|---|---|---|
| Nodule size (mm) | 13.3 ± 8.0 | 14.4 ± 9.7 | .220 |
| <10 mm | 45 (5.1%) | 843 (94.9%) | .803 |
| ≥10 mm | 72 (4.8%) | 1416 (95.2%) | |
| Composition (No.) (%) | |||
| Solid (cystic component <50%) | 98 (4.4%) | 2135 (95.6%) | <.001 |
| Cyst (cystic component ≥50%) | 19 (13.3%) | 124 (86.7%) | |
| Calcification (No.) (%) | |||
| None | 82 (4.9%) | 1576 (95.1%) | .941 |
| Macrocalcification | 15 (4.1%) | 349 (95.9%) | .441 |
| Microcalcification | 20 (5.6%) | 334 (94.4%) | .494 |
| Location (No.) (%) | |||
| Upper/lower | 61 (6.4%) | 896 (93.6%) | .007 |
| Mid | 56 (3.9%) | 1363 (96.1%) |
Inconclusive results accounted for 11.9% (283/2376), whereas 88.1% (2093/2376) generated conclusive outcomes. According to our subgroup analysis, the orientation, margin, and echogenicity of the nodules were associated with the conclusiveness of CNB results. The composition, size, and calcification of nodules were not associated with the conclusiveness of CNB results (Table 4).
Table 4:
Univariate analysis for factors associated with conclusive and inconclusive results on CNB
| Study Outcomes | Conclusive | Inconclusive | P Value |
|---|---|---|---|
| Age (mean) (yr) | 51.2 ± 12.6 | 48.5 ± 12.2 | <.001 |
| Sex (M/F) | 440:1653 | 44:293 | .032 |
| Nodule size (mm) | 14.2 ± 9.4 | 15.4 ± 10.3 | .064 |
| <10 mm | 788 (88.7%) | 100 (11.3%) | .450 |
| ≥10 mm | 1305 (87.7%) | 183 (12.3%) | |
| Composition (No.) (%) | |||
| Solid | 1336 (87.4%) | 192 (12.6%) | .186 |
| Predominantly solid | 95 (84.8%) | 17 (15.2%) | .274 |
| Predominantly cystic | 99 (84.6%) | 18 (15.4%) | .234 |
| Cystic | 4 (66.7%) | 2 (33.3%) | .154 |
| Shape (No.) (%) | |||
| Ovoid to round | 2033 (87.9%) | 279 (12.1%) | .156 |
| Irregular | 60 (93.8%) | 4 (6.3%) | |
| Orientation (No.) (%) | |||
| Parallel | 1728 (87.5%) | 247 (12.5%) | .047 |
| Nonparallel | 365 (91.0%) | 36 (9.0%) | |
| Margin (No.) (%) | |||
| Smooth | 1360 (85.6%) | 228 (14.4%) | <.001 |
| Spiculated | 366 (94.6%) | 21 (5.4%) | <.001 |
| Ill-defined | 367 (91.5%) | 34 (8.5%) | .020 |
| Echogenicity (No.) (%) | |||
| Isoechoic | 767 (88.5%) | 100 (11.5%) | .667 |
| Hypoechoic | 913 (86.0%) | 149 (14.0%) | .004 |
| Markedly hypoechoic | 402 (92.6%) | 32 (7.4%) | .001 |
| Hyperechoic | 11 (84.6%) | 2 (15.4%) | .662 |
| Calcification (No.) (%) | |||
| None | 1456 (87.8%) | 202 (12.2%) | .533 |
| Macrocalcification | 321 (88.2%) | 43 (11.8%) | .950 |
| Microcalcification | 316 (89.3%) | 38 (10.7%) | .459 |
| Location (No.) (%) | |||
| Upper/lower | 1100 (87.6%) | 156 (12.4%) | .417 |
| Mid | 993 (88.7%) | 127 (11.3%) |
Correlation of CNB Results with Surgical Findings
Of the 1200 verifiable diagnoses, 813 (67.8%) were benign and 387 (32.2%) were malignant. Five hundred seventy-two nodules (47.7%) were surgically resected; these procedures confirmed 379 as malignant and 193 as benign (On-line Table 2).
All 339 nodules considered malignant or suspicious for malignancy by CNB were confirmed as malignancies at surgery. Of the 24 nodules viewed as follicular neoplasms by CNB, 2 were adenomatous hyperplasia. Thus, unnecessary surgery was performed for only 2 nodules (2/363, 0.6%).
Motives for resecting nodules with benign CNB results were image-pathology discordance (benign by CNB but suspicious US features) (n = 21), malignancy on follow-up FNA or CNB (n = 5), coexistent nodules with a resected nodule (n = 40), and patient preference or aesthetic concerns. Of the 21 nodules with image-pathology discordance, 3 proved to be papillary carcinomas. Nodules (n = 48) interpreted as AUS/FLUS by CNB were resected to exclude papillary carcinoma. Among them, 12 (63.2%) were confirmed as malignant (11 papillary carcinomas, 1 follicular variant of papillary carcinoma) in the AUS group (n = 19) and 4 nodules (13.8%) were confirmed malignant (2 papillary carcinomas, 1 follicular carcinoma, and 1 follicular variant of papillary carcinoma) in the FLUS group (n = 29).
Complications
There were no major complications or hospitalizations associated with interventions in our patient cohort. Twelve patients developed minor complications. There was no difference according to nodule size. All minor complications were successfully managed by manual compression. No needle-tract seeding occurred in association with CNB.
Discussion
This present study validates the usefulness of CNB as a first-line option for assessing thyroid nodules, accruing a higher rate of conclusive results (88.1%) with low inconclusive (11.9%) and nondiagnostic (4.9%) rates compared with conventional FNA. The diagnostic accuracy of CNB was high (96.7%), with a PPV of 100% and no false-positive results. Moreover, the diagnostic performance of this study was consistent with that in previous studies (On-line Table 3).15,17–19,24,25 The unnecessary surgery (0.6%) rate was also compatible with that in a previous study (0.5%).19 There were low rates of minor complications (0.5%) without any major complication in the course of biopsy procedures. These findings indicate that CNB is a safe and reliable method and that repeated biopsies or unnecessary operations are likely to be avoided through this approach.
For small nodules (<10 mm), the diagnostic performance and the inconclusive or nondiagnostic rate showed no significant difference compared with nodules of >10 mm in this study. These findings are similar to the results reported in previous studies,15,19 and they suggest that CNB is a reliable and effective method for evaluating small and large thyroid nodules.
US-guided FNA is safe, relatively accurate, and cost-effective, but inconclusive or false-negative results of FNA are problematic. The inconclusive results of up to 25%–30% (nondiagnostic, 5%–17%; AUS/FLUS readings, 3%–18%) and false-negative results (17%–21%) of FNA are the major drawback of this technique.2,3,26–28 Recently, several studies have suggested that CNB is more useful than repeat FNA for nodules with prior nondiagnostic FNA results, especially if CNB and FNA are combined.4,5,15,24 Some sources have also indicated that CNB could be more useful for management decisions than repeat FNA in nodules with prior AUS/FLUS.4,29,30
Several studies have reported factors associated with nondiagnostic FNA results. The following factors were associated with nondiagnostic results of FNA: errors during tissue sampling (experience or skill of the operator, processing errors); interpretation errors; and the nature of the lesions, including cyst dominancy, small size, type of calcification, vascularity, and benign pathology.31–33 Performing repeat FNA for a nodule with a previously nondiagnostic FNA was significantly associated with a repeat nondiagnostic result.34 Distinct from FNA, the size of nodules and the presence or type of calcification did not affect the nondiagnostic and inconclusive results of CNB. However, nodules with cystic components, which represented >50% of the nodules, showed significantly higher nondiagnostic CNB results. It is important to aspirate the internal fluid of any cystic lesion before the CNB procedure. The location of the nodule was associated with nondiagnostic results in our study. This association might be caused by the level of the operator's skill or experience. Most of the CNB procedures were performed via a craniocaudal approach, which could restrict accurate targeting when obstructed by the clavicle or mandible. Although it has been previously reported that the operator's experience does not affect the conclusive results on CNB,19 the operator's experience or skill might be a factor.
In our study, there were 8 false-negative cases (1.1%) with benign CNB results consistent with previous studies (0%–1%).4,15,24,29 This rate remains superior to the false-negative results of FNA, reported up to 17%–21%.35,36 A recent study reported that one-third of sonographically suspicious nodules with initially benign cytology were upgraded after CNB, and among them, about 32% were proved malignant.37 False-negative FNA diagnoses may be explained by the nature of the lesions, intrinsic procedural limitations, levels of operator skill/experience,38,39 and interpretation errors.40 Unlike false-negative findings on FNA, the false-negative results of CNB in our study may reflect inaccurate targeting (6 nodules confirmed malignant at follow-up CNB or FNA, 1 nodule at the posterior margin of lower isthmus, and 1 nodule in a case of lymphocytic thyroiditis) due to procedural inexperience. An advantage of CNB is less operator dependency if the biopsy device successfully penetrates the nodule.15 Our study suggested that the ability of accurate targeting of the nodule might be important to reduce false-negative and nondiagnostic results on CNB. Awareness and expertise in several approach methods (transisthmic, craniocaudal, and lateral approaches) might be necessary.41
According to the Bethesda system for reporting thyroid cytopathology, the category of AUS/FLUS is related to a FNA specimen that manifests as scenarios of nuclear atypia, architectural atypia, and an oncocytic pattern in paucicellular aspirates.42 Although this category is regarded as having inconclusive results, nodules with AUS on FNA showed a significantly higher risk of malignancy than nodules with FLUS on FNA.29 Repeat FNA has been recommended for this subcategory, but inconclusive results, including nondiagnostic and AUS/FLUS readings, occur in 20%–49.1% of nodules with prior AUS/FLUS FNA results.4,6,27 Recently, several studies have shown that CNB is more useful than repeat FNA in cases with previous AUS/FLUS results.4,29,30 Although it is possible to get larger tissue samples through CNB procedures, there was still a low rate of AUS/FLUS on CNB for thyroid nodules in this study. It might be caused by the variable heterogeneity of this group and the lack of standardized diagnostic CNB categorization. In our study, malignancy was diagnosed significantly higher in nodules with AUS on CNB (63.2%) than nodules with FLUS (13.8%). Further investigations are needed to manage AUS/FLUS on CNB.
Although CNB conducted by experienced radiologists is safe and well-tolerated, there are still safety concerns.6,13,14,43 However, we encountered no major complications. To minimize the potential for complications and patient discomfort, technical provisions are in place, including strict color Doppler US monitoring and immediate compression of biopsy sites after CNB procedures. Compared with FNA, CNB may be technically unfeasible or difficult at times (typically in small nodules at the posterior thyroid margin).15 Furthermore, CNB can be uncomfortable for the patient, requiring local anesthesia and greater experience in image-guided thyroid interventions.
Our study has several limitations. First, it was a retrospective study performed during a relatively long period. This feature may cause selection bias. This study involved multiple radiologists and pathologists performing US-guided CNB and histopathologic interpretation. Second, up to 50% of total enrolled cases do not have final results. This lack of results might be due to loss of follow-up or lack of final surgery in 1 (general hospital) of 2 participating hospitals. Finally, we did not apply the standardized diagnostic CNB categorization of a recent publication.22
Conclusions
We have demonstrated that the first-line use of CNB may improve the diagnostic accuracy in thyroid nodules, reducing nondiagnostic or inconclusive results. The high PPV and NPV of CNB for a diagnosis of malignancy could prevent repeat biopsy or unnecessary surgery. Such benefits underscore the promising role of CNB in managing thyroid nodules and optimizing related surgical decision-making.
Supplementary Material
ABBREVIATIONS:
- AUS
atypia of undetermined significance
- CNB
core needle biopsy
- FLUS
follicular lesion of undetermined significance
- FNA
fine-needle aspiration
- NPV
negative predictive value
- PPV
positive predictive value
- US
ultrasonography
Footnotes
This work was supported by the Konyang University Myunggok Research Fund of 2015.
Paper previously presented at: Annual Meeting of the European Congress of Radiology, March 2–6, 2016; Vienna, Austria.
References
- 1. Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. J Endocrinol Invest 2010;33(5 suppl):51–56 10.1007/BF03346541 [DOI] [PubMed] [Google Scholar]
- 2. Cooper DS, Doherty GM, Haugen BR, et al. ; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167–214 10.1089/thy.2009.0110 [DOI] [PubMed] [Google Scholar]
- 3. Baloch ZW, Cibas ES, Clark DP, et al. The National Cancer Institute thyroid fine needle aspiration state of the science conference: a summation. Cytojournal 2008;5:6 10.1186/1742-6413-5-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Na DG, Kim JH, Sung JY, et al. Core-needle biopsy is more useful than repeat fine-needle aspiration in thyroid nodules read as nondiagnostic or atypia of undetermined significance by the Bethesda system for reporting thyroid cytopathology. Thyroid 2012;22:468–75 10.1089/thy.2011.0185 [DOI] [PubMed] [Google Scholar]
- 5. Samir AE, Vij A, Seale MK, et al. Ultrasound-guided percutaneous thyroid nodule core biopsy: clinical utility in patients with prior nondiagnostic fine-needle aspirate. Thyroid 2012;22:461–67 10.1089/thy.2011.0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park KT, Ahn SH, Mo JH, et al. Role of core needle biopsy and ultrasonographic finding in management of indeterminate thyroid nodules. Head Neck 2011;33:160–65 10.1002/hed.21414 [DOI] [PubMed] [Google Scholar]
- 7. Hryhorczuk AL, Stephens T, Bude RO, et al. Prevalence of malignancy in thyroid nodules with an initial nondiagnostic result after ultrasound-guided fine needle aspiration. Ultrasound Med Biol 2012;38:561–67 10.1016/j.ultrasmedbio.2011.12.026 [DOI] [PubMed] [Google Scholar]
- 8. Lubitz CC, Nagarkatti SS, Faquin WC, et al. Diagnostic yield of nondiagnostic thyroid nodules is not altered by timing of repeat biopsy. Thyroid 2012;22:590–94 10.1089/thy.2011.0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jo VY, Vanderlaan PA, Marqusee E, et al. Repeatedly nondiagnostic thyroid fine-needle aspirations do not modify malignancy risk. Acta Cytol 2011;55:539–43 10.1159/000333230 [DOI] [PubMed] [Google Scholar]
- 10. Bartolazzi A, Orlandi F, Saggiorato E, et al. Galectin-3-expression analysis in the surgical selection of follicular thyroid nodules with indeterminate fine-needle aspiration cytology: a prospective multicentre study. Lancet Oncol 2008;9:543–49 10.1016/S1470-2045(08)70132-3 [DOI] [PubMed] [Google Scholar]
- 11. Mathur A, Weng J, Moses W, et al. A prospective study evaluating the accuracy of using combined clinical factors and candidate diagnostic markers to refine the accuracy of thyroid fine-needle aspiration biopsy. Surgery 2010;148:1170–77 10.1016/j.surg.2010.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cibas ES, Ali SZ; NCI Thyroid FNA. The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol 2009;132:658–65 10.1309/AJCPPHLWMI3JV4LA [DOI] [PubMed] [Google Scholar]
- 13. Renshaw AA, Pinnar N. Comparison of thyroid fine-needle aspiration and core needle biopsy. Am J Clin Pathol 2007;128:370–74 10.1309/07TL3V58337TXHMC [DOI] [PubMed] [Google Scholar]
- 14. Screaton NJ, Berman LH, Grant JW. US-guided core-needle biopsy of the thyroid gland. Radiology 2003;226:827–32 10.1148/radiol.2263012073 [DOI] [PubMed] [Google Scholar]
- 15. Sung JY, Na DG, Kim KS, et al. Diagnostic accuracy of fine-needle aspiration versus core-needle biopsy for the diagnosis of thyroid malignancy in a clinical cohort. Eur Radiol 2012;22:1564–72 10.1007/s00330-012-2405-6 [DOI] [PubMed] [Google Scholar]
- 16. Nasrollah N, Trimboli P, Guidobaldi L, et al. Thin core biopsy should help to discriminate thyroid nodules cytologically classified as indeterminate: a new sampling technique. Endocrine 2013;43:659–65 10.1007/s12020-012-9811-z [DOI] [PubMed] [Google Scholar]
- 17. Trimboli P, Nasrollah N, Guidobaldi L, et al. The use of core needle biopsy as first-line in diagnosis of thyroid nodules reduces false negative and inconclusive data reported by fine-needle aspiration. World J Surg Oncol 2014;12:61 10.1186/1477-7819-12-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paja M, del Cura JL, Zabala R, et al. Ultrasound-guided core-needle biopsy in thyroid nodules: a study of 676 consecutive cases with surgical correlation. Eur Radiol 2016;26:1–8 10.1007/s00330-015-3821-1 [DOI] [PubMed] [Google Scholar]
- 19. Suh CH, Baek JH, Lee JH, et al. The role of core-needle biopsy as a first-line diagnostic tool for initially detected thyroid nodules. Thyroid 2016;26:395–403 10.1089/thy.2015.0404 [DOI] [PubMed] [Google Scholar]
- 20. Kwak JY, Jung I, Baek JH, et al. ; Korean Society of Thyroid Radiology (KSThR), Korean Society of Radiology. Image reporting and characterization system for ultrasound features of thyroid nodules: multicentric Korean retrospective study. Korean J Radiol 2013;14:110–17 10.3348/kjr.2013.14.1.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moon W, Baek JH, Jung SL, et al. ; Korean Society of Thyroid Radiology (KSThR), Korean Society of Radiology. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean J Radiol 2011;12:1–14 10.3348/kjr.2011.12.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jung CK, Min HS, Park HJ, et al. ; Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group. Pathology reporting of thyroid core needle biopsy: a proposal of the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group. J Pathol Transl Med 2015;49:288–99 10.4132/jptm.2015.06.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burke DR, Lewis CA, Cardella JF, et al. ; Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. J Vasc Interv Radiol 2003;14:S243–46 [PubMed] [Google Scholar]
- 24. Yeon JS, Baek JH, Lim HK, et al. Thyroid nodules with initially nondiagnostic cytologic results: the role of core-needle biopsy. Radiology 2013;268:274–80 10.1148/radiol.13122247 [DOI] [PubMed] [Google Scholar]
- 25. Ha EJ, Baek JH, Lee JH, et al. Core needle biopsy can minimise the non-diagnostic results and need for diagnostic surgery in patients with calcified thyroid nodules. Eur Radiol 2014;24:1403–09 10.1007/s00330-014-3123-z [DOI] [PubMed] [Google Scholar]
- 26. Nayar R, Ivanovic M. The indeterminate thyroid fine-needle aspiration: experience from an academic center using terminology similar to that proposed in the 2007 National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Cancer 2009;117:195–202 10.1002/cncy.20029 [DOI] [PubMed] [Google Scholar]
- 27. Yassa L, Cibas ES, Benson CB, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer Cytopathol 2007;111:508–16 10.1002/cncr.23116 [DOI] [PubMed] [Google Scholar]
- 28. Yang J, Schnadig V, Logrono R, et al. Fine-needle aspiration of thyroid nodules: a study of 4703 patients with histologic and clinical correlations. Cancer Cytopathol 2007;111:306–15 10.1002/cncr.22955 [DOI] [PubMed] [Google Scholar]
- 29. Choi YJ, Baek JH, Ha EJ, et al. Differences in risk of malignancy and management recommendations in subcategories of thyroid nodules with atypia of undetermined significance or follicular lesion of undetermined significance: the role of ultrasound-guided core-needle biopsy. Thyroid 2014;24:494–501 10.1089/thy.2012.0635 [DOI] [PubMed] [Google Scholar]
- 30. Na DG, Min HS, Lee H, et al. Role of core needle biopsy in the management of atypia/follicular lesion of undetermined significance thyroid nodules: comparison with repeat fine-needle aspiration in subcategory nodules. Eur Thyroid J 2015;4:189–96 10.1159/000437051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Degirmenci B, Haktanir A, Albayrak R, et al. Sonographically guided fine-needle biopsy of thyroid nodules: the effects of nodule characteristics, sampling technique, and needle size on the adequacy of cytological material. Clin Radiol 2007;62:798–803 10.1016/j.crad.2007.01.024 [DOI] [PubMed] [Google Scholar]
- 32. Choi SH, Han KH, Yoon JH, et al. Factors affecting inadequate sampling of ultrasound-guided fine-needle aspiration biopsy of thyroid nodules. Clin Endocrinol (Oxf) 2011;74:776–82 10.1111/j.1365-2265.2011.04011.x [DOI] [PubMed] [Google Scholar]
- 33. Moon HJ, Kwak JY, Kim EK, et al. Ultrasonographic characteristics predictive of nondiagnostic results for fine-needle aspiration biopsies of thyroid nodules. Ultrasound Med Biol 2011;37:549–55 10.1016/j.ultrasmedbio.2011.01.015 [DOI] [PubMed] [Google Scholar]
- 34. Choi SH, Baek JH, Lee JH, et al. Thyroid nodules with initially non-diagnostic, fine-needle aspiration results: comparison of core-needle biopsy and repeated fine-needle aspiration. Eur Radiol 2014;24:2819–26 10.1007/s00330-014-3325-4 [DOI] [PubMed] [Google Scholar]
- 35. Raab SS, Vrbin CM, Grzybicki DM, et al. Errors in thyroid gland fine-needle aspiration. Am J Clin Pathol 2006;125:873–82 10.1309/7RQE37K6439T4PB4 [DOI] [PubMed] [Google Scholar]
- 36. Yeh MW, Demircan O, Ituarte P, et al. False-negative fine-needle aspiration cytology results delay treatment and adversely affect outcome in patients with thyroid carcinoma. Thyroid 2004;14:207–15 10.1089/105072504773297885 [DOI] [PubMed] [Google Scholar]
- 37. Ha EJ, Baek JH, Lee JH, et al. Sonographically suspicious thyroid nodules with initially benign cytologic results: the role of a core needle biopsy. Thyroid 2013;23:703–08 10.1089/thy.2012.0426 [DOI] [PubMed] [Google Scholar]
- 38. Ylagan LR, Farkas T, Dehner LP. Fine needle aspiration of the thyroid: a cytohistologic correlation and study of discrepant cases. Thyroid 2004;14:35–41 10.1089/105072504322783821 [DOI] [PubMed] [Google Scholar]
- 39. Wu HH, Jones JN, Osman J. Fine-needle aspiration cytology of the thyroid: ten years experience in a community teaching hospital. Diagn Cytopathol 2006;34:93–96 10.1002/dc.20389 [DOI] [PubMed] [Google Scholar]
- 40. Baloch ZW, Sack MJ, Yu GH, et al. Fine-needle aspiration of thyroid: an institutional experience. Thyroid 1998;8:565–69 10.1089/thy.1998.8.565 [DOI] [PubMed] [Google Scholar]
- 41. Baek JH, Lee JH, Valcavi R, et al. Thermal ablation for benign thyroid nodules: radiofrequency and laser. Korean J Radiol 2011;12:525–40 10.3348/kjr.2011.12.5.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bongiovanni M, Krane JF, Cibas ES, et al. The atypical thyroid fine-needle aspiration: past, present, and future. Cancer Cytopathol 2012;120:73–86 10.1002/cncy.20178 [DOI] [PubMed] [Google Scholar]
- 43. Harvey JN, Parker D, De P, et al. Sonographically guided core biopsy in the assessment of thyroid nodules. J Clin Ultrasound 2005;33:57–62 10.1002/jcu.20092 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.