Abstract
BACKGROUND AND PURPOSE:
IRIS occurs in a small percentage of patients with AIDS following the initiation of HAART. Because PML lesions have a characteristic DWI/ADC appearance, our purpose was to determine if DWI/ADC measurements of PML lesions can be used to follow HAART treatment response and/or identify patients at risk for IRIS.
MATERIALS AND METHODS:
Six patients with AIDS and PML who had recently started HAART were retrospectively identified. On the basis of clinical history, patients were classified as having slow (non-IRIS) or rapid (IRIS) progression. Images were obtained at pre-HAART (time point 1) and post-HAART (time point 2). ADC parameters were measured and compared by using the 2-tailed t test.
RESULTS:
Seven lesions (4 rapidly progressing, 3 slowly progressing) were identified. Lesions from patients with rapid clinical progression had higher maximal ADC ratios at time point 1. There were also significant correlations between ADC parameters, time to clinical deterioration, and JCV titers.
CONCLUSIONS:
The ADC parameters of PML lesions were different for patients with rapid-versus-slow clinical progression. In our preliminary experience, ADC was helpful in diagnosing rapid clinical progression and IRIS. ADC values may correlate with the pathologic changes in PML lesions following HAART therapy.
PML is a demyelinating disease caused by the JCV in immunocompromised hosts. Approximately 5%–14% of patients with AIDS develop PML during the course of their disease,1 and PML is the cause of death in 0.7% of them.2 Untreated, the disease has a rapid and fatal course, with an average life expectancy of 4 months.3
The use of HAART in the treatment of PML has increased the 1-year survival rate by 10%–50%.4 However, even when HAART successfully restores immune function, 50% of patients with PML die.5 Approximately 6% of these patients show rapid clinical deterioration following HAART.6 This paradoxical deterioration in clinical status, termed IRIS, is thought to be secondary to the restoration of the immune system.7 The effect of IRIS on long-term survival is uncertain, with many but not all case reports suggesting that it results in short-term morbidity but heralds favorable long-term outcome.7 While clinical deterioration after HAART therapy is the sine qua non of IRIS, there are no unanimous diagnostic criteria.8 A number of clinical and imaging features have been associated with both IRIS and treatment response to HAART.9,10 Clinically, a low CD4 count and early initiation of treatment are thought to increase the risk for IRIS, though the specificity of these findings is limited.2 On imaging, lesion contrast enhancement has been associated with both IRIS and response to therapy,11 but because this enhancement is typically transient, its sensitivity as a marker for therapy response is limited. Therefore, there is a need to identify more accurate imaging characteristics of HAART treatment in PML, especially in regard to the diagnosis and development of IRIS.
DWI and ADC detect changes in diffusion of water in brain parenchyma. Several studies have reported ADC/DWI imaging changes in PML lesions. In particular, PML lesions demonstrate patchy restricted diffusion at their periphery correlating with areas of lesion expansion.12–15 High central ADC may reflect the evolution of PML lesions, indicating both how long they have existed and how likely they are to expand,16 and ADC/DWI changes are thought to correlate directly with the pathophysiology of the disease.13 Furthermore, initial studies of PML lesions following HAART therapy suggest that ADC/DWI may correlate with treatment outcomes.14
The purpose of our study was to determine whether ADC/DWI measurements of PML lesions before and after initiation of HAART can be used to follow treatment response, in particular, the development of IRIS.
Materials and Methods
All patients with AIDS seen at the New York Presbyterian Hospital between 2003 and 2005 were retrospectively reviewed, and 6 cases were found that fit the following criteria: 1) HIV positive, 2) diagnosis of PML established through JCV in CSF, 3) off HAART at the time of diagnosis or recently initiated HAART before the diagnosis of PML, and 4) T2WI and DWI with or without contrast performed before (time point 1) and initially after (time point 2) the commencement of HAART. The clinical history of all patients was reviewed for the first 60 days following the introduction of HAART. Patients who showed signs of significant clinical deterioration (new symptoms, worsening of initial symptoms related to PML requiring re-hospitalization, or death) within the first 60 days following initiation of HAART were classified as “rapidly progressive.” Patients who demonstrated no significant progression of symptoms in the first 60 days or who were admitted for reasons thought to be unrelated to their PML were deemed “slowly progressive.” All data were obtained in accordance with the Health Insurance Portability and Accountability Act, and the study was approved by the institutional review board.
MR imaging was performed on 1.5T systems by using T1 (TR/TE, 500/15 ms; matrix, 288 × 192; FOV, 22 × 22 cm) and T2WI (TR/TE, 4000/85 ms; matrix, 288 × 192; FOV, 22 × 22 cm) sequences in at least 2 planes. Axial DWI and ADC maps were acquired by using echo-planar sequences (TR/TE, 8000/85 ms; matrix, 128 × 128; FOV, 22 × 22 cm) at b-values of 0, 500, and 1000 s/mm2. Imaging was performed at 2 time points, separated by an average of 40 days (range, 11–91 days; the “scan interval”).
To facilitate comparison between images acquired at different time points, we used a spatial normalization algorithm to align the coordinates of the ADC and T2WI sequences at time points 1 and 2. After spatial normalization, the same coordinate corresponded to the same anatomic location for every image in the series, regardless of the time point or the image sequence (ADC, T2WI).
Image analysis was performed by using the MRICro system (http://www.sph.sc.edu/comd/rorden/mricro.html). Consistent with prior descriptions,11 PML lesions were defined as continuous asymmetric scalloped lesions in the white matter on axial T2WI with areas of restricted diffusion on DWI/ADC in patients with clinical, pathologic, and/or laboratory evidence for PML. Using MRICro, we drew individual regions of interest freehand on ADC images at the border of normal-appearing white matter determined by visual inspection by using a window centered at 130 × 10−5 s/mm2 with a width of 120 × 10−5 mm2/s in each axial image in which the lesion was present. The overall lesion region of interest was the composite of these individual axial regions of interest. The standard error in the freehand estimate of a region of interest was estimated at 10% on the basis of the differences between repeated measurements.
Quantitative data were obtained for both components of lesion progression, lesion expansion and lesion evolution. Lesion expansion was defined as the number of pixels within the PML lesion with region-of-interest methods as described above. Lesions with fewer than 80 pixels could not be reliably identified and were excluded from analysis. By definition, expansion was diagnosed when the lesion area at time point 2 was greater than the lesion area at time point 1, with the opposite being true for lesion regression. Lesion expansion was also correlated with the total area and average ADC values within regions of restricted diffusion at the periphery of the lesions. Regions of restricted diffusion were identified on DWIs and quantified by using regions of interest placed on corresponding ADC maps. Data analysis for lesion expansion took into account the interval in days between time points 1 and 2 (the scan interval) by measuring the rate of expansion as follows:
Lesion evolution was evaluated by comparing ADC parameters and their changes between time points 1 and 2. Three parameters were measured by using statistics obtained from regions of interest drawn around the entire lesion (average and maximal ADC) and, when present, a central area of ADC values > 160 × 10−5 mm2/s.
Data analysis for lesion evolution took into account variations in ADC of normal brain, known to vary ≤15% between patients.17 To control for variations in ADC of presumed normal brain tissue, we measured the ADC of CLWM by using an initial 3D region of interest in MRICro (difference from origin, 16; difference at edge, 16; radius of region of interest, 32) and then manually modified it to emulate the contour and position of the lesion. The average and maximal ADC ratios were then calculated as follows:
The third parameter—high central ADC area—was calculated as the percentage of pixels within the lesion with ADC ≥ 160 × 10−5 mm2/s. While patients with HIV can have a global decrease in white matter ADC due to diffuse leukoencephalopathy, which could cause the overestimation of measured ADC ratios, average contralateral ADC for the patients in this study was 80 × 10−5 mm2/s, similar to the average established by Sener18 for normal controls of 84 × 10−5 mm2/s.
ADC parameters for lesions associated with rapid and slow clinical progression were compared and were correlated with clinical and imaging variables, including rate of lesion growth, days to rehospitalization, presence of clinical deterioration, JCV titers when available, and immunologic status (CD4 count and viral load). In both cases, significance was determined by using a 2-sample 2-tailed t test with an assumption of unequal variances and a significance threshold of .05.
Results
Most patients meeting the selection criteria were new to the hospital, presenting with PML, with limited documentation of their HIV history. All patients were off HAART at the time of presentation. One patient had toxoplasmosis a year before admission, but there were no concurrent AIDS-defining illnesses at the time of presentation. The duration of illness was unknown in half of the patients and ranged from 1 month to 6 years in the others. Pertinent available clinical data are presented in Table 1.
Table 1:
Clinical and demographic characteristics of the 6 patients
| Patient No. | Age | Sex | Scan Interval (Days) | Clinical Progression |
|---|---|---|---|---|
| A | 40 | F | 60 | Yes, rapidly progressive dysphagia |
| B | 46 | M | 42 | Yes, altered mental status, psychosis |
| C | 35 | M | 34 | Yes, worsening altered mental status |
| D | 53 | M | 53 | No |
| E | 39 | M | 43 | No |
| F | 55 | M | 91 | No |
A total of 7 lesions were identified in 6 patients, 4 in the posterior fossa and 3 in the subcortical white matter of the frontal and parietal lobes.
Lesion Expansion Measurements
For patients with slow clinical progression (n = 3), a total of 3 separate lesions were identified. For patients with rapid clinical progression (n = 3), 4 lesions were identified. Rapid-progressing lesions expanded at an average rate of 168 ± 78 pixels/day, 9 times faster than slow-progressing lesions (20 ± 78 pixels/day). All lesions showed areas of restricted diffusion at their periphery, and none were associated with significant mass effect.
Lesion Evolution Measurements
ADC parameters for each lesion are presented in Table 2. Representative data is shown in Figures 1–3. There was a statistically significant difference between maximal ADC ratios for the rapid and slow clinical progression groups (P < .05; 95% CI, 1.9–2.6) but not for the average ADC ratio (P = .16; 95% CI, 1.4–1.7) or lesion size (P = .13; 95% CI, 1260–11 000). Maximal ADC ratios greater than 2.2 at time point 1 were unique to rapidly progressing lesions with a positive predictive value of 100% and a negative predictive value of 75%. Maximal ADC ratio was also correlated with lesion size (r = +0.83, P < .01).
Table 2:
Immunologic, virologic, time point 1 ADC parameters, and growth of PML lesions
| No. | Viral Load | CD4 Count | JCV Titer (Copies/mL) | Max ADC Ratio | Size (Pixels) | High Central ADC Area (%) | Rate of Growth (Pixels/Day) |
|---|---|---|---|---|---|---|---|
| A1 | 19 800 | 147 | + | 2.2 | 6535 | 4.5 | 78 |
| A2 | 19 800 | 147 | + | 1.8 | 1095 | 0 | 98 |
| B | 96 444 | 62 | 700 | 2.4 | 4094 | 3.2 | 403 |
| C | 101 026 | 3 | 10 100 | 2.7 | 12 797 | 1.6 | 94 |
| D | 1068 | 437 | 14 800 | 1.8 | 480 | 0 | 1.9 |
| E | 537 000 | 78 | + | 1.8 | 1442 | 0 | 7.3 |
| F | 713 | 30 | 1200 | 1.6 | 1341 | 0 | 51 |
Note:—+ indicates positive CSF JCV titer with quantitative titers not performed.
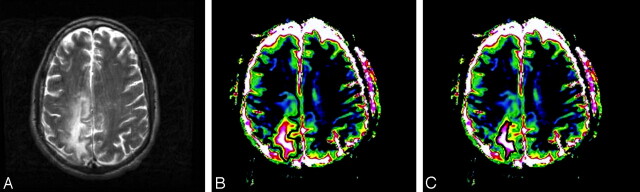
Fig 1.
MR imaging appearance of PML. T2 (A) and ADC maps with total (B) and central high (>160 × 10−5 mm2/s, indicated by pink and white in C) ADC values with corresponding regions of interest. The lesion demonstrates a typical pattern of high ADC in the center with decreasing values toward the periphery.
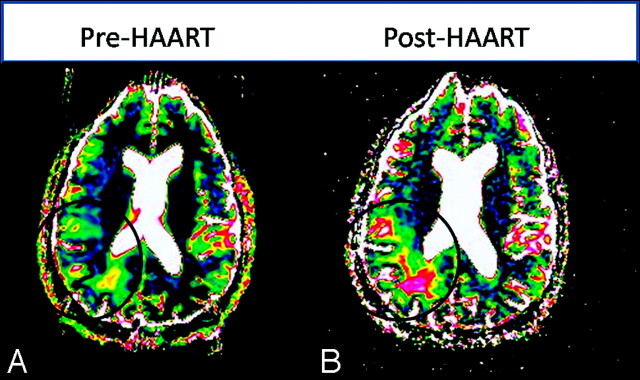
Fig 3.
Changing ADC/DWI measurements in patients with slow lesion progression. A, The initial lesion has no area of central high ADC (values >160 × 10−5 mm2/s, indicated by pink and white). B, One month after therapy, ADC values in the lesion center have increased but only slightly and to a lesser magnitude than lesions in patients with rapid progression.
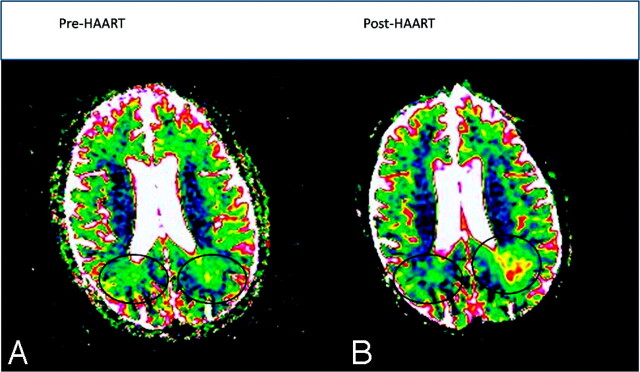
Fig 2.
Changing ADC/DWI measurements in patients with rapid lesion progression and IRIS. A, A small initial lesion has no area of central high ADC (values >160 × 10−5 mm2/s, indicated by pink and white). B, One month after therapy, areas of both the total lesion and central high ADC core have substantially increased.
There was no significant correlation between ADC parameters and immunologic status as measured by both CD4 and viral load. However, there was a significant correlation between JCV titers and high central ADC area at time point 1 (r = +0.96, P < .01).
Time to clinical deterioration was also correlated with both maximal ADC ratio (r = −0.89, P < .01) and high central ADC (r = −0.94, P < .01) at time point 1.
Discussion
ADC/DWI characteristics of PML lesions differ in patients with rapid and slow clinical progression following the initiation of HAART therapy, and these findings may aid in the diagnosis and evaluation of PML and IRIS after treatment.
Consistent with prior reports,12,13 foci of patchy restricted diffusion were present at the periphery of all PML lesions studied. This robust characteristic of PML is less common in other demyelinating disorders like multiple sclerosis, acute disseminated encephalomyelitis, and lymphoma and so may be helpful in differentiating PML from other white matter disorders. The finding does not appear to be helpful in predicting the development of IRIS because it was present in the lesions of patients with PML with both rapid and slow progression. In addition, the significance of this finding is less certain, with 1 study12 suggesting a correlation between the extent of peripheral diffusion abnormalities and clinical outcome and a larger study failing to find an association between areas of restricted diffusion and clinical performance.19
ADC may also help to diagnose IRIS and identify patients at risk. Lesions with higher maximum ADC ratios before initiation of HAART therapy were more likely to progress rapidly, with an ADC ratio of 2.2 having positive and negative predictive values of 100% and 75% for the development of rapid clinical progression. ADC parameters were also correlated with time to clinical progression and JCV titers. If confirmed by larger sample sizes, high ADC values within PML lesions before the initiation of HAART may be considered a risk factor for IRIS. The importance of high central ADC values in predicting clinical outcome is further supported by a recent study of 8 patients demonstrating a positive correlation between increased intralesional ADC and the duration and severity of disease.19
What is the significance of the ADC changes within PML lesions that occur with HAART therapy? The pathology of untreated PML lesions is well described20 and involves a circumferential margin of swollen oligodendrocytes with intranuclear inclusions surrounding a layer of active demyelination. As the disease progresses, demyelinated regions are removed by foamy macrophages; this process results in a disorganized central region with decreased cellularity and bizarre astrocytes without evidence of perivascular inflammation. Therefore, an elevated ADC at the center of slowly progressing lesions is associated with the loss of spatial organization and an increase in extracellular space, while reduced ADC at the lesion periphery is associated with cytotoxic edema and oligodendrocyte swelling in areas of active expansion.13 ADC changes, therefore, appear to correspond with the pathologic features of PML.
The pathologic correlates of ADC changes in PML lesions provide a basis for understanding the changes that may occur in lesion ADC after HAART both in the IRIS/rapid progression and non-IRIS/slow progression situations. High central ADC may predispose patients to IRIS because it indicates more extensive demyelination and more advanced initial disease. This is supported by a strong correlation between ADC values and JCV titers. Concerning the pathophysiology and diagnosis of IRIS, both rapid expansion and increased maximal ADC ratios within central portions of lesions could be due to increased extracellular fluid caused by vasogenic edema from the perivascular lymphocytic infiltrates typical of IRIS. It is also conceivable that immune reconstitution reactivates dormant viruses, accelerating the underlying disease process and resulting in greater demyelination, more advanced disease, and correspondingly higher central ADC. Limited available histopathologic and serial virologic correlation provides evidence for both mechanisms. In 1 screened patient who underwent a brain biopsy after rapid clinical progression following initiation of HAART (ultimately excluded from the study for a lack of initial MR imaging), elevated central intralesional ADC ratios post-HAART corresponded to areas of contrast enhancement and extensive perivascular lymphocytic infiltrates. In support of the second mechanism, increased ADC parameters in another rapidly progressing patient (number 3) correlated with an explosion in serial JCV titers from 700 to 322 000 copies/mL despite treatment with HAART, which increased the patient's CD4 count from 400 to 96 000. Hence, the increase in ADC parameters seen in IRIS may correlate with both immunologic and virologic mechanisms. Additional radiologic-pathologic correlation for PML lesions with both rapid and slow progression is needed to test these preliminary hypotheses.
Finally, ADC changes in the non-IRIS treatment response to PML may correspond to improved lesion stability and possibly remyelination. Although patients in this study failed to show significant improvement, HAART treatment may nevertheless slow progression of the disease to more extensive demyelination and correspondingly higher central ADC. Decreased central ADC seen in 1 patient with a favorable response to therapy14 could be seen with increased anisotropy and possibly indicates remyelination; however, confirmation of this hypothesis with measures of fractional anisotropy and brain biopsy has yet to be obtained. While further studies are needed, the ADC/DWI findings seen after HAART therapy in this study may reflect true pathologic changes that accompany both IRIS and non-IRIS treatment response.
The effect of PML-associated IRIS on overall mortality is controversial, with some studies suggesting rates of mortality similar to those of non-IRIS patients; the condition is often associated with temporary and sometimes significant morbidity.21 Recent work by Tan et al22 suggests that patients with PML, like those in the present study who develop IRIS after the initiation of HAART therapy (PML-d-IRIS), have earlier onset of symptoms, greater lesion loads, and shorter durations of survival compared with patients who develop IRIS and PML concurrently (PML-s-IRIS). Further studies comparing ADC changes in PML-d and PML-s lesions would be helpful to test the findings and hypothesis of the present study. Furthermore, there is some evidence that IRIS can be effectively treated with steroids.22,23 Because both prognosis and treatment may be altered by the presence of IRIS after HAART therapy, imaging tools that can identify patients at risk or detect early occurrence of the condition have important potential clinical benefits.
Our study has limitations. Its design is retrospective. While clinical deterioration after HAART therapy is the sine qua non of IRIS and there are no unanimous diagnostic criteria,8 correlation with contrast-enhanced MR imaging at time point 2 for patients with rapid progression would further support the diagnosis of IRIS over clinical progression as a part of the natural course of PML. A prospective design with routine contrast-enhanced MR imaging, while difficult given the rarity of the disease, would be helpful. ADC maps were computed by using only 3 b-values (0, 500, and 1000 s/mm2), though only small differences (0.84% in 1 study)24 existed between these and ADC values calculated by using 6 b-values. The use of DWI and scalars of anisotropic diffusion such as fractional anisotropy would give additional important insights in the type, progression, and extent of white matter injury. While standard methods were used for drawing regions of interest, they were drawn by a single observer not blinded to the results.
The small sample size significantly limits the power of the results to detect minor differences between rapid- and slow-progressing lesions. Two clinical limitations are the lack of patients with a favorable long-term response to HAART and potential difficulties assessing patient compliance with HAART, given the retrospective nature of the clinical information. Longer follow-up by using serial DWI of a larger number of patients with PML after the initiation of HAART will be helpful in testing and extending the observations and hypothesis made in this case series. For these reasons, we believe that our observations should be considered as preliminary only and will necessitate confirmation with a larger number of patients.
Conclusions
The ADC values of PML lesions were different for patients demonstrating rapid-versus-slow clinical progression after treatment with HAART. In our preliminary experience, ADC may be helpful in diagnosing IRIS. ADC values may correlate with the pathologic changes in PML lesions that follow HAART therapy.
Abbreviations
- ADC
apparent diffusion coefficient
- AIDS
acquired immunodeficiency syndrome
- CI
confidence interval
- CLWM
contralateral white matter
- DWI
diffusion-weighted imaging
- HAART
highly active antiretroviral therapy
- HIV
human immunodeficiency virus
- IRIS
immune reconstitution inflammatory syndrome
- JCV
JC virus
- Max
maximum
- PML
progressive multifocal leukoencephalopathy
- T2WI
T2-weighted imaging
Footnotes
Paper previously presented at: Annual Meeting of the American Society of Neuroradiology, May 31–June 5, 2008; New Orleans, Louisiana.
References
- 1. Antinori A, Cingolani A, Lorenzini P, et al. , for the Italian Registry Investigative Neuro AIDS Study Group. Clinical epidemiology and survival of progressive multifocal leukoencephalopathy in the era of highly active antiretroviral therapy: data from the Italian Registry Investigative Neuro AIDS (IRINA). J Neurovirol 2003; (9 suppl 1):47–53 [DOI] [PubMed] [Google Scholar]
- 2. Antinori A, Ammassari A, Giancola ML, et al. Epidemiology and prognosis of AIDS-associated progressive multifocal leukoencephalopathy in the HAART era. J Neurovirol 2001;7:323–28 [DOI] [PubMed] [Google Scholar]
- 3. Bergui M, Bradac GB, Oguz KK, et al. Progressive multifocal leukoencephalopathy: diffusion-weighted imaging and pathological correlations. Neuroradiology 2004;46:22–25 [DOI] [PubMed] [Google Scholar]
- 4. Clifford DB, Yiannoutsos C, Glicksman M, et al. HAART improves prognosis in HIV-associated progressive multifocal leukoencephalopathy. Neurology 1999;52:623–25 [DOI] [PubMed] [Google Scholar]
- 5. Du Pasquier RA, Koralnik IJ. Inflammatory reaction in progressive multifocal leukoencephalopathy: harmful or beneficial? J Neurovirol 2003; (9 suppl):125–31 [DOI] [PubMed] [Google Scholar]
- 6. Miralles P, Berenguer J, Lacruz C, et al. Inflammatory reactions in progressive multifocal leukoencephalopathy after highly active antiretroviral therapy. AIDS 2001;15:1900–02 [DOI] [PubMed] [Google Scholar]
- 7. Wyen C, Lehmann C, Fätkenheuer G, et al. AIDS-related progressive multifocal leukoencephalopathy in the era of HAART: report of two cases and review of the literature. AIDS Patient Care STDS 2005;19:486–94 [DOI] [PubMed] [Google Scholar]
- 8. Riedel DJ, Pardo CA, McArthur J, et al. Therapy insight: CNS manifestations of HIV-associated immune reconstitution inflammatory syndrome. Nat Clin Pract Neurol 2006;2:557–65 [DOI] [PubMed] [Google Scholar]
- 9. Thurnher MM, Post MJ, Rieger A, et al. Initial and follow-up MR imaging findings in AIDS-related progressive multifocal leukoencephalopathy treated with highly active antiretroviral therapy. AJNR Am J Neuroradiol 2001;22:977–84 [PMC free article] [PubMed] [Google Scholar]
- 10. Cinque P, Bossolasco S, Brambilla AM, et al. The effect of highly active antiretroviral therapy-induced immune reconstitution on development and outcome of progressive multifocal leukoencephalopathy: study of 43 cases with review of the literature. J Neurovirol 2003;(9 suppl 1):73–80 [DOI] [PubMed] [Google Scholar]
- 11. Post MJ, Yiannoutsos C, Simpson D, et al. Progressive multifocal leukoencephalopathy in AIDS: are there any MR findings useful to patient management and predictive of patient survival? AIDS Clinical Trials Group. AJNR Am J Neuroradiol 1999;20:1896–906 [PMC free article] [PubMed] [Google Scholar]
- 12. Küker W, Mader I, Nägele T, et al. Progressive multifocal leukoencephalopathy: value of diffusion-weighted and contrast-enhanced magnetic resonance imaging for diagnosis and treatment control. Eur J Neurol 2006;13:819–26 [DOI] [PubMed] [Google Scholar]
- 13. Mader I, Herrlinger U, Klose U, et al. Progressive multifocal leukoencephalopathy: analysis of lesion development with diffusion-weighted MRI. Neuroradiology 2003;45:717–21 [DOI] [PubMed] [Google Scholar]
- 14. Usiskin SI, Bainbridge A, Miller RF, et al. Progressive multifocal leukoencephalopathy: serial high-b-value diffusion-weighted MR imaging and apparent diffusion coefficient measurements to assess response to highly active antiretroviral therapy. AJNR Am J Neuroradiol 2007;28:285–86 [PMC free article] [PubMed] [Google Scholar]
- 15. Ohta K, Obara K, Sakauchi M, et al. Lesion extension detected by diffusion-weighted magnetic resonance imaging in progressive multifocal leukoencephalopathy. J Neurol 2001;248:809–11 [DOI] [PubMed] [Google Scholar]
- 16. Buckle CE, Chou D, Ogedegbe A. Progressive multifocal leukoencephalopathy: correlation with the apparent diffusion coefficient (ADC). Presented at: 24th International Congress of Radiology, Cape Town, South Africa. September 12–16, 2006 [Google Scholar]
- 17. Gaudinski MR, Henning EC, Miracle A, et al. Establishing final infarct volume: stroke lesion evolution past 30 days is insignificant. Stroke 2008;39:2765–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sener RN. Diffusion MRI: apparent diffusion coefficient (ADC) values in the normal brain and a classification of brain disorders based on ADC values. Comput Med Imaging Graph 2001;25:299–326 [DOI] [PubMed] [Google Scholar]
- 19. Cosottini M, Tavarelli C, Del Bono L, et al. Diffusion-weighted imaging in patients with progressive multifocal leukoencephalopathy. Eur Radiol 2008;18:1024–30 [DOI] [PubMed] [Google Scholar]
- 20. Aksamit AJ, Jr. Progressive multifocal leukoencephalopathy:a review of the pathology and pathogenesis. Microsc Res Tech 1995;32:302–11 [DOI] [PubMed] [Google Scholar]
- 21. Falcó V, Olmo M, del Saz SV, G, et al. Influence of HAART on the clinical course of HIV-1-infected patients with progressive multifocal leukoencephalopathy: results of an observational multicenter study. J Acquir Immune Defic Syndr 2008;49:26–31 [DOI] [PubMed] [Google Scholar]
- 22. Tan K, Roda R, Ostrow L, et al. PML-IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology 2009;72:1458–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berger JR. Steroids for PML-IRIS: a double-edged sword? Neurology 2009;72:1454–55 [DOI] [PubMed] [Google Scholar]
- 24. Burdette JH, Elster AD, Ricci PE. Calculation of apparent diffusion coefficients (ADCs) in brain using two-point and six-point methods. J Comput Assist Tomogr 1998;22:792–94 [DOI] [PubMed] [Google Scholar]