Abstract
BACKGROUND AND PURPOSE:
Hippocampal abnormalities are known as highly epileptogenic precursor lesions in the general population, primarily manifesting as MTS. The purpose of this study was to evaluate the occurrence of hippocampal abnormalities on MR imaging in patients with TS to explore the possible underlying mechanisms of the abnormalities and to identify the relationship between an abnormal HF and epilepsy.
MATERIALS AND METHODS:
We studied MR images and clinical data from 31 patients with TS. The MR imaging protocol was identical for all patients and included tilted coronal images of their temporal lobes. The diagnosis of TSC was made according to established criteria. The HFs of the patients were evaluated from coronal images according to size, morphology, and signal intensity. The data were submitted to statistical analysis, and P values ≤ .05 were considered significant.
RESULTS:
We found HF abnormalities in 5 patients. Four had typical MTS, and 1 had HIMAL. We found a positive correlation between childhood febrile seizures and MTS in patients with TS. We also confirmed that patients with altered hippocampi had a tendency to exhibit more cortical tubers.
CONCLUSIONS:
Analysis of this series of patients demonstrated the presence of HF abnormalities, mainly MTS in patients with TS. We also found that the occurrence of febrile seizures during the first year of life appears to be one of the determining factors for MTS development in these patients.
Tuberous sclerosis is a neurocutaneous genetically inherited disease characterized by dysplasias and hamartomas that affect multiple organs.1 Epilepsy is the most prevalent clinical manifestation, occurring in >70%–80% of patients. Virtually all subtypes of seizures (simple partial, complex partial, and generalized tonic-clonic) have been reported.2,3
Hippocampal abnormalities are also recognized as highly epileptogenic precursor lesions in the general population, and they mainly manifest as MTS, which is the most common cause of partial complex seizures. It has been reported in 40%–60% of adults4 and less frequently in children5 undergoing neurosurgical treatment of epilepsy. In 15% of patients with epilepsy undergoing surgery, MTS is combined with another potentially epileptogenic extrahippocampal abnormality referred to as “dual pathology.”6,7 Moreover, MTS has also been described in individuals without epilepsy.8,9 Conversely, hippocampal developmental abnormalities, characterized by changes in the shape and/or position of the hippocampus, have a reduced association with epilepsy10 but are found in many patients with congenital malformations such as TS.11 Furthermore, the dual pathology is associated with a less favorable prognosis and requires different management than either condition alone. Despite reports of hippocampal developmental abnormalities and a recently isolated case of MTS in a patient with TS,12 to our knowledge, a systematic study in a representative series of patients has not been conducted to verify this association.
Our aim was to evaluate the co-occurrence of HF abnormalities with structural lesions in a series of patients with TS by using MR imaging. EEG and clinical features were also analyzed to explore the relationship between HF abnormalities and epilepsy in these patients.
Materials and Methods
We reviewed the MR images and clinical data of 31 patients with TS evaluated from 2002 to 2008 (17 females; age range, 8 months to 34 years 11 months; mean age, 10.8 years). Inclusion criteria were patients with TS who underwent routine MR imaging of the brain during the study period. The exclusion criteria were the presence of other concurrent CNS diseases or contraindications in the MR imaging examinations and intravenous contrast injections.
All MR imaging studies were performed on a 1T unit by using a protocol that included axial SE T1-weighted sequences (TR, 509 ms; TE, 14 ms), before and after intravenous administration of gadopentetate dimeglumine; an axial FLAIR sequence (TR, 11,000 ms; TE, 140 ms; TI, 2600 ms); an axial T1 SE sequence with additional resonance MTC (TR, 509 ms; TE, 14 ms/MTC); and an axial T2 fast SE sequence (TR, 4576 ms; TE, 100 ms). The section thickness was 5 mm, with an intersection gap of 0.5 mm and a 256 × 512 matrix. Evaluation of HF was performed with tilted coronal FLAIR and T2-weighted images by using a section thickness of 3 mm, an intersection gap of 0.3 mm, and a matrix of 256 × 512.
The diagnosis of the TSC was made according to established criteria.13 The brain images were scrutinized for structural lesions of TS (cortical tubers, radial bands, subependymal nodules, and subependymal giant cell astrocytomas). Lesions were quantified with the most appropriate sequence for each patient, according to previously described methods.14 The hippocampi were qualitatively evaluated on coronal MR images with respect to size, morphology, signal intensity, and the side of the abnormality. MTS was characterized by hyperintensity on FLAIR and T2-weighted images, atrophy, and loss of the internal architecture of the hippocampus.15 Hippocampal developmental abnormalities, represented by HIMAL, were characterized by unilateral involvement and incomplete rotation of a hippocampus that was of normal size and signal intensity but was abnormally rounded.16
Assessment of MR images was done in consensus between 2 experienced neuroradiologists (A.J.d.R. and H.P.P.G.).
The clinical data and interictal EEGs obtained from interviews and medical records were examined by an experienced neurologist with a specialization in epilepsy (R.M.F.V.) and were correlated with the MR imaging findings. The data were submitted to a Fisher exact test for statistical analysis of qualitative variables and a Student t test or a Mann-Whitney U test for quantitative variables. P values ≤ .05 were considered statistically significant. This study was approved by the ethics and research committee of our institution.
Results
The MR imaging, interictal EEG, and clinical findings from the 31 subjects with TS are shown in the On-line Table.
We found HF abnormalities in 5 patients. Four had typical MTS (patients 17, 18, 22, and 31) equally present on both sides (Figs 1 and 2) (ie, 2 patients with right and 2 with left MTS). All 4 patients had normal contralateral HFs. All patients with MTS had epilepsy, and 2 (patients 18, 31) had childhood febrile seizures with an onset before they were 1 year of age. Two other patients (patients 24 and 28), who did not have MTS, also had childhood febrile seizures that occurred after their first year. We found a positive correlation between childhood febrile seizures initiated in the first year of life and MTS in patients with TS (P = .018). No other risk factors that could contribute to MTS development in patients 17 and 22 were identified.
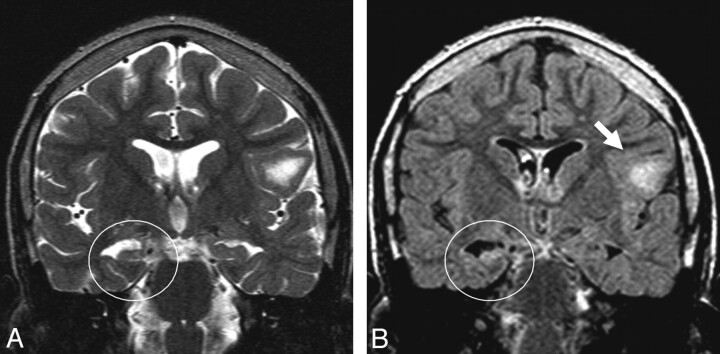
Fig 1.
Patient 18. A and B, Coronal T2-weighted (A) and FLAIR (B) images show the right MTS characterized by hyperintensity, atrophy, and loss of internal architecture. A cortical tuber is observed in the left frontal lobe (arrow).
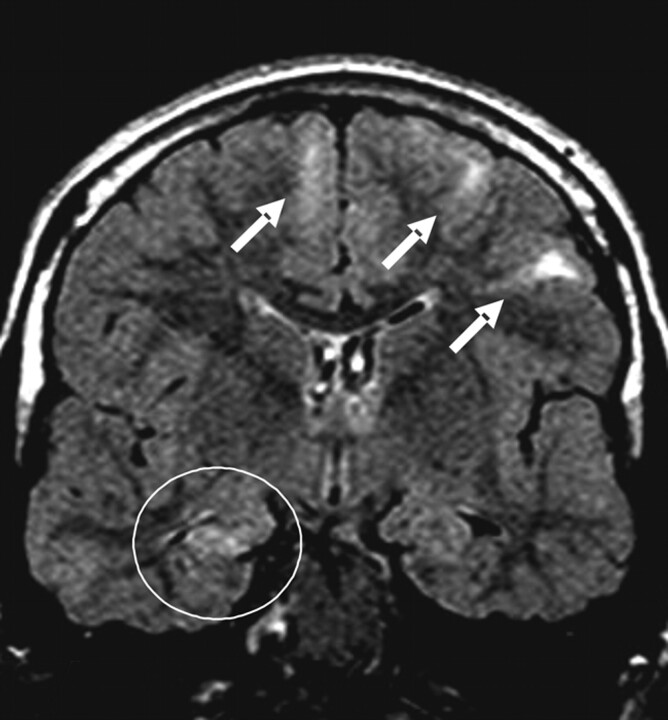
Fig 2.
Patient 22. Coronal FLAIR image shows the right MTS. Cortical tubers are observed in the bilateral frontal lobes (arrows).
Another patient had a left HIMAL (not shown), diagnosed according to the currently established criteria.16 The HF was vertically oriented and without signal-intensity abnormality or atrophy. There was also an abnormal angle of the collateral sulcus and an enlargement of the temporal horn. This patient had epilepsy as well. The remaining 26 patients did not show any HF abnormalities.
Seizures were observed in all except 5 patients (patients 4, 6, 16, 25, and 29), and several subtypes of seizures were recognized (On-line Table). Each patient with MTS showed a distinct type of seizure (generalized tonic-clonic, complex partial, simple partial, or atonic), and the patient with the HIMAL showed a simple partial crisis. Six patients had West syndrome (patients 7, 10, 11, 12, 13, and 15), characterized by infantile spasms, resistance to drug therapy, and hypsarrhythmia in the EEG recordings. None of these patients had HF abnormalities. We also observed 2 patients with behavioral disturbances consistent with autism (patients 14 and 18), and 1 of these (patient 18) had MTS.
The mean number of tubers in the patients with HF abnormalities was 39.0. The remaining patients had an average of 22.2 tubers. We observed that patients with altered HF tended to exhibit more cortical tubers (P < .05). No significant difference, however, was observed in cortical tuber distribution among the cerebral lobes, including the temporal ones. In all patients, the most tubers were present in the frontal and parietal lobes.
Regarding the interictal EEG results, no significant association was observed. The EEG results from the patients with MTS were focal (patients 18 and 22), multifocal (patient 17), or normal (patient 31). Regarding the 2 patients with focal results, only 1 (patient 18) showed an HF abnormality/EEG relationship (ie, a right MTS and a right temporal focus in the EEG). The patient with left HIMAL had a multifocal interictal EEG profile.
Discussion
The co-occurrence of MTS with another potentially epileptogenic extrahippocampal abnormality was observed in 15% of the patients. Cortical dysplasia was the most common association. Other extratemporal conditions have been described in the literature, including neuronal migration disorders, low-grade tumors, vascular malformations, porencephalic cysts, and gliotic lesions resulting from early-life cerebral insults.7 The combination with TS was only described in 1 recent report and was associated with polymicrogyria.12 Another report also described the association of MTS with the forme fruste of TS in 2 patients.17
Abnormalities of the HF are not usually grouped with the typical structural lesions of TSC. The major lesions of this disease include cortical tubers, radial white matter bands, subependymal nodules, and subependymal giant cell astrocytomas. Other uncommon lesions have been shown, including parenchymal cysts and cortical calcifications.18 The association of TS with other cortical dysplasias (hemimegalencephaly and focal cortical dysplasia) is rare.19
The etiology of MTS remains controversial. Genetic and acquired factors are involved, but it likely results from a multifactorial process. MTS was found in association with prolonged febrile seizures, head trauma, infection, birth trauma, recurrent seizures, and suspected genetic predisposition.20
In contrast, the etiology of TSC is well known. It is determined by mutations in tumor suppressor genes (TSC1 or TSC2) and results in abnormal differentiation, migration, and proliferation of germinal matrix cells.21,22 Two-thirds of the known TSC cases were due to spontaneous mutations, and an autosomal dominant trait was found in a small group of patients.
In our study, we had 5 patients with TS with hippocampal abnormalities (16.2%), including MTS (4/31, 12.9%), and 1 patient with HIMAL (1/31, 3.3%). Moreover, we found a correlation between febrile seizures with an onset in the first year of life and MTS in patients with TS. Notably, 2 patients with MTS showed this relationship, indicating that multiple predisposing factors may cause MTS. We argue that MTS may be a secondary outcome of a multifactorial process, such as structural abnormalities of TS and recurrent epileptic activity. The occurrence of febrile seizures, particularly during the first year of life, represents an aggravating factor that could potentially damage the HF.
There are some theoretic mechanisms to explain the dual pathology identified in our study. First, the hippocampal injury may result from repeated seizures during the course of the TS syndrome. It can promote secondary damage to the hippocampus and anatomic connections within the temporal lobe. This phenomenon is called kindling.17,23 The second possibility is related to a common pathogenetic mechanism during gestational development of the brain.24 CNS alterations in patients with TS result from abnormal differentiation/proliferation of germinal matrix cells and migrational arrest of dysgenetic neurons. It is, therefore, possible that the HF abnormalities are markers of a more widespread expression of this disturbance or susceptibility. The cerebral cortex and the HF develop from the neocortex and archicortex, respectively. A disturbance occurring early in gestation could explain the involvement of structures derived from different germinal matrices. This is consistent with the presence of cortical tubers.25 The association of mechanisms such as repeated stimuli in a vulnerable region may produce irreversible tissue damage, resulting in MTS. We argue that MTS is acquired and is not part of the inherited syndrome. MTS was observed in the older children in our series, and except for 1 patient (patient 17), none of our younger children presented with MTS.
It is possible that early febrile seizures damage the hippocampus and cause MTS.26
It is interesting that patients with MTS presented with more cortical tubers in our study. It is possible that these patients have more epileptogenic foci, thus causing secondary damage to the hippocampus. Although higher numbers of tubers were not observed in the temporal lobes of patients with MTS, hippocampal abnormality was present in the temporal lobes of patients with more tubers, except for patient 31, who had the same number of tubers in both lobes. This indicated an increased probability of epileptogenic activity in the temporal lobe ipsilateral to the damaged hippocampus; however, this was only observed in 1 patient (patient 18) by interictal EEG. As expected for patients with TS, the cortical tubers predominated in the frontal and parietal lobes, likely because of their larger size.27
Regarding the patient with HIMAL, a pathogenetic factor may be involved. Although this type of developmental abnormality is not a frequent cause of epilepsy, the demonstration of a multifocal EEG discharge could link it to a potentially epileptogenic focus.
Someone might argue that an anterior temporal cortical tuber may simulate a lesion in the hippocampus; however, the imaging pattern would be very different and the occurrence is low. The cortical tuber produces gyral expansion or distortion with subcortical signs of abnormality, while classic MTS presents with atrophy and hyperintensity on a T2-weighted image.28
Autistic behavior was noted in 2 patients from our series, 1 of them with MTS. The occurrence of autism in TSC has been described in the literature and likely results from generalized epilepsy in early life and functional deficits in the temporal neocortices.29 The 2 patients with autism presented with seizures before 1 year of age, which corroborates the hypothesis presented above. We did not, however, find other significant differences between these and the other patients with TS, including those with MTS. Moreover, 6 patients were identified with West syndrome. This association also occurs commonly in children with TSC and has a grave prognosis for cognitive and seizure outcomes.30 None of these patients had MTS, despite the frequent seizures. This finding supports the hypothesis that epileptic activity is not solely responsible for the development of MTS.
Finally, interictal EEG was not useful for predicting the association of MTS and TS or detecting HF abnormalities in patients with TS. A relationship between the EEG focus and MTS was found in only 1 patient, and it was not possible to assess whether this abnormality was related to the hippocampal lesion or to a cortical tuber in the temporal lobe. Modern neuroimaging techniques, therefore, play an important role in this context. Interictal 2-deoxy-2 fluorine-[18F]fluorodeoxyglucose PET with MR imaging (PET/MR imaging fusion imaging) and α-11C-labeled-methyl-L-tryptophan PET could identify the epileptogenic tuber, confirm the lateralization of the MTS, and predict an association between MTS and TSC, without invasive EEG or intraoperative electrocorticography.31–34
The results of our study in a large series of patients confirm the occurrence of MTS in TSC and support previously published findings.12 We failed to establish a clinical correlation showing seizures arising from the abnormal HF. This requires an appropriate clinical approach and needs further study. Nonetheless, we believe that use of an appropriate MR imaging protocol dedicated to the HF, including tilted coronal FLAIR and T2-weighted images of the temporal lobes in patients with TS, may help detect this abnormality. Our results also identified a minor relevance of the interictal EEG. The lack of a surgical correlation and intracranial electrocorticography limited our ability to interpret the involved mechanism of MTS development.
Conclusions
Analysis of this series of patients demonstrated the presence of HF abnormalities, consisting mainly of MTS in patients with TS. The occurrence of hippocampal abnormalities in patients with TS in our study is not commonly observed and could be considered an incidental finding; however, the dual pathology found here is likely a result of a multifactorial process. In this context, an association between TSC and febrile seizures during the first year of life appears to be one of the determining factors for MTS development. We believe that the search for additional abnormalities must continue, even after detection of a possible epileptogenic lesion by MR imaging, and especially in patients with TS.
Supplementary Material
Abbreviations
- A
atonic
- CNS
central nervous system
- CP
complex partial
- EEG
electroencephalography
- FLAIR
fluid-attenuated inversion recovery
- GTC
generalized tonic-clonic
- HF
hippocampal formation
- HIMAL
hippocampal malrotation
- LF
left frontal
- LO
left occipital
- LP
left parietal
- LT
left temporal
- MTC
magnetization transfer contrast
- MTS
mesial temporal sclerosis
- N
no
- PET
positron-emission tomography
- RF
right frontal
- RO
right occipital
- RP
right parietal
- RT
right temporal
- SE
spin-echo
- SP
simple partial
- TS
tuberous sclerosis
- TSC
tuberous sclerosis complex
- Y
yes
Footnotes
Indicates article with supplemental on-line table.
References
- 1. Roach ES, Smith M, Huttenlocher P, et al. Diagnostic criteria: tuberous sclerosis complex—Report of the Diagnostic Criteria Committee of the National Tuberous Sclerosis Association. J Child Neurol 1992;7:221–24 [DOI] [PubMed] [Google Scholar]
- 2. de Vries PJ, Prather PA. The tuberous sclerosis complex. N Engl J Med 2007;356:92, author reply 93–94 [DOI] [PubMed] [Google Scholar]
- 3. Curatolo P, Bombardieri R, Verdecchia M, et al. Intractable seizures in tuberous sclerosis complex: from molecular pathogenesis to the rationale for treatment. J Child Neurol 2005;20:318–25 [DOI] [PubMed] [Google Scholar]
- 4. Wiebe S, Blume WT, Girvin JP, et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001;345:311–18 [DOI] [PubMed] [Google Scholar]
- 5. Wyllie E, Comair YG, Kotagal P, et al. Seizure outcome after epilepsy surgery in children and adolescents. Ann Neurol 1998;44:740–48 [DOI] [PubMed] [Google Scholar]
- 6. Falconer MA. Mesial temporal (Ammon's horn) sclerosis as a common cause of epilepsy: aetiology, treatment, and prevention. Lancet 1974;2:767–70 [DOI] [PubMed] [Google Scholar]
- 7. Cendes F, Cook MJ, Watson C, et al. Frequency and characteristics of dual pathology in patients with lesional epilepsy. Neurology 1995;45:2058–64 [DOI] [PubMed] [Google Scholar]
- 8. Benbadis SR, Wallace J, Reed Murtagh F. MRI evidence of mesial temporal sclerosis in subjects without seizures. Seizure 2002;11:340–43 [DOI] [PubMed] [Google Scholar]
- 9. Moore KR, Swallow CE, Tsuruda JS. Incidental detection of hippocampal sclerosis on MR images: is it significant? AJNR Am J Neuroradiol 1999;20:1609–12 [PMC free article] [PubMed] [Google Scholar]
- 10. Baulac M, De Grissac N, Hasboun D, et al. Hippocampal developmental changes in patients with partial epilepsy: magnetic resonance imaging and clinical aspects. Ann Neurol 1998;44:223–33 [DOI] [PubMed] [Google Scholar]
- 11. Sato N, Hatakeyama S, Shimizu N, et al. MR evaluation of the hippocampus in patients with congenital malformations of the brain. AJNR Am J Neuroradiol 2001;22:389–93 [PMC free article] [PubMed] [Google Scholar]
- 12. Helbok R, Kuchukhidze G, Unterberger I, et al. Tuberous sclerosis complex with unilateral perisylvian polymicrogyria and contralateral hippocampal sclerosis: a case report. Seizure 2009;18:303–05 [DOI] [PubMed] [Google Scholar]
- 13. Roach ES, Gomez MR, Northrup H. Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol 1998;13:624–28 [DOI] [PubMed] [Google Scholar]
- 14. Pinto Gama HP, da Rocha AJ, Braga FT, et al. Comparative analysis of MR sequences to detect structural brain lesions in tuberous sclerosis. Pediatr Radiol 2006;36:119–25 [DOI] [PubMed] [Google Scholar]
- 15. Bote RP, Blazquez-Llorca L, Fernandez-Gil MA, et al. Hippocampal sclerosis: histopathology substrate and magnetic resonance imaging. Semin Ultrasound CT MR 2008;29:2–14 [DOI] [PubMed] [Google Scholar]
- 16. Barsi P, Kenez J, Solymosi D, et al. Hippocampal malrotation with normal corpus callosum: a new entity? Neuroradiology 2000;42:339–45 [DOI] [PubMed] [Google Scholar]
- 17. Raymond AA, Fish DR, Stevens JM, et al. Association of hippocampal sclerosis with cortical dysgenesis in patients with epilepsy. Neurology 1994;44:1841–45 [DOI] [PubMed] [Google Scholar]
- 18. Baron Y, Barkovich AJ. MR imaging of tuberous sclerosis in neonates and young infants. AJNR Am J Neuroradiol 1999;20:907–16 [PMC free article] [PubMed] [Google Scholar]
- 19. Christophe C, Sekhara T, Rypens F, et al. MRI spectrum of cortical malformations in tuberous sclerosis complex. Brain Dev 2000;22:487–93 [DOI] [PubMed] [Google Scholar]
- 20. Sisodiya S, Cross JH, Blumcke I, et al. Genetics of epilepsy: epilepsy research foundation workshop report. Epileptic Disord 2007;9:194–236 [DOI] [PubMed] [Google Scholar]
- 21. Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med 2006;355:1345–56 [DOI] [PubMed] [Google Scholar]
- 22. Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet 2008;372:657–68 [DOI] [PubMed] [Google Scholar]
- 23. Cavazos JE, Sutula TP. Progressive neuronal loss induced by kindling: a possible mechanism for mossy fiber synaptic reorganization and hippocampal sclerosis. Brain Res 1990;527:1–6 [DOI] [PubMed] [Google Scholar]
- 24. Kier EL, Kim JH, Fulbright RK, et al. Embryology of the human fetal hippocampus: MR imaging, anatomy, and histology. AJNR Am J Neuroradiol 1997;18:525–32 [PMC free article] [PubMed] [Google Scholar]
- 25. Ho SS, Kuzniecky RI, Gilliam F, et al. Temporal lobe developmental malformations and epilepsy: dual pathology and bilateral hippocampal abnormalities. Neurology 1998;50:748–54 [DOI] [PubMed] [Google Scholar]
- 26. Cendes F. Febrile seizures and mesial temporal sclerosis. Curr Opin Neurol 2004;17:161–64 [DOI] [PubMed] [Google Scholar]
- 27. Houser OW, Gomez MR. CT and MR imaging of intracranial tuberous sclerosis. J Dermatol 1992;19:904–08 [DOI] [PubMed] [Google Scholar]
- 28. Jackson GD, Berkovic SF, Duncan JS, et al. Optimizing the diagnosis of hippocampal sclerosis using MR imaging. AJNR Am J Neuroradiol 1993;14:753–62 [PMC free article] [PubMed] [Google Scholar]
- 29. Asano E, Chugani DC, Muzik O, et al. Autism in tuberous sclerosis complex is related to both cortical and subcortical dysfunction. Neurology 2001;57:1269–77 [DOI] [PubMed] [Google Scholar]
- 30. Husain AM, Foley CM, Legido A, et al. West syndrome in tuberous sclerosis complex. Pediatr Neurol 2000;23:233–35 [DOI] [PubMed] [Google Scholar]
- 31. Luat AF, Makki M, Chugani HT. Neuroimaging in tuberous sclerosis complex. Curr Opin Neurol 2007;20:142–50 [DOI] [PubMed] [Google Scholar]
- 32. Chugani DC, Chugani HT, Muzik O, et al. Imaging epileptogenic tubers in children with tuberous sclerosis complex using alpha-[11C]methyl-L-tryptophan positron emission tomography. Ann Neurol 1998;44:858–66 [DOI] [PubMed] [Google Scholar]
- 33. Kalantari BN, Salamon N. Neuroimaging of tuberous sclerosis: spectrum of pathologic findings and frontiers in imaging. AJR Am J Roentgenol 2008;190:W3040–49 [DOI] [PubMed] [Google Scholar]
- 34. Wu JY, Sutherling WW, Koh S, et al. Magnetic source imaging localizes epileptogenic zone in children with tuberous sclerosis complex. Neurology 2006;66:1270–72 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.