Abstract
BACKGROUND AND PURPOSE:
Periventricular pseudocysts are cystic cavities that lack the ependymal cell lining found in true cysts. The aim of this study was to characterize periventricular pseudocysts and related findings and their neurodevelopmental outcome.
MATERIALS AND METHODS:
This was a retrospective study of periventricular pseudocysts detected prenatally on fetal MR imaging in 26 fetuses. The fetuses were divided into group A (n = 8), which included cases with isolated periventricular pseudocysts, and group B (n = 18), which included cases of periventricular pseudocysts with additional findings. Cases were further subdivided into connatal cysts and subependymal pseudocysts. Data collected included prenatal history, MR imaging features, sonographic follow-up, and neurodevelopmental outcome.
RESULTS:
All cases in group A (n = 8) had a normal outcome. In group B (n = 18), 6 pregnancies were terminated and 2 had an abnormal outcome. Both cases with an abnormal outcome involved patients with subependymal pseudocysts. No significant association was found between the morphologic features on MR imaging and the neurodevelopmental outcome.
CONCLUSIONS:
Neurodevelopmental outcome in cases of isolated periventricular pseudocysts detected prenatally appears to be normal. A detailed evaluation should be performed to rule out additional brain findings, chromosomal aberration, and fetal malformation. This evaluation should include the following: maternal TORCH status, detailed fetal sonographic anatomic evaluation, fetal echocardiogram, fetal brain MR imaging, amniocentesis and karyotyping/comparative genomic hybridization, and genetic counseling. Additional findings on MR imaging, including mild-to-moderate dilated ventricles, asymmetric ventricles, or T2 hyperintense signal in the white matter without other findings or major fetal abnormality, appear to be benign. Connatal cysts appear to be benign.
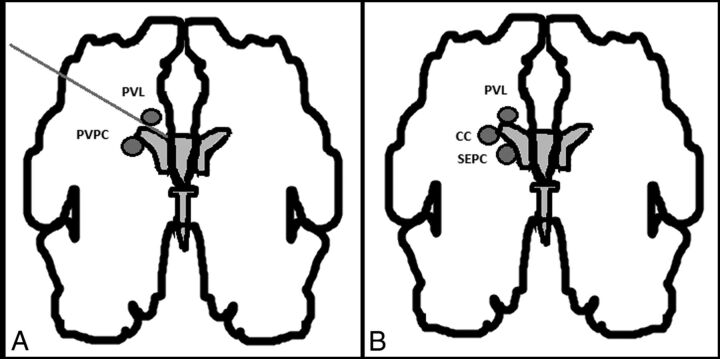
Periventricular pseudocysts (PVPC) are cystic cavities that lack the ependymal cell lining found in true cysts.1 They are found in 0.5%–5% of healthy term neonates by using transfontanellar sonography in the first days of life.2,3 For a long time, periventricular leukomalacia and PVPC have been confused. Malinger et al4 and Rademaker et al5 differentiated PVPC, which are found below the external angle of the lateral ventricles, from periventricular leukomalacia, which is located above it and has a different prognosis (Fig 1A).4 PVPC occur in the germinal matrix during the time of its exponential development in the beginning of the second trimester and during its rapid lysis toward its end. Therefore, Rademaker et al5 suggested that they should be referred to as “germinolytic cysts.” Epelman et al 6 further divided PVPC into connatal cysts, also known as frontal horn cysts, and subependymal pseudocysts (SEPC). Connatal cysts are located at the external angle, anterior to the foramina of Monro. SEPC are located posterior to the foramina of Monro (Fig 1B).6
Fig 1.
A, Schematic representation of the differential diagnosis between periventricular pseudocysts and periventricular leukomalacia. Originally published by Malinger et al.4 B, Differential diagnosis between the cystic lesions seen in periventricular leukomalacia (PVL), connatal cysts (CC), and subependymal cysts (SC). Malinger et al4 original publication modified by Epelman et al.6
There are 2 types of pathogenesis in the formation of PVPC: germinal matrix hemorrhage and germinolysis, which are associated with congenital infections,7–9 metabolic disorders,10 and chromosomal aberration.11 Nevertheless, PVPC have been reported as isolated findings.4,11–13
There are scarce data regarding the neurodevelopmental outcome of infants with PVPC. The studies conducted are of small cohorts and describe mostly neonates and premature infants. They have suggested that isolated PVPC have a good prognosis.4,12–16
The growing use of prenatal imaging such as fetal ultrasound (US) and MR imaging led to an increase in prenatal detection of PVPC. However, the significance of these findings is not well-established. Malinger et al4 were the first to describe the characteristics and outcome of prenatally detected PVPC in 2002. Since then, only a few case reports have been added.17,18 Recently, Esteban et al13 described an association between SEPC morphologic features and clinical outcome. To our knowledge, the characteristics of connatal cysts and outcome were only described in neonates, and they are considered benign.5,12,19–25
The objectives of the present study were to determine whether findings in addition to PVPC affect the neurodevelopmental outcome, characterize PVPC-related factors, and describe the differences between connatal cysts and SEPC. The strength of this work is in the wide range of parameters recorded, including PVPC-related clinical prenatal and perinatal factors; morphologic characteristics of fetal MRI; and the long-term follow-up of the cases.
Materials and Methods
Population and Setting
This was an observational retrospective study that included all pregnant women who underwent fetal brain MR imaging in which PVPC were detected at Sheba Medical Center, Israel, between 2011 and 2014.
Data obtained from the records include the following: maternal history (age, medical history, previous pregnancy outcome, and the presence of known risk factors associated with PVPC), abnormal pregnancy events, prenatal history (sex, prenatal testing, fetal echocardiogram, and TORCH [toxoplasmosis, rubella, cytomegalovirus, and herpes virus] serology), US and MR imaging features of PVPC, associated findings, perinatal history (gestational age, birth weight, Apgar score, and mode of delivery), sonographic follow-up, and clinical follow-up. Fetal cytomegalovirus (CMV) infection was confirmed by amniocentesis.
Following genetic and parental counseling, termination of pregnancy (TOP) was performed in several cases. Postmortem examination and MR imaging were performed on the fetuses of patients who went through TOP in our institution.
To establish whether isolated PVPC have a better prognosis as suggested by several studies,4,12–16 cases were divided into 2 groups (Fig 2):
Group A (isolated PVPC): cases with PVPC as a single finding on MR imaging and with no additional fetal abnormalities such as intrauterine growth restriction (IUGR), fetal infection, abnormal fetal echocardiogram findings, or chromosomal aberration.
Group B (nonisolated PVPC): cases with additional pathologic findings on fetal MR imaging, additional fetal abnormalities, or both.
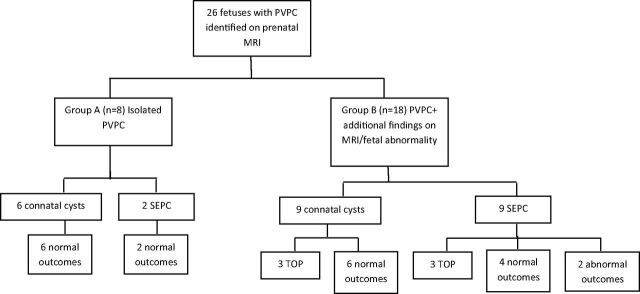
Fig 2.
Flowchart illustrating the study design and outcome. Cases are divided to 2 groups: fetuses in group A had only PVPC on MR imaging, while fetuses on group B had additional findings on MR imaging or fetal abnormality. Fetal abnormality is defined as the presence of fetal infection, chromosomal abnormality, IUGR, abnormal echocardiogram findings, or other fetal malformation. The groups were further subdivided into connatal cysts or subependymal pseudocysts.
Each group was further subdivided, according to the location of the PVPC, into connatal cysts and SEPC.
Sonography
Patients were referred to our institution when diagnosed by an US structural survey performed by specialized gynecologists or as part of the routine pregnancy follow-up. Additional focused US examinations were performed in most patients by a dedicated fetal sonographer by using a multiplanar approach (4 coronal and 3 sagittal planes) to evaluate the brain selectively and precisely according to the International Society of Ultrasound in Obstetrics and Gynecology guidelines.26
MR Imaging
All patients were referred for fetal brain MR imaging due to a specific suspected anomaly demonstrated by US or evidence of maternal CMV or toxoplasma infection. The preferred timing for fetal brain MR imaging at our institution is in the 32nd week of gestation due to the ability to assess brain maturation (sulcation and gyration) and parenchyma, in addition to a comprehensive brain structure scan.27,28 MR imaging was performed for the following reasons: suspected PVPC on US (n = 13), dilated or asymmetric lateral ventricles on US (n = 3), suspected PVPC and dilated or asymmetric lateral ventricles on US (n = 3), asymmetric lateral ventricles with multiple extracerebral malformations (n = 1), maternal infection (n = 4), follow-up after fetoscopic laser coagulation in twin-to-twin transfusion syndrome (n = 1), and IUGR with a previous child with white matter disease (n = 1).
Fetal MR imaging was performed and evaluated by an obstetrician who specializes in fetal US and MR imaging (E.K.), an expert MR imaging neuroradiologist (C.H.), and an experienced pediatric neurologist (O.B.-Y.) in a group analysis. A 1.5T MR imaging system (Optima 1.5T; GE Healthcare, Milwaukee, Wisconsin) was used. Single-shot fast spin-echo T2-weighted sequences in 3 orthogonal planes were used with section thicknesses of 3–4 mm, no gap, and a flexible coil (8-channel cardiac coil). The FOV was determined by the size of the fetal head, 24 cm for smaller fetuses and up to 30 cm for larger ones. Other parameters were a matrix of 320/224, TE of 90 ms, and TR of 1298 ms. The fast spoiled gradient-echo T1 sequence was performed only in the axial plane with a larger FOV of 40 cm, 4-mm section thickness, 0.5-mm gap, a TR of 160 ms, and a TE of 2.3 ms.
Mildly dilated ventricles were defined as an atrial measurement of 10–12 mm, and moderately dilated ventricles, as 13–15 mm.29
PVPC Imaging Features
PVPC were defined according to Malinger et al4 (Fig 1A) and were subdivided into 2 groups: connatal cysts and SEPC according to Epelman et al (Figs 1A and 3).6
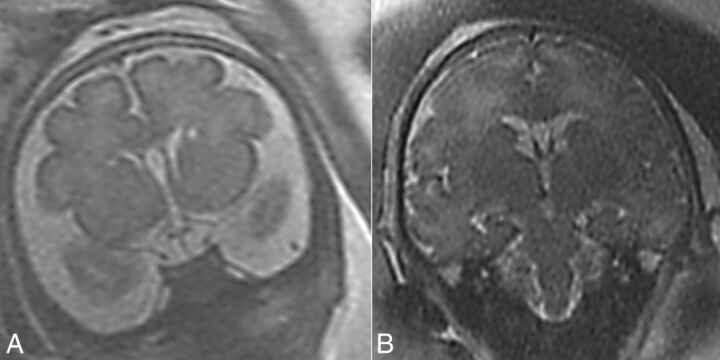
Fig 3.
T2 MR imaging coronal view. A, Case 5, bilateral connatal cysts located at the external angle, anterior to the foramina of Monro. B, Case 17, bilateral subependymal pseudocysts located posterior to the foramina of Monro.
Features and morphology of PVPC were described according to Esteban et al13 and included the following: uni-/bilaterality, uni-/multilocularity, and size and anatomic location in relation to the frontal, temporal, and occipital horns and the caudothalamic notch. Morphology features included the anteroposterior diameter and height of the pseudocysts that were evaluated on parasagittal sections and the shape and margins of the PVPC. PVPC were considered atypical when they showed ill-defined margins, were square (as opposed to oval), or had a height greater than the anteroposterior diameter.
Neurodevelopmental Outcome
Children were evaluated by using Vineland II Adaptive Behavior Scale,30 which examines 4 areas of development: communication, daily living skills, socialization, and motor skills. The score derived from this instrument has a mean of 100 ± 15. The published Israeli version of the Vineland II Adaptive Behavior Scale was administered, but no Israeli norms are available for the full age range. Because there was no reason to believe that Israeli and US children would develop mental and motor skills differently, we used US norms. Scores of children were considered abnormal if the standard score was <70. The mean age of children evaluated by using the Vineland II Adaptive Behavior Scale was 2.07 ± 0.86 years.
In addition, records of neurologic clinical follow-up were collected retrospectively.
Statistical Analysis
The Fisher exact test was used for categoric variables, and a t test, for continuous variables. The data were analyzed by using GraphPad QuickCalcs software (GraphPad Software, San Diego, California).
Ethics Approval
The research was approved by the hospital research ethics board.
Results
Population
Between 2011 and 2014, 1849 patients underwent a fetal brain MR imaging examination due to brain anomalies detected on fetal US and maternal TORCH infection. In 26 cases (1.41%), fetal PVPC were detected. TORCH serology was documented for 23/26 patients. Amniocentesis and karyotyping were performed in 14/26 patients. None of the patients were known to have a drug addiction or were documented as HIV-positive. The pregnancy and neonatal characteristics are described in Table 1. Abnormal pregnancy events included twin-to-twin transfusion syndrome with laser coagulation treatment and fetal reduction (case 5) and TIA during the pregnancy (case 15). There was no apparent difference in the incidence between male and female fetuses. Seven fetuses (26.9%) had additional fetal abnormalities: Three had IUGR, 2 had fetal CMV infection, 1 had chromosomal aberration, and 1 had IUGR in addition to chromosomal aberration and a ventricular septal defect detected on echocardiography. The chromosomal aberrations found included a 1P36 microdeletion and a translocation in chromosomes 11 and 22 (Emanuel syndrome; Online Mendelian Inheritance in Man No. 609029; http://omim.org/). The median birth gestational age was 38 weeks (range, 35–41 weeks), and the median birth weight was 3210 g (range, 2445–4060 g).
Table 1:
Main population, neonatal, and delivery characteristics (n = 26)a
| Characteristics | |
|---|---|
| Pregnancy and maternal | |
| Maternal age (yr) | 32.5 (21–42) |
| In vitro fertilization | 3/26 (11.5%) |
| Multiple pregnancies | 2/26 (7.7%) |
| Maternal infection | 5/26 (19.2%) |
| CMV | 4/26 (15.3%) |
| Toxoplasma | 1/26 (3.8%) |
| Maternal medical treatment | 5/26 (19.2%) |
| Maternal hypercoagulative disorder | 4/26 (15.3%) |
| TOP | 6/26 (23.1%) |
| Fetal and neonatal | |
| Male/female ratio (15:11) | 1.4:1 |
| Fetal abnormalities | |
| IUGR | 4/26 (15.4%) |
| CMV infection | 2/26 (7.7%) |
| Chromosomal aberration | 2/26 (7.7%) |
| Abnormal fetal echocardiogram findings | 1/26 (3.8%) |
| GA at MRI diagnosis (wk) | 33 (29–38) |
| Birth GA (wk) | 38 (35–41) |
| BW (g) | 3210 (2445–4060) |
| Apgar score | |
| At 1 min | 9 (8–9) |
| At 5 min | 10 (8–10) |
| Mode of delivery (n = 20) | |
| Vaginal delivery | 11/20 (55%) |
| Cesarean delivery | 7/20 (35%) |
| Assisted vaginal delivery | 2/20 (10%) |
Note:—GA indicates gestational age, BW, birth weight.
Data are expressed as median (range) or number (percentage).
Termination of pregnancy was performed in 6 cases: Two had fetal CMV infection, 2 had porencephalic cysts, and 2 had PVPC with additional abnormal MR imaging findings. Four patients went through TOP in our institution; 3 of them had a postmortem examination and postmortem MR imaging. One patient underwent a fetal reduction in a twin pregnancy; as a result, no postmortem examination was performed. Characteristics of pregnancy and MR imaging data of patients who underwent TOP are described in On-line Table 1.
Imaging Findings
Five (19.2%) cases of PVPC were not identified on US examination and were diagnosed only on MR imaging. The mean gestational age at MR imaging diagnosis was 33.4 ± 2.6 weeks.
Morphologic Features of PVPC on MR Imaging
Fifteen cases had connatal cysts and 11 cases had SEPC.
PVPC were bilateral in 24 cases (92.3%), all of which were multilocular. Mean PVPC height was 5.36 ± 0.89 mm, and mean anteroposterior diameter was 8.71 ± 2.46 mm. In 25 cases (96.1%), PVPC were located along the frontal horns, while in only 1 case were they located along the frontal and occipital horns. In 4 cases (15.4%), PVPC were extended posterior to the caudothalamic notch. One case (3.84%) had an atypical morphology. MR imaging morphologic features subdivided into connatal cysts and SEPC are described in Table 2.
Table 2:
MRI morphologic features and neurodevelopmental outcome of connatal cysts and subependymal pseudocysts
| MRI Morphologic Feature | Connatal Cysts | SEPC | P Value |
|---|---|---|---|
| Bilateral (No.) | 15/15 (100%) | 9/11 (82%) | .17 |
| Multilocular (No.) | 14/15 (93.3%) | 10/11 (91%) | 1.00 |
| Mean height | 5.17 ± 1.03 | 5.61 ± 0.63 | .22 |
| Mean AP diameter | 8.53 ± 2.76 | 8.97 ± 2.10 | .65 |
| Near the occipital horns (No.) | 0 | 1/11 (9%) | .42 |
| Posterior to the caudothalamic notch (No.) | 0 | 4/11 (36%) | .02 |
| Atypical morphology (No.) | 1/15 (6.6%) | 0 | .42 |
| Abnormal neurodevelopmental outcome (No.) | 0 | 2/11 (18%) | .15 |
| TOP (No.) | 3/15 (20%) | 3/11 (27%) | – |
Note:—AP indicates anteroposterior.
Sixteen cases from group B had additional MR imaging findings, including asymmetric lateral ventricles with or without dilation (n = 5), T2 hyperintense signal in the white matter (n = 2), a combination of asymmetric or dilated lateral ventricles and T2 hyperintense signal in the white matter (n = 4), mildly dilated lateral ventricles with evidence of GM bleeding (n = 1), porencephalic cyst (n = 2), a small cerebellum and an abnormal structure of the fourth ventricle (n = 1), and asymmetric lateral ventricles in addition to T2 hyperintense signal in the white matter and an abnormal structure of the fourth ventricle (n = 1).
Two cases in group B did not have additional findings on MR imaging. However, they had fetal abnormalities, including fetal CMV infection and IUGR.
Postnatal Sonographic Follow-Up
Postnatal sonographic follow-up was performed in 13/20 neonates (65%). The mean follow-up was 3.32 ± 2.51 months. In 4/13 (30.7%) neonates, PVPC did not appear on an US performed during the first 3 days of life. In 8/13 (61.5%) neonates, the PVPC resolved at up to 8 months of age. In 1 neonate, PVPC were demonstrated on the first day of life with no other sonographic follow-up.
Neurodevelopmental Outcome
Group A included 8 cases (On-line Table 2), all of which had a normal outcome. Group B included 18 cases (On-line Tables 3 and 4): 6 pregnancies were terminated (On-line Table 1), 10 had a normal outcome, and 2 had an abnormal outcome (Fig 2):
Case 19 was 2 years 2 months of age at the time of the study and was evaluated by using the Vineland II Adaptive Behavior Scale. His score was 58, and he had a low adaptive level. He had a low standard score in all 4 areas of development. He also had vision problems and seizures.
Case 20 was 1 year 9 months of age at the time of the study and was evaluated by using the Vineland II Adaptive Behavior Scale. His score was 63, and he had a low adaptive level. He had a low standard score in all 4 areas of development and was diagnosed postnatally with Emanuel syndrome (Online Mendelian Inheritance in Man, No. 609029).
The Vineland II adaptive behavior composite standard scores are presented in On-line Table 5.
Morphologic Features of PVPC on MR Imaging and Neurodevelopmental Outcome
Laterality and locularity of PVPC were not significantly associated with an abnormal outcome (P = 1, P = 1, respectively; Fisher exact test). Mean height and mean anteroposterior diameter of PVPC were not significantly different between cases with normal and abnormal findings (P = .71, P = .31, respectively; t test) or between groups A and B (P = .32, P = .34, respectively; t test). The association between occipital horn location and an abnormal outcome was not significant (P = .1, Fisher exact test). No significant association was found between the relation to the caudothalamic notch and an abnormal outcome (P = .36, Fisher exact test).
Eighty percent of the cases in the connatal cyst (12/15) subgroup had a normal outcome; the remaining 20% (3/15) underwent TOP. In the SEPC subgroup, only 55% of the cases (6/11) had a normal outcome, 18% (2/11) had an abnormal outcome, and 27% (3/11) underwent TOP. The association between PVPC subtype (connatal versus SEPC) and an abnormal outcome showed a trend but was not statistically significant (P = .15, Fisher exact test).
Discussion
The aim of this study was to describe characteristics of prenatally diagnosed PVPC and additional findings and to determine their association with neurodevelopmental outcome. In the literature, PVPC have been associated with a variable outcome. Most of the studies reported a good neurodevelopmental outcome in the presence of isolated PVPC.4,11–16 Makhoul et al,11 in a meta-analysis of the literature on infants, concluded that in the absence of additional factors, including IUGR, fetal infections, malformations, and chromosomal aberrations or persistence of PVPC, a favorable outcome is expected. Cevey-Macherel et al16 described the biggest cohort, which included 74 neonates. They concluded that isolated PVPC are associated with normal neurodevelopment and suggested that a neurologic examination at birth is a good outcome predictor. There are scarce data of prenatally diagnosed PVPC. Malinger et al4 described 9 cases of prenatally detected PVPC, of which 5 were isolated findings. One of those underwent TOP, and the other 4 were reported to have normal neurodevelopmental outcome. Esteban et al13 found that prenatal cases with isolated PVPC had a normal outcome. In accordance with the literature, all of the cases with isolated PVPC in our study, regardless of their subtype (connatal versus SEPC), had a normal neurodevelopment.
Two cases in the group of nonisolated PVPC had an abnormal outcome with developmental delay. In both cases, the PVPC subtype was SEPC, and both had additional findings on MR imaging and chromosomal aberrations.
Evaluation of the nonisolated PVPC with a normal outcome group (n = 10) revealed 5 cases with asymmetric lateral ventricles with or without mild-to-moderate ventriculomegaly; 3 cases with T2 hyperintense signal in the white matter; and 2 cases with both characteristics, one of them with an additional abnormal structure of the fourth ventricle. These findings might suggest that the combination of PVPC with mildly or moderately dilated or asymmetric lateral ventricles or with T2 hyperintense signal in the white matter is benign. The outcome of asymmetric lateral ventricles detected prenatally seems to be benign, but it is not well-established because there are very limited data.31–33 Mild isolated lateral ventriculomegaly is associated with a good outcome.34 To our knowledge, the significance of an isolated T2 hyperintense signal in the white matter detected prenatally is yet to be determined. Further investigation with a larger series is needed to establish the significance of these findings.
In the literature, several studies suggested an association between the morphologic features of PVPC and the etiology. Bilateral and multilocular PVPC were associated with an etiology that involves >1 part of the brain, such as viral infection or genetic anomalies rather than a focal cerebrovascular insult.14,20,35,36 However, in accordance with our findings, Cevey-Macherel et al16 and Esteban et al13 found no association between laterality and those etiologies.
Esteban et al13 suggested that further investigations should be performed when the great axis of the cyst is ≥9 mm, when PVPC face the temporal horns, when PVPC are located posterior to the caudothalamic notch, and in the presence of PVPC with atypical morphology. In our study, no association was found between the size of PVPC and their location in relation to the caudothalamic notch and adverse outcome. We hypothesized that the different timing of the occurrence of PVPC explains the differences between our study and that of Esteban et al.13 The occurrence of PVPC in the germinal matrix before the initiation of its lysis (26 weeks) or after could have an effect on morphology and outcome. This hypothesis was not supported by our data because the characteristics of PVPC and neurodevelopmental outcome were equally distributed over the gestational age at diagnosis.
PVPC in the occipital horns were associated with CMV infection in the literature.37 The 1 case with PVPC in the occipital horns in our study had CMV infection. The only case with atypical PVPC morphology underwent TOP.
Studies regarding connatal cysts, also known as frontal horn cysts, suggest that they are benign,5,12,15,19–24 but the number of cases described is very limited. Unger et al,25 in their review of the literature, found 87 cases of connatal cysts reported so far. From those cases, 49 (56%) had a normal outcome, 21 had an abnormal outcome, and 17 were lost to follow-up. In our study, all cases with connatal cysts had a normal outcome.
In this study, 6 cases underwent TOP. At present, there are no established indications for TOP when PVPC are diagnosed. The traditional approach to CMV in pregnancy is to consider termination of pregnancy in cases of viral transfer to the fetus. However, recent studies suggest that normal fetal imaging findings rule out almost entirely the presence of any major neurologic damage other than varying degrees of hearing impairment and minor neurologic sequelae.38,39 Isolated findings such as PVPC were suggested to have a better prognosis, 37,40 but this statement is not well-established. In our study, 2 cases with CMV infection underwent TOP at another institution. The remaining 2 cases had normal outcome. This should raise the question of whether TOP is justified in those cases with CMV or PVPC as an isolated finding. Further studies are needed to resolve this question. Within the limits of this study, we suggest that TOP should be considered only when PVPC are part of a major brain anomaly or when significant fetal malformation or genetic abnormalities exist. When PVPC are detected on US, the following evaluations should be made: maternal TORCH status, detailed fetal sonographic anatomic evaluation, fetal echocardiogram, fetal brain MR imaging, amniocentesis, and karyotyping/comparative genomic hybridization, and genetic counseling.
The strength of our study is by adding to the limited data of prenatally detected PVPC and by its relatively large number of cases (n = 26) and high follow-up rate (n = 20). Moreover, the patients in this study were extensively evaluated for additional findings in the prenatal period. Twenty-three patients (88.5%) had TORCH serologies, 17 patients (65.4%) had a fetal echocardiogram, 15 patients (57.7%) had amniocentesis with karyotyping, and 7 patients (26.9%) had comparative genomic hybridization testing.
Conclusions
Neurodevelopmental outcome in cases of isolated PVPC detected prenatally was normal. Additional findings on MR imaging, including mild-to-moderate dilated ventricles, asymmetric ventricles, or T2 hyperintense signal in the white matter without other findings or major fetal abnormality, appear to be benign. Associated fetal abnormalities may have a poor outcome. Connatal cysts appear to have a better prognosis than SEPC. Further large prospective research is needed to confirm our findings.
Supplementary Material
ABBREVIATIONS:
- CMV
cytomegalovirus
- IUGR
intrauterine growth restriction
- PVPC
periventricular pseudocysts
- SEPC
subependymal pseudocysts
- TOP
termination of pregnancy
- US
ultrasound
- TORCH
toxoplasmosis, rubella, cytomegalovirus, and herpes virus
References
- 1. Larroche JC. Sub-ependymal pseudo-cysts in the newborn. Biol Neonate 1972;21:170–83 10.1159/000240506 [DOI] [PubMed] [Google Scholar]
- 2. Shen EY, Huang FY. Subependymal cysts in normal neonates. Arch Dis Child 1985;60:1072–74 10.1136/adc.60.11.1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heibel M, Heber R, Bechinger D, et al. Early diagnosis of perinatal cerebral lesions in apparently normal full-term newborns by ultrasound of the brain. Neuroradiology 1993;35:85–91 10.1007/BF00593960 [DOI] [PubMed] [Google Scholar]
- 4. Malinger G, Lev D, Ben Sira L, et al. Congenital periventricular pseudocysts: prenatal sonographic appearance and clinical implications. Ultrasound Obstet Gynecol 2002;20:447–51 10.1046/j.1469-0705.2002.00840.x [DOI] [PubMed] [Google Scholar]
- 5. Rademaker KJ, De Vries LS, Barth PG. Subependymal pseudocysts: ultrasound diagnosis and findings at follow-up. Acta Paediatr 1993;82:394–99 10.1111/j.1651-2227.1993.tb12705.x [DOI] [PubMed] [Google Scholar]
- 6. Epelman M, Daneman A, Blaser SI, et al. Differential diagnosis of intracranial cystic lesions at head US: correlation with CT and MR imaging. Radiographics 2006;26:173–96 10.1148/rg.261055033 [DOI] [PubMed] [Google Scholar]
- 7. Stadlan EM, Sung JG. Congenital rubella encephalopathy. J Neuropathol Exp Neurol 1967;26:115 10.1097/00005072-196701000-00009 [DOI] [PubMed] [Google Scholar]
- 8. Gilles F. Congenital rubella encephalopathy. J Neuropathol Exp Neurol 1967;26:116. [PubMed] [Google Scholar]
- 9. Bale JF Jr, Sato Y, Eisert D. Progressive postnatal subependymal necrosis in an infant with congenital cytomegalovirus infection. Paediatr Neurol 1986;2:367–70 10.1016/0887-8994(86)90081-0 [DOI] [PubMed] [Google Scholar]
- 10. Russel IM, van Sonderen L, van Straaten HL, et al. Subependymal germinolytic cysts in Zellweger syndrome. Pediatr Radiol 1995;25:254–55 10.1007/BF02011090 [DOI] [PubMed] [Google Scholar]
- 11. Makhoul IR, Zmora O, Tamir A, et al. Congenital subependymal pseudocysts: own data and meta-analysis of the literature. Isr Med Assoc J 2001;3:178–83 [PubMed] [Google Scholar]
- 12. Zorzi C, Angonese I. Subependymal pseudocysts in the neonate. Eur J Pediatr 1989;148:462–64 10.1007/BF00595915 [DOI] [PubMed] [Google Scholar]
- 13. Esteban H, Blondiaux E, Audureau E, et al. Prenatal features of isolated subependymal pseudocysts associated with adverse pregnancy outcome. Ultrasound Obstet Gynecol 2015;46:678–87 10.1002/uog.14820 [DOI] [PubMed] [Google Scholar]
- 14. Larcos G, Gruenewald SM, Lui K. Neonatal subependymal cysts detected by sonography: prevalence, sonographic findings, and clinical significance. Am J Roentgenol 1994;162:953–56 10.2214/ajr.162.4.8141023 [DOI] [PubMed] [Google Scholar]
- 15. Ramenghi LA, Domizio S, Quartulli L, et al. Prenatal pseudocysts of the germinal matrix in preterm infants. J Clin Ultrasound 1997;25:169–73 [DOI] [PubMed] [Google Scholar]
- 16. Cevey-Macherel M, Forcada Guex M, Bickle Graz M, et al. Neurodevelopment outcome of newborns with cerebral subependymal pseudocysts at 18 and 46 months: a prospective study. Arch Dis Child 2013;98:497–502 10.1136/archdischild-2012-303223 [DOI] [PubMed] [Google Scholar]
- 17. Bats AS, Molho M, Senat MV, et al. Subependymal pseudocysts in the fetal brain: prenatal diagnosis of two cases and review of the literature. Ultrasound Obstet Gynecol 2002;20:502–05 10.1046/j.1469-0705.2002.00848.x [DOI] [PubMed] [Google Scholar]
- 18. Zoppi MA, Luculano A, Peltz MT, et al. Prenatal detection of periventricular pseudocysts by ultrasound: diagnosis and outcome. Case Reports in Perinatal Medicine 2012;1:75–78 10.1515/crpm-2012-0008 [DOI] [Google Scholar]
- 19. Chang CL, Chiu NC, Ho CS, et al. Frontal horn cysts in normal neonates. Brain Dev 2006;28:426–30 10.1016/j.braindev.2006.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu JH, Emons D, Kowalewski S. Connatal periventricular pseudocysts in the neonate. Pediatr Radiol 1992;22:55–58 10.1007/BF02011609 [DOI] [PubMed] [Google Scholar]
- 21. Pal BR, Preston PR, Morgan ME, et al. Frontal horn thin walled cysts in preterm neonates are benign. Arch Dis Child Fetal Neonatal Ed 2001;85:F187–93 10.1136/fn.85.3.F187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sudakoff GS, Mitchell DG, Stanley C, et al. Frontal periventricular cysts on the first day of life: a one-year clinical follow up and its significance. J Ultrasound Med 1991;10:25–30 [DOI] [PubMed] [Google Scholar]
- 23. Thun-Hohenstein L, Forster I, Künzle C, et al. Transient bifrontal solitary periventricular cysts in term neonates. Neuroradiology 1994;36:241–44 10.1007/BF00588143 [DOI] [PubMed] [Google Scholar]
- 24. Wong F, Fraser S, Kelly E, et al. Clinical significance of isolated paraventricular cysts on cranial ultrasonography. J Paediatr Child Health 2004;40:552–55 10.1111/j.1440-1754.2004.00462.x [DOI] [PubMed] [Google Scholar]
- 25. Unger S, Salem S, Wylie L, et al. Newborn frontal horn cysts: cause for concern? J Perinatol 2011;31:98–103 10.1038/jp.2010.79 [DOI] [PubMed] [Google Scholar]
- 26. International Society of Ultrasound in Obstetrics & Gynecology Education Committee. Sonographic examination of the fetal central nervous system: guidelines for performing the ‘basic examination’ and the ‘fetal neurosonogram.’ Ultrasound Obstet Gynecol 2007;29:109–16 10.1002/uog.3909 [DOI] [PubMed] [Google Scholar]
- 27. Salomon LJ, Garel C. Magnetic resonance imaging examination of the fetal brain. Ultrasound Obstet Gynecol 2007;30:1019–32 10.1002/uog.5176 [DOI] [PubMed] [Google Scholar]
- 28. Vazquez E, Mayolas N, Delgado I, et al. Fetal neuroimaging: US and MRI. Pediatr Radiol 2009;39(suppl 3):422–35 10.1007/s00247-009-1221-x [DOI] [PubMed] [Google Scholar]
- 29. Signorelli M, Tiberti A, Valseriati D, et al. Width of the fetal lateral ventricular atrium between 10 and 12 mm: a simple variation of the norm? Ultrasound Obstet Gynecol 2004;23:14–18 10.1002/uog.941 [DOI] [PubMed] [Google Scholar]
- 30. Sparrow SS, Cicchetti VD, Balla AD. Vineland Adaptive Behavior Scales. 2nd ed. Circle Pines: American Guidance Service; 2005 [Google Scholar]
- 31. Sadan S, Malinger G, Schweiger A, et al. Neuropsychological outcome of children with asymmetric ventricles or unilateral mild ventriculomegaly identified in utero. BJOG 2007;114:596–602 10.1111/j.1471-0528.2007.01301.x [DOI] [PubMed] [Google Scholar]
- 32. Achiron R, Yagel S, Rotstein Z, et al. Cerebral lateral ventricular asymmetry: is this a normal ultrasonographic finding in the fetal brain? Obstet Gynecol 1997;89:233–37 10.1016/S0029-7844(96)00506-6 [DOI] [PubMed] [Google Scholar]
- 33. Atad-Rapoport M, Schweiger A, Lev D, et al. Neuropsychological follow-up at school age of children with asymmetric ventricles or unilateral ventriculomegaly identified in utero. BJOG 2015;122:932–38 10.1111/1471-0528.12976 [DOI] [PubMed] [Google Scholar]
- 34. Pagani G, Thilaganathan B, Prefumo F. Neurodevelopmental outcome in isolated mild fetal ventriculomegaly: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2014;44:254–60 10.1002/uog.13364 [DOI] [PubMed] [Google Scholar]
- 35. Beltinger C, Saule H. Sonography of subependymal cysts in congenital rubella syndrome. Eur J Pediatr 1988;148:206–07 10.1007/BF00441403 [DOI] [PubMed] [Google Scholar]
- 36. Alvarez JF, Amess PN, Gandhi RS, et al. Diagnostic value of subependymal pseudocysts and choroid plexus cysts on neonatal cerebral ultrasound: a meta-analysis. Arch Dis Child Fetal Neonatal Ed 2009;94:F443–46 10.1136/adc.2008.155028 [DOI] [PubMed] [Google Scholar]
- 37. Malinger G, Lev D, Lerman-Sagie T. Imaging of fetal cytomegalovirus infection. Fetal Diagn Ther 2011;29:117–26 10.1159/000321346 [DOI] [PubMed] [Google Scholar]
- 38. Lipitz S, Yinon Y, Malinger G, et al. Risk of cytomegalovirus-associated sequelae in relation to time of infection and findings on prenatal imaging. Ultrasound Obstet Gynecol 2013;41:508–14 10.1002/uog.12377 [DOI] [PubMed] [Google Scholar]
- 39. Amir J, Atias J, Linder N, et al. Follow-up of infants with congenital cytomegalovirus and normal fetal imaging. Arch Dis Child Fetal Neonatal Ed 2016. January 18. [Epub ahead of print] 10.1136/archdischild-2015-308357 [DOI] [PubMed] [Google Scholar]
- 40. Capretti MG, Lanari M, Tani G, et al. Role of cerebral ultrasound and magnetic resonance imaging in newborns with congenital cytomegalovirus infection. Brain Dev 2014;36:203–11 10.1016/j.braindev.2013.04.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.