Abstract
Recent studies have recognized several risk factors for cardiopulmonary bypass- (CPB-) associated acute kidney injury (AKI). However, the lack of early biomarkers for AKI prevents practitioners from intervening in a timely manner. We reviewed the literature with the aim of improving our understanding of the risk factors for CPB-associated AKI, which may increase our ability to prevent or improve this condition. Some novel early biomarkers for AKI have been introduced. In particular, a combinational use of these biomarkers would be helpful to improve clinical outcomes. Furthermore, we discuss several interventions that are aimed at managing CPB-associated AKI, may increase the effect of renal replacement therapy (RRT), and may contribute to preventing CPB-associated AKI. Collectively, the conclusions of this paper are limited by the availability of clinical trial evidence and conflicting definitions of AKI. A guideline is urgently needed for CPB-associated AKI.
1. Introduction
Cardiopulmonary bypass (CPB) is a form of extracorporeal circulation that temporarily replaces the function of the heart and lungs during surgery to maintain the circulation of blood and oxygen in the patient, which has benefited thousands of patients since its introduction nearly 60 years ago [1, 2]. The 2017 Heart Disease and Stroke Statistics from the American Heart Association reported outcomes for approximately 400,000 patients undergoing cardiac surgical procedures each year, and more than 80% of these procedures were performed using CPB [3]. However, CPB is not a benign procedure, and a number of associated problems remain, including hemolysis [4], capillary leak syndrome [5], and acute kidney injury (AKI) [6–8]. AKI occurs in 18.2% to 30% of patients who undergo CPB surgery [7, 9–11] and is an important predictor of morbidity and mortality after cardiac surgery [12–15].
AKI is defined as the rapid deterioration of kidney function within 48 hours after the initiating event [16, 17] and exerts a separate independent effect on the risk of death [18, 19]. Generally, AKI could be caused by a decrease in the renal blood flow, renal inflammation, or pigment nephropathy resulting from any reason. Septic shock and CPB surgery are the two of the most common factors that contribute to AKI [20]. In patients undergoing CPB, AKI is associated with poor outcomes, prolonged hospital stays, increased mortality, and stroke [6–8, 21, 22]. In particular, CPB-associated AKI was associated with an 8.2-fold increase in in-hospital mortality [11].
Until a decade ago, a uniform definition of AKI was lacking, creating problems in comparing published results. In 2007, the AKI Network (AKIN) modified the risk, injury, failure, loss, end-stage renal disease (RIFLE) criteria established in 2004 by including an absolute change in the serum creatinine (sCr) level, which also decreased the time over which AKI was to be diagnosed from 7 days to 48 hours [16, 17]. The recent 2012 Kidney Disease: Improving Global Outcomes (KDIGO) criteria combined RIFLE and AKIN, providing clear guidelines for the timing of AKI ascertainment and severity staging based on changes in sCr levels and urine output [23]. Based on accumulating evidence from meta-analyses, a consistency of prognostic estimates exists across AKI definitions. Notably, if the expected hospital stay is less than 7 days, the AKIN definition may be the most suitable [7].
Renal replacement therapy (RRT) is necessary for treating CPB associated-AKI [24], which is experienced by approximately 1% to 2.9% of all patients who undergo CPB surgery [9, 21]. Additionally, the use of RRT is a marker of early mortality and long-term mortality [25], similar to CPB-associated RRT [7, 21, 26]. The thirty-day mortality rate of patients with CPB-associated AKI who require RRT is 42% [21]. An estimate of the variation in the risk factors associated with clinical outcomes is needed to contribute to the prevention of CPB-associated AKI [7]. The detection of biomarkers is useful to prevent CPB-associated AKI [27]. We must improve our understanding of the mechanisms involved in CPB-associated AKI to prevent this disease and to develop comprehensive interventions for managing CPB-associated AKI when it occurs.
The focus of this review is to provide a comprehensive evaluation of CPB-associated AKI. First, the risk factors for CPB-associated AKI are summarized. Then, we present a review of the methods currently used to manage AKI following CPB, including RRT and other interventions. Finally, potential directions for future CPB-associated AKI are discussed. Collectively, the compiled information should serve as a comprehensive repository of the evidence that is currently available in this area, which should aid in the design of future studies. This review should contribute to the prevention and management of CPB-associated AKI.
2. Risk Factors for Acute Renal Dysfunction following Cardiopulmonary Bypass
Generally, the relative risk of AKI has decreased by 8% per year beginning in 2000 [28], and AKI-associated mortality is also decreasing [29]. Several risk factors have been identified to predict the development of CPB-associated AKI. The risk factors are classified as patient-related and procedure-related factors, and a comprehensive understanding of these factors and AKI should further contribute to our ability to control CPB-associated AKI.
2.1. Patient Conditions
An older age is an independent risk factor for developing AKI [30, 31]; for example, an age > 70 years is an independent risk factor for postoperative AKI, with a relative risk ranging from 2 to 2.232 [95% confidence interval (CI), 1.326-3.757; P < 0.005] [32, 33]. Female sex is another established independent patient-related risk factor for the development of CPB-associated AKI. Most meta-analyses have revealed that women are more likely than men to develop AKI postoperatively [odds ratio (OR), 1.21; 95% CI, 1.09-1.33; P < 0.001] [34]. A smoking history is also an independent factor (OR, 2.008; 95% CI, 1.144-3.524; P = 0.0151) [34]. A left ventricular ejection fraction (LVEF) < 35% was shown to be an independent risk factor for postoperative AKI (OR, 1.25; 95% CI, 1.01-2.2; P = 0.01) [33]. Furthermore, controlled clinical trials revealed a high risk of the development of AKI in children with congenital heart disease [26, 35, 36], with reported incidences ranging from 29% to 86% [36].
The presence of any extent of preoperative renal dysfunction (which is defined as a sCr level ≥ 1.2 mg/dL) increases the risk of CPB-associated AKI compared with the very low risk (<2%) for a patient with normal renal function under the same conditions [37, 38]. The preoperative estimated glomerular filtration rate (eGFR) is the best indicator of postoperative renal dysfunction. Based on several lines of evidence, a baseline eGFR < 60 mL/min/1.73 m2 is associated with an increased risk of CPB-associated AKI [38–42]. Furthermore, diabetes is also suggested to be independently associated with AKI [43], which may result from the deterioration of already borderline renal function [44, 45]. A decreased eGFR and borderline renal function may therefore be collectively associated with the incidence of CPB-associated AKI. Patient-related factors must be carefully evaluated to obtain a better risk stratification. Preventive approaches, such as glucose control, are suggested to be administered to reduce the burden of AKI and prevent CPB-associated AKI [33, 43]. Thoughtful and individualized decisions regarding the treatment of patients with multiple risk factors are necessary.
2.2. Creatinine
An elevated preoperative sCr level is the most significant predictive risk factor for postoperative AKI following CPB described to date [46, 47], as also evidenced by higher peak postoperative creatinine levels within 48 h of arrival in the intensive care unit (ICU) associated with persistent AKI [32]. The risk of AKI is increased 4.8-folds for each 1 mg/dL increase in the sCr level [38]. The patient's risk for postoperative dialysis after AKI reaches 10–20% with a baseline creatinine concentration of 2.0-4.0 mg/dL and approximately 25% when the baseline creatinine concentration is greater than 4.0 mg/dL [48]. In addition to inducing the development of AKI, preoperative creatinine levels greater than 2.5 mg/dL increase the risk of mortality and prolong the length of hospital stay following CPB surgery [49]. Collectively, these data indicate the value of monitoring and focusing on increased sCr levels throughout the perioperative period.
2.3. Genetic Polymorphisms
The clinical predictors and biochemical markers that have been identified as being associated with the development of AKI only partially explain the individual risk [20]. Another tool for predicting the risk of AKI and improving individualized patient care is focused on identifying the genetic risk factors that might be involved in the development of AKI. Several genetic polymorphisms have been identified to play roles in the occurrence and progression of AKI after cardiac surgery with CPB. As shown in the study by Leaf et al. [50], patients with a longer allele genotype in the heme oxygenase-1 (HO-1) gene (HMOX1) promoter exhibited an increased risk of postoperative AKI after cardiac surgery with CPB (OR, 1.26; 95% CI, 1.05-1.503; P = 0.01). This finding is consistent with heme toxicity as a pathogenic feature of cardiac surgery-associated AKI, suggesting the potential of HO-1 as a therapeutic target in the future. Additionally, Popov et al. [51] examined SNP rs1617640 in the promoter of the erythropoietin (EPO) gene using DNA sequencing and found that the risk allele rs1617640 (T) plays a role in the development of AKI after cardiac surgery with CPB. Patients with the TT risk allele produced increased concentrations of EPO and required more frequent acute RRT. A patient's ability to produce more EPO may be associated with thromboembolic events and therefore affects morbidity and mortality after CPB. Furthermore, Stafford-Smith et al. [52] identified two novel susceptibility loci (chr3p21.6 and BBS9) for AKI after cardiac surgery with CPB. These data provide candidate regions for future genetic research on cardiac surgery-associated AKI and may ultimately contribute to improvements in preoperative screening and the development of novel prevention and intervention options to decrease AKI and associated morbidity and mortality. Despite the substantial progress, formidable challenges regarding the widespread clinical application of genetic testing remain due to its high cost and time-consuming process.
2.4. Hemoglobin Concentration
Based on accumulating evidence, the hemoglobin concentration measured during CPB surgery is associated with the incidence of AKI after CPB surgery. According to a study by Haase et al. [11], a decreased hemoglobin concentration during CPB surgery is an independent risk factor for AKI, with an effect cut-off value of <9 g/dL (<5.6 mmol/L) (OR, 1.16 per 1 g/dL decrease; 95% CI, 1.05-1.31; P = 0.018), which is not altered by systemic arterial oxygen saturation and pressure values. Other studies also support that the low hemoglobin concentration even within the normal range, as well as the nadir hemoglobin level, is associated with increased incidence of CPB-associated AKI [53, 54]. Strategies that improve the hemoglobin concentration, such as the conservative use of red blood cell (RBC) transfusion, are recommended, since circulating free iron-mediated nephrotoxicity with hemolysis and free hemoglobin are likely to lead to AKI in patients undergoing cardiac surgery with CPB. However, the volume of transfused RBCs represents a specific additional risk factor if this treatment is administered to patients with hemoglobin levels > 8 g/dL (>5 mmol/L). Therefore, future studies should further investigate if modified blood conservation strategies or the restriction of RBC transfusion to patients with a hemoglobin concentration < 8 g/dL (<5 mmol/L) will improve renal outcomes. Is increasing the hemoglobin concentration with an EPO supplement and other methods more beneficial for patients with decreased hemoglobin concentrations?
2.5. Hemodilution
Hemodilution is another independent risk factor for developing renal injury (including AKI), with the lowest predictive cut-off value being a hematocrit < 24% [37]. A multivariate analysis revealed a 7% increase in the relative risk of AKI per 1% decrease in the nadir hematocrit value during CPB [28]. Hemodilution-induced renal injury was exacerbated when CPB was prolonged by the use of intraoperative packed RBC transfusions [37], suggesting a conflict between maintaining adequate hydration and using extended diuretic therapy and avoiding hemodilution [35]. Improvements in oxygen delivery conditions may contribute to solving this problem [55]. Moreover, the hemodilution-related AKI risk has been limited by many improvements to CPB technology that have been proposed in the past decade, including the redesign of the circuit to minimize the volume, the use of retrograde autologous priming, and the management of intraoperative fluid administration [28].
Notably, Svenmarker et al. [56] observed a decrease in the sCr concentration following CPB-induced hemodilution, which significantly hampered the creatinine-based diagnosis of AKI. Thus, the use of sCr levels to monitor renal function during CPB should be employed with caution to avoid underestimating the risk of AKI.
2.6. Oxygen Delivery
According to an increasing number of studies, the kidneys may suffer from an imbalance between the amount of oxygen that is available and the amount of oxygen they need during CPB [57]. As shown in the study by Ranucci et al. [58], the best predictor for AKI and peak postoperative sCr levels was the lowest oxygen delivery during CPB, with a critical threshold of <272 mL/min/m2. The effects of decreased hemoglobin concentrations and hemodilution on the incidence of AKI during CPB are potentially attributed to a decrease in the oxygen carrying capacity [57]. A decreased oxygen carrying capacity related to hemodilution might be compensated by increasing CPB oxygen delivery using an adequately increased pump flow. Interestingly, in patients with severe anemia (<25th percentile for the lowest hemoglobin level), the independent effect of hypotension (>75th percentile of the area under the curve for MAP < 50 mmHg) on AKI was pronounced [OR, 3.36 (95% CI 1.34-8.41); P = 0.010] [11]. After correcting for the need for transfusions, only the lowest level of oxygen delivery remained an independent risk factor for AKI during CPB [58]. Excursions of mean arterial blood pressure that were below the limit of autoregulation were independently associated with AKI, but not the absolute mean arterial blood pressure [43]. Inadequate oxygen delivery during CPB was associated with lactate production, and hyperlactatemia appeared when oxygen delivery decreased below 260 mL/min/m2 under normothermic conditions [59]. Thus, monitoring the cerebral oximetry index using near-infrared spectroscopy signals may represent a novel method for precisely guiding procedures aimed at maintaining a mean arterial blood pressure target during CPB, which further reflects the level of renal perfusion [43, 60]. In addition, pump flow should be coupled with hematocrit monitoring to avoid a decrease below the critical level of oxygen delivery. However, the extent to which the dose for oxygen delivery can be decreased remains to be estimated.
Different interventions aimed at preserving oxygen delivery during CPB have been reported to exert beneficial effects on goal-directed perfusion applications, and the incidence of AKI has been reduced from 5.8% to 3.1% [28]. Four elements of the formula for oxygen delivery under CPB, including pump flow, hemoglobin, oxygen saturation, and arterial oxygen tension, can be carefully monitored and properly modified, thus promoting the likelihood of adequate oxygen delivery [61]. The ability to maintain oxygen delivery and satisfy all these components requires cooperation and coordination among the members of the cardiac surgical team. In addition, Bennett et al. [62] compared the average oxygen delivery during bypass for a miniaturized CPB and for a conventional CPB circuit and did not observe differences in the average oxygen delivery. The association between oxygen delivery and postoperative changes in plasma creatinine levels was clear in both groups. However, further studies are needed to determine whether a particular cohort of patients benefits (or are put at risk) from each method of CPB. Furthermore, oxygen preconditioning has been shown to prevent nephropathy in patients [63] and AKI [64], renal ischemia/reperfusion injury [65], and nephrotoxicity in rats [66]. Accordingly, the use of hyperbaric oxygen therapy and oxygen preconditioning may be beneficial for preventing AKI following CPB.
2.7. Use of Angiographic Examinations
Contrast agent has been suggested to induce AKI in patients undergoing coronary angiography or percutaneous coronary interventions [44]. Ranucci et al. [28] showed that a policy restricting angiographic examination on the day of operation reduced the AKI rate from 4.8% to 3.7%. Performing cardiac surgery on the day of cardiac catheterization and using a higher dose of contrast agent were independently associated with an increased risk of postoperative AKI [67]. Interestingly, the administration of contrast to cyanotic patients with CHD within the 48 h prior to CPB was not an additional risk factor for developing AKI [46]. In conclusion, the angiographic examination may be not associated with an increased AKI rate. However, delaying cardiac surgery for more than 24 h after exposure to contrast agents (when feasible) and minimizing the use of contrast agents may significantly decrease the incidence of postoperative AKI in patients undergoing CPB surgery.
2.8. Time on CPB
Time on CPB was associated with the risk of developing AKI requiring dialysis [41, 68–71] In one study, after adjusting for confounders, the association between the time on CPB and AKI requiring dialysis lost its statistical significance [71]. Thus, an accurate risk assessment might be more important than the time on CPB in predicting the occurrence of AKI requiring dialysis. Nonetheless, the CPB time should be minimized to the greatest extent possible to reduce the risk of AKI following CPB in cyanotic patients with congenital heart disease [35].
Collectively, these risk factors could be converted into a simple, accurate, and reliable bedside risk tool, which should promote improved clinician-patient discussions about risks of CPB-associated AKI. High-risk patients (defined as an age > 70 years, female gender, smoking history, left ventricular ejection fraction < 35%, worse borderline renal function, increased sCr levels around the perioperative period, decreased hemoglobin concentration, hemodilution, and genetic susceptibility) should be targeted for renal protective strategies, and clinicians should focus on preventing the occurrence of AKI following CPB in these patients (Figure 1 and Table 1). Taking these factors into account, improvements to CPB technologies have been proposed to limit the risk of occurrence of AKI. Long et al. have proposed that perfusion techniques may be associated with the incidence of AKI [8]. Furthermore, a set of interventions that are mainly aimed at limiting and improving these risk factors may be effective at reducing the AKI rate. These interventions include improving patient conditions, avoiding the use of contrast agents before CPB, avoiding any decrease in the hemoglobin concentration, reasonably using hemodilution, improving oxygen delivery, and controlling the surgery time.
Figure 1.
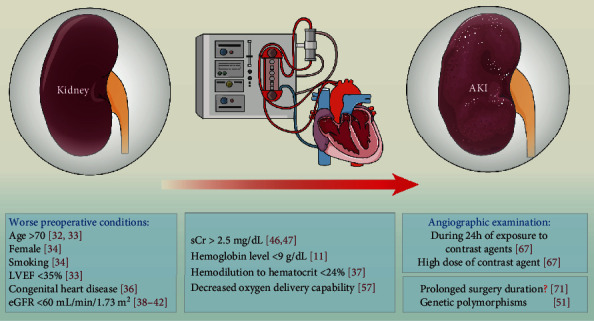
Risk factors for CPB-associated AKI. In addition to a set of independent risk factors that has been widely accepted and included in some predictive models, other risk factors are related to CPB-associated AKI, including genetic polymorphisms, a decreased hemoglobin concentration, hemodilution, and a decreased oxygen delivery capability. AKI: acute kidney injury; LVEF: left ventricular ejection fraction; eGFR: estimated glomerular filtration rate; sCr: serum creatinine.
Table 1.
Risk factors for acute renal dysfunction following cardiopulmonary bypass.
| Factors | Significance | References |
|---|---|---|
| Age > 70 | An independent risk factor for AKI following CPB | [32, 33] |
| Female | An independent risk factor for AKI following CPB | [34] |
| Smoking | An independent risk factor for AKI following CPB | [34] |
| LVEF < 35% | An independent risk factor for AKI following CPB | [33] |
| Congenital heart disease | Children with congenital heart disease are at high risk of AKI happening | [36] |
| eGFR < 60 mL/min/1.73 m2 | An independent risk factor for AKI following CPB | [38–42] |
| sCr > 2.5 mg/dL | An independent risk factor for AKI following CPB | [46, 47] |
| Genetic polymorphisms | Patients with the rs1617640 TT risk allele are more likely to develop AKI following CPB | [51] |
| Hemoglobin level < 9 g/dL | An independent risk factor for AKI following CPB | [11] |
| Hemodilution to hematocrit < 24% | An independent risk factor for AKI following CPB | [37] |
| Oxygen delivery | Increasing oxygen might be protective against AKI following CPB | [57] |
| During 24 h of exposure to contrast agents | Restricting angiographic examination on the day of operation reduced the AKI rate | [67] |
| High dose of contrast agent | An independent risk factor for AKI following CPB | [67] |
| Prolonged surgery duration | Prolonged time on CPB might be associated with increased risk of developing AKI following CPB | [71] |
LVEF: left ventricular ejection fraction; eGFR: estimated glomerular filtration rate; sCr: serum creatinine.
3. Renal Replacement Therapy
A meta-analysis of cohort studies reported that RRT is administered to 2.1% of patients with CPB-associated AKI [7]. According to another study, 2.2% of patients required long-term renal support [21]. The early institution of both peritoneal dialysis (PD) for AKI and low cardiac output after cardiac operations removes fluid, thus easing the fluid restriction and improving cardiopulmonary function [72]. In infants at high risk of developing AKI, PD catheter placement has also been shown to be safe, and it is associated with an earlier negative fluid balance, earlier extubation, improved inotrope scores, fewer electrolyte imbalances requiring correction, and improved clinical outcomes [73]. In general, dialysis-associated complications have not been observed during PD [24, 74]. When PD was contraindicated, the use of two small single-lumen catheters in separate veins enables consistent and effective hemodiafiltration in neonates and infants with challenging vascular access, allowing an excellent normalization of the blood flow of metabolic derangements and significant fluid removal [75].
In addition to PD, continuous RRT (CRRT) and hemodialysis (HD) are other suitable RRTs for the treatment of AKI [76]. CRRT is a safe and effective method for fluid and electrolyte homeostasis that allows hyperalimentation in infants and children after cardiac operations [77]. According to a multivariate analysis, intraoperative CRRT preserves postoperative renal function in patients with moderate renal dysfunction before surgery (OR, 0.8; 95% CI, 0.71-0.99; P = 0.02) [33]. However, the utilization of CRRT is limited by its high cost. As shown in the study by Sugahara and Suzuki [78], the early initiation of HD therapy (as soon as the urine output decreased to <30 mL/hour) might increase the survival of patients with AKI following cardiac surgery, which was obviously superior to the late-HD group (P < 0.01). In addition, perioperative prophylactic HD also decreases operative mortality and morbidity rates in high-risk patients [49]. Furthermore, modern CRRT and HD machines are equipped with exact volumetric systems that direct fluid removal and online solute clearance monitoring, providing obvious superiority and improving physician “comfort” compared with PD that contributes to potentially unpredictable fluid removal rates and possible inadequate solute clearances [76]. Notably, due to the hemodynamic instability of children, RRT has been improved to reduce the cost and enhance the therapeutic effects on neonatal populations (this issue has been discussed in another excellent review [79]).
The early institution of ultrafiltration in the operating room and RRT during the postoperative period may decrease the activity of the proinflammatory milieu and the resulting systemic effects [80]. The early initiation of RRT may prevent fluid overload and result in improved infant outcomes. Despite the theoretical advantages of using RRT and the effective control of uremia, the mortality associated with AKI following CPB remains high, and it is most likely determined by the number of failed organ systems [81]. Thus, the management of CPB-associated AKI should be aimed at relying on more comprehensive interventions.
4. Other Interventions
The early mortality of patients utilizing RRT is still very high (RR, 5.3; 95% CI, 3.4-8.1) [7]. Combinations of more effective interventions with RRT are urgently needed to prevent and manage CPB-associated AKI.
Statin therapy is reported to be effective at reducing the risk of contrast-induced AKI [82–84]. Several studies have focused on the effects of statins on CPB-associated AKI. Interestingly, in these studies, patients treated postoperatively with statins showed a significant reduction in sCr levels. However, some studies did not show an association between preoperative statin usage and a decreased incidence of AKI in adults who underwent surgery that required CPB [6, 85]. A recent meta-analysis by Wang and colleagues [86] revealed that preoperative statin therapy reduced the incidence of postoperative AKI by 13% and 7% in subgroups of patients whose AKI was evaluated using the AKIN or the RIFLE criteria, respectively, without significant heterogeneity. Collectively, statins might be a promising therapy for reducing renal complications and the incidence of AKI in patients undergoing CPB surgery [87], and this topic warrants further investigation.
Postoperative AKI has been shown to be associated with the increased intraoperative release of hemeprotein [88, 89]. Hemeprotein release following a mechanical injury is inevitable, but the association between increased plasma free hemoglobin (fHb) levels and renal injury provides new insights into the pathophysiology of AKI [89, 90].
5. Further Perspectives
The incidence and prognosis of CPB-associated AKI are influenced by multiple factors. Hence, predictive risk models must be established to comprehensively assess the general condition of patients [91, 92]. Several predictive risk models have been established, including the use of Mehta scores with the C statistic of 0.83 (10 variables: preoperative sCr level, age, race, type of surgery, diabetes, shock, New York Heart Association class, lung disease, recent myocardial infarction, and prior cardiovascular surgery are included in this bedside tool that is aimed at evaluating the need for postoperative dialysis) [93], Cleveland Clinic scores with an overall area under the receiver operating characteristic (ROC) curve of 0.81 (10 variables have been validated for a maximum score of 17: female gender, congestive heart failure, LVEF, preoperative use of intra-aortic balloon pump, COPD, diabetes, previous cardiac surgery, emergency surgery, type of surgery, and preoperative creatinine level) [94], and Simplified Renal Index scores with an overall area under the ROC curve of 0.81 (7 variables are identified for a maximum score of 8: GFR, diabetes, ejection fraction, previous cardiac surgery, procedure other than coronary artery bypass grafting, intra-aortic balloon pump, and nonelective case) [95]. Among these three models, the Cleveland scoring system offers the best discriminative value for predicting postoperative RRT and covers most patients undergoing CPB surgery [96]. It can also be used to predict the composite end point for severe AKI, which enables its broader application in patients at risk of postoperative AKI. Some other predictive scoring systems exist for postoperative RRT [97–99], but these scales have limitations in assessing CPB-associated AKI. Some other models have also been established to estimate CPB-associated AKI but are limited by their use of small derivation cohorts [100, 101]. The addition of novel biomarkers, particularly when used in combinations, significantly increased the predictive value of the model in another study [10]. The ability to accurately assess the risk of developing CPB-associated AKI before surgery should improve clinical management, lead to the earlier involvement of specialist services, and allow more informed decision making; however, further studies are needed to develop a more comprehensive and accurate assessment system.
While the sCr level, which is accepted as a delayed marker of AKI, remains within normal limits [102], other biomarkers are able to identify tubular and glomerular damage. The urine and serum biomarkers for CPB-associated AKI have been separately summarized in Tables 2 and 3. Combinations of two or more of these biomarkers may provide increased diagnostic sensitivity and specificity for evaluating AKI following CPB. Urine neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL-18), liver fatty acid-binding protein (L-FABP), and kidney injury molecule- (KIM-) 1 levels are sequential predictive biomarkers of AKI that correlate with disease severity and clinical outcomes in pediatric patients who undergo CPB [103, 104]. These biomarkers, particularly when used in combination, may help to establish the timing of injury and allow earlier intervention for AKI [10, 105]. The currently available studies included only limited urinary biomarkers without including other promising biomarkers, such as cystatin C, asymmetric dimethylarginine (ADMA), and NGAL [106]. Thus, a comprehensive “panel” of promising biomarkers that can be used individually and in combination should be developed to optimize both the sensitivity and specificity of predicting and diagnosing CPB-associated AKI. Parikh et al. [107] also proposed that a combination including NGAL and IL-18 might be used for the reliable early diagnosis and prognosis of AKI at all time points after CPB and substantially before an increase in sCr levels is considered to be diagnostic. Some novel predictors, such as elevated levels of plasma renin and IL-8, were recently shown to be associated with development of CPB-associated AKI [108–110], whereas elevated concentration of the serum macrophage migration inhibitory factor is associated with decreased risk of CPB-associated AKI [111]. These factors could be further included in these predictive models. Collectively, a more comprehensive examination of the relevant novel biomarkers both individually and in combination is urgently needed.
Table 2.
Summary of urine biomarkers for CPB-associated AKI.
| Novel biomarkers | Significance | Reference |
|---|---|---|
| Urine NGAL | Predictive time point of NGAL for CPB-associated AKI can be advanced as early as 2 h postoperatively. | [10, 107, 122] |
| Urine IL-18 | Urine IL-18 increased at 4-6 h after CPB, peaked at over 25-folds at 12 h, and remained markedly elevated up to 48 h in AKI patients after CPB. | [107] |
| Urine AIM | Urinary AIM peaks within 2 hours in children who developed AKI after CPB surgery. | [102] |
| Urine AAG | Urinary AAG peaks within 2 hours in children who developed AKI after CPB surgery. | [102] |
| Urine Alb | Urinary Alb peaks within 2 hours in children who developed AKI after CPB surgery. | [102] |
| Urine NAG | During surgery urinary excretion of NAG increased in patients with AKI, reaching peak levels at 15 min after reperfusion. | [89] |
| Urine hepcidin-25 | Elevated urinary hepcidin-25 at 24 h is a strong predictor of avoidance of AKI beyond postoperative day 1. | [123] |
| Urine TIMP-2 | Urine TIMP-2 and IGFBP7 are significantly higher in patients with AKI 1 hour after CPB start. | [124] |
| Urine IGFBP7 | ||
| Urine vasopressinase activity | For patients undergoing CPB surgery, their urine vasopressinase activity peaks at the time of arrival to the ICU. In patients who were diagnosed with AKI, urine vasopressinase activity peaked 30 minutes into CPB. | [125] |
NGAL: neutrophil gelatinase-associated lipocalin; IL-18: interleukin-18; AIM: α(1)-microglobulin; AAG: α(1)-acid glycoprotein; Alb: albumin; NAG: N-acetyl-beta-D-glucosaminidase.
Table 3.
Summary of serum biomarkers for CPB-associated AKI.
| Novel biomarkers | Significance | Reference |
|---|---|---|
| Serum cystatin C | The 12 h cystatin C strongly correlated with severity and duration of AKI as well as length of hospital stay. In multivariable analysis, 12 h cystatin C remained a powerful independent predictor of AKI. The discriminatory capacity of plasma cystatin C measured preoperatively and 2 hours after the conclusion of CPB was modest. | [126] |
| Serum ADMA | Patients with elevated ADMA before surgery were more likely to have prolonged mechanical ventilation, develop LCOS, require an extended length of stay, and require reoperation. ADMA levels inversely correlated with eGFR, but did not predict AKI. Preoperative serum ADMA appears to identify pediatric cardiac surgery patients at risk of poor postoperative outcomes following CPB. | [127] |
| Plasma fHb | During surgery, plasma fHb increased in patients with AKI, reaching peak levels at 2 h after reperfusion. | [89] |
| Serum vasopressinase activity | For patients undergoing CPB surgery, their serum vasopressinase activity peaks at the time of arrival to the ICU. In patients who were diagnosed with AKI, serum vasopressinase activity peaked 30 minutes into CPB. | [125] |
| Plasma micro-RNAs and microvesicle | A number of micro-RNAs (miR-192-5p, miR-487a-3p, miR-490-3p, and miR-501-3p) and microvesicles were differentially expressed in AKI children at 6–12 h following CPB, which might serve as tools for stratification of children at risk of AKI. | [128] |
| Serum miR-494 | Serum level of miR-494 in the death group due to AKI was more than fourfold higher than that in the survival group. Furthermore, its level was identified as an independent risk factor for death due to CPB-associated AKI. | [129] |
| Serum NGAL | The serum levels of NGAL in the death group were significantly higher than those in the survival group. Furthermore, the expression level of serum NGAL was positively correlated with that of miR-494 in the death group. | [129] |
| Serum KIM-1 | The serum levels of KIM-1 in the death group were significantly higher than those in the survival group. The expression level of serum KIM-1 was positively correlated with that of miR-494 in the death group. | [129] |
ADMA: asymmetrical dimethylarginine; LCOS: low cardiac output syndrome; eGFR: estimated glomerular filtration rate; fHb: free hemoglobin; NGAL: neutrophil gelatinase-associated lipocalin; KIM: kidney injury molecule.
Novel biomarkers indicate that free iron-mediated toxicity is an important mechanism of AKI in patients receiving cardiac surgery with CPB [112]. Haase et al. [113] analyzed the pathophysiological implications of some novel renal biomarkers in relation to CPB-associated AKI and found that NGAL, L-FABP, and alpha-1 microglobulin predict the development of CPB-associated AKI, while hepcidin isoforms appeared to predict protection from AKI [114]. However, all of these biomarkers are involved in iron metabolism. A free iron-related, reactive oxygen species-mediated type of kidney injury appears to be the unifying pathophysiological connection between these biomarkers. The effects of deferoxamine, a sequestering agent used to complex iron ions, when used in combination with N-acetylcysteine was inferior to the use of N-acetylcysteine alone in treating gentamicin-induced AKI in adult male Wistar rats [115]. Further studies are needed to determine whether deferoxamine improves the control of AKI following CPB. Billings et al. [88] reported an association between postoperative AKI and both increased intraoperative hemeprotein release and increased lipid peroxidation, indicating a potential role for hemeprotein-induced oxidative damage in the pathogenesis of postoperative AKI. The role of excessive oxidative stress-induced AKI has been widely recognized, and it has been ameliorated by the application of antioxidants [113, 115–117]. Thus, approaches aimed at inhibiting excessive oxidative stress may be an attractive strategy for preventing CPB-associated AKI. CPB-associated AKI is associated with endothelial dysfunction, regional tissue hypoxia, and proximal tubular epithelial cell stress [118]. Antagonism of the endothelin-1A receptor reversed these changes and may therefore represent a therapeutic target for strategies aimed at preventing AKI after CPB surgery (Figure 2).
Figure 2.
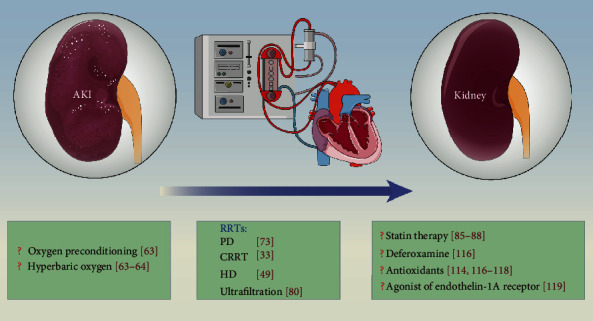
Potential interventions for CPB-associated AKI. RRT is efficient at managing CPB-associated AKI, and other potential interventions may further benefit patients undergoing CPB surgery. AKI: acute kidney injury; RRT: renal replacement therapy; PD: peritoneal dialysis; CRRT: continuous renal replacement therapy; HD: hemodialysis.
In a retrospective study, after adjusting for covariates and propensity scores, a multivariate analysis showed that off-pump surgery preserved postoperative renal function in patients with moderate renal dysfunction before surgery (OR, 0.9; 95% CI, 0.87-0.99; P = 0.04) [33]. A difference between off-pump and on-pump coronary artery bypass graft surgery was identified in a randomized clinical trial [119]. The off-pump surgery reduced the risk of postoperative AKI by 17%, but the authors did not provide evidence for improved preservation of renal functions at 1 year. Other studies have also supported the hypothesis that off-pump coronary artery bypass graft surgery reduces the risk of postoperative AKI [120, 121]. Based on these studies, interventions are required to reduce the risk of mild to moderate AKI following CPB without altering longer-term renal functions.
6. Conclusions
CPB-associated AKI remains a challenging problem with numerous risk factors, including age (>70 years), female gender, smoking history, left ventricular ejection fraction of <35%, borderline renal function, increased sCr levels around the perioperative period, decreased hemoglobin concentrations, hemodilution, and genetic susceptibility (Figure 1 and Table 1). Improvements in our understanding of these factors and establishing models predicting mortality would be beneficial for strategies aimed at predicting and preventing CPB-associated AKI. Some novel biomarkers that may be used to predict and diagnose AKI have attracted attention, and combinations of these biomarkers, including urine (Table 2) and serum biomarkers (Table 3), may provide additional value. The development of a comprehensive “panel” of these risk factors and biomarkers would be useful. The importance and beneficial effect of using RRT for AKI after CPB was validated. Other interventions are needed to assist and increase the effects of RRT (Figure 2). The role of statins in preventing AKI in patients undergoing CPB surgery shows promise and should be further investigated. Furthermore, several studies have investigated the possible mechanisms underlying CPB-associated AKI and found that fHb, free iron, excess oxidative stress, and endothelial dysfunction may be new therapeutic targets for reducing the incidence of AKI and improving the clinical outcomes of patients after CPB surgery.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (81700236).
Contributor Information
Baohui Liu, Email: baohuiliubz@163.com.
Yang Yang, Email: yang200214yy@163.com.
Wei Hu, Email: weihu555@126.com.
Data Availability
None.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Dianxiao Liu, Baohui Liu and Zhenxing Liang contributed equally to this work.
References
- 1.Gibbon J. H. Application of a mechanical heart and lung apparatus to cardiac surgery. Minnesota Medicine. 1954;37(3):171–185. [PubMed] [Google Scholar]
- 2.Kirklin J., Dushane J., Patrick R., et al. Intracardiac surgery with the aid of a mechanical pump-oxygenator system (gibbon type): report of eight cases. Proceedings of the Staff Meetings. Mayo Clinic. 1955;30(10):201–206. [PubMed] [Google Scholar]
- 3.Benjamin E. J., Blaha M. J., Chiuve S. E., et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vercaemst L. Hemolysis in cardiac surgery patients undergoing cardiopulmonary bypass: a review in search of a treatment algorithm. The Journal of Extra-Corporeal Technology. 2008;40(4):257–267. [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang S., Wang S., Li Q., et al. Capillary leak syndrome in children with C4A-deficiency undergoing cardiac surgery with cardiopulmonary bypass: a double-blind, randomised controlled study. Lancet. 2005;366(9485):556–562. doi: 10.1016/S0140-6736(05)67099-7. [DOI] [PubMed] [Google Scholar]
- 6.Lewicki M., Ng I., Schneider A. G. HMG CoA reductase inhibitors (statins) for preventing acute kidney injury after surgical procedures requiring cardiac bypass. Cochrane Database of Systematic Reviews. 2015;3(11, article CD010480) doi: 10.1002/14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickering J. W., James M. T., Palmer S. C. Acute Kidney Injury and Prognosis After Cardiopulmonary Bypass: A Meta- analysis of Cohort Studies. American Journal of Kidney Diseases. 2015;65(2):283–293. doi: 10.1053/j.ajkd.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Long D. M., Jenkins E., Griffith K. Perfusionist techniques of reducing acute kidney injury following cardiopulmonary bypass: an evidence-based review. Perfusion. 2015;30(1):25–32. doi: 10.1177/0267659114544395. [DOI] [PubMed] [Google Scholar]
- 9.Rosner M. H., Okusa M. D. Acute kidney injury associated with cardiac surgery. Clinical Journal of the American Society of Nephrology. 2005;1(1):19–32. doi: 10.1016/j.pedneo.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Krawczeski C. D., Goldstein S. L., Woo J. G., et al. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. Journal of the American College of Cardiology. 2011;58(22):2301–2309. doi: 10.1016/j.jacc.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haase M., Bellomo R., Story D., et al. Effect of mean arterial pressure, haemoglobin and blood transfusion during cardiopulmonary bypass on post-operative acute kidney injury. Nephrology, Dialysis, Transplantation. 2012;27(1):153–160. doi: 10.1093/ndt/gfr275. [DOI] [PubMed] [Google Scholar]
- 12.Zakeri R., Freemantle N., Barnett V., et al. Relation between mild renal dysfunction and outcomes after coronary artery bypass grafting. Circulation. 2005;112(9 Suppl):I270–1275. doi: 10.1161/CIRCULATIONAHA.104.522623. [DOI] [PubMed] [Google Scholar]
- 13.Hobson C. E., Yavas S., Segal M. S., et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119(18):2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 14.Bahar I., Akgul A., Ozatik M. A., et al. Acute renal failure following open heart surgery: risk factors and prognosis. Perfusion. 2005;20(6):317–322. doi: 10.1191/0267659105pf829oa. [DOI] [PubMed] [Google Scholar]
- 15.Hein O. V., Birnbaum J., Wernecke K. D., Konertz W., Jain U., Spies C. Three-year survival after four major post-cardiac operative complications. Critical Care Medicine. 2006;34(11):2729–2737. doi: 10.1097/01.CCM.0000242519.71319.AD. [DOI] [PubMed] [Google Scholar]
- 16.Mehta R. L., Kellum J. A., Shah S. V., et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical Care. 2007;11(2):p. R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellomo R., Ronco C., Kellum J. A., Mehta R. L., Palevsky P., Acute Dialysis Quality Initiative workgroup Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the Acute Dialysis Quality Initiative (ADQI) group. Critical Care. 2004;8(4):R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coca S. G., Yusuf B., Shlipak M. G., Garg A. X., Parikh C. R. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. American Journal of Kidney Diseases. 2009;53(6):961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bihorac A., Yavas S., Subbiah S., et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Annals of Surgery. 2009;249(5):851–858. doi: 10.1097/SLA.0b013e3181a40a0b. [DOI] [PubMed] [Google Scholar]
- 20.Haase-Fielitz A., Haase M., Bellomo R., Dragun D. Genetic polymorphisms in sepsis- and cardiopulmonary bypass-associated acute kidney injury. Contributions to Nephrology. 2007;156:75–91. doi: 10.1159/000102072. [DOI] [PubMed] [Google Scholar]
- 21.Luckraz H., Gravenor M. B., George R., et al. Long and short-term outcomes in patients requiring continuous renal replacement therapy post cardiopulmonary bypass. European Journal of Cardio-Thoracic Surgery. 2005;27(5):906–909. doi: 10.1016/j.ejcts.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 22.Kertai M. D., Zhou S., Karhausen J. A., et al. Platelet counts, acute kidney injury, and mortality after coronary artery bypass grafting surgery. Anesthesiology. 2016;124(2):339–352. doi: 10.1097/ALN.0000000000000959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellum J. A., Lameire N., for the KDIGO AKI Guideline Work Group Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Critical Care. 2013;17(1):p. 204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kist-van Holthe tot Echten J. E., Goedvolk C. A., Doornaar M. B., et al. Acute renal insufficiency and renal replacement therapy after pediatric cardiopulmonary bypass surgery. Pediatric Cardiology. 2001;22(4):321–326. doi: 10.1007/s002460010238. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg R., Dennen P. Long-term outcomes of acute kidney injury. Advances in Chronic Kidney Disease. 2008;15(3):297–307. doi: 10.1053/j.ackd.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Baskin E., Gulleroglu K. S., Saygili A., Aslamaci S., Varan B., Tokel K. Peritoneal dialysis requirements following open-heart surgery in children with congenital heart disease. Renal Failure. 2010;32(7):784–787. doi: 10.3109/0886022X.2010.493980. [DOI] [PubMed] [Google Scholar]
- 27.Meersch M., Schmidt C., Hoffmeier A., et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Medicine. 2017;43(11):1551–1561. doi: 10.1007/s00134-016-4670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ranucci M., Aloisio T., Carboni G., et al. Acute kidney injury and hemodilution during cardiopulmonary bypass: a changing scenario. The Annals of Thoracic Surgery. 2015;100(1):95–100. doi: 10.1016/j.athoracsur.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 29.Ostermann M. E., Taube D., Morgan C. J., Evans T. W. Acute renal failure following cardiopulmonary bypass: a changing picture. Intensive Care Medicine. 2000;26(5):565–571. doi: 10.1007/s001340051205. [DOI] [PubMed] [Google Scholar]
- 30.Rosner M. H. Acute kidney injury in the elderly. Clinics in Geriatric Medicine. 2013;29(3):565–578. doi: 10.1016/j.cger.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Helgason D., Helgadottir S., Ahlsson A., et al. Acute kidney injury after acute repair of type a aortic dissection. The Annals of Thoracic Surgery. 2020;S0003-4975(20):31505–31508. doi: 10.1016/j.athoracsur.2020.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Ge Ng R. R., Huey Chew S. T., Liu W., Kah Ti L. Persistent kidney injury at hospital discharge after cardiac surgery with cardiopulmonary bypass in patients with normal preoperative serum creatinine and normal estimated glomerular filtration rate. Journal of Cardiothoracic and Vascular Anesthesia. 2014;28(6):1453–1458. doi: 10.1053/j.jvca.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Roscitano A., Benedetto U., Goracci M., Capuano F., Lucani R., Sinatra R. Intraoperative continuous venovenous hemofiltration during coronary surgery. Asian Cardiovascular & Thoracic Annals. 2009;17(5):462–466. doi: 10.1177/0218492309348504. [DOI] [PubMed] [Google Scholar]
- 34.Neugarten J., Sandilya S., Singh B., Golestaneh L. Sex and the risk of AKI following cardio-thoracic surgery: a meta-analysis. Clinical Journal of the American Society of Nephrology. 2016;11(12):2113–2122. doi: 10.2215/CJN.03340316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dittrich S., Kurschat K., Dähnert I., et al. Renal function after cardiopulmonary bypass surgery in cyanotic congenital heart disease. International Journal of Cardiology. 2000;73(2):173–179. doi: 10.1016/S0167-5273(00)00217-5. [DOI] [PubMed] [Google Scholar]
- 36.Toda Y., Sugimoto K. AKI after pediatric cardiac surgery for congenital heart diseases-recent developments in diagnostic criteria and early diagnosis by biomarkers. Journal of Intensive Care. 2017;5(1):p. 49. doi: 10.1186/s40560-017-0242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Habib R. H., Zacharias A., Schwann T. A., et al. Role of hemodilutional anemia and transfusion during cardiopulmonary bypass in renal injury after coronary revascularization: implications on operative outcome. Critical Care Medicine. 2005;33(8):1749–1756. doi: 10.1097/01.CCM.0000171531.06133.B0. [DOI] [PubMed] [Google Scholar]
- 38.Ortega-Loubon C., Fernandez-Molina M., Carrascal-Hinojal Y., Fulquet-Carreras E. Cardiac surgery-associated acute kidney injury. Annals of Cardiac Anaesthesia. 2016;19(4):687–698. doi: 10.4103/0971-9784.191578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu H. Y., Chou N. K., Chen Y. S., Yu H. Y. Risk factor for acute kidney injury in patients with chronic kidney disease receiving valve surgery with cardiopulmonary bypass. Asian Journal of Surgery. 2021;44(1):229–234. doi: 10.1016/j.asjsur.2020.05.024. [DOI] [PubMed] [Google Scholar]
- 40.Ortega-Loubon C., Fernandez-Molina M., Paneda-Delgado L., Jorge-Monjas P., Carrascal Y. Predictors of postoperative acute kidney injury after coronary artery bypass graft surgery. Brazilian Journal of Cardiovascular Surgery. 2018;33(4):323–329. doi: 10.21470/1678-9741-2017-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yue Z., Yan-Meng G., Ji-Zhuang L. Prediction model for acute kidney injury after coronary artery bypass grafting: a retrospective study. International Urology and Nephrology. 2019;51(9):1605–1611. doi: 10.1007/s11255-019-02173-7. [DOI] [PubMed] [Google Scholar]
- 42.Reazaul Karim H. M., Yunus M., Dey S. A retrospective comparison of preoperative estimated glomerular filtration rate as a predictor of postoperative cardiac surgery associated acute kidney injury. Annals of Cardiac Anaesthesia. 2020;23(1):53–58. doi: 10.4103/aca.ACA_156_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ono M., Arnaoutakis G. J., Fine D. M., et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Critical Care Medicine. 2013;41(2):464–471. doi: 10.1097/CCM.0b013e31826ab3a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han Y., Zhu G., Han L., et al. Short-term rosuvastatin therapy for prevention of contrast-induced acute kidney injury in patients with diabetes and chronic kidney disease. Journal of the American College of Cardiology. 2014;63(1):62–70. doi: 10.1016/j.jacc.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 45.Moschopoulou M., Ampatzidou F. C., Loutradis C., et al. Diabetes mellitus does not affect the incidence of acute kidney injury after cardiac surgery; a nested case-control study. Journal of Nephrology. 2016;29(6):835–845. doi: 10.1007/s40620-016-0281-x. [DOI] [PubMed] [Google Scholar]
- 46.Huggins N., Nugent A., Modem V., et al. Incidence of acute kidney injury following cardiac catheterization prior to cardiopulmonary bypass in children. Catheterization and Cardiovascular Interventions. 2014;84(4):615–619. doi: 10.1002/ccd.25405. [DOI] [PubMed] [Google Scholar]
- 47.the TACS Investigators, Karkouti K., Rao V., Chan C. T., Wijeysundera D. N. Early rise in postoperative creatinine for identification of acute kidney injury after cardiac surgery. Canadian Journal of Anesthesia. 2017;64(8):801–809. doi: 10.1007/s12630-017-0899-8. [DOI] [PubMed] [Google Scholar]
- 48.Kumar A. B., Suneja M., Riou B. Cardiopulmonary bypass-associated acute kidney injury. Anesthesiology. 2011;114(4):964–970. doi: 10.1097/ALN.0b013e318210f86a. [DOI] [PubMed] [Google Scholar]
- 49.Durmaz I., Yagdi T., Calkavur T., et al. Prophylactic dialysis in patients with renal dysfunction undergoing on-pump coronary artery bypass surgery. The Annals of Thoracic Surgery. 2003;75(3):859–864. doi: 10.1016/S0003-4975(02)04635-0. [DOI] [PubMed] [Google Scholar]
- 50.Leaf D. E., Body S. C., Muehlschlegel J. D., et al. Length polymorphisms in heme oxygenase-1 and AKI after cardiac surgery. Journal of the American Society of Nephrology. 2016;27(11):3291–3297. doi: 10.1681/ASN.2016010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Popov A. F., Schulz E. G., Schmitto J. D., et al. Relation between renal dysfunction requiring renal replacement therapy and promoter polymorphism of the erythropoietin gene in cardiac surgery. Artificial Organs. 2010;34(11):961–968. doi: 10.1111/j.1525-1594.2010.01108.x. [DOI] [PubMed] [Google Scholar]
- 52.Stafford-Smith M., Li Y. J., Mathew J. P., et al. Genome-wide association study of acute kidney injury after coronary bypass graft surgery identifies susceptibility loci. Kidney International. 2015;88(4):823–832. doi: 10.1038/ki.2015.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perek B., Maison D., Budnick S., et al. Preoperative blood morphology and incidence of acute kidney injury after on-pump coronary artery bypass grafting - a single-center preliminary report. Polish Journal of Cardio-Thoracic Surgery. 2018;15(1):18–22. doi: 10.5114/kitp.2018.74670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soh S., Shim J. K., Song J. W., Kang B., Kwak Y. L. Perioperative nadir hemoglobin concentration and outcome in off-pump coronary artery bypass surgery- a retrospective review. Circulation Journal. 2020;85(1):37–43. doi: 10.1253/circj.CJ-20-0694. [DOI] [PubMed] [Google Scholar]
- 55.Wanderer J. P., Rathmell J. P. Cardiopulmonary bypass, renal oxygenation, & acute kidney injury. Anesthesiology. 2017;126(2):p. A21. doi: 10.1097/aln.0000000000001503. [DOI] [Google Scholar]
- 56.Svenmarker S., Haggmark S., Holmgren A., Naslund U. Serum markers are not reliable measures of renal function in conjunction with cardiopulmonary bypass. Interactive Cardiovascular and Thoracic Surgery. 2011;12(5):713–717. doi: 10.1510/icvts.2010.259432. [DOI] [PubMed] [Google Scholar]
- 57.Lannemyr L., Bragadottir G., Krumbholz V., Redfors B., Sellgren J., Ricksten S. E. Effects of cardiopulmonary bypass on renal perfusion, filtration, and oxygenation in patients undergoing cardiac surgery. Anesthesiology. 2017;126(2):205–213. doi: 10.1097/ALN.0000000000001461. [DOI] [PubMed] [Google Scholar]
- 58.Ranucci M., Romitti F., Isgrò G., et al. Oxygen delivery during cardiopulmonary bypass and acute renal failure after coronary operations. The Annals of Thoracic Surgery. 2005;80(6):2213–2220. doi: 10.1016/j.athoracsur.2005.05.069. [DOI] [PubMed] [Google Scholar]
- 59.Ranucci M. Perioperative renal failure: hypoperfusion during cardiopulmonary bypass? Seminars in Cardiothoracic and Vascular Anesthesia. 2007;11(4):265–268. doi: 10.1177/1089253207311141. [DOI] [PubMed] [Google Scholar]
- 60.Prough D. S., Esenaliev R. Monitoring the brain to save the kidneys. Critical Care Medicine. 2013;41(2):671–672. doi: 10.1097/CCM.0b013e318274247e. [DOI] [PubMed] [Google Scholar]
- 61.Kramer R. S., Herron C. R., Groom R. C., Brown J. R. Acute kidney injury subsequent to cardiac surgery. The Journal of Extra-Corporeal Technology. 2015;47(1):16–28. [PMC free article] [PubMed] [Google Scholar]
- 62.Bennett M. J., Rajakaruna C., Bazerbashi S., Webb G., Gomez-Cano M., Lloyd C. Oxygen delivery during cardiopulmonary bypass (and renal outcome) using two systems of extracorporeal circulation: a retrospective review. Interactive Cardiovascular and Thoracic Surgery. 2013;16(6):760–764. doi: 10.1093/icvts/ivt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sekiguchi H., Ajiro Y., Uchida Y., et al. Oxygen pre-conditioning prevents contrast-induced nephropathy (OPtion CIN study) Journal of the American College of Cardiology. 2013;62(2):162–163. doi: 10.1016/j.jacc.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 64.Ayvaz S., Aksu B., Kanter M., et al. Preventive effects of hyperbaric oxygen treatment on glycerol-induced myoglobinuric acute renal failure in rats. Journal of Molecular Histology. 2012;43(2):161–170. doi: 10.1007/s10735-012-9391-5. [DOI] [PubMed] [Google Scholar]
- 65.Solmazgul E., Uzun G., Cermik H., Atasoyu E. M., Aydinoz S., Yildiz S. Hyperbaric oxygen therapy attenuates renal ischemia/reperfusion injury in rats. Urologia Internationalis. 2007;78(1):82–85. doi: 10.1159/000096941. [DOI] [PubMed] [Google Scholar]
- 66.Aydinoz S., Uzun G., Cermik H., et al. Effects of different doses of hyperbaric oxygen on cisplatin-induced nephrotoxicity. Renal Failure. 2007;29(3):257–263. doi: 10.1080/08860220601166487. [DOI] [PubMed] [Google Scholar]
- 67.Ranucci M., Ballotta A., Kunkl A., et al. Influence of the timing of cardiac catheterization and the amount of contrast media on acute renal failure after cardiac surgery. The American Journal of Cardiology. 2008;101(8):1112–1118. doi: 10.1016/j.amjcard.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 68.Wittlinger T., Maus M., Kutschka I., Baraki H., Friedrich M. G. Risk assessment of acute kidney injury following cardiopulmonary bypass. Journal of Cardiothoracic Surgery. 2021;16(1):p. 4. doi: 10.1186/s13019-020-01382-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang Y. Y., Kong X. R., Xue F. L., et al. Incidence, risk factors and clinical outcomes of acute kidney injury after heart transplantation: a retrospective single center study. Journal of Cardiothoracic Surgery. 2020;15(1):p. 302. doi: 10.1186/s13019-020-01351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma X., Li J., Yun Y., et al. Risk factors analysis of acute kidney injury following open thoracic aortic surgery in the patients with or without acute aortic syndrome: a retrospective study. Journal of Cardiothoracic Surgery. 2020;15(1):p. 213. doi: 10.1186/s13019-020-01257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mancini E., Caramelli F., Ranucci M., et al. Is time on cardiopulmonary bypass during cardiac surgery associated with acute kidney injury requiring dialysis? Hemodialysis International. 2012;16(2):252–258. doi: 10.1111/j.1542-4758.2011.00617.x. [DOI] [PubMed] [Google Scholar]
- 72.Werner H. A., Wensley D. F., Lirenman D. S., LeBlanc J. G. Peritoneal dialysis in children after cardiopulmonary bypass. The Journal of Thoracic and Cardiovascular Surgery. 1997;113(1):64–70. doi: 10.1016/S0022-5223(97)70400-8. [DOI] [PubMed] [Google Scholar]
- 73.Kwiatkowski D. M., Menon S., Krawczeski C. D., et al. Improved outcomes with peritoneal dialysis catheter placement after cardiopulmonary bypass in infants. The Journal of Thoracic and Cardiovascular Surgery. 2015;149(1):230–236. doi: 10.1016/j.jtcvs.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 74.Book K., Ohqvist G., Bjork V. O., Lundberg S., Settergren G. Peritoneal dialysis in infants and children after open heart surgery. Scandinavian Journal of Thoracic and Cardiovascular Surgery. 2009;16(3):229–233. doi: 10.1016/s1875-9572(09)60077-2. [DOI] [PubMed] [Google Scholar]
- 75.El Masri K., Jackson K., Borasino S., Law M., Askenazi D., Alten J. Successful continuous renal replacement therapy using two single-lumen catheters in neonates and infants with cardiac disease. Pediatric Nephrology. 2013;28(12):2383–2387. doi: 10.1007/s00467-013-2578-5. [DOI] [PubMed] [Google Scholar]
- 76.Cullis B., Abdelraheem M., Abrahams G., et al. Peritoneal dialysis for acute kidney injury. Peritoneal Dialysis International. 2014;34(5):494–517. doi: 10.3747/pdi.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paret G., Cohen A. J., Bohn D. J., et al. Continuous arteriovenous hemofiltration after cardiac operations in infants and children. The Journal of Thoracic and Cardiovascular Surgery. 1992;104(5):1225–1230. doi: 10.1016/S0022-5223(19)34609-4. [DOI] [PubMed] [Google Scholar]
- 78.Sugahara S., Suzuki H. Early start on continuous hemodialysis therapy improves survival rate in patients with acute renal failure following coronary bypass surgery. Hemodialysis International. 2004;8(4):320–325. doi: 10.1111/j.1492-7535.2004.80404.x. [DOI] [PubMed] [Google Scholar]
- 79.Sinha R., Sethi S. K., Bunchman T., Lobo V., Raina R. Prolonged intermittent renal replacement therapy in children. Pediatric Nephrology. 2018;33(8):1283–1296. doi: 10.1007/s00467-017-3732-2. [DOI] [PubMed] [Google Scholar]
- 80.Picca S., Ricci Z., Picardo S. Acute kidney injury in an infant after cardiopulmonary bypass. Seminars in Nephrology. 2008;28(5):470–476. doi: 10.1016/j.semnephrol.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 81.Baudouin S. V., Wiggins J., Keogh B. F., Morgan C. J., Evans T. W. Continuous veno-venous haemofiltration following cardio-pulmonary bypass. Indications and outcome in 35 patients. Intensive Care Medicine. 1993;19(5):290–293. doi: 10.1007/BF01690550. [DOI] [PubMed] [Google Scholar]
- 82.Ukaigwe A., Karmacharya P., Mahmood M., et al. Meta-Analysis on Efficacy of _Statins_ for Prevention of Contrast-Induced Acute Kidney Injury in Patients Undergoing Coronary Angiography. The American Journal of Cardiology. 2014;114(9):1295–1302. doi: 10.1016/j.amjcard.2014.07.059. [DOI] [PubMed] [Google Scholar]
- 83.Lee J. M., Park J., Jeon K. H., et al. Efficacy of short-term high-dose statin pretreatment in prevention of contrast-induced acute kidney injury: updated study-level meta-analysis of 13 randomized controlled trials. PLoS One. 2014;9(11, article e111397) doi: 10.1371/journal.pone.0111397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Quintavalle C., Fiore D., de Micco F., et al. Impact of a high loading dose of atorvastatin on contrast-induced acute kidney injury. Circulation. 2012;126(25):3008–3016. doi: 10.1161/CIRCULATIONAHA.112.103317. [DOI] [PubMed] [Google Scholar]
- 85.Park J. H., Shim J. K., Song J. W., Soh S., Kwak Y. L. Effect of atorvastatin on the incidence of acute kidney injury following valvular heart surgery: a randomized, placebo-controlled trial. Intensive Care Medicine. 2016;42(9):1398–1407. doi: 10.1007/s00134-016-4358-8. [DOI] [PubMed] [Google Scholar]
- 86.Wang J., Gu C., Gao M., Yu W., Yu Y. Preoperative Statin Therapy and Renal Outcomes After Cardiac Surgery: A Meta- analysis and Meta-regression of 59,771 Patients. The Canadian Journal of Cardiology. 2015;31(8):1051–1060. doi: 10.1016/j.cjca.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 87.Kandula P. Statins in perioperative prevention of acute kidney injury in patients undergoing cardiac surgery. European Heart Journal. 2009;30(2):p. 250. doi: 10.1093/eurheartj/ehn545. [DOI] [PubMed] [Google Scholar]
- 88.Billings F. T., 4th, Ball S. K., Roberts L. J., 2nd, Pretorius M. Postoperative acute kidney injury is associated with hemoglobinemia and an enhanced oxidative stress response. Free Radical Biology & Medicine. 2011;50(11):1480–1487. doi: 10.1016/j.freeradbiomed.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vermeulen Windsant I. C., Snoeijs M. G., Hanssen S. J., et al. Hemolysis is associated with acute kidney injury during major aortic surgery. Kidney International. 2010;77(10):913–920. doi: 10.1038/ki.2010.24. [DOI] [PubMed] [Google Scholar]
- 90.Haase M., Haase-Fielitz A., Bagshaw S. M., Ronco C., Bellomo R. Cardiopulmonary bypass-associated acute kidney injury: a pigment nephropathy? Contributions to Nephrology. 2007;156:340–353. doi: 10.1159/000102125. [DOI] [PubMed] [Google Scholar]
- 91.Hu P., Chen Y., Wu Y., et al. Development and validation of a model for predicting acute kidney injury after cardiac surgery in patients of advanced age. Journal of Cardiac Surgery. 2021;36(3):806–814. doi: 10.1111/jocs.15249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin H., Hou J., Tang H., et al. A novel nomogram to predict perioperative acute kidney injury following isolated coronary artery bypass grafting surgery with impaired left ventricular ejection fraction. BMC Cardiovascular Disorders. 2020;20(1):p. 517. doi: 10.1186/s12872-020-01799-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mehta R. H., Grab J. D., O’Brien S. M., et al. Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114(21):2208–2216. doi: 10.1161/CIRCULATIONAHA.106.635573. [DOI] [PubMed] [Google Scholar]
- 94.Thakar C. V., Arrigain S., Worley S., Yared J. P., Paganini E. P. A clinical score to predict acute renal failure after cardiac surgery. Journal of the American Society of Nephrology. 2005;16(1):162–168. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 95.Wijeysundera D. N., Karkouti K., Dupuis J. Y., et al. Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. JAMA. 2007;297(16):1801–1809. doi: 10.1001/jama.297.16.1801. [DOI] [PubMed] [Google Scholar]
- 96.Englberger L., Suri R. M., Li Z., et al. Validation of clinical scores predicting severe acute kidney injury after cardiac surgery. American Journal of Kidney Diseases. 2010;56(4):623–631. doi: 10.1053/j.ajkd.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 97.Rahmanian P. B., Filsoufi F., Castillo J. G., et al. Predicting postoperative renal failure requiring dialysis, and an analysis of long-term outcome in patients undergoing valve surgery. The Journal of Heart Valve Disease. 2008;17(6):657–665. [PubMed] [Google Scholar]
- 98.Aronson S., Fontes M. L., Miao Y., Mangano D. T. Risk index for perioperative renal dysfunction/failure: critical dependence on pulse pressure hypertension. Circulation. 2007;115(6):733–742. doi: 10.1161/CIRCULATIONAHA.106.623538. [DOI] [PubMed] [Google Scholar]
- 99.Brown J. R., Cochran R. P., Leavitt B. J., et al. Multivariable prediction of renal insufficiency developing after cardiac surgery. Circulation. 2007;116(11 Suppl):I139–I143. doi: 10.1161/CIRCULATIONAHA.106.677070. [DOI] [PubMed] [Google Scholar]
- 100.Palomba H., de Castro I., Neto A. L., Lage S., Yu L. Acute kidney injury prediction following elective cardiac surgery: AKICS score. Kidney International. 2007;72(5):624–631. doi: 10.1038/sj.ki.5002419. [DOI] [PubMed] [Google Scholar]
- 101.Neyra J. A., Hu M. C., Minhajuddin A., et al. Kidney tubular damage and functional biomarkers in acute kidney injury following cardiac surgery. Kidney International Reports. 2019;4(8):1131–1142. doi: 10.1016/j.ekir.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Devarajan P., Krawczeski C. D., Nguyen M. T., Kathman T., Wang Z., Parikh C. R. Proteomic identification of early biomarkers of acute kidney injury after cardiac surgery in children. American Journal of Kidney Diseases. 2010;56(4):632–642. doi: 10.1053/j.ajkd.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cooper D. S., Claes D., Goldstein S. L., et al. Follow-up renal assessment of injury long-term after acute kidney injury (FRAIL-AKI) Clinical Journal of the American Society of Nephrology. 2016;11(1):21–29. doi: 10.2215/CJN.04240415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moriyama T., Hagihara S., Shiramomo T., Nagaoka M., Iwakawa S., Kanmura Y. Comparison of three early biomarkers for acute kidney injury after cardiac surgery under cardiopulmonary bypass. Journal of Intensive Care. 2016;4(1):p. 41. doi: 10.1186/s40560-016-0164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dong L., Ma Q., Bennett M., Devarajan P. Urinary biomarkers of cell cycle arrest are delayed predictors of acute kidney injury after pediatric cardiopulmonary bypass. Pediatric Nephrology. 2017;32(12):2351–2360. doi: 10.1007/s00467-017-3748-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haase M., Haase-Fielitz A. Can novel biomarkers complement best possible clinical assessment for early acute kidney injury diagnosis?⁎. Journal of the American College of Cardiology. 2011;58(22):2310–2312. doi: 10.1016/j.jacc.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 107.Parikh C. R., Mishra J., Thiessen-Philbrook H., et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney International. 2006;70(1):199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 108.Küllmar M., Saadat-Gilani K., Weiss R., et al. Kinetic changes of plasma renin levels predict acute kidney injury in cardiac surgery patients. American Journal of Respiratory and Critical Care Medicine. 2020 doi: 10.1164/rccm.202005-2050OC. [DOI] [PubMed] [Google Scholar]
- 109.Greenberg J. H., Zappitelli M., Jia Y., et al. Biomarkers of AKI progression after pediatric cardiac surgery. Journal of the American Society of Nephrology. 2018;29(5):1549–1556. doi: 10.1681/ASN.2017090989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.de Fontnouvelle C. A., Greenberg J. H., Thiessen-Philbrook H. R., et al. Interleukin-8 and tumor necrosis factor predict acute kidney injury after pediatric cardiac surgery. The Annals of Thoracic Surgery. 2017;104(6):2072–2079. doi: 10.1016/j.athoracsur.2017.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stoppe C., Averdunk L., Goetzenich A., et al. The protective role of macrophage migration inhibitory factor in acute kidney injury after cardiac surgery. Science Translational Medicine. 2018;10(441, article eaan4886) doi: 10.1126/scitranslmed.aan4886. [DOI] [PubMed] [Google Scholar]
- 112.Shah S. V., Rajapurkar M. M., Baliga R. The role of catalytic iron in acute kidney injury. Clinical Journal of the American Society of Nephrology. 2011;6(10):2329–2331. doi: 10.2215/CJN.08340811. [DOI] [PubMed] [Google Scholar]
- 113.Haase M., Bellomo R., Haase-Fielitz A. Novel biomarkers, oxidative stress, and the role of labile iron toxicity in cardiopulmonary bypass-associated acute kidney injury. Journal of the American College of Cardiology. 2010;55(19):2024–2033. doi: 10.1016/j.jacc.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 114.Ho J., Lucy M., Krokhin O., et al. Mass spectrometry-based proteomic analysis of urine in acute kidney injury following cardiopulmonary bypass: a nested case-control study. American Journal of Kidney Diseases. 2009;53(4):584–595. doi: 10.1053/j.ajkd.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 115.Petronilho F., Constantino L., de Souza B., et al. Efficacy of the combination of N-acetylcysteine and desferrioxamine in the prevention and treatment of gentamicin-induced acute renal failure in male Wistar rats. Nephrology, Dialysis, Transplantation. 2009;24(7):2077–2082. doi: 10.1093/ndt/gfn774. [DOI] [PubMed] [Google Scholar]
- 116.Palipoch S. A review of oxidative stress in acute kidney injury: protective role of medicinal plants-derived antioxidants. African Journal of Traditional, Complementary, and Alternative Medicines. 2013;10(4):88–93. doi: 10.4314/ajtcam.v10i4.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu S., Chen Y. H., Tan Z. X., et al. Vitamin D3 pretreatment alleviates renal oxidative stress in lipopolysaccharide-induced acute kidney injury. The Journal of Steroid Biochemistry and Molecular Biology. 2015;152:133–141. doi: 10.1016/j.jsbmb.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 118.Patel N. N., Toth T., Jones C., et al. Prevention of post-cardiopulmonary bypass acute kidney injury by endothelin A receptor blockade. Critical Care Medicine. 2011;39(4):793–802. doi: 10.1097/CCM.0b013e318206d563. [DOI] [PubMed] [Google Scholar]
- 119.Garg A. X., Devereaux P. J., Yusuf S., et al. Kidney function after off-pump or on-pump coronary artery bypass graft surgery: a randomized clinical trial. JAMA. 2014;311(21):2191–2198. doi: 10.1001/jama.2014.4952. [DOI] [PubMed] [Google Scholar]
- 120.Sidaway P. IgG reactivity to apoptotic cells--role in presensitization revealed. Nature Reviews. Nephrology. 2014;10(8):p. 423. doi: 10.1038/nrneph.2014.118. [DOI] [PubMed] [Google Scholar]
- 121.Seabra V. F., Alobaidi S., Balk E. M., Poon A. H., Jaber B. L. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized controlled trials. Clinical Journal of the American Society of Nephrology. 2010;5(10):1734–1744. doi: 10.2215/CJN.02800310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vives M., Lockwood G., Punjabi P. P., Krahne D. Neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery. Anesthesiology. 2012;116(2):490–491. doi: 10.1097/ALN.0b013e31823ed5ba. [DOI] [PubMed] [Google Scholar]
- 123.Ho J., Reslerova M., Gali B., et al. Urinary hepcidin-25 and risk of acute kidney injury following cardiopulmonary bypass. Clinical Journal of the American Society of Nephrology. 2011;6(10):2340–2346. doi: 10.2215/CJN.01000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mayer T., Bolliger D., Scholz M., et al. Urine biomarkers of tubular renal cell damage for the prediction of acute kidney injury after cardiac surgery--a pilot study. Journal of Cardiothoracic and Vascular Anesthesia. 2017;31(6):2072–2079. doi: 10.1053/j.jvca.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 125.Kim N., Dai S. Y., Pang V., Mazer C. D. Vasopressinase activity: a potential early biomarker for detecting cardiopulmonary bypass-associated acute kidney injury? The Thoracic and Cardiovascular Surgeon. 64(7):555–560. doi: 10.1055/s-0035-1564446. [DOI] [PubMed] [Google Scholar]
- 126.Wald R., Liangos O., Perianayagam M. C., et al. Plasma cystatin C and acute kidney injury after cardiopulmonary bypass. Clinical Journal of the American Society of Nephrology. 2010;5(8):1373–1379. doi: 10.2215/CJN.06350909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hassinger A. B., Wainwright M. S., Lane J. C., Haymond S., Backer C. L., Wald E. Elevated preoperative serum asymmetrical dimethylarginine (ADMA) is associated with poor outcomes after pediatric cardiac surgery. Intensive Care Medicine. 2012;38(10):1697–1704. doi: 10.1007/s00134-012-2657-2. [DOI] [PubMed] [Google Scholar]
- 128.Sullo N., Mariani S., JnTala M., et al. An observational cohort feasibility study to identify microvesicle and micro-RNA biomarkers of acute kidney injury following pediatric cardiac surgery. Pediatric Critical Care Medicine. 19(9):816–830. doi: 10.1097/PCC.0000000000001604. [DOI] [PubMed] [Google Scholar]
- 129.Wu R., Wu Y., Yang L., Deng Y., Chen D. Value of serum level of microRNA-494 in predicting prognosis of acute renal injury after cardiac surgery in children. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 31(12):1469–1473. doi: 10.3760/cma.j.issn.2095-4352.2019.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
None.


