Abstract
BACKGROUND AND PURPOSE:
Obtaining safe and effective closure of the femoral access site following neurointerventional procedures can sometimes be challenging, especially in patients on anti-coagulation or anti-platelet therapy. The purpose of this study was to evaluate the safety and efficacy of a novel percutaneous closure device that employs a nitinol clip–mediated extravascular closure strategy following neurointerventional procedures.
MATERIALS AND METHODS:
We performed a retrospective review of all patients who underwent neurointerventional procedures at our institution between January 1, 2006 and December 31, 2008. We evaluated the safety and efficacy of the StarClose device in patients undergoing first and repeat procedures. Groin complications were classified as self-limited hematoma, hematoma requiring transfusion, other/minor (pseudoaneurysm, infection), and other/major (vascular complication).
RESULTS:
StarClose device use was attempted in 281 of 352 cases (79.8%) with success reported in 269 cases (95.7%). Minor and major complications occurred in 0.7% and 0.4% of patients, respectively. There was one major vascular complication. Repeat use was performed in 84 patients with 100% success and a 2.3% minor complication rate. Time to reaccess ranged from 1 to 1036 days (mean, 105 days).
CONCLUSIONS:
The StarClose device achieves rapid and safe femoral arterial closure in patients, both for primary closure and after reaccess.
Femoral arterial access is common and the number of catheter-based neurointerventional procedures is increasing. Although manual compression of the femoral artery access site is standard, it is limited by additional operator time and prolonged patient immobilization following the procedure. Additionally, hemostasis following placement of larger sheaths, especially in patients who are anticoagulated, can be difficult. Finally, our patient population often requires multiple procedures for diagnosis, treatment, and follow-up, and therefore repeat access is the norm.
Several percutaneous closure devices are available and rely on either 1) passive closure with enhanced manual compression by using external patches with prothrombotic coatings (Syvtek Patch; Marine Polymer Technologies, Danvers, Massachusetts) or 2) active closure by using suture-mediated closure (Perclose; Abbott Vascular, Redwood City, California), creation of a collagen plug external to the punctured artery (Angio-Seal; St Jude Medical, St Paul, Minnesota), or suture-collagen combinations (Angio-Seal VIP; St Jude Medical). The most recent active closure device employs a nitinol clip–mediated extravascular closure strategy (StarClose; Abbott Vascular).
The purported advantages of the StarClose device are rapid hemostasis, reduced procedural time, improved patient comfort, early ambulation, and “through-the-sheath” delivery, which aims to avoid skin contact and thereby reduce infection rates. Additionally, the StarClose device permits immediate reaccess to the femoral artery. The purpose of our study is to evaluate the safety and efficacy of the StarClose device in patients undergoing neurointerventional procedures.
Materials and Methods
Local institutional review board approval was obtained for retrospective review. All patients who underwent neurointerventional procedures at our institution between January 1, 2006 and December 31, 2008 were included. Pediatric patients, those undergoing purely diagnostic angiographic procedures, and patients with nonfemoral access were excluded.
The neurointerventional procedures were performed under general anesthesia or conscious sedation. Systemic heparinization to achieve an activated clotting time 2–3 times normal was used in almost all cases and was not reversed before groin closure by either method. Antibiotics were not used and were not routinely used before our use of the StarClose vascular closure device. Sheath exchange was performed only for 2 acute stroke patients requiring 9F sheaths. At the conclusion of the procedure, we exchanged the 9F sheath for a 7F or 8F dilator, after which pressure was held for 5 minutes. An exchange was then performed for the 6F StarClose sheath, and closure was performed in the usual fashion.
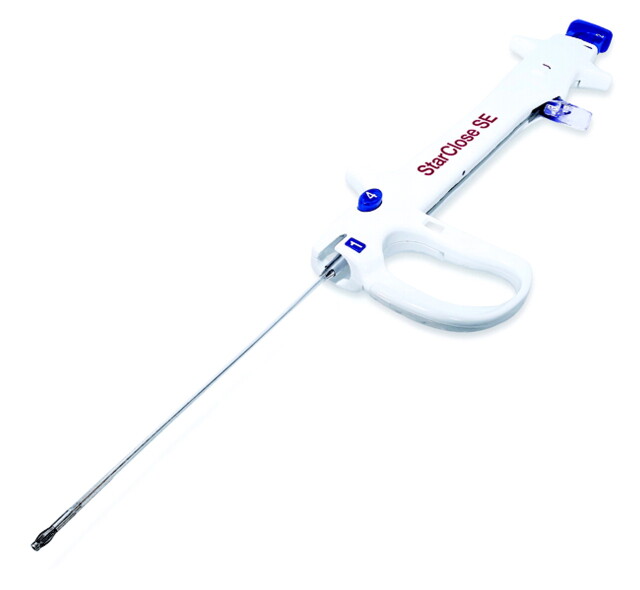
Before groin closure, angiographic evaluation of the femoral access site was performed in all cases. StarClose deployment was considered in all patients, and manual compression (typically 20 minutes) was used for all patients found unsuitable for StarClose deployment. Exclusion criteria for StarClose deployment were punctures located at or distal to the bifurcation of the common femoral artery and significant atherosclerosis at the puncture site. The StarClose and StarClose SE (second generation) devices were used during the study period (Figs 1 and 2).
Fig 1.
The StarClose SE vascular closure system (6F introducer sheath not shown).
Fig 2.
Mechanism of StarClose “through-the-sheath” circumferential clip closure. A, The vessel locator wings are deployed and positioned against the arteriotomy. The clip delivery tube delivers the clip to the arteriotomy within the introducer sheath. B, The trigger is depressed and the clip is released as vessel locator wings are recovered. C, The tines of the clip grasp the tissue and close the arteriotomy in a purse-string fashion.
Patients were instructed to remain flat for 2 hours following the procedure regardless of anticoagulation strategy. Patients undergoing manual compression were to remain flat for 6 hours, after which time they were permitted activity.
To capture vascular and bleeding complications following closure, review of medical records and relevant follow-up imaging studies (vascular sonography, abdominal or pelvic CT, angiography) were performed. Groin complications were classified as self-limited hematoma, hematoma requiring transfusion, other/minor (pseudoaneurysm, infection), or other/major (vascular complication).
Additionally, patients in whom repeat vascular access was performed were also identified. In these patients, time to reaccess was noted, as was the presence of any abnormality on the femoral artery angiograms performed on vessels previously treated with StarClose.
Results
Between January 1, 2006 and December 31, 2008, 385 procedures were performed in 314 patients. Twelve cases in 8 pediatric patients were excluded. Twenty patients were excluded because there was no mention of groin hemostasis method in the angiogram report or medical record. Finally, 1 patient was excluded because the brachial artery was accessed. Thus, 352 cases performed in 285 patients were included in our analysis. The mean age of the patients was 59 years (range, 18–93 years) and 188 (53.4%) were female.
Procedure types included ruptured aneurysms (n = 82, 23.3%), unruptured aneurysms (n = 55, 15.6%), carotid artery stents (n = 63, 17.9%), embolization of vascular malformations (n = 38, 10.8%), acute ischemic stroke (n = 19, 5.4%) intracranial angioplasty or stent placement for atherosclerotic disease (n = 14, 4.0%), and other interventions (n = 81, 23%). Nearly all patients were systemically heparinized. Additionally, stroke patients received either abciximab (n = 1), tissue plasminogen activator (n = 14), or both (n = 3).
The right groin was accessed for 336 procedures and the left groin was accessed for 16 procedures. Procedural sheath sizes included 5F (n = 146), 6F (n = 199), and 9F (n = 2). Sheath size was unknown in 5 cases. Percutaneous closure of the femoral access site with the StarClose device was attempted in 281 cases (79.8%), with success reported in 269 cases (95.7%). Self-limited hematomas were seen in 4 (1.4%) StarClose patients and 3 (4.2%) patients in whom hemostasis was achieved with manual compression. One patient (0.4%) in the StarClose group suffered a hematoma requiring a transfusion. No minor complications occurred.
One major groin complication occurred in our series. A 53-year-old man had undergone intracranial angioplasty and stent placement for a symptomatic, high-grade stenosis of his right middle cerebral artery. This patient had an aortobifemoral bypass graft 4 years before the procedure. StarClose was deployed without difficulty. However, in the recovery room, the patient was noted to have a cool extremity with diminished pulses. A lower extremity angiogram demonstrated an occluded superficial femoral artery and poor filling of the profunda femoris artery. The patient subsequently had successful open thrombectomy and patch angioplasty.
The femoral artery was reaccessed in 84 patients (82 of 84 cases were right side). Eleven patients were reaccessed multiple times (range, 3–6 times). Time to reaccess ranged from 1 to 1036 days (<90 days, n = 57; 90–180 days, n = 9; >180 days, n = 18). All follow-up angiograms of the treated femoral artery were normal. Two self-limited hematomas occurred in patients who were reaccessed, occurring 1 and 380 days after prior StarClose use.
Discussion
We found the StarClose device to be safe and efficacious in patients undergoing neurointerventional procedures. Procedural success was 95.7% and complication rates are in keeping with those reported in studies of patients undergoing cardiac and peripheral vascular procedures.1–8 Rates of groin hematomas in both the manual closure (4.2%) and StarClose (1.4%) groups were similar.
Our cohort represents the largest published series of StarClose deployments. Our results with the StarClose device are in keeping with the largest prospective trial of the same device in a general interventional radiology practice that reported a 96% success rate and an 8% small hematoma rate in 222 patients.4 Major vascular complications, especially those requiring surgery, are rare in published series. However, major vascular complications requiring a femoro-femoral bypass graft5 and surgical evacuation of hematoma3 have been reported. Our patient requiring open thrombectomy and patch angioplasty is another instance.
Also important in a neurointerventional practice is the ability to reaccess the same groin. Many of these patients will need multiple angiograms, often in short order. We successfully deployed this device 2 or more times in the same groin in 84 patients. No deployment failures were noted in the repeat closure patients. Eighteen patients were reaccessed after 180 days. While scarring at the groin site is a theoretic concern in these patients, it was not an issue in our small subgroup of delayed reaccess patients. There were no major complications and only 2 small self-limited hematomas in that subgroup. Tay et al reported their experience with repeat closure in 25 patients undergoing coronary interventions.7 The device success (96%) and low complication rates (8%) were encouraging.
The StarClose SE (the second-generation device) was introduced to our department in June 2008, after which the first-generation device was no longer used. One noticeable difference between the first- and second-generation device is the enhanced ergonomic design to allow operators with smaller hands to easily use the device. Additionally, the second-generation device provides numbered steps and windows for visual confirmation of step completion, as well as an improved safety release and addition of access ports for patient safety and clinical confidence. The footplate (vessel locator wings) may not retract properly when the soft tissue is impinged between the footplate and clip delivery tube. If soft tissue is impacted between the vessel locator wings and clip delivery tube, the use of the access ports with a 6F dilator allows the clip delivery tube to be retracted and the vessel locator wings to passively retract within the delivery sheath.
Although our study is the first to report the use of the StarClose device, Khaghany et al reported their experience with the Closer S device (Perclose, Redwood City, California) in 337 patients undergoing neurointerventional procedures.9 Success was similar between the device group and manual compression group (95% vs 96%). As in our study, they reported the use of the device in patients with previous device procedures (n = 138). The success of the Perclose device declined in vessels with 3 or more prior procedures.
Our study has limitations as well. First, this is a retrospective review culled from our neuroendovascular database. As such, it is possible that some patients may have been missed. Additionally, we relied on medical records to assess for groin complications. It is therefore possible that minor groin hematomas could have been missed as well. However, all transfusions were captured and any more serious complications are unlikely to have been missed.
Conclusions
The StarClose device can be used to achieve rapid closure of femoral access site in patients undergoing neuroendovascular procedures with a failure rate of <5% and a low complication rate. Furthermore, it appears that this extraluminal clip system allows for safe repeat access and closure of the same femoral artery.
References
- 1. Desideri A, Tonello D, Coscarelli S, et al. Early mobilization after percutaneous catheterization and vascular closure with a novel device (Star-Close). Am J Cardiol 2005;96:1408–09 [DOI] [PubMed] [Google Scholar]
- 2. Hermiller J, Simonton C, Hinohara T, et al. Clinical experience with a circumferential clip-based vascular closure device in diagnostic catheterization. J Invasive Cardiol 2005;17:504–10 [PubMed] [Google Scholar]
- 3. Hermiller JB, Simonton C, Hinohara T, et al. The StarClose vascular closure system: interventional results from the CLIP study. Catheter Cardiovasc Interv 2006;68:677–83 [DOI] [PubMed] [Google Scholar]
- 4. Imam A, Carter RM, Phillips-Hughes J, et al. StarClose vascular closure device: prospective study on 222 deployments in an interventional radiology practice. Cardiovasc Intervent Radiol 2007;30:738–42 [DOI] [PubMed] [Google Scholar]
- 5. Ratnam LA, Raja J, Munneke GJ, et al. Prospective nonrandomized trial of manual compression and Angio-Seal and StarClose arterial closure devices in common femoral punctures. Cardiovasc Intervent Radiol 2007;30:182–88 [DOI] [PubMed] [Google Scholar]
- 6. Ruygrok PN, Ormiston JA, Stewart JT, et al. Initial experience with a new femoral artery closure device following percutaneous coronary intervention with glycoprotein IIb/IIIa inhibition. Catheter Cardiovasc Interv 2005;66:185–91 [DOI] [PubMed] [Google Scholar]
- 7. Tay EL, Co M, Tai BC, et al. Clinical experience of StarClose vascular closure device in patients with first and recurrent femoral punctures. J Interv Cardiol 2008;21:67–73 [DOI] [PubMed] [Google Scholar]
- 8. Veasey RA, Large JK, Silberbauer J, et al. A randomised controlled trial comparing StarClose and AngioSeal vascular closure devices in a district general hospital: the SCOAST study. Int J Clin Pract 2008;62:912–18 [DOI] [PubMed] [Google Scholar]
- 9. Khaghany K, Al-Ali F, Spigelmoyer T, et al. Efficacy and safety of the Perclose Closer S device after neurointerventional procedures: prospective study and literature review. AJNR Am J Neuroradiol 2005;26:1420–24 [PMC free article] [PubMed] [Google Scholar]